Abstract
Spasticity, contracture, and muscle weakness are commonly observed poststroke in muscles crossing the ankle. However, it is not clear how biomechanical properties of the Achilles tendon change poststroke, which may affect functions of the impaired muscles directly. Biomechanical properties of the Achilles tendon, including the length and cross-sectional area, in the impaired and unimpaired sides of 10 hemiparetic stroke survivors were evaluated using ultrasonography. Elongation of the Achilles tendon during controlled isometric ramp-and-hold and ramping up then down contractions was determined using a block-matching method. Biomechanical changes in stiffness, Young's modulus, and hysteresis of the Achilles tendon poststroke were investigated by comparing the impaired and unimpaired sides of the 10 patients. The impaired side showed increased tendon length (6%; P = 0.04), decreased stiffness (43%; P < 0.001), decreased Young's modulus (38%; P = 0.005), and increased mechanical hysteresis (1.9 times higher; P < 0.001) compared with the unimpaired side, suggesting Achilles tendon adaptations to muscle spasticity, contracture, and/or disuse poststroke. In vivo quantitative characterizations of the tendon biomechanical properties may help us better understand changes of the calf muscle-tendon unit as a whole and facilitate development of more effective treatments.
Keywords: stroke, mechanical property, ultrasound, muscle
the achilles tendon plays an important role in force transmission and energy storage and return during functional activities such as walking and running, (26) and it interacts with the calf muscle directly (32). Recent studies show that the metabolic activity in human tendon is remarkably high, which affords the tendon the ability to adapt to changing demands (3, 4, 15, 16). Stroke survivors often develop spasticity, contracture, and/or muscle weakness at the ankle, which contribute considerably to their disabilities poststroke (5, 12, 14, 34). About 34% of stroke survivors develop ankle contracture (27, 28, 34). The impaired plantar flexors and associated foot drop poststroke makes it hard for the foot to clear the ground during locomotion, and the lack of ankle plantar flexion power results in reduced forward propulsion (34, 35). Although a number of studies have been done on the changes of muscle fascicle mechanical properties poststroke (5, 8–10) (e.g., shortened muscle fascicles have been found in the gastrocnemius muscle poststroke), the associated changes of Achilles tendon mechanical properties and how the tendon interacts with the architectural/functional changes of the calf muscles in stroke survivors are not clear.
One study proposed that the increase of passive ankle stiffness was due to the increase of Achilles tendon stiffness, based on the assumption that Achilles tendon was more compliant than muscle (31). In another study, Achilles tendon stiffness poststroke was evaluated indirectly during eccentric-concentric muscle actions based on a muscle-tendon complex model (30). With the B-mode ultrasonography, the mechanical properties of human tendon can be evaluated in vivo and noninvasively (7). A number of ultrasonic studies have been carried out to investigate tendon properties and their changes associated with aging, disuse, exercise, and paralysis (1, 11, 13, 17, 23, 37). Tendon mechanical properties were commonly evaluated with the subject performing maximum voluntary contractions (MVC). The ultrasound images of the muscle-tendon junction (MTJ) before and after contraction were recorded and manually compared with measure the tendon elongation. However, because of muscle weakness and lack of control, the impaired ankle of stroke survivors can only generate a fraction of a healthy subject's MVC. The MTJ displacement in the impaired ankle is therefore considerably smaller and requires accurate and objective measurement.
The purpose of the present study was to investigate changes of the Achilles tendon biomechanical properties poststroke. An automatic block-matching method based on minimum sum of absolute differences (MSAD) algorithm (19) was used to continuously track the displacement of the soleus-Achilles MTJ. The hypotheses were that the impaired side had longer, thinner, and more compliant Achilles tendon with lower energy efficiency than the unimpaired side.
METHODS
Subjects.
Ten stroke survivors participated in the study (5 right side impaired and 5 left side impaired; both the left side- and right side-impaired groups contained 1 female subject and 4 male subjects). The inclusion criteria for the patients were having hemiparesis following a stroke at least 1 yr earlier, able to walk with a cane or without any mechanical aid, and able to generate plantar flexion torque using the calf muscles. Exclusion criteria were: having other severe disease, leg musculoskeletal injuries, and/or orthopedic surgeries on the leg. The patients had a mean age of 60.3 ± 8.4 (mean ± SD, range 52.8–78.8) yr, stroke duration of 12.0 ± 5.8 (range 4.5–20.8) yr, a mean height of 170.8 ± 10.2 (range 147.3–181.6) cm, body mass 80.6 ± 15.3 (range 59.9–99.9) kg. Their modified Ashworth scale was 1.6 ± 1.3 (range 0–3). Their passive range of motion was 11.0 ± 4.8 (range 5–19) ° in dorsiflexion, and 42.6 ± 4.5 (range 37–51) ° in plantar flexion, respectively. Three subjects wore an ankle foot orthosis, and one subject was using antispasticity medication. Three subjects used the straight cane and one subject used the quad cane. All participants gave informed consent to participate in this study, which was approved by the Institution Review Board.
