Abstract
The respiratory and cerebrovascular reactivity to changes in arterial Pco2 (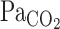 ) is an important mechanism that maintains CO2 or pH homeostasis in the brain. It remains unclear, however, how cerebrovascular CO2 reactivity might influence the respiratory chemoreflex. The purpose of the present study was therefore to examine the interaction between onset responses of the respiratory chemoreflex and middle cerebral artery (MCA) mean blood velocity (Vmean) to hypercapnia (5.0% CO2-40% O2-balance N2) at rest and during dynamic exercise (∼1.0 l/min O2 consumption). Each onset response was evaluated using a single-exponential regression model consisting of the response time latency [CO2-response delay (t0)] and time constant (τ). At rest, t0 and τ data indicated that the MCA Vmean onset response was faster than the ventilatory (V̇e) response (P < 0.001). In contrast, during exercise, t0 of V̇e and MCA Vmean onset responses were decreased. In addition, despite the enhanced
) is an important mechanism that maintains CO2 or pH homeostasis in the brain. It remains unclear, however, how cerebrovascular CO2 reactivity might influence the respiratory chemoreflex. The purpose of the present study was therefore to examine the interaction between onset responses of the respiratory chemoreflex and middle cerebral artery (MCA) mean blood velocity (Vmean) to hypercapnia (5.0% CO2-40% O2-balance N2) at rest and during dynamic exercise (∼1.0 l/min O2 consumption). Each onset response was evaluated using a single-exponential regression model consisting of the response time latency [CO2-response delay (t0)] and time constant (τ). At rest, t0 and τ data indicated that the MCA Vmean onset response was faster than the ventilatory (V̇e) response (P < 0.001). In contrast, during exercise, t0 of V̇e and MCA Vmean onset responses were decreased. In addition, despite the enhanced 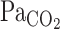 response to CO2 administration (P = 0.014), τ of MCA Vmean tended to increase during exercise (P = 0.054), whereas τ of V̇e decreased (P = 0.015). These findings indicate that 1) at rest, faster washout of CO2 via cerebral vasodilation results in a reduced activation of the central chemoreflex and subsequent reduced V̇e onset response, and 2) during exercise, despite higher rates of increasing
response to CO2 administration (P = 0.014), τ of MCA Vmean tended to increase during exercise (P = 0.054), whereas τ of V̇e decreased (P = 0.015). These findings indicate that 1) at rest, faster washout of CO2 via cerebral vasodilation results in a reduced activation of the central chemoreflex and subsequent reduced V̇e onset response, and 2) during exercise, despite higher rates of increasing 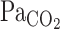 , the lack of change in the onset response of cerebral blood flow and reduced washout of CO2 may act to augment the V̇e onset response.
, the lack of change in the onset response of cerebral blood flow and reduced washout of CO2 may act to augment the V̇e onset response.
Keywords: chemoreflex, breathing, cerebral perfusion
the regulation of pH or CO2 is a vital homeostatic function, because numerous enzyme and ion channels that influence neural activity are modulated by changes in pH (5). In the brain, there are two strong CO2 regulatory mechanisms: the central respiratory chemoreflex and cerebrovascular CO2 reactivity. Respiration is tightly controlled by changes in arterial Pco2 (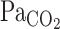 ) via the central chemoreflex (3) to maintain CO2 homeostasis. In addition, cerebral blood flow (CBF) and its distribution are highly sensitive to changes in
) via the central chemoreflex (3) to maintain CO2 homeostasis. In addition, cerebral blood flow (CBF) and its distribution are highly sensitive to changes in 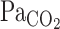 (16, 18, 26, 31). This CBF response, termed cerebrovascular CO2 reactivity, is a vital homeostatic function that helps regulate and maintain constant pH (5). Previous studies (19, 25), however, have only considered the steady-state changes in cerebrovascular CO2 reactivity and have not identified the potential dynamic nature of this response. Early reports (30) indicated that the CBF response started within 30 s of CO2 inhalation and that ∼2 min were required to reach peak values (9). Recent studies (23, 24), incorporating transcranial Doppler (TCD) and more sophisticated methods to manipulate end-tidal gases, have demonstrated that the CBF response to step changes in CO2 in humans was much faster (6-s delay). Because the response of
(16, 18, 26, 31). This CBF response, termed cerebrovascular CO2 reactivity, is a vital homeostatic function that helps regulate and maintain constant pH (5). Previous studies (19, 25), however, have only considered the steady-state changes in cerebrovascular CO2 reactivity and have not identified the potential dynamic nature of this response. Early reports (30) indicated that the CBF response started within 30 s of CO2 inhalation and that ∼2 min were required to reach peak values (9). Recent studies (23, 24), incorporating transcranial Doppler (TCD) and more sophisticated methods to manipulate end-tidal gases, have demonstrated that the CBF response to step changes in CO2 in humans was much faster (6-s delay). Because the response of 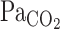 immediately after CO2 administration is rapidly increased (3), the onset (or dynamic) CBF response to changes in CO2 is likely to be an important mechanism in the maintenance of CO2 homeostasis in the brain.
immediately after CO2 administration is rapidly increased (3), the onset (or dynamic) CBF response to changes in CO2 is likely to be an important mechanism in the maintenance of CO2 homeostasis in the brain.
Cerebrovascular CO2 reactivity seems to influence the ventilatory response to CO2 at rest (1, 4, 6, 21, 33, 34) and during exercise (19). However, these previous findings were based on the CBF (or CBF velocity) response to a steady-state change in 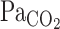 . Therefore, it remains unclear how the onset (or dynamic) CBF response to changes in CO2 might modulate dynamic ventilatory control via the respiratory chemoreflex, especially during exercise. The ventilatory-
. Therefore, it remains unclear how the onset (or dynamic) CBF response to changes in CO2 might modulate dynamic ventilatory control via the respiratory chemoreflex, especially during exercise. The ventilatory-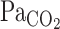 onset response is reported to be enhanced by exercise (17). In addition, exercise-induced hyperpnea may alter the ventilatory onset response and the subsequent interaction with CBF. Nevertheless, the dynamic response of CBF to changes in
onset response is reported to be enhanced by exercise (17). In addition, exercise-induced hyperpnea may alter the ventilatory onset response and the subsequent interaction with CBF. Nevertheless, the dynamic response of CBF to changes in 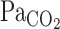 , especially during exercise, and the subsequent integration with the respiratory chemoreflex have not been characterized.
