Abstract
Inflammatory brain disease may damage cerebral vascular endothelium leading to cerebral blood flow dysregulation. The proinflammatory cytokine TNF-α causes oxidative stress and apoptosis in cerebral microvascular endothelial cells (CMVEC) from newborn pigs. We investigated contribution of major cellular sources of reactive oxygen species to endothelial inflammatory response. Nitric oxide synthase and xanthine oxidase inhibitors (Nω-nitro-l-arginine and allopurinol) had no effect, while mitochondrial electron transport inhibitors (CCCP, 2-thenoyltrifluoroacetone, and rotenone) attenuated TNF-α-induced superoxide (O2•−) and apoptosis. NADPH oxidase inhibitors (diphenylene iodonium and apocynin) greatly reduced TNF-α-evoked O2•− generation and apoptosis. TNF-α rapidly increased NADPH oxidase activity in CMVEC. Nox4, the cell-specific catalytic subunit of NADPH oxidase, is highly expressed in CMVEC, contributes to basal O2•− production, and accounts for a burst of oxidative stress in response to TNF-α. Nox4 small interfering RNA, but not Nox2, knockdown prevented oxidative stress and apoptosis caused by TNF-α in CMVEC. Nox4 is colocalized with HO-2, the constitutive isoform of heme oxygenase (HO), which is critical for endothelial protection against TNF-α toxicity. The products of HO activity, bilirubin and carbon monoxide (CO, as a CO-releasing molecule, CORM-A1), inhibited Nox4-generated O2•− and apoptosis caused by TNF-α stimulation. We conclude that Nox4 is the primary source of inflammation- and TNF-α-induced oxidative stress leading to apoptosis in brain endothelial cells. The ability of CO and bilirubin to combat TNF-α-induced oxidative stress by inhibiting Nox4 activity and/or by O2•− scavenging, taken together with close intracellular compartmentalization of HO-2 and Nox4 in cerebral vascular endothelium, may contribute to HO-2 cytoprotection against inflammatory cerebrovascular disease.
Keywords: inflammatory brain disease, brain endothelium, blood-brain barrier, cerebral vascular injury, heme oxygenase isoform-2, carbon monoxide-releasing molecule-A1, reduced nicotinamide adenine dinucleotide phosphatase oxidase isoforms 2 and 4, bilirubin, antioxidants
inflammation plays an important role in pathogenesis of neonatal brain diseases, including meningitis, septic shock, brain trauma, asphyxia, ischemia, and seizures. Inflammatory brain diseases may damage cerebral vessels and cause cerebral blood flow dysregulation thus adding to a secondary neurological sequelae. Of the various proinflammatory cytokines, tumor necrosis factors-α (TNF-α) plays a pivotal role in inflammatory brain disease (4, 6, 52). Recently, we showed that TNF-α targets cerebral vascular endothelial cells, increases formation of reactive oxygen species (ROS), and causes apoptosis (5) that may lead to inflammatory cerebral vascular dysfunction. Superoxide anion (O2•−) appears to be a primary mediator of TNF-α-induced oxidative stress in cerebral vascular endothelium. ROS production is provided by numerous enzyme complexes, including NADPH oxidase, nitric oxide (NO) synthase, xanthine oxidase, and the mitochondrial respiratory chain. However, the cellular sources generating proapoptotic O2•− in response to TNF-α in cerebral vascular endothelium are not known.
NADPH oxidase (Nox family), which was originally discovered in phagocytes, is a potent cellular source of O2•− in the cardiovascular system (7, 20, 22, 25, 38, 45) and in the brain (2, 19, 26, 27, 33, 50). NADPH oxidase components include flavocytochrome b558, an integral membrane heterodimer composed of the large catalytic Nox1–Nox5 subunits (homologues of phagocytic gp91phox/Nox2) associated with the small anchoring p22phox subunit, and the cytoplasmic regulatory subunits p47phox, p67phox, p40phox, and small GTPase Rac1/2 (7, 17, 22). NADPH oxidase is posttranslationally activated to produce oxidative bursts in response to various stimuli. Posttranslational activation of NADPH oxidase occurs in a cell- and signal-specific manner and may include phosphorylation and spatial relocation of the cytosolic subunits to the catalytic cytochrome to form the active enzyme complex (14, 22, 29).
