Abstract
Reissner's membrane epithelium forms much of the barrier that produces and sustains the large ionic differences between cochlear endolymph and perilymph. We have reported that Reissner's membrane contributes to normal cochlear function by absorbing Na+ from endolymph via amiloride-sensitive channels in gerbil inner ear. We used mouse Reissner's membrane to 1) identify candidate genes involved in the Na+ transport pathway, 2) determine whether their level of expression was regulated by the synthetic glucocorticoid dexamethasone, and 3) obtain functional evidence for the physiological importance of these genes. Transcripts were present for α-, β-, and γ-subunits of epithelial Na+ channel (ENaC); corticosteroid receptors GR (glucocorticoid receptor) and MR (mineralocorticoid receptor); GR agonist regulator 11β-hydroxysteroid dehydrogenase (HSD) type 1 (11β-HSD1); Na+ transport control components SGK1, Nedd4-2, and WNKs; and K+ channels and Na+-K+-ATPase. Expression of the MR agonist regulator 11β-HSD2 was not detected. Dexamethasone upregulated transcripts for α- and β-subunits of ENaC (∼6- and ∼3-fold), KCNK1 (∼3-fold), 11β-HSD1 (∼2-fold), SGK1 (∼2-fold), and WNK4 (∼3-fold). Transepithelial currents from the apical to the basolateral side of Reissner's membrane were sensitive to amiloride (IC50 ∼0.7 μM) and benzamil (IC50 ∼0.1 μM), but not EIPA (IC50 ∼34 μM); amiloride-blocked transepithelial current was not immediately changed by forskolin/IBMX. Currents were reduced by ouabain, lowered bath Na+ concentration (from 150 to 120 mM), and K+ channel blockers (XE-991, Ba2+, and acidification from pH 7.4 to 6.5). Dexamethasone-stimulated current and gene expression were reduced by mifepristone, but not spironolactone. These molecular, pharmacological, and functional observations are consistent with Na+ absorption by mouse Reissner's membrane, which is mediated by apical ENaC and/or other amiloride-sensitive channels, basolateral Na+-K+-ATPase, and K+-permeable channels and is under the control of glucocorticoids. These results provide an understanding and a molecular definition of an important transport function of Reissner's membrane epithelium in the homeostasis of cochlear endolymph.
Keywords: inner ear, corticosteroids, endolymph homeostasis, gene expression, vibrating probe
the volume of the luminal compartment of the cochlea is thought to be controlled by net solute fluxes, and alterations in luminal volume have been associated with genetic and toxicological hearing disorders. The most important osmolytes in cochlear fluids and their luminal/abluminal concentrations are as follows (in mM): 157/4 K+, 1/148 Na+, 31/21 HCO3−, and 132/119 Cl− (57). The large differences in K+, Ca2+, and Na+ between luminal and abluminal fluids provide the ionic milieu needed to sustain transduction of sound into nerve impulses necessary for normal hearing.
Transepithelial transport systems for K+, Ca2+, and HCO3− secretion and for Na+ and Ca2+ absorption have been identified in the cochlea (57). Net transepithelial movement of solutes provides a driving force for an accompanying diffusive movement of water, and the cochlear epithelium is known to be highly permeable to water (51). Reduced activity of the Na+ absorption mechanisms could produce an endolymphatic hydrops, whereas stimulation in Na+-loaded, hydropic cochleae could provide a corrective function.
Two Na+ absorptive epithelial cell types have been identified in the cochlea: outer sulcus and Reissner's membrane epithelia (30, 33). The former is mediated by constitutively open and purinergically activated nonselective cation channels and the latter by amiloride-sensitive channels in the apical membrane. In both types of cells, transepithelial transport occurs when endolymphatic Na+ diffuses into the cell through the apical ion channels and is subsequently removed across the basolateral membrane by the Na+ pump (Na+-K+-ATPase). K+ brought into the cell by the pump recycles into the abluminal fluid through basolateral K+-permeable channels. The molecular identity of these transporters in Reissner's membrane is not known, although the apical entry pathway is thought to be mediated by the epithelial Na+ channel (ENaC) (30), which can occur in several isoform stoichiometries (34, 35).
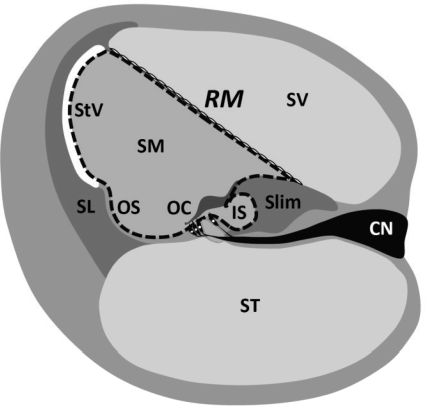
Na+ absorption mediated by amiloride-sensitive ENaCs is known in other epithelial cells to be under the control of mineralocorticoids or glucocorticoids (e.g., renal and vestibular epithelia) (27). Glucocorticoid-regulated Na+ transport was recently demonstrated in rat semicircular canal epithelium of the vestibular labyrinth, where expression levels of the transport and regulatory genes were controlled by dexamethasone and other glucocorticoids (41, 42). Although the nonselective cation channel system of the cochlear outer sulcus is also capable of absorbing Na+, this relatively small epithelial domain is thought to primarily provide a parasensory shunt for K+ efflux (28). Among the cochlear epithelial cells, Reissner's membrane comprises most of the luminal surface (Fig. 1) and is thought to be primarily responsible for endolymphatic Na+ homeostasis.
Fig. 1.
Cochlear cross section. Reissner's membrane (RM) epithelium forms the border between endolymphatic space [scala media (SM), luminal compartment] and perilymphatic space [scala vestibuli (SV), abluminal compartment]. Scala media is bounded by ∼12 epithelial cell types; apical surface is indicated by dashed line. Endolymph of scala media is high in K+ (∼160 mM) and low in Na+ (∼1 mM); abluminal perilymph is low in K+ (∼4 mM) and high in Na+ (∼150 mM). Stria vascularis (StV) generates a lumen-positive voltage of about +80 to +100 mV with respect to perilymph. Reissner's membrane is composed of 2 cell layers: a discontinuous layer of mesothelial cells lies beneath the epithelial cells and faces the scala vestibuli. OC, organ of Corti; SL, spiral ligament; Slim, spiral limbus; CN, cochlear nerve; OS, outer sulcus; IS, inner sulcus.
The present study was conducted to 1) determine the molecular identities of membrane transporters and key regulatory genes in Reissner's membrane, 2) determine whether their level of expression was regulated by corticosteroids by microarray and quantitative real-time RT-PCR (qRT-PCR) analysis, and 3) obtain functional evidence for the physiological importance of these genes by electrophysiological and pharmacological means.
METHODS
Tissue samples.
For each qRT-PCR experiment, 4–10 C57BL/6 mice (4–8 wk old) were anesthetized with 4% tribromoethanol (0.016 ml/g body wt ip) and killed by transcardial perfusion with Cl−-free solution to reduce cell swelling and the possible contamination of tissue samples by blood cells (49). The temporal bones were removed, and Reissner's membrane (Fig. 1) was obtained by microdissection in Cl−-free solution. The dissection medium was changed twice during microdissection to minimize cross-contamination. The isolated Reissner's membrane was divided into two groups and incubated for 24 h in DMEM-F12 medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum, 100 U/ml penicillin (Sigma, St. Louis, MO), and 100 μg/ml streptomycin (Sigma) at 37°C in a 5% CO2 atmosphere with saturated humidity. Each group of tissues was incubated in the presence and absence of cyclodextrin-encapsulated dexamethasone (Sigma) at 100 nM, a concentration that corresponds to the physiological and therapeutic concentration of dexamethasone (2, 6) and to the concentration that produced a peak response from semicircular canal epithelial cells (42). Some cultures were treated with 100 nM dexamethasone in the presence and absence of the glucocorticoid receptor (GR) antagonist mifepristone (100 nM; Sigma) or the mineralocorticoid receptor (MR) antagonist spironolactone (100 nM; Sigma).
For microarray analyses, the tissue was dissected and incubated with and without 100 nM dexamethasone (see above), without the transcardial perfusion. For electrophysiological studies, C57BL/6 mice (4–8 wk old) were anesthetized as described above and killed by decapitation under deep anesthesia. The temporal bones were removed, and the bony walls of the cochlea were peeled off. The cochleae were then incubated in a culture dish for 24 h in the medium and solutions described above. Reissner's membrane attached to the lateral wall was isolated after 24 h as described previously (30). Reissner's membrane samples were dissected and used immediately without incubation for measurement of K+ channel-dependent current but incubated with dexamethasone for measurement of pH-dependent current. All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Kansas State University.
RNA isolation.
Total RNA was extracted from Reissner's membrane using the RNeasy Micro Kit according to the manufacturer's protocol, including the use of carrier RNA (Qiagen, Valencia, CA). Positive controls for ion channels and corticosteroid regulatory genes utilized total RNA (Ambion, Austin, TX) from mouse kidney and brain. Total RNA was extracted from mouse stria vascularis using the RNeasy Micro Kit, from mouse cochlear nerve, thyroid, and bone using the RNeasy Mini Kit (Qiagen), and from mouse blood using the Mouse RiboPure Blood RNA Isolation Kit (Ambion) according to the manufacturers’ protocols for use in tests of cross-contamination of Reissner's membrane from neighboring tissues.
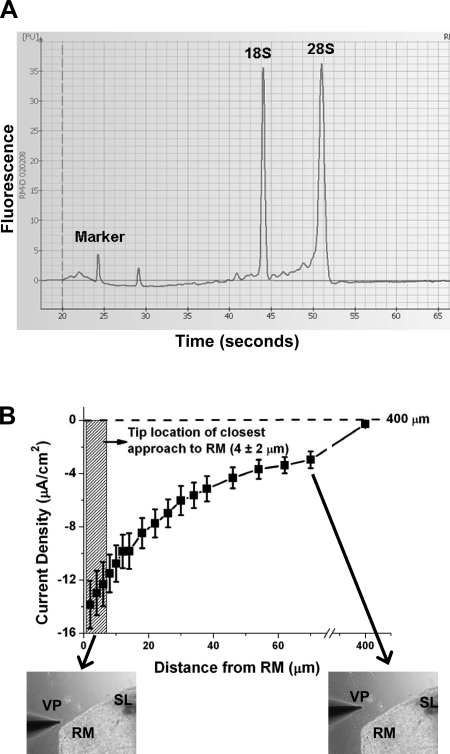
The quality of isolated RNA was examined in two ways. First, an Agilent Bioanalyzer 2100 with RNA 6000 Pico Assay Kits (Agilent Technology, Palo Alto, CA) was used to determine the quality and approximate quantity of total RNA as described previously (58). Isolated RNA showed good quality and minimal degradation on the electropherogram (Fig. 2A). Second, possible cross-contamination of isolated Reissner's membrane from neighboring tissues was examined by qRT-PCR. Possible cross-contamination of Reissner's membrane from the rupture of neighboring cells during dissection was determined by an evaluation of genes that are thought to be tissue specific and are known to be expressed in adjacent tissues. The selected genes were tyrosinase, prestin, synaptophysin, osteocalcin, and T cell receptor-α, which are expressed in stria vascularis (21), organ of Corti (outer hair cells) (21), cochlear nerve (16), bone (31), and blood T cell (21), respectively (Table 1).