, especially during exercise, and the subsequent integration with the respiratory chemoreflex have not been characterized.
The purpose of this study was therefore to examine the onset responses of the respiratory chemoreflex and middle cerebral artery (MCA) mean blood velocity (Vmean) to hypercapnia at rest and during exercise. We examined the hypothesis that the onset response of CBF influences respiratory control via a central chemoreflex and exercise modifies the interaction between CBF and ventilatory onset responses.
METHODS
Seven healthy men were recruited to participate in the study as approved by the Human Subjects Committee of Morinomiya University of Medical Sciences (no. 001). The subjects were not performing endurance training on a regular basis. In addition, they were free of any known cardiovascular and pulmonary disorders and were not using any prescribed or over-the-counter medications. Before the experiment, each subject gave written informed consent and visited the laboratory for familiarization with the techniques and procedures. Subjects were requested to abstain from caffeinated beverages for 12 h and strenuous physical activity and alcohol for ≥24 h before the day of the experiment.
Measurements
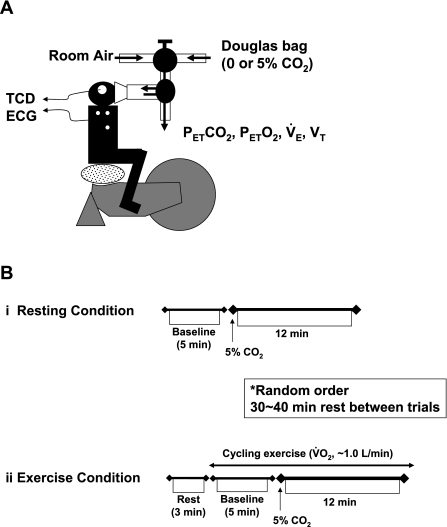
All studies were performed at room temperature (23–24°C) with minimal external stimuli. A diagram of the experimental setup is presented in Fig. 1A. Heart rate (HR) was monitored using a lead II electrocardiogram. The MCA blood velocity was obtained by TCD ultrasonography (WAKI, Atys Medical, St. Genislaval, France). A 2-MHz Doppler probe was placed over the temporal ultrasound window and fixed with an adjustable headband and adhesive ultrasonic gel (Tensive, Parker Laboratories, Orange, NJ). To gain an optimum Doppler signal, the position and angle of the TCD probe were first adjusted at the same depth (5 cm from the skin surface of the temple window) for all subjects; optimization of the gain and power intensity of the signal was then modified accordingly for each subject. Ventilatory responses were measured using an open-circuit apparatus. The subjects breathed through a face mask attached to a low-resistance one-way valve with a flowmeter. The valve mechanism allowed subjects to inspire room air or a selected gas mixture from a 200-liter Douglas bag containing 0.0 or 5.0% CO2-40% O2-balance N2. High O2 concentration in the inspiration gas avoids peripheral chemoreflex influence (10). The total instrumental dead space was 200 ml. Respiratory and metabolic data during the experiments were recorded by an automatic breath-by-breath gas-analyzing system consisting of a differential pressure transducer, sampling tube, filter, suction pump, and mass spectrometer (model ARCO2000-MET, Arcosystem, Chiba, Japan). We digitized expired flow and CO2 and O2 concentrations and derived tidal volume (Vt), minute ventilation (V̇e), and end-tidal Po2
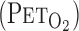 and Pco2
and Pco2
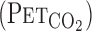 . We computed flow signals to single breath data and matched them to gas concentrations identified as single breaths using the peak
. We computed flow signals to single breath data and matched them to gas concentrations identified as single breaths using the peak 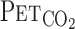 , after accounting for the time delay in gas concentration measurements. The corresponding O2 uptake (V̇o2) and CO2 output values for each breath were calculated from inspired-expired gas concentration differences and by expired ventilation (V̇e), with inspired ventilation being calculated by N2 correction. During each protocol, HR, V̇e,
, after accounting for the time delay in gas concentration measurements. The corresponding O2 uptake (V̇o2) and CO2 output values for each breath were calculated from inspired-expired gas concentration differences and by expired ventilation (V̇e), with inspired ventilation being calculated by N2 correction. During each protocol, HR, V̇e, 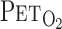 ,
, 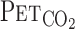 , and MCA blood velocity (V) were recorded continuously at 200 Hz.
, and MCA blood velocity (V) were recorded continuously at 200 Hz.
Fig. 1.
A: experimental setup. Each subject breathed through a face mask attached to a low-resistance 1-way valve with a built-in hot-wire flowmeter. Valve mechanism allowed subjects to inspire room air or a selected gas mixture from a 200-liter Douglas bag. TCD, transcranial Doppler; 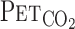 , end-tidal Pco2;
, end-tidal Pco2; 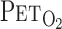 , end-tidal Po2; V̇e, minute ventilation; Vt, tidal volume. B: experimental protocol. CO2 was administered to each subject at rest (i) and during exercise (ii). Order of the rest and exercise trials was randomized for each subject. V̇o2, O2 uptake.
, end-tidal Po2; V̇e, minute ventilation; Vt, tidal volume. B: experimental protocol. CO2 was administered to each subject at rest (i) and during exercise (ii). Order of the rest and exercise trials was randomized for each subject. V̇o2, O2 uptake.
Experimental Protocol
Exercise capacity.
On day 1, the subjects performed maximal cycling exercise for the measurement of maximal V̇o2 (V̇o2max). V̇o2max was assessed with an incremental protocol on a cycle ergometer (model Corival1000SS, Lode, Groningen, Holland). The workload was set at 20 W and increased by 20 W every minute until the subjects could no longer maintain the pedaling frequency of ≥60 rpm, despite strong verbal encouragement. The subjects breathed through a face mask attached to a volume transducer, while gases were continuously sampled for analysis of fractional concentrations of O2, CO2, and N2. The respiratory gas analysis system was calibrated before each test using known standard gases.
CO2 administration protocol.