Experimental setup.
The experimental setup consisted of four major parts: a custom knee-ankle joint-driving device, an ultrasound imaging system, an electromyography (EMG) system, and a personal computer.
A custom knee-ankle joint-driving device was used to position the leg and the ankle and to measure the joint torque. The motor at the knee was fixed rigidly to a frame anchored to the ground, and a leg linkage was mounted to the knee motor through a torque sensor. The ankle motor was mounted at the distal end of the leg linkage, and a footplate was mounted to the ankle motor through another torque sensor. The ankle motor and footplate could be adjusted along the leg linkage so that the ankle and knee motors were aligned with the ankle and knee flexion axes, respectively (Fig. 1).
Fig. 1.
A custom knee-ankle joint-driving device used for the study. This knee-ankle evaluation device consisted of 2 motors and a linkage in between. The torque sensors were mounted on the motor shaft at both joints to measure the ankle and knee joint torques. With the knee flexion axis aligned with the knee motor, the ankle motor can be adjusted along the leg linkage to align it with the ankle flexion axis.
The ankle plantar flexion torque was measured and displayed on the screen in real time as a visual feedback. Bagnoli EMG system (Delsys, Boston, MA) was used to monitor the EMG signal from the tibialis anterior muscle. A trigger signal was sent to the ultrasound imaging system to initiate the ultrasound image recording. The sampling rate for both torque and EMG data acquisition was 1,000 samples/s.
A commercial ultrasound imaging system, GE LOGIQ-9 with a 12-MHz high-resolution matrix probe M12L (GE Healthcare, Waukesha, WI), was used to obtain images of the Achilles tendon and monitor the movement of the soleus-Achilles MTJ.
Experimental design.
The experiment for every subject consisted of two visits. Each of the legs was tested in one of the two visits, following the same protocol. The sequence of the side being tested was selected randomly. Time between the two visits for each subject was within 1 wk. For the patients who were taking antispastic medications, the experiment was done at least 4 h after the medicine was taken.
The subject was seated upright with the thigh and trunk secured using Velcro straps. The leg and foot were securely attached to the leg linkage and footplate, respectively (Fig. 1), with the knee and ankle at full extension and 0° dorsiflexion, respectively. After several warm-up isometric contractions in plantar flexion, the subject gradually increased the plantar flexion torque until reaching a specified level and then held this for a period of time (ramp-and-hold), following the target and actual torques displayed in real time on the monitor. The ramping speed was set so that the subjects could carry out the task properly and the MTJ displacement could be tracked in the ultrasound images. The task was repeated three times with a 1-min rest in between. The subject then performed a ramp up and down (gradually increased the torque to a specified level and then gradually decreased it) isometric plantar flexion following the target torque for three times separated by a 1-min rest. The torque levels for both tasks were set (∼15 N·m) so that the subject could finish the task properly without losing control or inducing much fatigue during the isometric contractions. The ultrasound video data, ankle joint torque, and EMG signals were recorded throughout both tasks.
Measurement of Achilles tendon length.
The Achilles tendon insertion into the calcaneus notch, and the soleus-Achilles MTJ were located with LOGIQ-9 and marked at the corresponding positions on the skin surface. The soleus-Achilles MTJ was defined as the location where Achilles tendon (or so-called “free tendon”) (25) divides into soleus aponeurosis and gastrocnemius tendon. In an ultrasound image (Fig. 2, bottom), the MTJ was the intersection between the most distal muscle fascicles of the soleus muscle and the Achilles tendon. A line (defined as “line A”) was drawn along the Achilles tendon on the skin surface to connect these two points (Fig. 2, top). The probe was placed perpendicular to the skin and moved smoothly along line A, scanning in the sagittal plane with the subject relaxed. A special function of LOGIQ-9, called LOGIQView, was used to extend the field of view and register the images covering the full tendon length (Fig. 2, bottom). The scan was repeated 10 times to minimize the measurement error. ImageJ (National Institutes of Health, Bethesda, MD) was used to measure the distance from the soleus-Achilles MTJ to the calcaneus notch insertion along the tendon line of action (LOA) in the LOGIQView images.