Fig. 2.
Quality of isolated RNA and relationship between position of vibrating probe and current density from Reissner's membrane. A: representative electropherogram showing high quality of total RNA obtained from Reissner's membrane. Sharp peaks representing 18S and 28S rRNA demonstrate high quality of RNA. B: distance-response curve of current density from Reissner's membrane detected by vibrating probe. Probe was nominally placed 4 μm from apical surface of the epithelium, with an estimated precision of ±2 μm (hatched vertical rectangle). Measurements therefore had variation that was intrinsic not only to different tissue samples, but also to precision of electrode placement. Current density was ∼0 at 400 μm from the tissue, the distance taken as reference. Values are means ± SE (n = 12).
Table 1.
Primers for quantitative real-time RT-PCR analysis
| Gene | GenBank Accession No. | Forward Primer | Reverse Primer | Amplicon Size, bp |
|---|---|---|---|---|
| 18SrRNA | BK000964 | 5′-GAGGTTCGAAGACGATCAGA-3′ | 5′-TCGCTCCACCAACTAAGAAC-3′ | 315 |
| Tyr | NM_011661 | 5′-GCCTGTGCCTCCTCTAAG-3′ | 5′-TTCTAATCAAGACTCGCTTCTC-3′ | 295 |
| Slc26a5 | NM_030727 | 5′-GACCTCACCCGCAACAAC-3′ | 5′-CTCGTTCACCCTCCAAATCG-3′ | 311 |
| Syp | NM_009305 | 5′-GCAGACAGGCAGGTGAAGAGG-3′ | 5′-GGCAGAGAAAGGGTGGAGAAGG-3′ | 350 |
| Bglap | NM_007541 | 5′-ACAAGTCCCACACAGCAG-3′ | 5′-TGTAGGCGGTCTTCAAGC-3′ | 304 |
| Tcra | U07662 | 5′-AGACCAACGCCACCTACC-3′ | 5′-TCTCCAGCAACCTTCCTCAC-3′ | 336 |
| Scnn1a | NM_011324 | 5′-AACGACCAAACGAACCGAACAC-3′ | 5′-GCTCAGAAGGCACACAAGAAGG-3′ | 315 |
| Scnn1b | NM_011325 | 5′-CTCGGTGCTGTGCCTCATTG-3′ | 5′-GCCTCAGGGAGTCATAGTTGGG-3′ | 278 |
| Scnn1g | NM_011326 | 5′-TGGTCCTCCTATCCTCGTTCTG-3′ | 5′-GTCACACCCATCAGGCAATAGC-3′ | 344 |
| Cftr | M69298 | 5′-AAGCAAAGAAGAAGGAAAGAGG-3′ | 5′-ACAATAAGCCGAATGACTAACC-3′ | 347 |
| Atp1a1 | NM_144900 | 5′-TGCCCGCCTCAACATTCC-3′ | 5′-GACACATCAGAGCCAACAATCC-3′ | 291 |
| Atp1a2 | NM_178405 | 5′-AGGACTTGGCTTGCTAAGG-3′ | 5′-ACTGACTTGGCTGTTGTGG-3′ | 278 |
| Atp1a3 | NM_144921 | 5′-TTCGGCTTGTTTGAGGAGAC-3′ | 5′-GAACGGACACATCTGAGAGG-3′ | 261 |
| Atp1a4 | AF164349 | 5′-GCCTTCTTCATCAGTATCGTG-3′ | 5′-TCTCCTTCTCCAGCCATCC-3′ | 301 |
| Atp1b1 | NM_009721 | 5′-GGCGGATACTACGGCTTC-3′ | 5′-GATTAAGTGTAGGTCCCATACG-3′ | 322 |
| Atp1b2 | NM_009721 | 5′-CCGCTGTCTTGGCTCTATC-3′ | 5′-AGGAATATGCTGCTCTGATGG-3′ | 330 |
| Atp1b3 | NM_007502 | 5′-AACAAGAATGAGCGTGACAAG-3′ | 5′-GTTCAAGGGCAGCAGAGG-3′ | 260 |
| Kcnj10 | NM_001039484 | 5′-GGCTATGGCTTCCGCTAC-3′ | 5′-CTGAGGCTGTGTCTACTTGG-3′ | 346 |
| Kcnq1 | NM_008434 | 5′-ATGCTGCTCTTCTGATGG-3′ | 5′-GGTGGACAGTGGACAATC-3′ | 348 |
| Kcnq3 | NM_152923 | 5′-GGAGAAGAAAGAAGACAACAG-3′ | 5′-AGTCCAGAAGAGTCAAGATG-3′ | 260 |
| Kcnb1 | NM_008420 | 5′-TCTGTCTGCCTCTGAGTG-3′ | 5′-GGAACCCTTTCTGGATTGC-3′ | 325 |
| Kcnc3 | NM_008422 | 5′-CTCCAGTTCCCTACACATATC-3′ | 5′-CCCTCTGCCAATCATTCC-3′ | 314 |
| Kcnk1 | NM_008430 | 5′-TCTGTGCTGGAGGATGAC-3′ | 5′-CAGTGACGGAGGAGAAGG-3′ | 307 |
| Kcnk2 | NM_010607 | 5′-ACTGTTCAGACTGGTATTGC-3′ | 5′-CTGTTAGAGACGACCTATGC-3′ | 345 |
| Kcnk5 | NM_021542 | 5′-GCTGCTGTATATGTCCTTG-3′ | 5′-CCTCTCACTGTCCTAACG-3′ | 320 |
| Kcne2 | NM_134110 | 5′-CAGGAGCAGTGGGAATAAATAG-3′ | 5′-TGTCCAGATGTCCAACTCAG-3′ | 287 |
| Hsd11b1 | BC_132364 | 5′-GTAGTGTCTCGCTGCCTTGAAC-3′ | 5′-TGCTGCCATTGCTCTGCTTC-3′ | 274 |
| Hsd11b2 | NM_008289 | 5′-GCCACGAAGCCGCTACTAC-3′ | 5′-GACGAGGCATCAGCAATAGAGG-3′ | 336 |
| Nr3c1 | DQ_504162 | 5′-AACCTGCTATGCTTTGCTCCTG-3′ | 5′-AACCGCTGCCAATTCTGACTG-3′ | 290 |
| Nr3c2 | NM_001083906 | 5′-TGACGGCGGATTGACAGTTG-3′ | 5′-AGCGAGAGATACCAGAAACCAC-3′ | 256 |
| Sgk1 | NM_011361 | 5′-GTGGCGTGAGTGTGCTATGC-3′ | 5′-AACGGCTTTGACTGACAACTGG-3′ | 284 |
| Nedd4l | NM_031881 | 5′-ACGAAGAGAACTTTGGGCAGAC-3′ | 5′-TGGATGACAGGGTGGTTGGG-3′ | 310 |
| Prkwnk1 | NM_198703 | 5′-TGCTTCCTCCTTCACCTTG-3′ | 5′-CCTGTAACCTGCTTCACTCC-3′ | 271 |
| Prkwnk2 | NM_029361 | 5′-CTGGTGGTGTGGCAAGTG-3′ | 5′-GCTTCTGCTGGCTCTGTAG-3′ | 264 |
| Prkwnk3 | XM_001481298 | 5′-GCACTTAGCCACCATCTTG-3′ | 5′-TTCCTTCCCGTCAAACTATTAG-3′ | 334 |
| Prkwnk4 | NM_175638 | 5′-ACTACAGACACTACAGAAGAAG-3′ | 5′-TTCACATCCTGCCAATATCC-3′ | 281 |
18SrRNA, 18S rRNA; Tyr, tyrosinase; Slc26a5, prestin; Syp, synaptophysin; Bglap, bone γ-carboxyglutamate protein (osteocalcin); Tcra, T cell receptor-α; Scnn1a, b, and g, Na+ channel, nonvoltage-gated, type I, α, β, and γ (epithelial Na+ channel α, β, and γ); Cftr, CFTR; Atp1a, ATPase, Na+-K+ transporting, α-polypeptide; Atp1b, ATPase, Na+/K+ transporting, β-polypeptide; Hsd11b, 11β-hydroxysteroid dehydrogenase; Nr3c1, nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor); Nr3c2, nuclear receptor subfamily 3, group C, member 2 (mineralocorticoid receptor); Sgk1, serum- and glucocorticoid-regulated kinase; Nedd4l, neural precursor cell-expressed developmentally downregulated 4-2; Prkwnk, protein kinase, lysine deficient (with no lysine kinase).
qRT-PCR of isolated Reissner's membrane RNA showed that the transcript expressions of genes known to be expressed in the neighboring tissues were not detected or were very low (Table 2). Transcript expression of tyrosinase was not detected in Reissner's membrane, and transcript expressions of prestin, synaptophysin (in the presence of dexamethasone), osteocalcin, and T cell receptor-α in Reissner's membrane was <0.5% of their respective positive controls, and transcript expression of synaptophysin in Reissner's membrane incubated without dexamethasone (control) was <1.5% of its positive control. In these experiments, the cycle threshold (Ct) values of 18S rRNA for positive controls were 12.6–20.1; the total RNA in each reaction was 0.5–10 ng, within the linear range of the assay. Also, in the microarray, none of the genes was found in Reissner's membrane except synpatophysin, which was detected by one of two probe sets in each of six chips. Taken together, isolated RNA was of good quality, and little contamination from neighboring tissues was detected.
Table 2.
Low level of cross-contamination of RNA isolated from Reissner's membrane
| Gene | Positive Control |
rER |
|
|---|---|---|---|
| RM without dexamethasone (n = 5) | RM with dexamethasone (n = 4) | ||
| Tyr | Stria vascularis | 0.000±0.000 | 0.000±0.000 |
| Slc26a5 | Organ of Corti | 0.004±0.002 | 0.005±0.005 |
| Syp | Cochlear nerve | 0.015±0.012 | 0.005±0.003 |
| Bglap | Bone | 0.002±0.001 | 0.002±0.001 |
| Tcra | Blood | 0.003±0.003 | 0.000±0.000 |
Values are means ± SE. Cross-contamination was determined by evaluation of genes that are known to be expressed in neighboring tissues that were potential sources of contamination. Tyrosinase, prestin, synaptophysin, osteocalcin, and T cell receptor-α are expressed in stria vascularis, organ of Corti (outer hair cell), cochlear nerve, bone, and blood T cells, respectively. Each transcript was normalized to 18S rRNA in each sample. rER, relative expression ratio; normalized expression level of each gene in Reissner's membrane was divided by normalized expression level in control tissue. Cycle threshold values of 18S rRNA were 12.6–20.1; total RNA in each reaction was 0.5–10 ng.
Microarray analysis.
Affymetrix microarrays were used to examine the expression of ion channels, transporters, and corticosteroid regulatory genes involved in the corticosteroid-stimulated Na+ transport pathway. K+ channel candidates were selected from the microarray data for subsequent qRT-PCR analyses.