On the experimental day, the subjects arrived at the laboratory ≥2 h after a light meal. A diagram of the experimental protocol is presented in Fig. 1B. After instrumentation, the subjects sat on a comfortable chair. For characterization of dynamic CBF and respiratory responses to hypercapnia, the subjects breathed through a face mask and inspired a selected gas mixture from a 200-liter Douglas bag containing 5.0% CO2-40% O2-balance N2 [fraction of inspired CO2
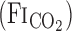 = 0.05] at rest and during cycling exercise (∼1.0 l/min V̇o2). Five minutes of baseline data were recorded during room air breathing from subjects wearing the face mask. After baseline recording, each hypercapnia trial was induced by a rapid change in the
= 0.05] at rest and during cycling exercise (∼1.0 l/min V̇o2). Five minutes of baseline data were recorded during room air breathing from subjects wearing the face mask. After baseline recording, each hypercapnia trial was induced by a rapid change in the 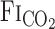 and lasted for 12 min, which is long enough to permit CO2 to reach its new steady-state value at the central chemoreceptors (22). During the interval between rest and exercise protocols, the subjects inspired room air. The order of the rest and exercise trials was randomized for each subject.
and lasted for 12 min, which is long enough to permit CO2 to reach its new steady-state value at the central chemoreceptors (22). During the interval between rest and exercise protocols, the subjects inspired room air. The order of the rest and exercise trials was randomized for each subject.
Data Analysis
From the 200-Hz sampling data, 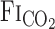 , Vt, V̇e,
, Vt, V̇e, 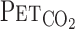 , and MCA Vmean were averaged at 0.2 Hz. Because
, and MCA Vmean were averaged at 0.2 Hz. Because 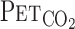 is known to not accurately reflect
is known to not accurately reflect 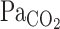 during conditions of exercise or hypercapnia (13),
during conditions of exercise or hypercapnia (13), 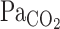 was calculated from
was calculated from 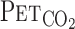 and Vt using the following equation
and Vt using the following equation
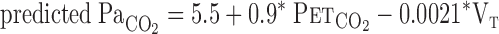 |
To address our hypothesis, onset responses of V̇e, predicted 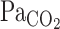 , and MCA Vmean were evaluated using a one-compartment nonlinear least-squares optimization method. The remaining data of onset responses of
, and MCA Vmean were evaluated using a one-compartment nonlinear least-squares optimization method. The remaining data of onset responses of 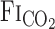 , V̇e, predicted
, V̇e, predicted 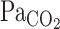 , and MCA Vmean were fitted to the following single-exponential regression equation consisting of the response time latency [CO2-response delay (t0)], baseline value, gain term (G), and time constant (τ) fitted to the CO2 administration protocol
, and MCA Vmean were fitted to the following single-exponential regression equation consisting of the response time latency [CO2-response delay (t0)], baseline value, gain term (G), and time constant (τ) fitted to the CO2 administration protocol
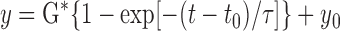 |
where y is response, t is time, and y0 is a baseline value. Time 0 was the beginning of CO2 administration.
Statistical Analysis
A paired t-test was used to assess the differences in G of MCA Vmean, V̇e, and predicted 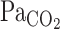 between rest and exercise. Hemodynamic values and t0, τ, and t0 + τ were compared using a two-way repeated-measures ANOVA, and a Student-Newman-Keuls test was employed post hoc to investigate main effects and interactions. Statistical significance was set at P < 0.05. Values are means ± SE, and analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL).
between rest and exercise. Hemodynamic values and t0, τ, and t0 + τ were compared using a two-way repeated-measures ANOVA, and a Student-Newman-Keuls test was employed post hoc to investigate main effects and interactions. Statistical significance was set at P < 0.05. Values are means ± SE, and analyses were conducted using SigmaStat (Jandel Scientific Software, SPSS, Chicago, IL).
RESULTS
Steady-State Responses to CO2 Administration: Rest and Exercise
Table 1 outlines the steady-state hemodynamic values at rest and during exercise before and after 5% CO2 administration. Averaged V̇o2 during the cycling exercise was 33 ± 6% of V̇o2max. This mild exercise increased HR, MCA Vmean, V̇e, Vt, 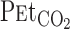 , and predicted
, and predicted 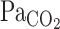 (Table 1). During 5% CO2 administration, as expected,
(Table 1). During 5% CO2 administration, as expected, 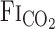 ,
, 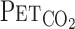 , and predicted
, and predicted 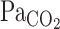 were elevated at rest and during exercise. Although hypercapnia elevated V̇e and MCA Vmean at rest and during exercise, these responses were much higher during exercise than at rest (P = 0.009 for V̇e, P = 0.010 for MCA Vmean).
were elevated at rest and during exercise. Although hypercapnia elevated V̇e and MCA Vmean at rest and during exercise, these responses were much higher during exercise than at rest (P = 0.009 for V̇e, P = 0.010 for MCA Vmean).
Table 1.
Ventilatory and hemodynamic variables before hypercapnia and after attainment of steady state at rest and during exercise
| Rest |
Exercise | P | |||||
|---|---|---|---|---|---|---|---|
| Pre | Steady state | Pre | Steady state | Pre vs. steady state | Condition | Interaction | |
| Fraction of inspired CO2, % | 0.07±0.01 | 5.32±0.01 | 0.05±0.01 | 5.37±0.08 | <0.001 | 0.604 | 0.356 |
| HR, beats/min | 70±6 | 73±6 | 97±8* | 112±9†‡ | 0.002 | <0.001 | 0.002 |
| MCA Vmean, cm/s | 51±4 | 64±4* | 60±4* | 83±4†‡ | <0.001 | <0.001 | 0.01 |
| Minute ventilation, l/min | 10.8±0.7 | 24.9±2.9* | 23.6±1.7* | 47.0±4.6†‡ | <0.001 | <0.001 | 0.009 |
| VT, ml | 910±118 | 1,512±196 | 1,357±103 | 2,100±113 | <0.001 | 0.006 | 0.445 |
| End-tidal PCO2, Torr | 37.7±1.8 | 50.2±1.0 | 44.5±2.2 | 59.7±3.2 | <0.001 | 0.003 | 0.183 |
| Predicted arterial PCO2, Torr | 37.4±1.4 | 47.5±0.8* | 42.9±2.0* | 54.7±2.7†‡ | <0.001 | 0.013 | 0.271 |
Values are means ± SE. HR, heart rate; MCA Vmean, middle cerebral artery mean blood velocity; VT, tidal volume.
P < 0.05 vs. Pre at rest.
P < 0.05 vs. steady state at rest.
P < 0.05 vs. Pre during exercise.