Fig. 2.
Achilles tendon length and moment arm measurements. Top: a picture of a human foot and ankle. Line A was drawn along the Achilles tendon from the calcaneus to soleus-Achilles muscle-tendon junction (MTJ). Line B was drawn from the inferior tip of malleolus perpendicular to the tendon line of action. The intersection of line A and line B is also shown. Bottom: a LOGIQView image of Achilles tendon in sagittal plane. The image was acquired by scanning the probe along line A. The tendon length was defined as the distance from the MTJ to the calcaneus notch along the tendon line of action. The tendon moment arm was calculated by subtracting the perpendicular distance between the intersection and the tendon LOA (mt; in bottom) from the distance between the center of rotation and the intersection mg in sagittal plane.
Measurement of moment arm.
A simple method was developed to measure in vivo the Achilles tendon moment arm, defined as the perpendicular distance from the center of rotation (inferior tip of the malleolus) (33) to the tendon LOA. Another line (defined as “line B”) from the center of rotation along the direction perpendicular to the tendon LOA was drawn on the skin surface (Fig. 2, top). The intersection of line A and line B was marked (Fig. 2, top), and the total distance from the center of rotation to the intersection projected in the sagittal plane (mg) was measured. The corresponding position of the intersection can be found in the LOGIQView image shown in Fig. 2, bottom, by using a marker to produce shadow in the ultrasound image. Then the distance from the intersection to the tendon LOA (mt) was measured in the LOGIQView image (Fig. 2, bottom), and the Achilles tendon moment arm (mA) was obtained by subtracting mt from mg (Eq. 1):
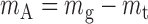 |
(1) |
Measurement of the cross-sectional area.
Three points dividing line A equally into five sections were marked on the skin surface. The transverse plane at each point was scanned three times using standard ultrasonography. Great care was taken to make the probe and the scanning plane perpendicular to the Achilles tendon. The direction of the transducer was adjusted gradually to get the minimum area, which corresponded to the cross-sectional area (CSA). A representative image of the Achilles tendon cross section is shown in Fig. 3. ImageJ was used to measure the CSA. The average value from the nine measurements was calculated as the Achilles tendon CSA.
Fig. 3.
An ultrasound image of Achilles tendon on transverse plane. The region inside the brighter boundary shows the cross section of Achilles tendon.
Displacement.
The ultrasound probe was securely attached to the lower leg with a customized probe holder with the soleus-Achilles MTJ in sagittal plane clearly visualized at rest. The probe holder was made of thermoplastic material and custom fit to the shape of the probe at one end and the shape of the posterior lower leg at the other end so that the probe can be mounted to the leg securely using Velcro straps. The properly strapped holder helped keep the probe perpendicular to the skin. When fixed properly, the probe would not move relative to the leg during the plantar flexion effort. The ultrasound video data were continuously recorded at 50 frames/s during the entire plantar flexion effort once LOGIQ-9 received the trigger signal from the personal computer.
The ultrasound video data were analyzed frame by frame to determine the soleus-Achilles MTJ displacement using the MSAD block-matching method, which was proposed to quantify two-dimensional velocities in human (2) and regional myocardial dysfunction in mice (19) and which has been validated using simulation and experimental data. Briefly, this method specifies a tracking pixel block from a previous image frame, and it moves the block around in the following frame to find the best match by comparing the sum of absolute differences. In the present study, a tracking pixel block size of ∼2 mm × 2 mm was used to search best match in a 6 mm × 6-mm surrounding region in the next frame. Parabolic interpolation was used to estimate subpixel displacements, and a three-tap median filter was used to smooth the estimated displacements between successive image frames. Four regions near soleus-Achilles MTJ were tracked in consecutive frames, and two representative ultrasound images without and with contraction were displayed together with the tracking result of one region in Fig. 4. The arrow points from the original position toward the displaced position based on MSAD block matching. Therefore, the average displacement of the four regions was regarded as the estimated displacement of the soleus-Achilles MTJ. The estimated MTJ displacement during a ramping up then down task was shown in Fig. 5, together with the corresponding ankle joint torque recorded.