NADPH oxidase has important functions in cerebral circulation that include regulation of vascular smooth muscle tone and modulation of endothelium-dependent dilator responses (10, 15, 27, 33, 36). NADPH oxidase activation has been also implicated in loss of neuronal coupling to cerebral blood flow (27), disruption of the blood-brain barrier following stroke (26), and in apoptosis (33, 36). Among other catalytic Nox homologues, high expression of Nox4 appears to be a specific characteristic of vascular cells (3, 13, 22, 28, 38, 39, 43, 49, 51), although Nox2 expression also has been reported (9, 43, 49). Nox4 upregulation has been implicated in the development of cardiovascular pathologies (43, 45). Current knowledge on the role of distinct Nox isoforms in cerebral circulation remains very limited. Nox4 in the human brain cortex is upregulated following cerebral ischemia (50). Recent reports indicate the importance of gp91phox/Nox2 and Nox4 in the regulation of cerebral vascular tone (27, 33, 36). However, the functions of Nox4 in cerebral vascular endothelium have not been investigated.
Heme oxygenase (HO), in cooperation with biliverdin reductase, catalyzes the degradation of intracellular heme to carbon monoxide (CO) and bilirubin and functions as an endogenous antioxidant defense system in various cells (1, 37, 40, 41, 48). Inducible HO-1 is important in cytoprotection against sustained oxidative stress caused by inflammatory disease (1, 40, 48), whereas constitutively expressed HO-2 may protect against damage caused by acute oxidative stress (37). In cerebral vascular endothelial cells, HO-2 is critical for immediate cytoprotection against apoptosis caused by TNF-α. Brain endothelial cells from HO-2 knockout mice are more susceptible to TNF-α-induced apoptosis than are wild-type cells (5). Pharmacological inhibition of HO-2 activity in brain endothelial cells from newborn pigs augments apoptosis caused by TNF-α (5). The HO-2-mediated mechanism of cytoprotection against apoptosis caused by inflammation is far from clear. Previously, we reported that the products of HO activity, bilirubin and CO, have antioxidant properties in cerebral vascular endothelium exposed to inflammatory stimulation (5). However, the ROS-producing targets for the antioxidant effects of the HO products in cerebral vascular endothelium have not been identified.
The present study using primary cultures of cerebral vascular endothelial cells addresses the hypotheses that 1) Nox4 NADPH oxidase is the primary source of proapoptotic ROS evoked by TNF-α and 2) CO and bilirubin protect against inflammation-induced apoptosis by inhibiting Nox4-derived O2•− radicals.
METHODS
Cerebral microvascular endothelial cells.
All protocols and procedures that involve animals were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee, Memphis. Cerebral microvessels (60–300 μm) obtained from the brain cortex of newborn pigs (1–5 days old) were digested by collagenase-dispase, and cerebral microvascular endothelial cells (CMVEC) were separated by density gradient centrifugation as we described elsewhere (5). CMVEC were plated on Matrigel-coated plates or glass coverslips and were cultured for 5–6 days until confluence in Dulbecco's modified Eagle's medium (DMEM) containing 20% fetal bovine serum (FBS), 30 μg/ml endothelial cell growth supplement, 1 U/ml heparin, and antibiotic-antimycotic mixture. CMVEC identified by von Willebrand factor accounted for >90% of the cell population. All experiments were performed on confluent quiescent cells in primary cultures. To achieve quiescence, CMVEC were exposed overnight to 0.1% FBS-DMEM.
Nox small interfering RNA-mediated gene silencing.
CMVEC were transfected with Nox4 small interfering RNA (siRNA), Nox2 siRNA, or nontargeting control siRNA (Dharmacon RNA Technologies, Lafayette, CO) according to standard protocols. Briefly, confluent CMVEC were replated to 12-well plates (3 × 105 cells/ml) and grown in 20% FBS-DMEM without antibiotic for 24 h to 60–70% confluence. To prepare the transfection complex, DharmaFECT-1 transfection reagent (3 μl/well) was incubated with Nox4 siRNA, Nox2 siRNA, or control siRNA (100 pmol/well) in antibiotic- and serum-free medium for 30 min at room temperature. For siRNA transfection, cells were incubated with the siRNA-DharmaFECT-1 complexes in antibiotic- and serum-free medium for 24 h at 37°C. For recovery, the cells were cultured in 20% FBS-DMEM (antibiotic free) for another 72 h. Before the experiment, CMVEC were serum deprived overnight in antibiotic-free 0.1% FBS-DMEM.
Experimental treatments.