Tissue was obtained as described above. Reissner's membrane was acutely isolated from four mice to obtain sufficient RNA for the gene microarray process and to minimize interanimal variation. One RNA sample was obtained from Reissner's membrane acutely isolated, and two RNA samples were extracted from Reissner's membrane incubated for 24 h in the presence or absence of dexamethasone (100 nM). The pooled samples were used to hybridize three gene array chips (mouse 430 2.0 gene chip, Affymetrix, Santa Clara, CA) for each pooled sample. Isolated RNA was evaluated for quality, concentrated, and frozen for shipment to the Biotechnology Support Facility at the University of Kansas Medical Center.
Sample processing for RNA amplification, cDNA synthesis, labeling, and hybridization were carried out using the Affymetrix Small Sample Labeling Protocol vII and Hybridization Protocol utilized by the University of Kansas Medical Center microarray facility (http://www2.kumc.edu/siddrc/microarray/protocols.html). This information on the methodology conforms to the Minimum Information about a Microarray Experiment guidelines (http://www.mged.org/Workgroups/MIAME/Miame.html), and details are deposited with the data in Gene Expression Omnibus (accession no. GSE6196). The quality metrics for the six gene chips were as follows: 56.6 ± 3.7 (SD) background, 2.9 ± 0.2 (SD) noise, and 2.32 RawQ 1.8 and scaling factor.
Signal intensities were quantified by pixel intensity, and expression signals were analyzed using Affymetrix Data Acquisition Software Gene Chip Operating Software 1.4. Statistical algorithms [detection, change call, and signal log ratio (SLR)] were then used to identify differential gene expression in control and experimental samples. Probe sets were assigned as present, absent, or marginal on the basis of detection P values (P < 0.05 for present, P = 0.05–0.065 for marginal, and P > 0.065 for absent). Additional analysis was conducted if and only if two of three samples in the control or dexamethasone-treated groups were called present. Pair-wise comparisons between individual dexamethasone-treated and control arrays were made to subsequently generate a median SLR value for each transcript. Student's t-test was performed between the SLR values to determine the significance of changes in expression of these genes (significant for P < 0.05). Fold change was calculated from the median of the SLR values and only considered physiologically significant when the fold change was ≥1.5. This analysis by SLR accounts for the differences in individual hybridization efficiencies at the individual probe pair level.
qRT-PCR.
qRT-PCR was used to test the changes in expression that were suggested by the microarray analysis for selected genes. qRT-PCR was performed on total RNA using 0.2× SYBR Green I (Molecular Probes), a One Step RT-PCR kit (Qiagen), and an iCycler iQ thermocycler (Bio-Rad, Hercules, CA). Transcripts of 18S rRNA and target genes were amplified using gene-specific primers (Table 1). RT was performed for 30 min at 50°C and 15 min at 95°C, and PCR consisted of 40 cycles of 1 min at 95°C, 1 min at 60°C, and 1 min at 72°C. PCR was followed by a melt at 60–95°C. Sample fluorescence was read at 78°C (3–5°C below the melting temperature peak of each specific cDNA) to exclude contributions from nonspecific sources such as primer dimers. To exclude the possibility of genomic DNA and nonspecific RNA amplification during RT-PCR, no-template controls were performed and accepted when the Ct value was at least nine cycles greater than the template run. Measurements were performed in duplicate and accepted if the difference of Ct value between the duplicates was <1. The generation of a single product of appropriate size was routinely checked by the presence of a single melt peak and by agarose gel electrophoresis. PCR products were then purified by a PCR purification kit (Qiagen), and purified PCR products were sequenced to verify the identity of the RT-PCR product. The specific gene expression was normalized to the level of 18S rRNA in each sample as described previously (58), with the fidelity of each PCR taken into account. In the absence of information on the efficiency of the RT step, no conclusions were drawn about relative gene expression levels among different genes.
Transepithelial current.
Transepithelial current under short-circuit condition was measured as described elsewhere with a vibrating probe, a technique well suited to the small dimensions of isolated Reissner's membrane (30). Briefly, the tissue was mounted in a perfusion chamber on the stage of an inverted microscope (model TE-300, Nikon, Tokyo, Japan) and continuously perfused at 37°C with an exchange rate of 1.1 times/s. The electrode tip of the probe was vibrated at 400–700 Hz by piezoelectric bimorph elements (Applicable Electronics, Forestdale, MA) and positioned 4 ± 2 μm from the apical surface of Reissner's membrane. The probe was positioned where the current density showed a maximum in the direction perpendicular to the tissue. The current decreased as the probe was moved away from the tissue, with nearly no current detected 400 μm (reference position) away from the tissue (Fig. 2B). A platinum-black electrode served as reference in the bath chamber. The signals from the phase-sensitive detectors were digitized (16 bit) at a rate of 0.5 Hz. The output was expressed as current density at the electrode and plotted with Origin software (version 7.0, Origin Lab Software, Northampton, MA). Current density from the suprastrial spiral ligament after removal of Reissner's membrane was not detected (data not shown); therefore, it did not contribute to the current density measured from Reissner's membrane. The output from the vibrating probe depends not only on the specific short-circuit current of the epithelium but also on the position of the probe relative to the surface of the tissue and the exact geometry of the tissue. The current density reported here refers to that at the position of the probe and represents only a fraction of the current crossing the epithelium.
Solutions and materials.
In all electrophysiological experiments, both sides of the epithelium were perfused with a perilymph-like physiological saline solution containing (in mM) 150 NaCl, 3.6 KCl, 1 MgCl2, 0.7 CaCl2, 5 glucose, and 10 HEPES (pH 7.4). Amiloride, benzamil, EIPA, forskolin, IBMX, ouabain, bumetanide, glibenclamide, clotrimazole, iberiotoxin, mifepristone, and spironolactone were purchased from Sigma and dissolved in DMSO (Sigma), which was then diluted to <0.1% DMSO in the physiological saline solution before application. DMSO at this concentration had no effect on current density or transcript expression (41). Dexamethasone (Sigma), tetraethylammonium (TEA)-Cl (Sigma), 4-aminopyridine (4-AP; Sigma), apamin (Sigma), BaCl2 dihydrate (Fluka), and XE-991 (Tocris, Ellisville, MO) were directly dissolved in physiological saline solution just before use. TEA-Cl was substituted on an equimolar basis for NaCl, and current density was corrected for the 4.5% increase in the resistivity of the solution. The effect of TEA-Cl (30 mM) was compared with that of equimolar N-methyl-d-glucamine (NMDG)-Cl to control for any effect of the reduced Na+ concentration ([Na+]). The current density measured with NMDG-Cl was corrected for the 6.8% increase in the resisitivity of the solution. For pH 6.5 physiological saline solution, MES buffer was substituted on an equimolar basis for HEPES buffer, and pH was adjusted to 6.5.
Data analysis.
Values are means ± SE from n observations. Significance of current density changes between the control and individual experimental conditions was calculated with the paired t-test. Significance among the control, dexamethasone-treated, dexamethasone + mifepristone-treated, and dexamethasone + spironolactone-treated groups was calculated with one-way ANOVA and Holm-Sidak post tests. Differences were considered significant for P < 0.05. Concentration dependence of the effects was analyzed using the Hill equation: I = Imax [Ch/(IC50h + Ch)] + Ioffset, where Imax is current in the presence of saturating concentration of drug, IC50 is the concentration that produces a half-maximal effect, C is the concentration of drug, h is the Hill coefficient, and Ioffset is the residual current during maximal inhibition by the drug. Data were fitted for each experiment separately, and then means ± SE were calculated.
RESULTS
Microarray analysis of Na+ transport pathway-related genes.
Gene microarrays were used to identify the presence or absence of transcripts that may be related to glucocorticoid-stimulated Na+ transport in Reissner's membrane and to compare gene expression differences between control and dexamethasone-treated groups. Table 3 shows mouse gene nomenclature and common protein name of the selected ion channels, transporters, and corticosteroid regulatory genes that are related to ENaC-mediated Na+ transport in the microarrays. For ion channels and transporters, transcripts of α-, β-, γ-subunits of ENaC, α1,2- and β1,2,3-isoforms of Na+-K+-ATPase, Na+-K+-2Cl− cotransporter (NKCC) type 1, and nine K+ channels (KCNJ10, KCNK1, KCNK2, KCNK5, KCNB1, KCNC3, KCNQ1, KCNQ3, and KCNE2) were present, but α3,4-isoforms of Na+-K+-ATPase and CFTR were not present, and KCNB1 was present only after dexamethasone exposure. Transcripts for α- and γ-subunits of ENaC, KCNJ10, KCNK1, and KCNQ3 were upregulated by 3-, 1.5-, 1.9-, 2.0-, and 1.5-fold after dexamethasone exposure, respectively. For corticosteroid regulatory genes, transcripts for GR, serum- and glucocorticoid-regulated kinase (SGK), neural precursor cell-expressed developmentally downregulated (Nedd) 4-2, with no lysine kinase (WNK) 1, and WNK4 were present, and WNK4 was upregulated by fourfold after dexamethasone exposure. Transcripts of 11β-HSD1 were present only in one of three chips in each condition, and transcripts of 11β-HSD2 and WNK3 were absent in all six chips. Probes for MR and WNK2 were not included in the microarray, so we examined the transcript expression and changes of those genes by qRT-PCR (Table 3).
Table 3.