Fig. 4.
Top: an ultrasound image of soleus-Achilles MTJ at rest. The zoomed block shows the region to be tracked during ramp-and-hold task. Bottom: an ultrasound image during the holding phase from the same task. The arrow is pointing from the original position to the tracked position after the tendon elongation took place. The zoomed blocks shows similar pattern as the block in the top.
Fig. 5.
The Achilles tendon force and MTJ displacement estimated during a ramping up-then down task. The force and displacement data displayed were decimated to 5 samples/s. From the trend of the curves, we can see how tendon force and MTJ displacement correlated.
Monitoring cocontraction and heel motion.
EMG signal from the tibialis anterior muscle was used to monitor the activities of dorsiflexors. The EMG signals recorded at rest were saved as the baseline. The trials with the EMG amplitude higher than the baseline mean amplitude plus three times baseline standard deviation (6) were rejected. A marker was placed on the heel and the motion of the marker was captured by a high-resolution (1,920 × 1,080 pixels) digital camcorder (model HDR-SR11, Sony). The camcorder was mounted on a tripod placed close to the marker on the heel. A ruler was placed at the same distance as the marker to show the scale of the picture. After each trial, the video was played back and trials with the heel motion >0.3 mm were discarded.
Calculation of tendon Young's modulus, stiffness, and mechanical hysteresis.
The MTJ displacement was determined as the Achilles tendon elongation. Achilles tendon strain was obtained by dividing the tendon elongation by the initial tendon length. Tendon force was calculated by dividing the ankle joint torque by the tendon moment arm. Tendon stress was acquired by dividing the tendon force by the average tendon CSA. With the stress-strain curve for the ramp-and-hold task determined, the slope of the curve at 3% strain was defined as the tendon Young's modulus. Similarly, the slope of the force-elongation curve at the displacement corresponding to 3% strain was defined as tendon stiffness. Force-displacement curves from the ramping up then down task were used to calculate the tendon mechanical hysteresis during loading and unloading, which was defined as the ratio of the area within the loop to the area below the loading curve (22). Representative force-displacement curves from both sides of the same subject are shown in Fig. 6.
Fig. 6.
Force-displacement curves collected from both sides of a subject in ramping up then down task. From the figure, we can conclude that 1) the tendon in the impaired side was more compliant than the unimpaired side and 2) the impaired side had higher mechanical hysteresis compared with the unimpaired side.
Statistics.
The various measurements were presented as means ± SD. Paired Student's t-test was used to test differences in the tendon dimension and biomechanical measures between the impaired and unimpaired sides. Statistical difference was set at a level of P < 0.05.
RESULTS
Achilles tendon length and CSA.
The Achilles tendon length measured in the impaired side (68.5 ± 13.3 mm) was significantly longer (P = 0.04) than in the unimpaired side (64.4 ± 11.4 mm; Fig. 7A). No significant difference (P = 0.19) was found between the Achilles tendon CSA measured in the impaired side (53.5 ± 11.2 mm2) and in the unimpaired side (56.1 ± 11.2 mm2; Fig. 7B).
Fig. 7.
Mean and standard deviation of Achilles tendon length (A), Achilles tendon cross-sectional area (CSA; B), Achilles tendon stiffness (C), Achilles tendon Young's modulus (D), and Achilles tendon mechanical hysteresis in the impaired (G1) and unimpaired (G2) sides, respectively (E).
Tendon young's modulus and stiffness.
The estimated Achilles tendon Young's modulus in the impaired side (136.4 ± 38.1 MPa) was significantly lower (P = 0.005) than that in the unimpaired side (220.2 ± 83.3 MPa; Fig. 7C). Similarly, the estimated Achilles tendon stiffness in the impaired side (105.0 ± 14.6 N/mm) was significantly lower (P < 0.001) than that of the unimpaired side (184.8 ± 48.4 N/mm; Fig. 7D).
Tendon mechanical hysteresis.
Three subjects were excluded in this test due to obvious cocontraction during the unloading (ramping down) task. From the remaining seven subjects, the tendon mechanical hysteresis calculated in the impaired side (19.6 ± 3.4%) was significantly higher (P < 0.001) than in the unimpaired side (6.8 ± 3.0%), as shown in Fig. 7E, indicating that the impaired limbs had lower energy efficiency than the unimpaired limbs.