CMVEC were untreated or treated with TNF-α (15 ng/ml) for 1–3 h at 37°C in the presence or absence of ROS inhibitors. To inhibit NADPH oxidase, we used diphenyliodonium (DPI, 5–200 μM) and apocynin (0.1–2 mM) (10, 24, 31, 53, 55, 56). To inhibit the Rac1 component of NADPH oxidase, we used the Rac1 inhibitor NSC-23766 (10–100 μM, 16, 42). To inhibit the mitochondrial electron transport chain, we used carbonyl cyanide 3-chlorophenylhydrazone (CCCP; respiratory uncoupling agent, 5–10 μM), 2-thenoyltrifluoroacetone (TTFA; complex II inhibitor, 5–10 μM), and rotenone (complex I inhibitor; 5–10 μM) (53, 55, 56). Nω-nitro-l-arginine (l-NNA; 0.5–2 mM) and allopurinol (50–100 μM) were used to inhibit NO synthase and xanthine oxidase, respectively (53, 56). To block overall O2•− content, we used the cell-permeable superoxide dismutase polyethylene glycol (PEG)-SOD (1,000 units), an O2•− scavenger (5, 10, 55). To select the maximally effective concentrations for our cell system, we conducted preliminary experiments using a wide concentration range of the inhibitor (5- to 50-fold over the Ki value).
Detection of O2•− generation.
Dihydroethidium (DHE) is oxidized by O2•− to ethidium and oxyethidium, intercalates with DNA in the nucleus, and emits red fluorescence. The O2•− production by cultured cells was quantitatively detected by fluorescence spectroscopy as we described previously (5). CMVEC grown on 12-well plates were incubated with 15 ng/ml TNF-α alone or with ROS inhibitors for 1 h at 37°C. DHE (20 μM) was added to the incubation media for an additional 20 min. TNF-α causes an apoptosis-related cell detachment and increases O2•− level in both attached and detached cells (5). To detect overall O2•− generation, we collected attached and floating cells by scraping and aspiration. The pooled cells were pelleted by centrifugation and washed twice with ice-cold Dulbecco's phosphate-buffered saline (DPBS). CMVEC were sonicated in ice-cold DPBS, and the lysates were cleared by centrifugation. Supernatants were transferred to Falcon 96-well black plates, and ethidium fluorescence (excitation/emission maxima, 485/590 nm) was measured by the Synergy HT multi-mode microplate reader (BioTek Instruments, Winooski, VT) and normalized to the protein amount.
Detection of DNA fragmentation.
DNA fragmentation was detected using a cell death detection kit (Roche Applied Science, Indianapolis, IN) by formation of cytoplasmic histone-associated DNA fragments (5). CMVEC were treated with TNF-α (15 ng/ml) for 3 h at 37°C. Detached floating cells were collected by aspiration for counting. Attached cells were lysed with the lysis buffer. Aliquots of the nuclei-free supernatant were placed in streptavidin-coated wells and incubated with anti-histone-biotin antibody and anti-DNA peroxidase-conjugated antibody for 2 h at room temperature. Immunobilized DNA fragments were visualized with 2,2′-azino-di(3-ethylbenzthiazolin-sulfonate), and the absorbance at 405 nm was normalized to the protein amount.
Protein detection.
To detect the amount of protein in samples dissolved in Laemmli sample buffer before Western immunoblotting, we used the amidoblack dot-blot method (5). For all other purposes, cell protein was measured by the bicinchoninic acid method (Pierce, Rockford, IL).
Western immunoblotting.
Proteins (10–20 μg/lane) were separated by 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and blocked with 5% milk-0.1% Tween 20. Cleaved caspase-3 fragments (17 kDa) were detected by anti-caspase-3-Asp 175 polyclonal antibodies (Cell Signaling, Beverly, MA). Nox4 was detected by anti-Nox4 (N-15) polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA) or by anti-Nox4 polyclonal antibodies to a COOH-terminal region of the human protein (Novus Biologicals, Littleton, CO). Nox2 was detected using polyclonal antibodies to a human gp91phox peptide (Upstate, Temecula, CA). Peroxidase-conjugated secondary antibodies were from Sigma (St. Louis, MO). The membranes were reprobed for actin as a loading control using monoclonal anti-actin (Roche Molecular Biochemicals, Indianapolis, IN). The immunocomplexes were visualized with the Western lightning chemiluminescence kit (Perkin Elmer Life Sciences, Shelton, CT) and digitally quantified using ImageJ 1.33 software (National Institutes of Health, Bethesda, MD).
Immunostaining.