Microarray analysis of glucocorticoid-stimulated Na+ transport pathway-related genes in Reissner's membrane
| GenBank Accession No. | Gene | Protein |
Call/Fold Change |
|
|---|---|---|---|---|
| Gene array* | qRT-PCR* | |||
| AF112185 | Scnn1a | α−ENaC | P (+3.0) | P (+5.8) |
| NM_011325 | Scnn1b | β−ENaC | P (NC) | P (+2.7) |
| NM_011326 | Scnn1 g | γ−ENaC | P (+1.5) | P (NC) |
| BC025618 | Atp1a1 | Na+-K+-ATPase-α1 | P (NC) | P (NC) |
| BC025807 | Atp1a2 | Na+-K+-ATPase-α2 | P (NC) | P (NC) |
| BC020177 | Atp1a3 | Na+-K+-ATPase-α3 | A | A |
| AI480619 | Atp1a4 | Na+-K+-ATPase-α4 | A | A |
| NM_009721 | Atp1b1 | Na+-K+-ATPase-β1 | P (NC) | P (NC) |
| BG261955 | Atp1b2 | Na+-K+-ATPase-β2 | P (NC) | P (NC) |
| BI080505 | Atp1b3 | Na+-K+-ATPase-β3 | P (NC) | P (NC) |
| AF322631 | Kcnj10 | KCNJ10 (Kir1.2) | P (+1.9) | P (NC) |
| NM_008430 | Kcnk1 | KCNK1 (K2P1.1) | P (+2.0) | P (+2.6) |
| NM_010607 | Kcnk2 | KCNK2 (K2P2.1) | P (−2.2) | P (−3.7) |
| AF319542 | Kcnk5 | KCNK5 (K2P5.1) | P (NC) | P (NC) |
| BB324482 | Kcnb1 | KCNB1 (Kv2.1) | P* | P (NC) |
| BE944832 | Kcnc3 | KCNC3 (Kv3.3) | P (NC) | A |
| NM_008434 | Kcnq1 | KCNQ1 (Kv7.1) | P (NC) | P (NC) |
| AW494964 | Kcnq3 | KCNQ3 (Kv7.3) | P (+1.5) | P (NC) |
| NM_134110 | Kcne2 | KCNE2 (MiRP1) | P (−2.5) | P (NC) |
| NM_008419 | Kcna5 | KCNA5 (Kv1.5) | A | NT |
| NM_011389 | Slc12a1 | NKCC2 | A | NT |
| BG069505 | Slc12a2 | NKCC1 | P (NC) | NT |
| NM_021050 | Cftr | CFTR | A | A |
| NM_008288 | Hsd11b1 | 11β-HSD1 | P† (NC) | P (+2.0) |
| BC014753 | Hsd11b2 | 11β-HSD2 | A | A |
| NM_008173 | Nr3c1 | GR | P (NC) | P (NC) |
| NM_008289 | Nr3c2 | MR | NI | P (NC) |
| NM_011361 | Sgk1 | SGK1 | P (NC) | P (+1.8) |
| B663717 | Nedd4l | Nedd4-2 | P (NC) | P (NC) |
| BM232571 | Prkwnk1 | WNK1 | P (NC) | P (NC) |
| NM_029361 | Prkwnk2 | WNK2 | NI | P (NC) |
| BB084132 | Prkwnk3 | WNK3 | A | P (NC) |
| BG074348 | Prkwnk4 | WNK4 | P (+4.0) | P (+2.8) |
| NM_008829 | Pgr | PGR | A | NT |
Call, gene detected as present (P) or absent (A); Fold change, change in expression of each gene due to exposure to dexamethasone as assayed by gene microarray and quantitative real-time RT-PCR (qRT-PCR). Cut-off value for fold change was 1.5 for up- or downregulated genes. Values in parentheses are fold changes: +, upregulated; −, downregulated; NC, no change.
Transcripts present only after dexamethasone exposure.
Transcripts were present only in 1 of 3 gene chips in each condition. ENaC, epithelial Na+ channel; MiRP, MinK-related peptides; NKCC, Na+-K+-2Cl− cotransporter; 11β-HSD, 11β-hydroxysteroid dehydrogenase; GR, glucocorticoid receptor; MR, mineralocorticoid receptor; PGR, progesterone receptor. NT, not tested; NI, not included on microarray chip.
Amiloride-sensitive current from Reissner's membrane.
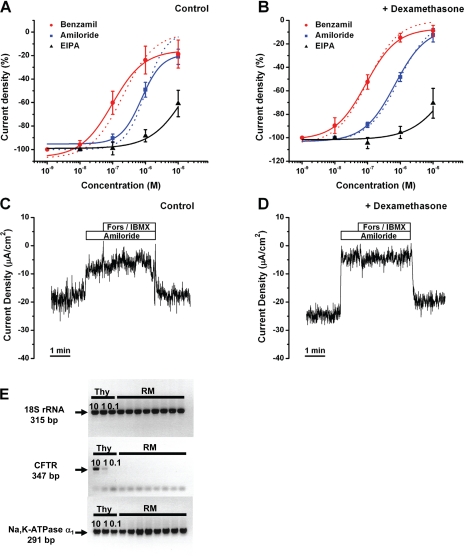
The amiloride-sensitive current density was greater after 24 h of incubation of Reissner's membrane with dexamethasone (100 nM): −14.4 ± 0.7 and −24.1 ± 1.6 μA/cm2 without and with dexamethasone, respectively (n = 10; Fig. 3, C and D, and Fig. 4A). In control and dexamethasone-treated tissues, perfusion of amiloride and its analogs (benzamil and EIPA) decreased the current density in a dose-dependent manner, with potency order as follows: benzamil > amiloride >> EIPA. The concentration-response curves were fitted with the Hill equation (Fig. 3, A and B, Table 4).
Fig. 3.
Inhibition of current density from Reissner's membrane by amiloride and amiloride analogs. A and B: concentration-response curves for decrease of current density by amiloride, benzamil, and EIPA in control and dexamethasone-treated tissue; curves are best fits to Hill equation. Dashed lines represent curves fitted with residual current during maximal inhibition by the drug (Ioffset) = 0. Values are means ± SE (n = 5). C: representative current traces with the effect of amiloride (10 μM) and forskolin (Fors, 10 μM)/IBMX (125 μM). D: representative current traces after dexamethasone exposure (24 h) with the effect of amiloride (10 μM) and forskolin (10 μM)/IBMX (125 μM). E: agarose gel electrophoresis of gene-specific PCR products for 18S rRNA, CFTR, and Na+-K+-ATPase α1-subunit from thyroid (Thy; positive control) and Reissner's membrane. Thyroid template RNA amounts were 10 ng (10), 1 ng (1), and 0.1 ng (0.1). Transcripts for 18S rRNA and Na+-K+-ATPase α1-subunit were expressed in thyroid and Reissner's membrane, whereas transcripts for CFTR were only expressed in 10 and 1 ng thyroid. Bands in Reissner's membrane CFTR are from primer dimers formed in the absence of target template.
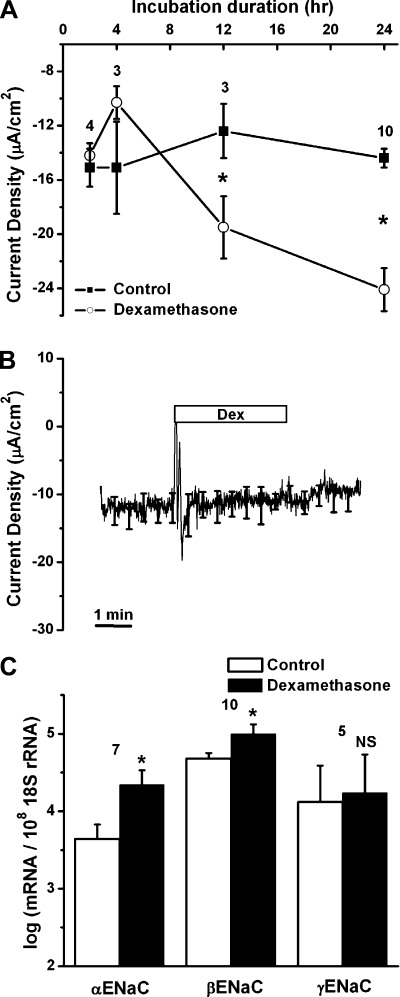
Fig. 4.
Increased current density and upregulation of transcripts of epithelial Na+ channel (ENaC) by genomic action of dexamethasone. A: time course of activation of current density in control and dexamethasone (Dex, 100 nM)-treated tissues. Values are means ± SE (n = 3–10); numbers above data points represent number of animal pairs. *P < 0.05, without vs. with dexamethasone. B: absence of an acute (nongenomic) effect of dexamethasone (100 nM) on current density from Reissner's membrane. Averages of all experiments are shown; SE is drawn only at intervals for clarity (n = 4). C: quantitative real-time RT-PCR (qRT-PCR) evaluation of transcript expression of α-, β-, and γ-subunits of ENaC without and with dexamethasone (100 nM for 24 h). Transcripts were quantified relative to 18S rRNA in Reissner's membrane. Values are means ± SE (n = 5–10); numbers above bars represent number of qRT-PCRs. NS, not significant. *P < 0.05 vs. control (absence of dexamethasone).
Table 4.
Potency of amiloride analogs for Reissner's membrane
| Drug | IC50, μM (pIC50, M) | *IC50, μM (pIC50, M) | Hill Coeff |
|---|---|---|---|
| Amiloride | |||
| Control | 0.75 (−6.12±0.08) | 1.03 (−5.99±0.11) | 1.39 |
| Dex | 0.68 (−6.17±0.14) | 0.89 (−6.05±0.06) | 0.92 |
| Benzamil | |||
| Control | 0.10 (−7.01±0.16) | 0.20 (−6.68±0.25) | 0.90 |
| Dex | 0.09 (−7.05±0.10) | 0.10 (−7.01±0.11) | 1.00 |
| EIPA | |||
| Control | 20.8 (−4.68±0.24) | 0.79 | |
| Dex | 47.5 (−4.32±0.44) | 0.73 |
Values are means ± SE (n = 5 for each drug). IC50, concentration required to produce half-maximal current density inhibition. Cont, control incubation without dexamethasone; Dex, incubation with dexamethasone.
IC50 from fit to Hill equation with residual current during maximal inhibition by drug (Ioffset) = 0.
IC50 ∼0.7 μM for amiloride was not changed by dexamethasone, and the Hill coefficient remained ∼1. Similarly, IC50 (∼0.1 μM) and the Hill coefficient (∼1) for benzamil were not changed by dexamethasone. Similar results were obtained for acutely isolated Reissner's membrane without incubation: IC50 = 1.7 ± 0.09 μM and Hill coefficient = 0.6 ± 0.01 for amiloride (n = 5) and IC50 = 0.18 ± 0.05 μM and Hill coefficient = 0.8 ± 0.2 for benzamil (n = 5). These results compare closely with the reported IC50 of amiloride for αβ-ENaC.
The fit of EIPA inhibition to the Hill equation was not well defined but clearly showed a low sensitivity of the currents to this drug. The benzamil data suggested a small amiloride-insensitive residual current at high drug concentration. Fits to the Hill equation were made by forcing the fit with no residual (dashed lines in Fig. 3, A and B) or allowing a residual current at high concentrations (solid lines in Fig. 3, A and B); there were no appreciable differences between the Hill parameters (Table 4).
Test for cAMP-mediated current from Reissner's membrane.
Evidence was sought for anion currents under control of cAMP. Reissner's membrane was superfused with forskolin (10 μM), an agonist for adenylyl cyclase, and IBMX (125 μM), an inhibitor of phosphodiesterase, in the continued presence of apical amiloride (10 μM) to elevate cytosolic cAMP levels in the absence of interfering Na+ currents. Superfusion of forskolin/IBMX did not cause a significant change of current in the absence of dexamethasone (Fig. 3C; from −6.0 ± 2.6 to −5.6 ± 2.5 μA/cm2, n = 3) or the presence of dexamethasone (Fig. 3D; from −5.8 ± 1.6 to −5.7 ± 2.2 μA/cm2, n = 3). The only molecularly identified Cl− channel that is stimulated by cAMP via protein kinase A is CFTR. Transcripts for CFTR were absent in the gene array (Table 3) and in qRT-PCR experiments in which thyroid RNA was used as a positive control (Fig. 3E). There is therefore no evidence for cAMP-stimulated anion currents in mouse Reissner's membrane.
Dexamethasone increased current density and transcript expression.