Onset Responses to CO2 Administration: Rest and Exercise
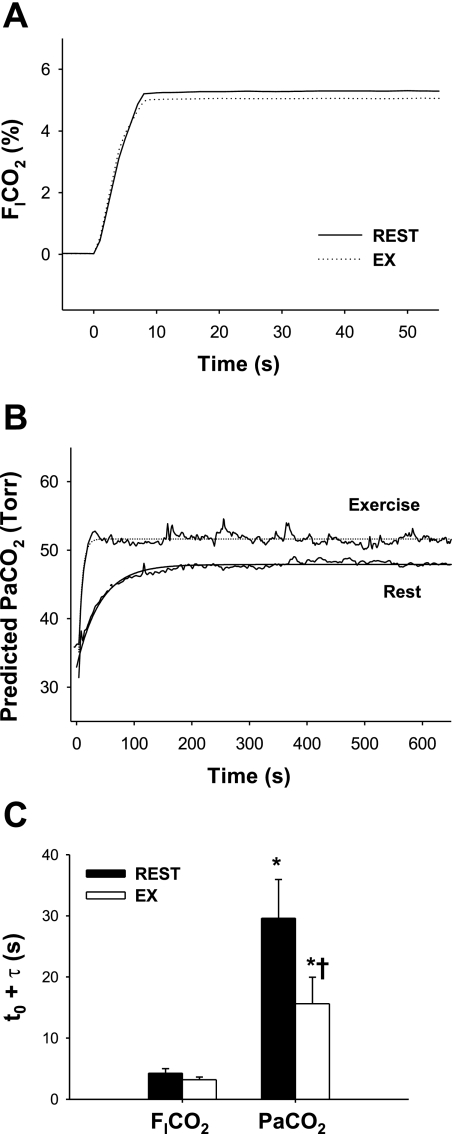
The onset response of 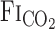 to CO2 administration was faster than that of other variables (i.e., MCA Vmean and V̇e), with no differences (P = 0.147) between rest and exercise (Fig. 2). Similarly, the response of predicted
to CO2 administration was faster than that of other variables (i.e., MCA Vmean and V̇e), with no differences (P = 0.147) between rest and exercise (Fig. 2). Similarly, the response of predicted 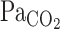 to these changes in
to these changes in 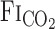 was faster than the responses of MCA Vmean and V̇e. At rest, the average of t0 and τ of
was faster than the responses of MCA Vmean and V̇e. At rest, the average of t0 and τ of 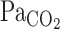 from the exponential fitting curve was 1.1 ± 0.83 and 22.2 ± 5.1 s, respectively. Exercise did not change t0 (1.4 ± 0.7 s, P = 0.703). In contrast, averaged τ was faster during exercise (11.9 ± 4.4 s vs. rest, P = 0.014).
from the exponential fitting curve was 1.1 ± 0.83 and 22.2 ± 5.1 s, respectively. Exercise did not change t0 (1.4 ± 0.7 s, P = 0.703). In contrast, averaged τ was faster during exercise (11.9 ± 4.4 s vs. rest, P = 0.014).
Fig. 2.
A and B: continuous recordings of fraction of inspired CO2 (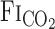 ) and predicted arterial Pco2 (
) and predicted arterial Pco2 (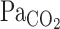 ) responses to hypercapnia (5% CO2) at rest and during exercise (Ex) in 1 representative subject. Hypercapnia was started at time 0. C: group-averaged sum of CO2-response delay (t0) and time constant (τ) of
) responses to hypercapnia (5% CO2) at rest and during exercise (Ex) in 1 representative subject. Hypercapnia was started at time 0. C: group-averaged sum of CO2-response delay (t0) and time constant (τ) of 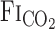 and predicted
and predicted 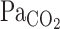 exponential fitting curves at rest and during exercise. Values are means ± SE. *P < 0.05 vs.
exponential fitting curves at rest and during exercise. Values are means ± SE. *P < 0.05 vs. 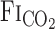 . †P < 0.05 vs. rest.
. †P < 0.05 vs. rest.
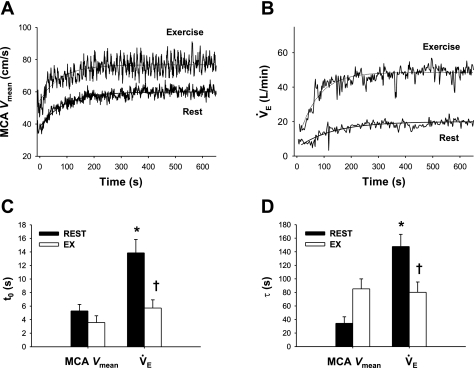
At rest, the average of t0 and τ of the MCA Vmean exponential fitting curve was 5.3 ± 1.0 and 34.3 ± 9.6 s, respectively (Fig. 3). These values were much faster than t0 (13.9 ± 2.0 s, P < 0.001) and τ (147.7 ± 18.0 s, P < 0.001) of V̇e. In addition, averaged τ of MCA Vmean was not different from that of 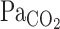 (P = 0.49). During exercise, t0 of MCA Vmean was unchanged from rest (−1.7 ± 1.0 s, P = 0.235), whereas t0 of V̇e was markedly reduced (−8.1 ± 1.6 s, P < 0.001). In addition, exercise increased τ of MCA Vmean from rest, although this did not quite reach statistical significance (+51 ± 18 s, P = 0.054). In contrast, exercise decreased τ of V̇e (−68 ± 28 s, P = 0.015). G of the exponential curves of the predicted
(P = 0.49). During exercise, t0 of MCA Vmean was unchanged from rest (−1.7 ± 1.0 s, P = 0.235), whereas t0 of V̇e was markedly reduced (−8.1 ± 1.6 s, P < 0.001). In addition, exercise increased τ of MCA Vmean from rest, although this did not quite reach statistical significance (+51 ± 18 s, P = 0.054). In contrast, exercise decreased τ of V̇e (−68 ± 28 s, P = 0.015). G of the exponential curves of the predicted 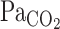 (P = 0.147) and MCA Vmean (P = 0.104) was unchanged during exercise, whereas G of V̇e was increased from 14.6 ± 2.2 to 25.8 ± 3.3 l/min (P = 0.008; Table 2).
(P = 0.147) and MCA Vmean (P = 0.104) was unchanged during exercise, whereas G of V̇e was increased from 14.6 ± 2.2 to 25.8 ± 3.3 l/min (P = 0.008; Table 2).
Fig. 3.
A and B: continuous recordings of middle cerebral artery (MCA) mean blood velocity (Vmean) and V̇e during hypercapnia at rest and during exercise in 1 representative subject. Hypercapnia was started at time 0. C and D: group-averaged t0 and τ of MCA Vmean and V̇e exponential fitting curves at rest and during exercise. Values are means ± SE. *P < 0.05 vs. MCA Vmean. †P < 0.05 vs. rest.