DISCUSSION
In the present study, changes of Achilles tendon mechanical properties post stroke were evaluated in vivo and noninvasively using ultrasonography combined with biomechanical measurements. Results showed significant changes in the tendon dimension and biomechanical properties, including increased tendon length, lower tendon stiffness and Young's modulus, and higher mechanical hysteresis in the impaired side compared with the matched unimpaired side of the stroke survivors.
It was important to monitor agonist-antagonist cocontraction and heel motion to achieve accurate evaluations in the present study. With potential dorsiflexors cocontraction, the estimated Achilles tendon force could be lower than the actual force. The subjects in this study were selected from those who still could control their plantar flexors and generate certain levels of plantar flexion torque (∼15 N·m) in their impaired lower limbs, and the subjects learned to minimize cocontraction after practicing the task multiple times with EMG signals from dorsiflexors displayed as visual feedback. As a result, the subjects managed to avoid cocontraction during the ramp-and-hold task, and there was not obvious cocontraction during the loading or holding phase, especially when the task was set at comfortable levels for the subjects. However, considerable cocontraction still occurred during the unloading phase of the ramp-then-down task in some subjects, and three subjects were excluded for the mechanical hysteresis evaluation due to the reason. In addition, some subjects also tended to lift their heels during plantar flexion effort, which could cause overestimation of tendon displacement. Therefore, the calcaneus motion was monitored using a digital camcorder, and the subject's foot was tightly secured to the footplate. The subjects were also asked to perform the plantar effort tasks without lifting the heel. Moreover, some subjects tended to flex or extend their knees during the tasks. So the knee and the leg were mounted to the leg linkage tightly to minimize knee movement.
Results showed that tendon length was slightly longer (6%) in the impaired side compared with the unimpaired side. This was probably due to the fact that all the subjects were chronic stroke patients (average 12 yr after stroke) who had developed spasticity/contracture in the plantar flexor muscles for a long time. In related studies, shortening of muscle fascicles was found in the plantar flexors of chronic stroke patients (8, 9, 18). Roughly, the calf muscles and the Achilles tendon act like two springs in series, with the total length of the two springs determined by the leg length. With the calf muscles becoming stiffer and shorter poststroke, the middle point between the two springs (the soleus MTJ) may shift proximally toward the calf muscles and the Achilles tendon gets elongated poststroke (Fig. 8). In the process of muscle stiffening and shortening poststroke, it may apply stress onto the Achilles tendon, and creep may also be a mechanism involved in the potential Achilles tendon elongation process. Of note is that such a possible adaptation poststroke involves relatively low level of loading, which is much lower than the failure loads in previous failure testing of Achilles tendon (36). Although no significant difference was found in the tendon CSA between the impaired and unimpaired sides in the present study, the average CSA in impaired side was slightly smaller (5%) than that of the unimpaired side. The difference might have become significant over a larger sample.
Fig. 8.
Illustration of the changes of the calf muscles and Achilles tendon (simplified and modeled as 2 springs in series, with the spring width representing its stiffness value) poststroke. As the calf muscles become stiffer and shorter poststroke, the middle point between the two springs (the soleus-Achilles MTJ) may shift toward the calf muscles with the Achilles tendon elongated.
Decreased tendon Young's modulus (38%) and stiffness (43%) were found in the impaired side of the stroke survivors in this study. Reductions of patella tendon Young's modulus and stiffness were reported in spinal cord-injured subjects compared with able-bodied controls (23). Although the amount of reductions were greater than what were found in our study, our comparisons were done between the closely matched impaired and unimpaired sides of the same patients poststroke. In addition, the tendon Young's moduli calculated in the present study were lower than values reported in some other studies with muscles under MVC (21, 22, 24), which may be related to the submaximal contractions within the comfortable torque-generation range of the stroke survivors in this study, considering that the stress-strain relation has an overall nonlinear curve with lower slope in the lower stress (“toe region”) of the tendon stress-strain curve. In the present study, Young's moduli and stiffness were calculated at 3% strain for fair comparison, which were consistent with those in the corresponding region of the stress-strain curve reported in previous studies (23, 25, 29).
Increased mechanical hysteresis was found in the impaired side (19.6%) compared with the unimpaired side (6.8%). Both values are within the range (3–38%) that has been reported based on tensile testing of isolated specimens, and both values are also comparable to the value of gastrocnemius tendon (18%) reported in Ref. 22. The difference between different studies might be due to the different tendon segments and measurement approaches. Higher hysteresis values showed more energy was dissipated during the loading-unloading cycle, indicating lower energy efficiency in the impaired limbs.