We investigated whether dexamethasone regulates currents by genomic and/or nongenomic action, which are characterized by slow (hours) and rapid (seconds to minutes) onset, respectively. First, we checked for genomic effects of dexamethasone by observing current density after 2, 4, 12, and 24 h of exposure to dexamethasone (Fig. 4A). Dexamethasone (100 nM) increased current density significantly at 12 h (−12.4 ± 2.0 and −19.5 ± 2.3 μA/cm2 without and with dexamethasone, respectively, n = 3) and increased current density further by 24 h (−14.4 ± 0.7 and −24.13 ± 1.6 μA/cm2 without and with dexamethasone, respectively, n = 10; Fig. 4A). Therefore, 24-h exposures were used in this study. Second, possible nongenomic effects, which take place within seconds to minutes, were investigated. Superperfusion of the nonincubated freshly prepared tissue for 3 min with dexamethasone did not change current density (from −14.6 ± 3.2 to −14.2 ± 3.7 μA/cm2, n = 4; Fig. 4B). These data indicate that stimulation of current density by dexamethasone is consistent with its genomic action.
We investigated whether transcripts for each subunit of ENaC are expressed and regulated by dexamethasone. All three subunits were present (gene microarray), and the transcript expression (qRT-PCR) of the α- and β-subunits was upregulated by approximately six- and threefold, respectively, whereas the γ-subunit was not significantly changed after dexamethasone application (Fig. 4C).
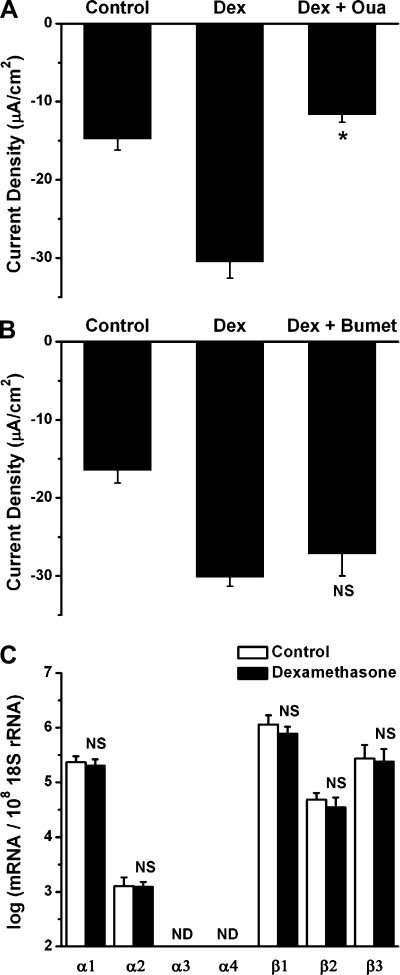
Contributions of Na+-K+-ATPase and NKCC to current density.
We investigated whether an ouabain-sensitive Na+-K+-ATPase and a bumetanide-sensitive NKCC could be involved in dexamethasone-mediated Na+ absorption. Application of ouabain (1 mM) for 3 min caused a decrease of current density in Reissner's membrane exposed to 100 nM dexamethasone (from −30.5 ± 2.1 to −11.7 ± 0.9 μA/cm2, n = 4; Fig. 5A), whereas 3 min of exposure to bumetanide (10 μM) caused no change (from −30.2 ± 1.1 to −27.2 ± 2.8 μA/cm2, n = 3; Fig. 5B). These results demonstrate the involvement of Na+-K+-ATPase, but not NKCC, in dexamethasone-stimulated Na+ transport. Transcripts for α1-, α2-, β1-, β2-, and β3-isoforms of Na+-K+-ATPase are expressed, and none of the transcripts were altered significantly by >1.5-fold after dexamethasone incubation (Fig. 5C). Transcripts for NKCC1 were present but were not altered by dexamethasone (gene microarray), whereas NKCC2 was absent; these results were not verified by qRT-PCR.
Fig. 5.
Dependence of dexamethasone-stimulated current density on Na+-K+-ATPase and regulation of Na+-K+-ATPase expression. A: decrease in dexamethasone (100 nM for 24 h)-stimulated current density by Na+-K+-ATPase blocker ouabain (Oua, 1 mM). Values are means ± SE (n = 4). *P < 0.05, before vs. after 2.5-min ouabain application. B: change in dexamethasone-stimulated current density by Na+-K+-2Cl− cotransporter blocker bumetanide (Bumet, 10 μM). Values are means ± SE (n = 3). C: qRT-PCR evaluation of transcript expression of subunits of Na+-K+-ATPase in the absence and presence of dexamethasone. Transcripts were quantified relative to 18S rRNA in Reissner's membrane. Values are means ± SE (n = 5). ND, no detectable transcript expression; NS, not significant (i.e., <1.5-fold difference between absence and presence of dexamethasone).
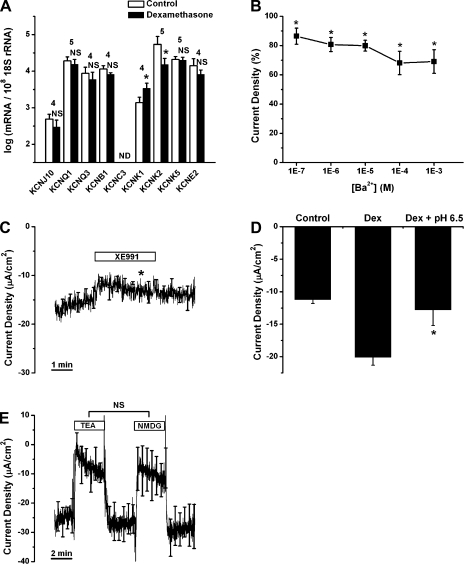
Regulation of K+ channel transcript expression and effects of K+ channel blockers on current density.
KCNJ10, KCNQ1, KCNQ3, KCNE2, KCNB1, KCNC3, KCNK1, KCNK2, and KCNK5 were selected as candidates of K+ channels that were identified as present in the microarray data (Table 3). We investigated whether transcripts for these channels are expressed and whether their levels are changed by dexamethasone. Transcripts of all the candidate genes except KCNC3 were detected by qRT-PCR (Fig. 6A). Transcripts for KCNK1 were upregulated by 2.6-fold and transcripts for KCNK2 were downregulated by 3.7-fold after dexamethasone exposure.
Fig. 6.
Regulation of K+ channel transcript expression by dexamethasone and effects of K+ channel blockers on current density. A: qRT-PCR evaluation of transcript expression of KCNJ10, KCNQ1, KCNQ3, KCNB1, KCNC3, KCNK1, KCNK2, KCNK5, and KCNE2 in Reissner's membrane in the absence and presence of dexamethasone (100 nM for 24 h). Transcripts were quantified relative to 18S rRNA in Reissner's membrane. Values are means ± SE (n = 4–5); numbers above bars represent number of qRT-PCRs. ND, no detectable transcript expression. NS, not significant (i.e., <1.5-fold change). *P < 0.05 (>1.5-fold difference between absence and presence of dexamethasone). B: concentration-response curve for decrease of current density by Ba2+. Values are means ± SE (n = 5). *P < 0.05, before vs. during Ba2+ application. C: effect of XE-991 (100 μM, n = 4) on current density. Averages of all experiments are shown; SE is drawn only at intervals for clarity. *P < 0.05, 1 min before vs. 2.5 min after XE-991 application. D: decrease in dexamethasone-stimulated current density caused by exposure to pH 6.5. Values are means ± SE (n = 4). *P < 0.05, 1 min before vs. 2.5 min after application of pH 6.5 solution. E: effect of tetraethylammonium Cl (TEA, 30 mM, n = 5) on current density compared with that of equimolar N-methyl-d-glucamine-Cl (NMDG). Averages of all experiments are shown; SE is drawn only at intervals for clarity.
A functional investigation of K+ channels was also performed using electrophysiological and pharmacological methods. We used Ba2+ (100 nM–1 mM), TEA (30 mM), XE-991 (100 μM), and pH 6.5 as blockers for candidate K+ channels: Ba2+ blocks KCNJ10 (18) and KCNK2 (40); TEA blocks KCNB1 (53), KCNC3 (55), and KCNQ3 (17); XE-991 blocks KCNQ1 and KCNQ3 (38); and pH 6.5 inhibits homomeric KCNQ1 (20), KCNK1 (43), and KCNK5 (37). Ba2+, TEA, and XE-991 were applied to nonincubated acutely isolated Reissner's membrane. Tissues incubated in the presence of dexamethasone were exposed to a change in bath pH (from 7.4 to 6.5), since the only candidate K+ channel upregulated by dexamethasone was KCNK1, a channel inhibited by acidic extracellular pH.
All these inhibitors except TEA decreased the current density significantly but did not fully block the current. Application of Ba2+ (Fig. 6B) decreased the current over the concentration range 0.1 μM–1 mM (decreased by 30.9 ± 8.1% at 1 mM, n = 5). XE-991 (Fig. 6C) decreased the current density by 12.2 ± 2.5% from −15.1 ± 1.3 to −13.3 ± 1.3 μA/cm2 (n = 4). The change of pH from 7.4 to 6.5 (Fig. 6D) decreased by 34.7 ± 9.8% the current density in Reissner's membrane exposed to dexamethasone (from −20.1 ± 1.2 to −13.1 ± 2.4 μA/cm2, n = 4). Substitution of 30 mM NMDG-Cl for NaCl (Fig. 6E) decreased the current density from −26.9 ± 5.4 to −9.5 ± 6.0 μA/cm2 (73.2 ± 11.4%), demonstrating a high sensitivity of the current to extracellular [Na+]. It is possible that the decrease in [Na+] may lower the intracellular pH via Na+/H+ exchange in the basolateral membrane, which may thereby inhibit apical ENaC activity (44). Substitution of 30 mM TEA-Cl for NaCl led to the same decrease of the current density (from −24.6 ± 5.1 to −8.0 ± 4.5 μA/cm2, 75.7 ± 10.1%). Other K+ channel blockers [4-AP (1 mM, n = 5), glibenclamide (10 μM, n = 5), clotrimazole (10 μM, n = 4), iberiotoxin (100 nM, n = 3), and apamin (100 nM, n = 4)] were used for screening of other K+ channels besides the candidate channels; however, they had no significant effect on the current density from Reissner's membrane.
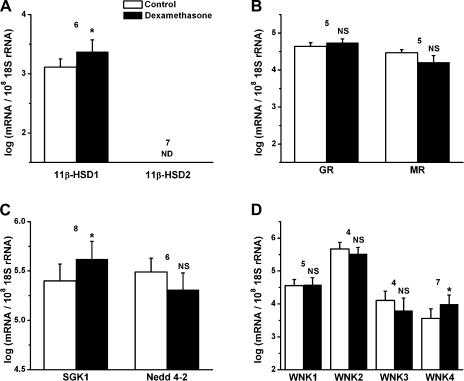
Dexamethasone dependence of corticosteroid regulatory genes.