Table 2.
Gain of MCA Vmean, minute ventilation, and predicted arterial PCO2 exponential fitting curves at rest and during exercise
| Rest | Exercise | P | |
|---|---|---|---|
| MCA Vmean, cm/s | 16.3±2.0 | 19.0±2.0 | 0.104 |
| Minute ventilation, l/min | 14.6±2.2 | 25.8±3.3* | 0.008 |
| Predicted arterial PCO2, Torr | 10.2±1.0 | 11.5±1.3 | 0.147 |
Values are means ± SE.
P < 0.05 vs. rest.
DISCUSSION
At rest, during hypercapnia, the MCA Vmean onset response was much faster than the V̇e onset response. However, although the V̇e onset response was augmented, exercise did not enhance the MCA Vmean onset response, despite the high rate of increasing 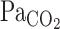 . These findings suggest that dynamic CBF regulation is tightly linked with respiratory control via a central chemoreflex.
. These findings suggest that dynamic CBF regulation is tightly linked with respiratory control via a central chemoreflex.
At rest, t0 and τ of the MCA Vmean exponential fitting curve were much smaller than those of V̇e (P < 0.001; Fig. 3). In addition, τ did not differ between 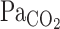 and MCA Vmean (22 ± 5 and 34 ± 10 s, respectively, P = 0.49). These findings indicate that the MCA Vmean onset response was much faster and more directly reflected changes in
and MCA Vmean (22 ± 5 and 34 ± 10 s, respectively, P = 0.49). These findings indicate that the MCA Vmean onset response was much faster and more directly reflected changes in 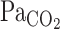 than the V̇e onset response via the central chemoreflex.
than the V̇e onset response via the central chemoreflex. 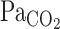 had increased to 51 ± 8% of the steady-state value after CO2 administration at t0 of V̇e (14 s) at rest. Thus the fast response of CBF is potentially an important mechanism that acts to cause the time delay in the V̇e response at the onset of hypercapnic stimulation.
had increased to 51 ± 8% of the steady-state value after CO2 administration at t0 of V̇e (14 s) at rest. Thus the fast response of CBF is potentially an important mechanism that acts to cause the time delay in the V̇e response at the onset of hypercapnic stimulation.
During exercise, the onset response of 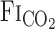 after hypercapnia was not different from that at rest (Fig. 2). Thus the differences in the
after hypercapnia was not different from that at rest (Fig. 2). Thus the differences in the 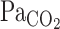 , MCA Vmean, and V̇e onset responses between rest and exercise were independent of changes in
, MCA Vmean, and V̇e onset responses between rest and exercise were independent of changes in 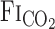 . During exercise, τ of the
. During exercise, τ of the 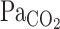 exponential fitting curve (12 s) was significantly decreased from rest (22 s, P = 0.014), indicating that the
exponential fitting curve (12 s) was significantly decreased from rest (22 s, P = 0.014), indicating that the 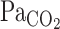 onset response was enhanced by exercise. This enhancement was attributed not only to the increased hemodynamics of exercise, but also to the possibility that, during exercise, the improved rapid changes in respiratory gas composition were transmitted to the arterial blood with greater fidelity than at rest (32). Cerebrovascular CO2 reactivity, especially to hypercapnia, is enhanced during steady-state exercise (19, 25). Although the onset responses of
onset response was enhanced by exercise. This enhancement was attributed not only to the increased hemodynamics of exercise, but also to the possibility that, during exercise, the improved rapid changes in respiratory gas composition were transmitted to the arterial blood with greater fidelity than at rest (32). Cerebrovascular CO2 reactivity, especially to hypercapnia, is enhanced during steady-state exercise (19, 25). Although the onset responses of 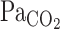 and cerebrovascular CO2 reactivity were augmented, the onset response of MCA Vmean was not enhanced during exercise. The τ of the MCA Vmean exponential fitting curve tended to increase (P = 0.054), rather than decrease, during exercise. However, τ includes information of not only response speed, but also G, which is the difference in steady-state value between pre- and post-CO2 administration. For example, a rise in G increases τ, despite no change in the response speed. Thus, increased τ of the MCA Vmean exponential fitting curve could be partly due to an increased G induced by an enhanced cerebral CO2 reactivity, rather than a reduction in the response speed. Interestingly, exercise-induced enhancement of cerebral CO2 reactivity (steady-state) (19, 25) did not correlate with the onset response of MCA Vmean (dynamic) to hypercapnia.
and cerebrovascular CO2 reactivity were augmented, the onset response of MCA Vmean was not enhanced during exercise. The τ of the MCA Vmean exponential fitting curve tended to increase (P = 0.054), rather than decrease, during exercise. However, τ includes information of not only response speed, but also G, which is the difference in steady-state value between pre- and post-CO2 administration. For example, a rise in G increases τ, despite no change in the response speed. Thus, increased τ of the MCA Vmean exponential fitting curve could be partly due to an increased G induced by an enhanced cerebral CO2 reactivity, rather than a reduction in the response speed. Interestingly, exercise-induced enhancement of cerebral CO2 reactivity (steady-state) (19, 25) did not correlate with the onset response of MCA Vmean (dynamic) to hypercapnia.
In contrast to rest, exercise markedly decreased the response time delay of V̇e (t0; −8.1 s, P < 0.001). In addition, exercise decreased τ of the V̇e exponential fitting curve (−68 s, P = 0.015). These time delays are comparable to those reported in the previous studies (3, 17). Bellville et al. (3) assumed that the transport delay time to the central chemoreflex was estimated as a model parameter by curve-fitted analysis and reported that this value at rest was 13.7 ± 3.4 s. In addition, MacFarlane and Cunningham (17) reported that central chemoreflex delay during exercise was 9.9 ± 1.7 s. The shortened central chemoreflex time delay during exercise seems to be associated with the faster onset response of 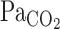 (Fig. 2), potentially caused by the exercise-induced fast transmission of CO2 from the lungs to the arterial blood (32). Moreover, G of the exponential curves of V̇e was increased from 14.6 ± 2.2 to 25.8 ± 3.3 l/min (P = 0.008; Table 2). Together, these findings highlight the marked enhancement of the V̇e onset response to hypercapnia during exercise.
(Fig. 2), potentially caused by the exercise-induced fast transmission of CO2 from the lungs to the arterial blood (32). Moreover, G of the exponential curves of V̇e was increased from 14.6 ± 2.2 to 25.8 ± 3.3 l/min (P = 0.008; Table 2). Together, these findings highlight the marked enhancement of the V̇e onset response to hypercapnia during exercise.