The method in this study could only be used to evaluate patients with the ability of repeatedly performing controlled submaximal isometric plantar flexion tasks, with the subjects trained to perform the tasks following the torque target while avoiding cocontraction. For patients who cannot generate sufficient plantar flexion torque, electric stimulation may be an alternative method for tendon property evaluation (20).
Conclusion.
In the present study, Achilles tendon dimension and mechanical properties of impaired and unimpaired sides of 10 hemiparetic stroke survivors were evaluated. Increased tendon length, decreased stiffness and Young's modulus, and increased mechanical hysteresis were found in the impaired side. These changes may reflect adaptations to the changes of calf muscle fascicles poststroke. A better understanding of the adaptations and changes of the tendon biomechanical properties may help us gain insight into spasticity/contracture and motor impairment poststroke, and this may facilitate development of rehabilitation procedures.
GRANTS
The authors acknowledge the support of the National Institutes of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Arampatzis A, Karamanidis K, Morey-Klapsing G, De Monte G, Stafilidis S. Mechanical properties of the triceps surae tendon and aponeurosis in relation to intensity of sport activity. J Biomech 40: 1946–1952, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Bohs LN, Friemel BH, McDermott BA, Trahey GE. A real time system for quantifying and displaying two-dimensional velocities using ultrasound. Ultrasound Med Biol 19: 751–761, 1993. [DOI] [PubMed] [Google Scholar]
- 3.Bojsen-Moller J, Kalliokoski KK, Seppanen M, Kjaer M, Magnusson SP. Low-intensity tensile loading increases intratendinous glucose uptake in the Achilles tendon. J Appl Physiol 101: 196–201, 2006. [DOI] [PubMed] [Google Scholar]
- 4.Christensen B, Dyrberg E, Aagaard P, Enehjelm S, Krogsgaard M, Kjaer M, Langberg H. Effects of long-term immobilization and recovery on human triceps surae and collagen turnover in the Achilles tendon in patients with healing ankle fracture. J Appl Physiol 105: 420–426, 2008. [DOI] [PubMed] [Google Scholar]
- 5.Chung SG, Van Rey E, Bai Z, Roth EJ, Zhang LQ. Biomechanic changes in passive properties of hemiplegic ankles with spastic hypertonia. Arch Phys Med Rehabil 85: 1638–1646, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Di Fabio RP Reliability of computerized surface electromyography for determining the onset of muscle activity. Phys Ther 67: 43–48, 1987. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga T, Ito M, Ichinose Y, Kuno S, Kawakami Y, Fukashiro S. Tendinous movement of a human muscle during voluntary contractions determined by real-time ultrasonography. J Appl Physiol 81: 1430–1433, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Grant TH, Roth EJ, Zhang LQ. Changes in passive mechanical properties of the gastrocnemius muscle at the muscle fascicle and joint levels in stroke survivors. Arch Phys Med Rehabil. In press. [DOI] [PubMed]
- 9.Gao F, Zhang LQ. Altered contractile properties of the gastrocnemius muscle Post Stroke. J Appl Physiol 105: 1802–1808, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Given JD, Dewald JPA, Rymer WZ. Joint dependent passive stiffness in paretic and contralateral limbs of spastic patients with hemiparetic stroke. J Neurol Neurosurg Psychiatry 59: 271–279, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen P, Aagaard P, Kjaer M, Larsson B, Magnusson SP. Effect of habitual running on human Achilles tendon load-deformation properties and cross-sectional area. J Appl Physiol 95: 2375–2380, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Harlaar J, Becher JG, Snijders CJ, Lankhorst GJ. Passive stiffness characteristics of ankle plantar flexors in hemiplegia. Clin Biomech (Bristol, Avon) 15: 261–270, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Karamanidis K, Arampatzis A. Mechanical and morphological properties of human quadriceps femoris and triceps surae muscle-tendon unit in relation to aging and running. J Biomech 39: 406–417, 2006. [DOI] [PubMed] [Google Scholar]
- 14.Katz RT, Rymer WZ. Spastic hypertonia: mechanisms and measurement. Arch Phys Med Rehabil 70: 144–155, 1989. [PubMed] [Google Scholar]