11β-HSD1, which regulates the intracellular concentration of active glucocorticoids in corticosteroid-responsive target tissues, was upregulated by approximately twofold after dexamethasone exposure (Fig. 7A). However, mRNA for 11β-HSD2, which converts active glucocorticoids to an inactive form to increase mineralocorticoid activity, was not expressed in the control or the dexamethasone-treated tissues (Fig. 7A). The transcripts of GR and MR were expressed. Neither of the transcripts was altered after dexamethasone exposure (Fig. 7B). The transcripts of SGK1 were upregulated by approximately twofold after dexamethasone exposure, whereas the transcripts of Nedd4-2 were not altered (Fig. 7C). The transcripts of all WNK isoforms were detected, and WNK4 was upregulated by approximately threefold after dexamethasone exposure (Fig. 7D). Taken together, 11β-HSD1, SGK1, and WNK4 are likely involved in the dexamethasone-stimulated Na+ transport pathway.
Fig. 7.
Changes in transcript expression of corticosteroid regulatory genes in the absence and presence of dexamethasone (100 nM, 24 h). A: qRT-PCR evaluation of transcript expression of 11β-hydroxysteroid dehydrogenase (11β-HSD) types 1 and 2. B: glucocorticoid receptor (GR) and mineralocorticoid receptor (MR). C: serum- and glucocorticoid-regulated kinase 1 (SGK1) and neural precursor cell-expressed developmentally downregulated 4-2 (Nedd4-2). D: with no lysine kinase (WNK) isoforms 1–4. Transcripts were quantified relative to 18S rRNA in Reissner's membrane. Values are means ± SE (n = 4–8); numbers above bars represent number of qRT-PCRs. NS, not significant (<1.5-fold change). *P < 0.05 vs. control.
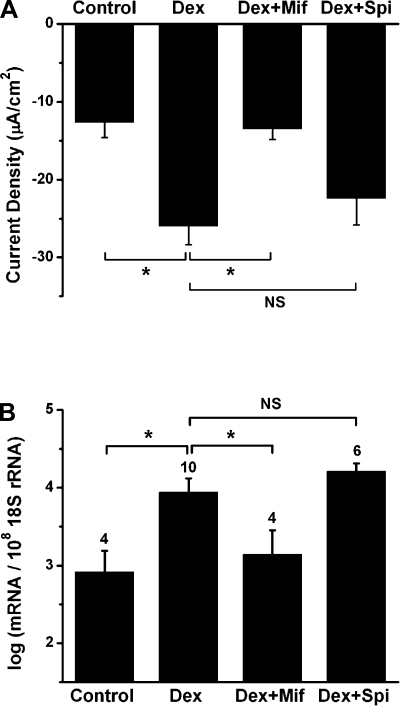
Dexamethasone acts at GR to regulate current density and transcript expression.
We investigated whether the current density and transcriptional changes in response to dexamethasone are via GR or MR, because GR and MR transcripts were expressed in Reissner's membrane and because these receptors and their agonists are promiscuous. The GR antagonist mifepristone (100 nM) significantly reduced the current density of dexamethasone-treated Reissner's membrane, whereas the MR antagonist spironolactone (100 nM) had no significant effect (−12.6 ± 2.0, −26.9 ± 5.4, −13.5 ± 1.4, and −22.4 ± 3.4 μA/cm2 for control, dexamethasone, dexamethasone + mifepristone, and dexamethasone + spironolactone, respectively, n = 6; Fig. 8A). In addition, dexamethasone-stimulated transcript expression of α-ENaC was significantly inhibited by mifepristone, but not spironolactone (Fig. 8B). Mifepristone is also known to be an antagonist of the progesterone receptor; however, the microarray data showed an absent detection call for progesterone receptor in Reissner's membrane (Table 3). These findings suggest that dexamethasone acts selectively at GR to exert changes in current density and gene expression.
Fig. 8.
Dexamethasone acts at GR to regulate current density and transcript expression. A: changes of current density from Reissner's membrane under control condition, dexamethasone (100 nM), dexamethasone + GR antagonist mifepristone (Mif, 100 nM), and dexamethasone + MR antagonist spironolactone (Spi, 100 nM). Values are means ± SE (n = 6). B: qRT-PCR evaluation of transcript expression of α-subunit of ENaC under control conditions and in the presence of dexamethasone, dexamethasone + mifepristone, and dexamethasone + spironolactone. Values are means ± SE (n = 4–10); numbers above bars represent number of qRT-PCRs. *P < 0.05. NS, not significant.
DISCUSSION
The present study was conducted to 1) determine the molecular identities of membrane transporters and key regulatory genes in Reissner's membrane, 2) determine whether their level of expression was regulated by corticosteroids using microarray and qRT-PCR, and 3) obtain functional evidence for the physiological importance of these genes by electrophysiological and pharmacological means. We have shown in this study that mouse Reissner's membrane absorbs Na+ from the cochlear lumen by electrogenic transepithelial transport, which is apparently mediated by apical ENaC, basolateral Na+-K+-ATPase, and basolateral K+ channels. The localization of these transporters was not investigated directly, but the expression of genes for all these transporters, the apical-to-basolateral direction of transepithelial current, the signal sequence on ENaC that directs it to the apical membrane (47), and the nearly complete blockade of transepithelial current by benzamil are consistent with this interpretation.
The rate of transport was found to be regulated by glucocorticoids through the GR, and the molecular identities of the transporters and regulatory genes were determined. The molecular evidence correlated with the functional electrophysiological and pharmacological results. The rate of Na+ absorption by the cochlear duct under normal conditions is slow (∼1%) compared with the rate of secretion of K+ (25), the ion species that carries the transduction current. The cochlear duct is therefore likely very resistant to Na+ leakage, requiring only a low absorption rate to maintain the low Na+ concentration. A low absorption rate is also consistent with the lack of direct vascular supply to Reissner's membrane, whereas the K+ secretory tissue, stria vascularis, is so named for its dense vasculature. Nonetheless, a pathological elevation of luminal [Na+] would be expected to load the sensory hair cells with Na+ through the nonselective cation transduction channels, leading to swelling, dysfunction, and deafness. Therefore, if the ion transport through the relatively large surface area of Reissner's membrane can be enhanced or inhibited by change of glucocorticoid levels, it could be a site of important therapeutic control for pathological symptoms related to endolymphatic hydrops as well as a physiological homeostatic mechanism.
There are unconfirmed reports of elevated endolymphatic [Na+] in an animal model of endolymphatic hydrops (36, 48), although whether this occurs in cases of Meniere's disease is not known. Nonetheless, in view of the extremely low [Na+] of endolymph, even a slightly increased leak would lead to sufficient excess Na+ in the lumen of the inner ear to draw significant quantities of water in and, thereby, lead to hydrops.
The multiplicity of subunits and genes for Na+-K+-ATPase and K+ channels is perhaps surprising but may reflect different requirements for function under various conditions. Primary cultures of semicircular canal epithelial cells ostensibly of a single cell type also expressed a multiplicity of genes involved in amiloride-sensitive transport (41). Alternatively, the multiplicity of genes in Reissner's membrane may partially reflect expression of some of these genes in the sparse mesothelial cells underlying the epithelial cell layer.
Glucocorticoids were previously reported to regulate ion homeostasis of inner ear fluids via genomic and nongenomic pathways in semicircular canal and stria vascularis (29, 41, 42). The involvement of a genomic pathway in Reissner's membrane epithelium shows that the cochlear and vestibular organs are controlled by glucocorticoids, with Reissner's membrane being a cochlear homolog of the vestibular semicircular canal in this respect.
Amiloride-sensitive channels.
The direction of current measured from the apical side of Reissner's membrane could be accounted for by cation absorption and/or anion secretion. However, only a cation-absorptive function was found in gerbil Reissner's membrane, even though strong adenylyl cyclase activity had been detected and earlier thought to stimulate Cl− secretion in Reissner's membrane (30). Results from the present study showed that the majority of current was decreased by amiloride analogs, where the order of potency was benzamil (∼10 times higher than amiloride) > amiloride >> EIPA, and there was no immediate response of current to forskolin/IBMX after Na+ absorption was blocked with amiloride. Furthermore, there was no change in current when genistein (30 μM, 3 min), a stimulator of cAMP-dependent Cl− currents via CFTR, was applied in the presence of 10 μM amiloride (data not shown). Transcripts for α-, β-, and γ-subunits of ENaC were detected in the microarray and qRT-PCR, whereas transcript for CFTR was not detected. These data suggest that the transepithelial current of mouse Reissner's membrane is primarily from Na+ absorption via ENaC, not cAMP-dependent Cl− secretion, findings similar to those from the gerbil. Nonetheless, Reissner's membrane may contain a significant Cl− conductive pathway, since whole cell reversal voltages were reported to be about −20 mV under presumptive physiological conditions (23) and transcripts for several anion transporters were reported by our gene microarray (data not shown). On the other hand, benzamil blockade of current left a small residual current (approximately −2 and approximately −1 μA/cm2 in control and dexamethasone-treated tissue, respectively), suggesting that other cation-absorbing or anion-secreting transporters may be coupled to transepithelial current.
The question of subunit composition of ENaC in Reissner's membrane is raised by the observation that the IC50 values of amiloride and benzamil were about 1 decade higher in Reissner's membrane than found for αβγ-ENaC. The potencies in Reissner's membrane are consistent with αβ-ENaC [IC50 ∼1–4 μM expressed in Xenopus oocytes (14, 34, 60)] but would also allow for an additional minor population of αβγ-ENaC, especially after incubation. Alternatively, the IC50 may be influenced by intracellular regulatory pathways, since it was reported that the IC50 of amiloride on Xenopus oocytes expressing rat αβγ-ENaC was increased over twofold by coexpression with hSGK, a kinase known to regulate ENaC (5).
It is conceivable that other amiloride-sensitive channels are also expressed in Reissner's membrane, such as cyclic nucleotide gated cation channels [amiloride IC50 ∼100 times that of αβγ-ENaC (13)] or heteromeric assembly of acid-sensitive ion channel subunits (ASICs) and ENaC, which increases the IC50 of amiloride when expressed in Xenopus oocytes (35). However, the participation of ASIC subunits in Reissner's membrane transepithelial transport is not likely, since ASICs are mostly inactive at the pH in our experiments and pH 6.5 led to a decrease in current, rather than the increase expected from acid-stimulated channels. Indeed, it was recently reported that H441 human lung epithelial cells contain ENaC and a nonselective cation channel of unknown molecular identity (but not cyclic nucleotide gated), both of which are sensitive to amiloride (1). The transepithelial current in those cells was sensitive to amiloride with an IC50 ∼0.6 μM, nearly identical to that in Reissner's membrane. Homomeric α-ENaC is amiloride sensitive and also thought to form nonselective cation channels (22, 24).
The transepithelial current from Reissner's membrane increased via genomic, but not nongenomic, action of dexamethasone, as determined by the long time scale (29). Dexamethasone increased the expression of transcripts for the α- and β-subunits of ENaC and without any change in the IC50 values for amiloride and benzamil, consistent with the notion that the active channel is composed of these two subunits and that additional channels are not involved in carrying the amiloride-sensitive currents in the presence or absence of glucocorticoid. The lack of regulation of γ-ENaC transcript expression by dexamethasone is consistent with reports of noncoordinate regulation of ENaC subunits in other cells and tissues at the mRNA and protein levels (8). The increased current in Reissner's membrane after exposure to dexamethasone was likely the result of increased expression of ENaC protein in the apical membrane of the epithelial cells due to increased insertion and reduced retrieval. Increased insertion would be indicated by the elevated transcript expression, whereas reduced retrieval would occur through the SGK1 signal pathway (52), which was also activated in Reissner's membrane.