There have been previous conflicting reports of an augmentation of the central chemoreflex during exercise. Poon and Greene (22) showed that the slope of the V̇e-Pco2 relationship was increased during exercise. In contrast, Asmussen and Nielsen (2) demonstrated that the V̇e-Pco2 relationship line shifted to the left without a change in its sensitivity (or gain). The findings of the present study indicate that the V̇e-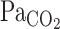 relationship was unchanged from rest to exercise (1.5 ± 0.3 vs. 2.3 ± 0.6 l·min−1·Torr−1). Thus, because of the lack of augmentation of the central chemoreflex during exercise, it seems unlikely that this mechanism might act to enhance the V̇e onset response.
relationship was unchanged from rest to exercise (1.5 ± 0.3 vs. 2.3 ± 0.6 l·min−1·Torr−1). Thus, because of the lack of augmentation of the central chemoreflex during exercise, it seems unlikely that this mechanism might act to enhance the V̇e onset response.
From the results of the present study, enhancement of the V̇e onset response during exercise seems to be related to unchanged CBF onset at this time point, despite high rates of increasing 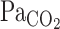 . For example, the faster elevations in
. For example, the faster elevations in 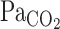 will activate the central chemoreflex to a greater extent during exercise than at rest because of the lack of “buffering” of brain tissue Pco2 via cerebral vasodilation and subsequent elevations in CBF. This notion is broadly consistent with previous investigations (1, 4, 6, 21, 33, 34), which indicate that cerebral CO2 reactivity is linked to the ventilatory response to CO2. Peebles et al. (21) reported that hypercapnic cerebral CO2 reactivity was inversely related to the increase in V̇e change. In other words, a reduced cerebral CO2 reactivity results in less central CO2 washout and a greater V̇e stimulus. This finding is consistent with the onset data of the present study.
will activate the central chemoreflex to a greater extent during exercise than at rest because of the lack of “buffering” of brain tissue Pco2 via cerebral vasodilation and subsequent elevations in CBF. This notion is broadly consistent with previous investigations (1, 4, 6, 21, 33, 34), which indicate that cerebral CO2 reactivity is linked to the ventilatory response to CO2. Peebles et al. (21) reported that hypercapnic cerebral CO2 reactivity was inversely related to the increase in V̇e change. In other words, a reduced cerebral CO2 reactivity results in less central CO2 washout and a greater V̇e stimulus. This finding is consistent with the onset data of the present study.
Because the starting time point of the attenuation of the elevation in 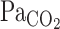 is around t0 of V̇e (Figs. 2 and 3), it seems possible that the increase in V̇e attenuated the elevations in
is around t0 of V̇e (Figs. 2 and 3), it seems possible that the increase in V̇e attenuated the elevations in 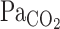 . The onset responses of MCA Vmean followed
. The onset responses of MCA Vmean followed 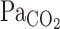 ; therefore, the increase in V̇e also might cause a marked attenuation of increasing MCA Vmean at rest as well as during exercise. Since hyperventilation constrains subsequent elevations in
; therefore, the increase in V̇e also might cause a marked attenuation of increasing MCA Vmean at rest as well as during exercise. Since hyperventilation constrains subsequent elevations in 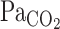 , the ventilatory chemoreflex seems to indirectly regulate MCA Vmean at rest and during exercise. Collectively, the onset responses of
, the ventilatory chemoreflex seems to indirectly regulate MCA Vmean at rest and during exercise. Collectively, the onset responses of 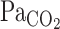 , MCA Vmean, and V̇e clearly indicate that CBF regulation is linked to respiratory control via the central chemoreflex.
, MCA Vmean, and V̇e clearly indicate that CBF regulation is linked to respiratory control via the central chemoreflex.
Technical Considerations
The changes in 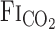 after CO2 administration are related to the dead space of respiratory equipment, the sampling and response time of gas analyzers, and the time in which the change in
after CO2 administration are related to the dead space of respiratory equipment, the sampling and response time of gas analyzers, and the time in which the change in 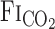 is reflected at the sampling site (i.e., the mouth), which is, in part, determined by pulmonary ventilation. Thus exercise-induced elevation of V̇e would be expected to increase the
is reflected at the sampling site (i.e., the mouth), which is, in part, determined by pulmonary ventilation. Thus exercise-induced elevation of V̇e would be expected to increase the 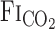 response time. However, because of the specially designed fast gas delivery system used in the present study, there was no difference in the
response time. However, because of the specially designed fast gas delivery system used in the present study, there was no difference in the 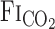 response between rest and exercise (Fig. 2). In contrast to the response time of
response between rest and exercise (Fig. 2). In contrast to the response time of 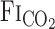 , the response time of
, the response time of 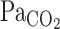 differed between rest and exercise.