Cells were fixed with 3.7% paraformaldehyde (20 min), permeabilized with 0.1% Triton X-100 (20 min), and blocked with 5% bovine serum albumin-DPBS. The cells were incubated with monoclonal antibodies against Nox4 (1:30), p22phox (1:30), or p47phox (1:30) (all from Santa Cruz Biotechnology) for 1 h at 37°C followed by FITC-conjugated secondary antibodies (Vector Laboratories, Burlingame, CA) for 1 h at 37°C. For F-actin staining, cells were additionally incubated with rhodamine-phalloidin (1:100, Molecular Probes, Eugene, OR). To detect colocalization of HO-2 and Nox4, cells were first incubated with anti-Nox4 monoclonal antibodies for 1 h (as above) followed by anti-HO-2 polyclonal antibodies (1:50; StressGen Biotechnologies, Victoria, BC, Canada) for 1 h. Primary antibodies were visualized with FITC- and Cy3-conjugated secondary antibodies (Vector Laboratories) for 1 h at 37°C. Coverslips were mounted using anti-fade mounting medium with a nuclear counterstain, 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories). Slides were viewed using Nikon Diaphot fluorescence microscope equipped with an image deconvolution system. Images were deconvolved and processed using IPLab Spectrum software and Adobe Photoshop (Adobe Systems).
NADPH oxidase activity.
NADPH oxidase activity in cell homogenates was measured by O2•− production from NADPH using the lucigenin-enhanced luminescence assay (21). CMVEC were lysed by sonication in 5 ml of ice-cold lysis buffer (20 mM KH2PO4, 1 mM EGTA) containing a protease inhibitor cocktail (Sigma). NADPH oxidase activity was measured in a reaction mixture containing 50 mM KH2PO4 (pH 7.0), 1 mM EGTA, 150 mM sucrose, and protease inhibitors (total volume, 300 μl). Cell homogenates (∼100 μg protein) were incubated with 100 μM NADPH in the absence or presence of TNF-α (15 ng/ml), ROS inhibitors (DPI, apocynin, and SOD), bilirubin, and CO-releasing molecule (CORM)-A1 for 40 min at 37°C. Lucigenin (50 μM) was added to the reaction mixture, and the incubation continued for 20 min. Luminescence indicative of O2•− concentration was measured by a Synergy HT microplate reader (BioTek Instruments) and normalized to the protein amount.
Statistical analysis.
Data are presented as means ± SE of absolute values or percentage of control. Data were analyzed by analysis of variance for repeated measurements, followed by Fisher's test for protected least significant difference to isolate differences between groups. A level of P < 0.05 was considered significant in all statistical tests.
Materials.
Cell culture reagents were purchased from Life Technologies (Gaithersburg, MD), Hyclone (South Logan, UT), and Amersham Pharmacia Biotech (Piscataway, NJ). Human TNF-α and Matrigel were from BD Biosciences (Bedford, MA). Apocynin and Rac1 inhibitor (NSC-23766) were from Calbiochem (San Diego, CA). Dihydroethidium was from Molecular Probes. CORM-A1 was a generous gift from Hector Knight from Tyco-Mallincrodt Medical (Petten, Holland). All other reagents were from Sigma.
RESULTS
Effects of ROS inhibitors on TNF-α-induced oxidative stress in CMVEC.
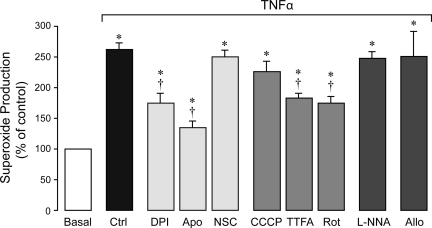
TNF-α (15 ng/ml, 1 h) increased O2•− formation two- to threefold above the baseline as detected by hydroethidium spectroscopy (Fig. 1). We investigated the contributions of distinct ROS-generating cellular systems, NADPH oxidase, mitochondria, NO synthase, and xanthine oxidase to TNF-α-induced oxidative stress (Fig. 1). NADPH oxidase inhibitors, DPI (5–100 μM) and apocynin (0.5–2 mM), inhibited TNF-α-induced O2•− production by 50–70%, whereas the inhibitor of the Rac1 component, NSC-23766 (10–100 μM), did not affect the oxidative stress. Mitochondrial electron transport chain inhibitors, CCCP (respiratory uncoupling reagent, 5–10 μM), TTFA (complex II inhibitor, 5–10 μM), and rotenone (complex I inhibitor, 5–10 μM), reduced TNF-α-evoked O2•− generation by 20–40%. The NO synthase inhibitor l-NNA (0.5–2 mM) and xanthine oxidase inhibitor allopurinol (50–100 μM) did not alter TNF-α-induced oxidative stress. Overall, these data suggest that NADPH oxidase, and, to a lesser extent, mitochondrial electron transport chain, are the cellular sources of O2•−generated by CMVEC in response to TNF-α stimulation.