Na+-K+-ATPase.
The driving force for Na+ absorption in gerbil Reissner's membrane was reported to be generated by the basolateral Na+-K+-ATPase in conjunction with a basolateral K+ channel (30). This was also found in the present study to occur in mouse Reissner's membrane from corroborative qRT-PCR and electrophysiological data. Dexamethasone-stimulated current was decreased by ouabain, an Na+-K+-ATPase blocker, but not by bumetanide, an NKCC blocker, demonstrating that dexamethasone-stimulated Na+ transport is dependent on Na+-K+-ATPase, but not NKCC. Since transcripts of NKCC1 were detected on the microarray, it is likely that NKCC1 is located in the mesothelial cells of Reissner's membrane or is only activated under other conditions, such as hypertonic challenge (4).
Transcripts for α1-, α2-, β1-, β2-, and β3-isoforms of Na+-K+-ATPase were detected, but none were upregulated as measured with the microarray and qRT-PCR. Dexamethasone was reported to upregulate transcript expression of the β1-subunit of Na+-K+-ATPase in rat lung and alveolar epithelial cells at 3 and 8 h, respectively, which then decreased until 48 h (10, 19). We incubated Reissner's membrane for 24 h; it is conceivable that transcripts of Na+-K+-ATPase might have peaked earlier and decreased at our time of collection, so that upregulation of Na+-K+-ATPase would not be detected. However, α1- and β1-subunits in INS-1 cells and β2-subunit in semicircular duct epithelial cells were upregulated or remained upregulated as late as 24 h (41, 54), and the time course of glucocorticoid-stimulated Na+-K+-ATPase regulation may vary with tissue and cell types.
K+ channels.
K+-permeable channels are needed in the basolateral membrane of Na+-absorbing epithelia to recycle K+ brought into the cell by the Na+-K+-ATPase; the molecular identity of K+ channel(s) in mouse Reissner's membrane is likely different from that in the gerbil, since the pharmacological sensitivities differ (30). We selected nine K+ channels as candidates for electrophysiological and qRT-PCR study on the basis of the microarray data. The Ba2+-, quinine-, and 4-AP-sensitive Kv1.5 K+ channels (KCNA5) were pharmacologically identified in Reissner's membrane of the gerbil (30), but not the mouse (Table 3). Our results point to the participation of multiple K+ channels in the establishment of the cellular K+ conductance.
The most significant K+ channel in glucocorticoid-stimulated transepithelial Na+ transport by mouse Reissner's membrane is likely to be KCNK1, since it is the only upregulated K+ channel after dexamethasone exposure and is sensitive to low pH. The low pH was not likely a direct effect on ENaC, since αβ-ENaC activity is not significantly decreased until pH values much lower than that tested here (60). However, none of the K+ channel blockers applied in this study fully decreased the current from Reissner's membrane. The findings that none of the K+ channel inhibitors fully decreased the current density may point to a nonselective cation channel in the basolateral membrane through which K+ recycled.
Glucocorticoid regulation of Na+ transport via regulatory genes.
Na+ transport is regulated through control of corticosteroid receptor agonist levels (via 11β-HSD1/2) and the numbers of ENaC channels inserted in the apical membrane by control of membrane traffic and protein degradation (via the kinases WNK, SGK, and ubiquitin ligase Nedd4-2) (7, 26, 50).
The transcripts for 11β-HSD1, but not 11β-HSD2, were found in Reissner's membrane in the present study and upregulated by dexamethasone, which supports the conclusion that Reissner's membrane is a glucocorticoid-selective responsive tissue. Substitution of 9α-fluor in synthetic glucocorticoids, such as dexamethasone, was reported to increase chemical reduction of glucocorticoids by 11β-HSD1 in glucocorticoid-selective responsive tissue (11). In addition, transcripts of 11β-HSD1 were reported to be increased in rat hippocampus tissue (32) and human osteoblast (9) by dexamethasone, and dexamethasone increased transcriptional activity of the 11β-HSD1 site in 2S FAZA cells (56).
Additional results support the conclusion that Na+ absorption in Reissner's membrane is stimulated by glucocorticoid activation of GR, and not MR. First, dexamethasone increased current density and transcript expression of ENaC via the GR, SGK1, and Nedd4-2 pathway in Reissner's membrane, as also observed in vestibular semicircular canal epithelial cells (41). Second, current density and transcripts of α-ENaC were not altered by dexamethasone in the presence of a GR blocker but were stimulated and upregulated in the presence of an MR blocker.
Recently, another class of kinases (WNKs) has been reported to be associated with the regulation of ENaC, with isoform-specific actions. WNK1 activates SGK1 to stimulate ENaC-mediated Na+ transport when expressed in Xenopus oocytes and Chinese hamster ovary cells (39, 59). By contrast, WNK4 decreases Na+ currents by accelerating degradation mediated by Nedd4-2 when expressed in Xenopus laevis oocytes (45). However, when WNK4 is phosphorylated by SGK1, the inhibition of Na+ transport is lessened when expressed in BL21(DE3) cells, HEK-293T cells, and X. laevis oocytes (46).
This is the first report to identify the involvement of WNK as a likely Na+ transport regulator in the inner ear. All four of these isoforms are expressed in Reissner's membrane. However, only WNK4 mRNA was upregulated after dexamethasone exposure in Reissner's membrane, which suggests that WNK4 may be involved in the dexamethasone-stimulated Na+ transport pathway. WNK4 has also been reported to be involved in the regulation of Na+ transport mediated by the Na+-Cl− cotransporter and NKCC1, but transcripts for the Na+-Cl− cotransporter were not found with the microarray (data not shown), and there was no apparent functional activity of NKCC in our experiments. We therefore propose that Na+ transport in Reissner's membrane operates at a reduced rate in the absence of steroid due in part to inhibition by WNK4. Increased expression of SGK1 (and WNK4) in the presence of steroid leads to increased phosphorylated WNK4 and, consequently, increased Na+ transport mediated by increased ENaC in the apical membrane.
Physiological and clinical significance.
Meniere's disease is a disorder of the auditory and vestibular periphery that is clinically characterized by fluctuating hearing loss with episodic vertigo and tinnitus. A frequent correlate of Meniere's disease is swelling of the luminal compartment of the inner ear (endolymphatic hydrops), which can be due to hypersecretion or hypoabsorption of the luminal fluid, endolymph. Channelopathies of inner ear epithelial cells have been suggested as one important etiology of endolymphatic hydrops (12, 15), and, in some studies, hypoabsorption of Na+ from the endolymph has been reported to be associated with endolymphatic hydrops (36, 48). Recently, synthetic glucocorticoids, such as dexamethasone and prednisolone, have been used to treat Meniere's disease and have been effective in controlling vertigo in ∼52–76% of cases (3). It is tempting to speculate that some cases of Meniere's disease result from increased endolymphatic [Na+] and volume and that effective treatment by glucocorticoids is a result of increased cochlear Na+ absorption by Reissner's membrane.
In conclusion, we demonstrated Na+ absorption by mouse Reissner's membrane that is mediated by apical ENaC, basolateral Na+-K+-ATPase, and K+-permeable channels and is under the control of the synthetic glucocorticoid dexamethasone and glucocorticoid regulatory genes such as 11β-HSD1, SGK1, Nedd4-2, and WNK4. These results provide an understanding and molecular definition of an important transport function of Reissner's membrane epithelium in the homeostasis of Na+ in cochlear endolymph.
GRANTS
This work was supported by National Institutes of Health Grants R01-DC-00212 and P20-RR-017686.
Acknowledgments
We thank Dr. Philine Wangemann for helpful discussions and suggestions and Dr. Daisuke Yamauchi, Dr. Kazuhiro Nakaya, Donald Harbidge, and Joel Sanneman for assistance with preliminary experiments.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Albert AP, Woollhead AM, Mace OJ, Baines DL. AICAR decreases the activity of two distinct amiloride-sensitive Na+-permeable channels in H441 human lung epithelial cell monolayers. Am J Physiol Lung Cell Mol Physiol 295: L837–L848, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balis FM, Lester CM, Chrousos GP, Heideman RL, Poplack DG. Differences in cerebrospinal fluid penetration of corticosteroids: possible relationship to the prevention of meningeal leukemia. J Clin Oncol 5: 202–207, 1987. [DOI] [PubMed] [Google Scholar]
- 3.Barrs DM, Keyser JS, Stallworth C, McElveen JT Jr. Intratympanic steroid injections for intractable Meniere's disease. Laryngoscope 111: 2100–2104, 2001. [DOI] [PubMed] [Google Scholar]
- 4.Bildin VN, Yang H, Crook RB, Fischbarg J, Reinach PS. Adaptation by corneal epithelial cells to chronic hypertonic stress depends on upregulation of Na:K:2Cl cotransporter gene and protein expression and ion transport activity. J Membr Biol 177: 41–50, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Bohmer C, Wagner CA, Beck S, Moschen I, Melzig J, Werner A, Lin JT, Lang F, Wehner F. The shrinkage-activated Na+ conductance of rat hepatocytes and its possible correlation to rENaC. Cell Physiol Biochem 10: 187–194, 2000. [DOI] [PubMed] [Google Scholar]
- 6.Braat MC, Oosterhuis B, Koopmans RP, Meewis JM, Van Boxtel CJ. Kinetic-dynamic modeling of lymphocytopenia induced by the combined action of dexamethasone and hydrocortisone in humans, after inhalation and intravenous administration of dexamethasone. J Pharmacol Exp Ther 262: 509–515, 1992. [PubMed] [Google Scholar]
- 7.Butterworth MB, Edinger RS, Frizzell RA, Johnson JP. Regulation of the epithelial sodium channel by membrane trafficking. Am J Physiol Renal Physiol 296: F10–F24, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterworth MB, Weisz OA, Johnson JP. Some assembly required: putting the epithelial sodium channel together. J Biol Chem 283: 35305–35309, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper MS, Rabbitt EH, Goddard PE, Bartlett WA, Hewison M, Stewart PM. Osteoblastic 11β-hydroxysteroid dehydrogenase type 1 activity increases with age and glucocorticoid exposure. J Bone Miner Res 17: 979–986, 2002. [DOI] [PubMed] [Google Scholar]
- 10.Dagenais A, Denis C, Vives MF, Girouard S, Masse C, Nguyen T, Yamagata T, Grygorczyk C, Kothary R, Berthiaume Y. Modulation of α-ENaC and α1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol 281: L217–L230, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Diederich S, Eigendorff E, Burkhardt P, Quinkler M, Bumke-Vogt C, Rochel M, Seidelmann D, Esperling P, Oelkers W, Bahr V. 11β-Hydroxysteroid dehydrogenase types 1 and 2: an important pharmacokinetic determinant for the activity of synthetic mineralo- and glucocorticoids. J Clin Endocrinol Metab 87: 5695–5701, 2002. [DOI] [PubMed] [Google Scholar]