differed between rest and exercise. 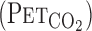 overestimates
overestimates 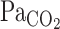 when metabolic CO2 production and V̇e are increased during exercise (13). In addition, cerebrovascular CO2 reactivity is underestimated by
when metabolic CO2 production and V̇e are increased during exercise (13). In addition, cerebrovascular CO2 reactivity is underestimated by 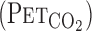 compared with
compared with 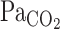 (21). Thus, to better reflect
(21). Thus, to better reflect 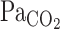 (i.e., the stimulus for ventilatory and CBF alterations), we corrected our
(i.e., the stimulus for ventilatory and CBF alterations), we corrected our 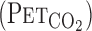 data to
data to 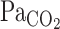 on the basis of established equations (13). CO2 is an important controlled variable or mediator, but it does not directly influence the central chemoreflex. For example, CO2 diffuses freely to the cerebrospinal fluid and influences H+ concentration, which drives ventilation via central chemoreceptors (19, 28, 29). Indexes of pH regulation were not measured in the present study; however, it is unfortunately impossible in humans to assess the dynamic regulation of cerebrospinal fluid H+ concentration. Another potential limitation of MCA V estimation using TCD ultrasonography is that changes in the diameter of the isonated vessels could modulate MCA V independently of flow. However, the MCA diameter appears to remain relatively constant in humans during moderate variations in blood pressure and CO2 (11), the physiological stimuli of moderate lower body negative pressure, or changes in
on the basis of established equations (13). CO2 is an important controlled variable or mediator, but it does not directly influence the central chemoreflex. For example, CO2 diffuses freely to the cerebrospinal fluid and influences H+ concentration, which drives ventilation via central chemoreceptors (19, 28, 29). Indexes of pH regulation were not measured in the present study; however, it is unfortunately impossible in humans to assess the dynamic regulation of cerebrospinal fluid H+ concentration. Another potential limitation of MCA V estimation using TCD ultrasonography is that changes in the diameter of the isonated vessels could modulate MCA V independently of flow. However, the MCA diameter appears to remain relatively constant in humans during moderate variations in blood pressure and CO2 (11), the physiological stimuli of moderate lower body negative pressure, or changes in 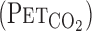 (27). Poulin et al. (23) reported that there is little change in total power of the Doppler signal with hypercapnic stimuli, indicating that MCA diameter changes little. However, this index has never been validated against a direct method, and the use of reflected power has been dismissed as a reliable indicator of vascular size changes (7). Nevertheless, the changes in MCA Vmean during submaximal dynamic exercise appear to be similar to the changes in CBF determined by other techniques, i.e., internal carotid artery blood flow (12) and 133Xe clearance (14, 15). Importantly, since determinations of cerebrovascular CO2 reactivity are based on stimulus-response principles, absolute CBF values are not as important as reliable and repeatable recordings with short (beat-to-beat) time resolution. For this purpose, TCD is a well-suited (and, indeed, the only) technique for this type of experimental research. Finally, when CBF increases during exercise or with hypercapnia (or both), the changes in cerebral vessel diameter occur downstream of the MCA. For example, the variable resistance to flow is encountered mostly in the cerebral arteriolar and capillary bed (8). In contrast, the large cerebral arteries (e.g., MCA) and veins are dedicated to blood distribution and the storage of blood volume and are, in principle, noncompliant and act merely as a conduit for the pulsatile arterial flow from the aorta to the brain (20). Taken together, cerebral vasoconstriction and vasodilatation occur via changes in the caliber of the smaller cerebral vessels, whereas MCA diameter likely remains unchanged.
(27). Poulin et al. (23) reported that there is little change in total power of the Doppler signal with hypercapnic stimuli, indicating that MCA diameter changes little. However, this index has never been validated against a direct method, and the use of reflected power has been dismissed as a reliable indicator of vascular size changes (7). Nevertheless, the changes in MCA Vmean during submaximal dynamic exercise appear to be similar to the changes in CBF determined by other techniques, i.e., internal carotid artery blood flow (12) and 133Xe clearance (14, 15). Importantly, since determinations of cerebrovascular CO2 reactivity are based on stimulus-response principles, absolute CBF values are not as important as reliable and repeatable recordings with short (beat-to-beat) time resolution. For this purpose, TCD is a well-suited (and, indeed, the only) technique for this type of experimental research. Finally, when CBF increases during exercise or with hypercapnia (or both), the changes in cerebral vessel diameter occur downstream of the MCA. For example, the variable resistance to flow is encountered mostly in the cerebral arteriolar and capillary bed (8). In contrast, the large cerebral arteries (e.g., MCA) and veins are dedicated to blood distribution and the storage of blood volume and are, in principle, noncompliant and act merely as a conduit for the pulsatile arterial flow from the aorta to the brain (20). Taken together, cerebral vasoconstriction and vasodilatation occur via changes in the caliber of the smaller cerebral vessels, whereas MCA diameter likely remains unchanged.
The onset response of MCA Vmean to hypercapnia was faster than that of respiratory chemoreflex. Dynamic exercise did not enhance the MCA Vmean onset response to hypercapnia, despite the greater elevation of 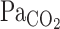 and subsequent elevation of the ventilatory onset response. These findings indicate that the CBF onset response influences respiratory control via a central chemoreflex and exercise modifies the interaction between CBF and ventilatory onset responses. Clinically, a range of pathological conditions alter the normal chemoreflex control of breathing (e.g., chronic lung disease, heart failure, and sleep apnea) and, therefore, may alter dynamic CBF regulation. In other words, in these patient groups, any alterations in the interaction between respiratory control and CBF regulation may be associated with adverse cerebral events and/or may increase the likelihood of abnormal breathing. Further studies, however, are needed to identify the extent to which dynamic CBF regulation may be associated with respiratory dysfunction in these pathological states. Application of our described model to integration of the onset and steady-state responses of ventilation and CBF provides a new tool for such investigations.
and subsequent elevation of the ventilatory onset response. These findings indicate that the CBF onset response influences respiratory control via a central chemoreflex and exercise modifies the interaction between CBF and ventilatory onset responses. Clinically, a range of pathological conditions alter the normal chemoreflex control of breathing (e.g., chronic lung disease, heart failure, and sleep apnea) and, therefore, may alter dynamic CBF regulation. In other words, in these patient groups, any alterations in the interaction between respiratory control and CBF regulation may be associated with adverse cerebral events and/or may increase the likelihood of abnormal breathing. Further studies, however, are needed to identify the extent to which dynamic CBF regulation may be associated with respiratory dysfunction in these pathological states. Application of our described model to integration of the onset and steady-state responses of ventilation and CBF provides a new tool for such investigations.
GRANTS
This study was supported in part by Japanese Ministry of Education, Culture, Sports, Science, and Technology Grant-in-Aid for Scientific Research 19500574, a grant from the Descente and Ishimoto Memorial Foundation for the Promotion of Sports Science, and a grant from the Kouzuki Foundation for Sports and Education.
Acknowledgments
The authors appreciate the time and effort of the volunteer subjects.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ainslie PN, Murrell C, Peebles K, Swart M, Skinner MA, Williams MJ, Taylor RD. Early morning impairment in cerebral autoregulation and cerebrovascular CO2 reactivity in healthy humans: relation to endothelial function. Exp Physiol 92: 769–777, 2007. [DOI] [PubMed] [Google Scholar]
- 2.Asmussen E, Nielsen M. Ventilatory response to CO2 during work at normal and at low oxygen tensions. Acta Physiol Scand 39: 27–35, 1957. [DOI] [PubMed] [Google Scholar]
- 3.Bellville JW, Whipp BJ, Kaufman RD, Swanson GD, Aqleh KA, Wiberg DM. Central and peripheral chemoreflex loop gain in normal and carotid body-resected subjects. J Appl Physiol 46: 843–853, 1979. [DOI] [PubMed] [Google Scholar]
- 4.Chapman RW, Santiago TV, Edelman NH. Effects of graded reduction of brain blood flow on chemical control of breathing. J Appl Physiol 47: 1289–1294, 1979. [DOI] [PubMed] [Google Scholar]
- 5.Chesler M Regulation and modulation of pH in the brain. Physiol Rev 83: 1183–1221, 2003. [DOI] [PubMed] [Google Scholar]
- 6.Dempsey JA Crossing the apnoeic threshold: causes and consequences. Exp Physiol 90: 13–24, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Deverson S, Evans DH. The effects of beam shape on the ability to predict changes in vessel size from Doppler signal power. Ultrasound Med Biol 26: 245–253, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Edvinsson L, Krause DN. Cerebral Blood Flow and Metabolism. Philadelphia: Lippincott, Williams & Wilkins, 2002.