Fig. 1.
Effects of reactive oxygen species (ROS) inhibitors on TNF-α-induced O2•− production in cerebral microvascular endothelial cells (CMVEC). CMVEC from newborn piglets were treated with TNF-α (15 ng/ml, 1 h) in the absence or presence of diphenyliodonium (DPI; 5 μM), apocynin (Apo, 500 μM), NSC-23766 (NSC, 50 μM), CCCP (5 μM), 2-thenoyltrifluoroacetone (TTFA; 5 μM), rotenone (Rot, 5 μM), Nω-nitro-l-arginine (l-NNA; 0.5 mM), or allopurinol (Allo, 50 μM). O2•− production was measured by ethidium fluorescence generated from dihydroethidium (DHE) and is expressed as percentage of the baseline control. Data represent average of 11 independent experiments. Values are means ± SE. *P < 0.05 compared with baseline control values. †P < 0.05 compared with TNF-α alone.
NADPH oxidase inhibitors attenuate apoptosis caused by TNF-α.
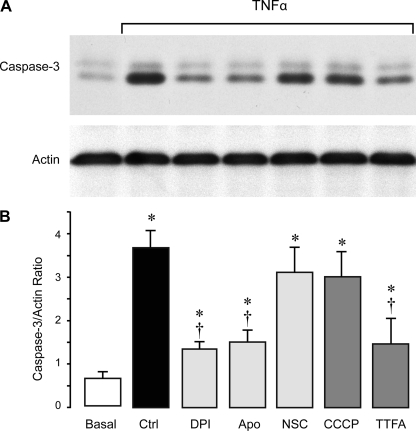
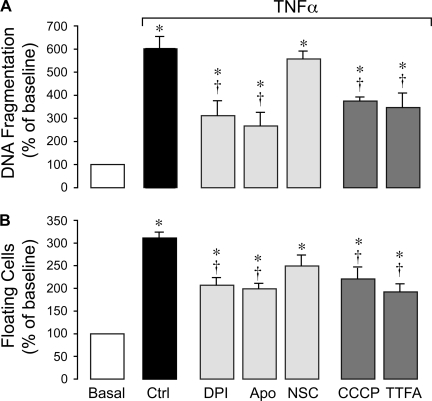
TNF-α is a potent inducer of apoptosis in CMVEC as evidenced by caspase-3 activation, DNA fragmentation, and loss of cell contacts (5). We investigated whether NADPH oxidase-derived O2•−anions contribute to TNF-α-evoked apoptosis. NADPH oxidase inhibitors DPI (5 μM) and apocynin (500 μM), but not the Rac1 inhibitor NSC-23766 (50 μM), reduced caspase-3 activation (Fig. 2, A and B), DNA fragmentation (Fig. 3A), and cell detachment (Fig. 3B) caused by TNF-α (15 ng/ml, 3 h). The mitochondrial electron transport chain inhibitors, CCCP (5 μM) and TTFA (5 μM), also partially reduced TNF-α-evoked apoptosis (Fig. 3, A and B). These data suggest that NADPH oxidase-derived O2•− greatly contributes to TNF-α-induced endothelial apoptosis.
Fig. 2.
Effects of ROS inhibitors on TNF-α-induced caspase-3 activation in CMVEC. CMVEC from newborn piglets were treated with TNF-α (15 ng/ml, 3 h) in the absence or presence of NADPH oxidase inhibitors DPI (5 μM), Apo (500 μM), and NSC (50 μM) and mitochondrial inhibitors CCCP (5 μM) and TTFA (5 μM). Caspase-3 activity was immunodetected in cell lysates by formation of active proteolitic fragment of caspase-3 (17 kDa). Blots were reprobed for actin as the loading control. A: representative blot. B: densitometry (n = 3 independent experiments). Values are means ± SE. *P < 0.05 compared with control values. †P < 0.05 compared with TNF-α alone.
Fig. 3.