- 15.Kjaer M, Langberg H, Miller BF, Boushel R, Crameri R, Koskinen S, Heinemeier K, Olesen JL, Dossing S, Hansen M, Pedersen SG, Rennie MJ, Magnusson P. Metabolic activity and collagen turnover in human tendon in response to physical activity. J Musculoskeletal Neuronal Interact 5: 41–52, 2005. [PubMed] [Google Scholar]
- 16.Kjaer M, Magnusson P, Krogsgaard M, Boysen Moller J, Olesen J, Heinemeier K, Hansen M, Haraldsson B, Koskinen S, Esmarck B, Langberg H. Extracellular matrix adaptation of tendon and skeletal muscle to exercise. J Anat 208: 445–450, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kubo K, Akima H, Kouzaki M, Ito M, Kawakami Y, Kanehisa H, Fukunaga T. Changes in the elastic properties of tendon structures following 20 days bed-rest in humans. Eur J Appl Physiol 83: 463–468, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Li L, Tong KY, Hu X. The effect of poststroke impairments on brachialis muscle architecture as measured by ultrasound. Arch Phys Med Rehabil 88: 243–250, 2007. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Garson CD, Xu Y, Beyers RJ, Epstein FH, French BA, Hossack JA. Quantification and MRI validation of regional contractile dysfunction in mice post myocardial infarction using high resolution ultrasound. Ultrasound Med Biol 33: 894–904, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maganaris CN, Paul JP. Hysteresis measurements in intact human tendon. J Biomech 33: 1723–1727, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Maganaris CN, Paul JP. In vivo human tendon mechanical properties. J Physiol 521: 307–313, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech 35: 1639–1646, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Maganaris CN, Reeves ND, Rittweger J, Sargeant AJ, Jones DA, Gerrits K, De Haan A. Adaptive response of human tendon to paralysis. Muscle Nerve 33: 85–92, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Magnusson SP, Aagaard P, Dyhre-Poulsen P, Kjaer M. Load-displacement properties of the human triceps surae aponeurosis in vivo. J Physiol 531: 277–288, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magnusson SP, Hansen P, Aagaard P, Brond J, Dyhre-Poulsen P, Bojsen-Moller J, Kjaer M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol Scand 177: 185–195, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Magnusson SP, Narici MV, Maganaris CN, Kjaer M. Human tendon behaviour and adaptation, in vivo. J Physiol 586: 71–81, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pfeffer MM, Reding MJ. Stroke rehabilitation. In: Principles of Neurologic Rehabilitation, edited by Lazar RB. New York: McGraw-Hill, 1998, p. 105–119.
- 28.Reding MJ, McDowell F. Stroke rehabilitation. Neurol Clin 5: 601–630, 1987. [PubMed] [Google Scholar]
- 29.Reeves ND, Maganaris CN, Narici MV. Effect of strength training on human patella tendon mechanical properties of older individuals. J Physiol 548: 971–981, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Svantesson U, Takahashi H, Carlsson U, Danielsson A, Sunnerhagen KS. Muscle and tendon stiffness in patients with upper motor neuron lesion following a stroke. Eur J Appl Physiol 82: 275–279, 2000. [DOI] [PubMed] [Google Scholar]
- 31.Thilmann AF, Fellows SJ, Ross HF. Biomechanical changes at the ankle joint after stroke. J Neurol Neurosurg Psychiatry 54: 134–139, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trestik CL, Lieber RL. Relationship between Achilles tendon mechanical properties and gastrocnemius muscle function. J Biomech Eng 115: 225–230, 1993. [DOI] [PubMed] [Google Scholar]
- 33.van den Bogert AJ, Smith GD, Nigg BM. In vivo determination of the anatomical axes of the ankle joint complex: an optimization approach. J Biomech 27: 1477–1488, 1994. [DOI] [PubMed] [Google Scholar]
- 34.Vattanasilp W, Ada L, Crosbie J. Contribution of thixotropy, spasticity, and contracture to ankle stiffness after stroke. J Neurology Neurosurg Psychiatry 69: 34–39, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Winter DA Biomechanics and Motor Control of Human Movement. New York: Wiley, 2004.
- 36.Wren TA, Lindsey DP, Beaupre GS, Carter DR. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng 31: 710–717, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Ying M, Yeung E, Li B, Li W, Lui M, Tsoi CW. Sonographic evaluation of the size of Achilles tendon: the effect of exercise and dominance of the ankle. Ultrasound Med Biol 29: 637–642, 2003. [DOI] [PubMed] [Google Scholar]