-
9.Ellingsen I, Hauge A, Nicolaysen G, Thoresen M, Walloe L. Changes in human cerebral blood flow due to step changes in
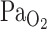 and
and 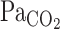 . Acta Physiol Scand
129: 157–163, 1987. [DOI] [PubMed] [Google Scholar]
. Acta Physiol Scand
129: 157–163, 1987. [DOI] [PubMed] [Google Scholar] - 10.Fitzgerald RS, Parks DC. Effect of hypoxia on carotid chemoreceptor response to carbon dioxide in cats. Respir Physiol 12: 218–229, 1971. [DOI] [PubMed] [Google Scholar]
- 11.Giller CA, Bowman G, Dyer H, Mootz L, Krippner W. Cerebral arterial diameters during changes in blood pressure and carbon dioxide during craniotomy. Neurosurgery 32: 737–741, 1993. [PubMed] [Google Scholar]
- 12.Hellstrom G, Fischer-Colbrie W, Wahlgren NG, Jogestrand T. Carotid artery blood flow and middle cerebral artery blood flow velocity during physical exercise. J Appl Physiol 81: 413–418, 1996. [DOI] [PubMed] [Google Scholar]
- 13.Jones NL, Robertson DG, Kane JW. Difference between end-tidal and arterial Pco2 in exercise. J Appl Physiol 47: 954–960, 1979. [DOI] [PubMed] [Google Scholar]
- 14.Jorgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physiol 73: 1825–1830, 1992. [DOI] [PubMed] [Google Scholar]
- 15.Jorgensen LG, Perko M, Hanel B, Schroeder TV, Secher NH. Middle cerebral artery flow velocity and blood flow during exercise and muscle ischemia in humans. J Appl Physiol 72: 1123–1132, 1992. [DOI] [PubMed] [Google Scholar]
- 16.Kety SS, Schmidt CF. The effects of altered arterial tensions of carbon dioxide and oxygen on cerebral blood flow and cerebral oxygen consumption of normal young men. J Clin Invest 27: 484–492, 1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacFarlane DJ, Cunningham DJ. Dynamics of the ventilatory response in man to step changes of end-tidal carbon dioxide and of hypoxia during exercise. J Physiol 457: 539–557, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markwalder TM, Grolimund P, Seiler RW, Roth F, Aaslid R. Dependency of blood flow velocity in the middle cerebral artery on end-tidal carbon dioxide partial pressure—a transcranial ultrasound Doppler study. J Cereb Blood Flow Metab 4: 368–372, 1984. [DOI] [PubMed] [Google Scholar]
- 19.Ogoh S, Hayashi N, Inagaki M, Ainslie P, Miyamoto T. Interaction between the ventilatory and cerebrovascular responses to hypo- and hypercapnia at rest and during exercise. J Physiol 586: 4327–4338, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olufsen MS, Nadim A, Lipsitz LA. Dynamics of cerebral blood flow regulation explained using a lumped parameter model. Am J Physiol Regul Integr Comp Physiol 282: R611–R622, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Peebles K, Celi L, McGrattan K, Murrell C, Thomas K, Ainslie PN. Human cerebrovascular and ventilatory CO2 reactivity to end-tidal, arterial and internal jugular vein Pco2. J Physiol 584: 347–357, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poon CS, Greene JG. Control of exercise hyperpnea during hypercapnia in humans. J Appl Physiol 59: 792–797, 1985. [DOI] [PubMed] [Google Scholar]
- 23.Poulin MJ, Liang PJ, Robbins PA. Dynamics of the cerebral blood flow response to step changes in end-tidal Pco2 and Po2 in humans. J Appl Physiol 81: 1084–1095, 1996. [DOI] [PubMed] [Google Scholar]
- 24.Poulin MJ, Liang PJ, Robbins PA. Fast and slow components of cerebral blood flow response to step decreases in end-tidal Pco2 in humans. J Appl Physiol 85: 388–397, 1998. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen P, Stie H, Nielsen B, Nybo L. Enhanced cerebral CO2 reactivity during strenuous exercise in man. Eur J Appl Physiol 96: 299–304, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Reivich M Arterial Pco2 and cerebral hemodynamics. Am J Physiol 206: 25–35, 1964. [DOI] [PubMed] [Google Scholar]
- 27.Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke 31: 1672–1678, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Severinghaus JW, Carcelen A. Cerebrospinal fluid in man native to high altitude. J Appl Physiol 19: 319–321, 1964. [DOI] [PubMed] [Google Scholar]
- 29.Severinghaus JW, Mitchell RA, Richardson BW, Singer MM. Respiratory control at high altitude suggesting active transport regulation of CSF pH. J Appl Physiol 18: 1155–1166, 1963. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro E, Wasserman AJ, Patterson JL Jr. Human cerebrovascular response time to elevation of arterial carbon dioxide tension. Arch Neurol 13: 130–138, 1965. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro W, Wasserman AJ, Patterson JL Jr. Mechanism and pattern of human cerebrovascular regulation after rapid changes in blood CO2 tension. J Clin Invest 45: 913–922, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ward DS, Bellville JW. Effect of intravenous dopamine on hypercapnic ventilatory response in humans. J Appl Physiol 55: 1418–1425, 1983. [DOI] [PubMed] [Google Scholar]
- 33.Xie A, Skatrud JB, Khayat R, Dempsey JA, Morgan B, Russell D. Cerebrovascular response to carbon dioxide in patients with congestive heart failure. Am J Respir Crit Care Med 172: 371–378, 2005. [DOI] [PubMed] [Google Scholar]
- 34.Xie A, Skatrud JB, Morgan B, Chenuel B, Khayat R, Reichmuth K, Lin J, Dempsey JA. Influence of cerebrovascular function on the hypercapnic ventilatory response in healthy humans. J Physiol 577: 319–329, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]