Effects of ROS inhibitors on TNF-α-induced apoptosis in CMVEC. CMVEC from newborn piglets were treated with TNF-α (15 ng/ml, 3 h) in the absence or presence of DPI (5 μM), Apo (500 μM), Rac1 inhibitor NSC (50 μM), CCCP (5 μM), and TTFA (5 μM). A: DNA fragmentation. B: cell detachment. Data represent average of 4 independent experiments. Values are means ± SE. *P < 0.05 compared with baseline control values. †P < 0.05 compared with TNF-α alone.
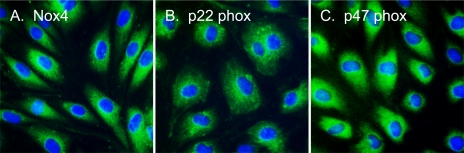
Immunofluorescence detection of Nox4 in CMVEC.
CMVEC highly express Nox4, the cell-specific catalytic subunit of NADPH oxidase (Fig. 4A). p22phox, the membrane-bound subunit, and p47phox, the cytosolic regulatory subunit of the enzyme, are also immunodetectable in CMVEC (Fig. 4, B and C). All subunits of NADPH oxidase are localized to the perinuclear compartment of endothelial cells that corresponds to endoplasmic reticulum.
Fig. 4.
Localization of NADPH oxidase subunits in CMVEC. Nox4 (A), p22phox (B), and p47phox (C) were visualized by immunofluorescence in confluent quiescent CMVEC.
Effect of Nox4 knockdown on TNF-α-induced apoptosis.
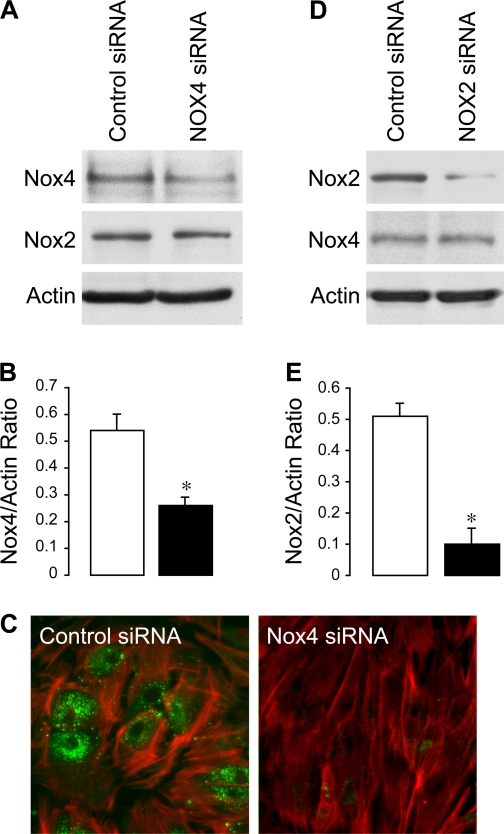
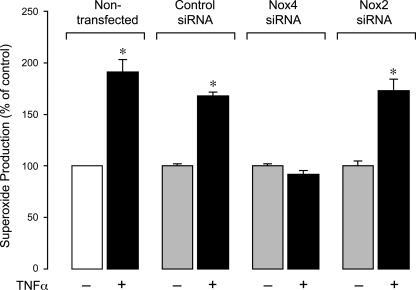
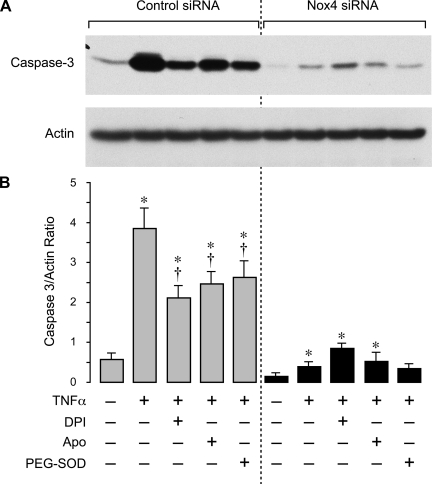
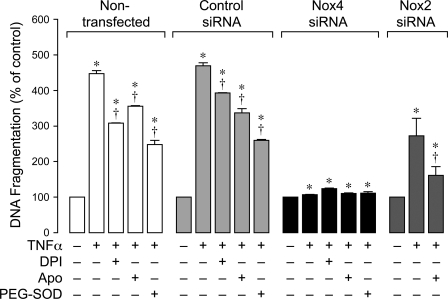
To delineate the role of Nox4 in inflammation-induced apoptosis in CMVEC, we downregulated Nox4 protein by posttranscriptional gene silencing using siRNAs. CMVEC transfected with Nox4 siRNA showed a significantly reduced Nox4 expression by immunoblotting (50–60% reduction) and immunofluorescence as compared with control nontransfected or transfected cells (Fig. 5, A–C). Nox2 expression in CMVEC was not reduced by the transfection (Fig. 5A), confirming the specificity of Nox4 siRNA. Nox4 knockdown resulted in 40% reduction in basal production of O2•− (4,950 ± 350 and 2,820 ± 170 fluorescence units/mg protein in control siRNA- and Nox4 siRNA-transfected cells; n = 4, P < 0.05), suggesting that Nox4 is constitutively active in unstimulated CMVEC. Most importantly, in Nox4 knockdown CMVEC, TNF-α completely failed to increase O2•− production, in contrast to nontransfected or control transfected cells (Fig. 6). The key apoptotic responses to TNF-α, including caspase-3 activation and DNA fragmentation, were also greatly reduced in Nox4 knockdown CMVEC (Figs. 7 and 8). Unlike control CMVEC, Nox4 knockdown cells stimulated by TNF-α were largely insensitive to DPI, apocynin, or PEG-SOD (Figs. 7 and 8). Overall, these data suggest that Nox4 is activated by TNF-α and is the major contributor to oxidative stress and apoptosis caused by TNF-α in CMVEC. Because PEG-SOD did not completely block apoptosis in control and Nox4 knockdown cells (Figs. 7 and 8), it appears that ROS-independent component(s) also contribute to TNF-α-induced caspase 3-mediated apoptosis in CMVEC.
Fig. 5.