- 12.Doi K, Sato T, Kuramasu T, Hibino H, Kitahara T, Horii A, Matsushiro N, Fuse Y, Kubo T. Meniere's disease is associated with single nucleotide polymorphisms in the human potassium channel genes, KCNE1 and KCNE3. ORL J Otorhinolaryngol Relat Spec 67: 289–293, 2005. [DOI] [PubMed] [Google Scholar]
- 13.Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. J Gen Physiol 100: 45–67, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fyfe GK, Canessa CM. Subunit composition determines the single channel kinetics of the epithelial sodium channel. J Gen Physiol 112: 423–432, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gates P Hypothesis: could Meniere's disease be a channelopathy? Intern Med J 35: 488–489, 2005. [DOI] [PubMed] [Google Scholar]
- 16.Gil-Loyzaga P, Pujol R. Synaptophysin in the developing cochlea. Int J Dev Neurosci 6: 155–160, 1988. [DOI] [PubMed] [Google Scholar]
- 17.Hadley JK, Noda M, Selyanko AA, Wood IC, Abogadie FC, Brown DA. Differential tetraethylammonium sensitivity of KCNQ1–4 potassium channels. Br J Pharmacol 129: 413–415, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagiwara S, Miyazaki S, Moody W, Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol 279: 167–185, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hao H, Wendt CH, Sandhu G, Ingbar DH. Dexamethasone stimulates transcription of the Na+-K+-ATPase β1 gene in adult rat lung epithelial cells. Am J Physiol Lung Cell Mol Physiol 285: L593–L601, 2003. [DOI] [PubMed] [Google Scholar]
- 20.Heitzmann D, Koren V, Wagner M, Sterner C, Reichold M, Tegtmeier I, Volk T, Warth R. KCNE β-subunits determine pH sensitivity of KCNQ1 potassium channels. Cell Physiol Biochem 19: 21–32, 2007. [DOI] [PubMed] [Google Scholar]
- 21.Jabba SV, Oelke A, Singh R, Maganti RJ, Fleming SD, Wall SM, Green ED, Wangemann P. Macrophage invasion contributes to degeneration of stria vascularis in Pendred syndrome mouse model. BMC Med 4: 37, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain L, Chen XJ, Malik B, Al Khalili OK, Eaton DC. Antisense oligonucleotides against the α-subunit of ENaC decrease lung epithelial cation-channel activity. Am J Physiol Lung Cell Mol Physiol 276: L1046–L1051, 1999. [DOI] [PubMed] [Google Scholar]
- 23.King M, Housley GD, Raybould NP, Greenwood D, Salih SG. Expression of ATP-gated ion channels by Reissner's membrane epithelial cells. Neuroreport 9: 2467–2474, 1998. [DOI] [PubMed] [Google Scholar]
- 24.Kizer N, Guo XL, Hruska K. Reconstitution of stretch-activated cation channels by expression of the α-subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA 94: 1013–1018, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konishi T, Hamrick PE, Walsh PJ. Ion transport in guinea pig cochlea. I. Potassium and sodium transport. Acta Otolaryngol 86: 22–34, 1978. [DOI] [PubMed] [Google Scholar]
- 26.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V. (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 86: 1151–1178, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Lang F, Vallon V, Knipper M, Wangemann P. Functional significance of channels and transporters expressed in the inner ear and kidney. Am J Physiol Cell Physiol 293: C1187–C1208, 2007. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Chiba T, Marcus DC. P2X2 receptor mediates stimulation of parasensory cation absorption by cochlear outer sulcus cells and vestibular transitional cells. J Neurosci 21: 9168–9174, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee JH, Marcus DC. Nongenomic effects of corticosteroids on ion transport by stria vascularis. Audiol Neurootol 7: 100–106, 2002. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Marcus DC. Endolymphatic sodium homeostasis by Reissner's membrane. Neuroscience 119: 3–8, 2003. [DOI] [PubMed] [Google Scholar]
- 31.Lian JB, Stein GS, Stewart C, Puchacz E, Mackowiak S, Aronow M, Von Deck M, Shalhoub V. Osteocalcin: characterization and regulated expression of the rat gene. Connect Tissue Res 21: 61–68, 1989. [DOI] [PubMed] [Google Scholar]
- 32.Low SC, Moisan MP, Noble JM, Edwards CR, Seckl JR. Glucocorticoids regulate hippocampal 11β-hydroxysteroid dehydrogenase activity and gene expression in vivo in the rat. J Neuroendocrinol 6: 285–290, 1994. [DOI] [PubMed] [Google Scholar]
- 33.Marcus DC, Chiba T. K+ and Na+ absorption by outer sulcus epithelial cells. Hear Res 134: 48–56, 1999. [DOI] [PubMed] [Google Scholar]
- 34.McNicholas CM, Canessa CM. Diversity of channels generated by different combinations of epithelial sodium channel subunits. J Gen Physiol 109: 681–692, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meltzer RH, Kapoor N, Qadri YJ, Anderson SJ, Fuller CM, Benos DJ. Heteromeric assembly of acid-sensitive ion channel and epithelial sodium channel subunits. J Biol Chem 282: 25548–25559, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Morgenstern C, Mori N, Amano H. Pathogenesis of experimental endolymphatic hydrops. Acta Otolaryngol Suppl 406: 56–58, 1984. [DOI] [PubMed] [Google Scholar]
- 37.Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci USA 102: 16102–16106, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser SL, Harron SA, Crack J, Fawcett JP, Cowley EA. Multiple KCNQ potassium channel subtypes mediate basal anion secretion from the human airway epithelial cell line Calu-3. J Membr Biol 221: 153–163, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Naray-Fejes-Toth A, Snyder PM, Fejes-Toth G. The kidney-specific WNK1 isoform is induced by aldosterone and stimulates epithelial sodium channel-mediated Na+ transport. Proc Natl Acad Sci USA 101: 17434–17439, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Connell AD, Morton MJ, Hunter M. Two-pore domain K+ channels-molecular sensors. Biochim Biophys Acta 1566: 152–161, 2002. [DOI] [PubMed] [Google Scholar]
- 41.Pondugula SR, Raveendran NN, Ergonul Z, Deng Y, Chen J, Sanneman JD, Palmer LG, Marcus DC. Glucocorticoid regulation of genes in the amiloride-sensitive sodium transport pathway by semicircular canal duct epithelium of neonatal rat. Physiol Genomics 24: 114–123, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Pondugula SR, Sanneman JD, Wangemann P, Milhaud PG, Marcus DC. Glucocorticoids stimulate cation absorption by semicircular canal duct epithelium via epithelial sodium channel. Am J Physiol Renal Physiol 286: F1127–F1135, 2004. [DOI] [PubMed] [Google Scholar]
- 43.Rajan S, Plant LD, Rabin ML, Butler MH, Goldstein SA. Sumoylation silences the plasma membrane leak K+ channel K2P1. Cell 121: 37–47, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Reddy MM, Wang XF, Quinton PM. Effect of cytosolic pH on epithelial Na+ channel in normal and cystic fibrosis sweat ducts. J Membr Biol 225: 1–11, 2008. [DOI] [PubMed] [Google Scholar]
- 45.Ring AM, Cheng SX, Leng Q, Kahle KT, Rinehart J, Lalioti MD, Volkman HM, Wilson FH, Hebert SC, Lifton RP. WNK4 regulates activity of the epithelial Na+ channel in vitro and in vivo. Proc Natl Acad Sci USA 104: 4020–4024, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ring AM, Leng Q, Rinehart J, Wilson FH, Kahle KT, Hebert SC, Lifton RP. An SGK1 site in WNK4 regulates Na+ channel and K+ channel activity and has implications for aldosterone signaling and K+ homeostasis. Proc Natl Acad Sci USA 104: 4025–4029, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rotin D, Bar-Sagi D, O'Brodovich H, Merilainen J, Lehto VP, Canessa CM, Rossier BC, Downey GP. An SH3 binding region in the epithelial Na+ channel (α-rENaC) mediates its localization at the apical membrane. EMBO J 13: 4440–4450, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Silverstein H, Takeda T. Endolymphatic sac obstruction. Biochemical studies. Ann Otol Rhinol Laryngol 86: 493–499, 1977. [DOI] [PubMed] [Google Scholar]
- 49.Singh R, Wangemann P. Free radical stress-mediated loss of KCNJ10 protein expression in stria vascularis contributes to deafness in Pendred syndrome mouse model. Am J Physiol Renal Physiol 294: F139–F148, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Snyder PM Regulation of epithelial Na+ channel trafficking. Endocrinology 146: 5079–5085, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Sterkers O, Saumon G, Tran Ba Huy P, Amiel C. K, Cl, and H2O entry in endolymph, perilymph, and cerebrospinal fluid of the rat. Am J Physiol Renal Fluid Electrolyte Physiol 243: F173–F180, 1982. [DOI] [PubMed] [Google Scholar]
- 52.Stockand JD New ideas about aldosterone signaling in epithelia. Am J Physiol Renal Physiol 282: F559–F576, 2002. [DOI] [PubMed] [Google Scholar]
- 53.Taglialatela M, VanDongen AM, Drewe JA, Joho RH, Brown AM, Kirsch GE. Patterns of internal and external tetraethylammonium block in four homologous K+ channels. Mol Pharmacol 40: 299–307, 1991. [PubMed] [Google Scholar]
- 54.Ullrich S, Zhang Y, Avram D, Ranta F, Kuhl D, Haring HU, Lang F. Dexamethasone increases Na+/K+ ATPase activity in insulin secreting cells through SGK1. Biochem Biophys Res Commun 352: 662–667, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Vega-Saenz de Miera EC, Moreno H, Fruhling D, Kentros C, Rudy B. Cloning of ShIII (Shaw-like) cDNAs encoding a novel high-voltage-activating, TEA-sensitive, type-A K+ channel. Proc Biol Sci 248: 9–18, 1992. [DOI] [PubMed] [Google Scholar]
- 56.Voice MW, Seckl JR, Edwards CR, Chapman KE. 11β-Hydroxysteroid dehydrogenase type 1 expression in 2S FAZA hepatoma cells is hormonally regulated: a model system for the study of hepatic glucocorticoid metabolism. Biochem J 317: 621–625, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wangemann P Cochlear homeostasis and homeostatic disorders. In: Handbook of Auditory Research. Auditory Trauma and Protection, edited by Schacht J, Popper AN, Fay RR. New York: Springer, 2008.
- 58.Wangemann P, Itza EM, Albrecht B, Wu T, Jabba SV, Maganti RJ, Lee JH, Everett LA, Wall SM, Royaux IE, Green ED, Marcus DC. Loss of KCNJ10 protein expression abolishes endocochlear potential and causes deafness in Pendred syndrome mouse model. BMC Med 2: 30, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xu BE, Stippec S, Chu PY, Lazrak A, Li XJ, Lee BH, English JM, Ortega B, Huang CL, Cobb MH. WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc Natl Acad Sci USA 102: 10315–10320, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang P, Fyfe GK, Grichtchenko II, Canessa CM. Inhibition of α,β epithelial sodium channels by external protons indicates that the second hydrophobic domain contains structural elements for closing the pore. Biophys J 77: 3043–3051, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]