Small interfering RNA (siRNA) knockdown of Nox4 and Nox2 in CMVEC. CMVEC were transfected with control siRNA, Nox4 siRNA (A–C), or Nox2 siRNA (D and E). A, B, D, and E: Nox4 and Nox2 detection by immunoblotting. A and D: representative blots. B and E: densitometry (n = 3 independent transfections; *P < 0.05 compared with control). C: Nox4 immunofluorescence in CMVEC transfected with control siRNA or Nox4 siRNA: Nox4 was visualized with FITC-conjugated secondary antibodies (green); F-actin was visualized by rhodamine-phalloidin (red).
Fig. 6.
Effects of TNF-α on O2•− production in Nox4 siRNA- and Nox2 siRNA-knockdown CMVEC. CMVEC (nontransfected and transfected with control siRNA, Nox4 siRNA, or Nox2 siRNA) were treated with TNF-α (15 ng/ml, 1 h). O2•− production was measured by ethidium fluorescence generated from DHE and is expressed as a percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with baseline control values.
Fig. 7.
Effects of TNF-α on caspase-3 activity in Nox4 knockdown CMVEC. CMVEC from newborn piglets transfected with control siRNA or Nox4 siRNA were treated with TNF-α (15 ng/ml, 3 h) in the absence or presence of DPI (5 μM), Apo (500 μM), or polyethylene glycol (PEG)-SOD (1,000 units). Active fragment of caspase-3 (17 kDa) was detected by immunoblotting and normalized to actin. A: representative blot. B: densitometry (n = 2 independent experiments). Values are means ± SE. *P < 0.05 compared with control values. †P < 0.05 compared with TNF-α alone.
Fig. 8.
Effects of TNF-α on DNA fragmentation in Nox4 siRNA- and Nox2 siRNA-knockdown CMVEC. CMVEC (nontransfected or transfected with control siRNA, Nox4 siRNA, or Nox2 siRNA) were treated with TNF-α (15 ng/ml, 3 h) in the absence or presence of DPI (5 μM), Apo (500 μM), or PEG-SOD (1,000 units). DNA fragmentation was detected by ELISA. Values are means ± SE (n = 2 independent experiments in triplicates). *P < 0.05 compared with baseline control values. †P < 0.05 compared with TNF-α alone.
Effect of Nox2 knockdown on TNF-α-induced oxidative stress and apoptosis.
gp91phox/Nox2 is also expressed in CMVEC from newborn piglets (Fig. 5). To investigate the role of Nox2 in CMVEC, we downregulated Nox2 expression using the siRNA technique. The transfection with Nox2 siRNA reduced Nox2 expression in CMVEC by 80% (Fig. 5, D and E). Nox4 expression was not altered by the transfection (Fig. 5D), confirming the Nox2 knockdown specificity. Knockdown of Nox2 did not produce any reduction in basal production of O2•− (2,180 ± 230 and 2,830 ± 430 fluorescence units/mg protein in control siRNA- and Nox2 siRNA-transfected cells; n = 3, P > 0.05) and did not alter the oxidative response to TNF-α (Fig. 6). Furthermore, Nox2 knockdown only partially attenuated TNF-α-induced DNA fragmentation, and this effect was greatly enhanced by apocynin (Fig. 8), indicating predominant contributions of other Nox homologues (presumably, Nox4) to inflammation-induced apoptosis. Overall, these data suggest that, in cerebral vascular endothelium, Nox2 is dormant under basal conditions and has only limited contribution to oxidative stress and apoptosis caused by TNF-α.
Colocalization of Nox4 and HO-2 in CMVEC.
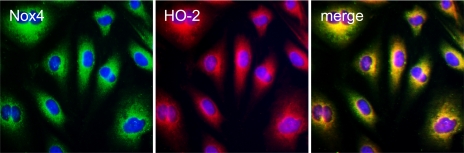
HO-2 is the endogenous constitutive anti-oxidant enzyme that is an important component of antiapoptotic protection against TNF-α (36). To establish potential interaction between the prooxidant and antioxidant enzymes, we determined whether Nox4 is colocalized with HO-2 in CMVEC (Fig. 9). Using double immunofluorescence staining, we detected that Nox4 and HO-2 are colocalized in the perinuclear compartment and the nuclear envelope, indicating potential functional interaction between the two enzymes.
Fig. 9.
Colocalization of Nox4 and heme oxygenase isoform 2 (HO-2) in CMVEC. Quiescent CMVEC were immunostained for Nox4 (green fluorescence) and HO-2 (red fluorescence); nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue).
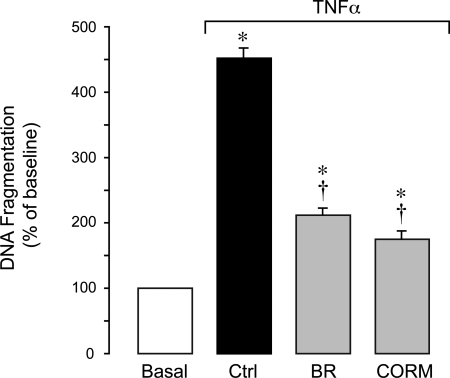
Bilirubin and CO prevent TNF-α-induced apoptosis in CMVEC.
We examined the effects of the products of HO-catalyzed heme degradation, bilirubin and CO, on endothelial apoptosis initiated by TNF-α. We used CORM-A1, a CO-releasing molecule that slowly releases gaseous CO in physiological solutions (35, 57). Bilirubin (1 μM) and CORM-A1 (50 μM) blocked DNA fragmentation caused in CMVEC by TNF-α (Fig. 10). CORM-A1 inactivated by overnight exposure to air failed to reduce DNA fragmentation caused by TNF-α (data not shown). These data suggest that bilirubin and CO are cytoprotective against apoptosis caused by TNF-α in cerebral vascular endothelium.
Fig. 10.
Protective effects of bilirubin (BR) and a CO-releasing molecule, CORM-A1 (CORM), on TNF-α-induced apoptosis in CMVEC. Confluent quiescent CMVEC from newborn piglets were treated with TNF-α (15 ng/ml, 3 h) in the absence or presence of BR (1 μM) or CORM (50 μM). Apoptosis was determined by DNA fragmentation. Values are means ± SE. *P < 0.05 compared with baseline control values. †P < 0.05 compared with TNF-α alone.
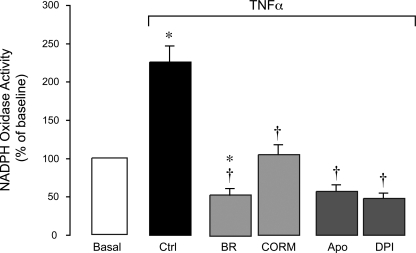
Effects of TNF-α, CO, and bilirubin on endothelial NADPH oxidase activity.
We investigated whether cytoprotective effects of the HO products against inflammation-induced apoptosis involve modulation of endothelial NADPH oxidase activity. NADPH oxidase activity in CMVEC was detected as O2•− production from 100 μM NADPH (21). TNF-α (15 ng/ml) rapidly (within 1 h) increased NADPH oxidase activity two- to threefold (Fig. 11). DPI (5 μM) and apocynin (500 μM) completely blocked the TNF-α-stimulated O2•− production, indicating the specificity of the method for NADPH oxidase activity detection. Given that TNF-α completely failed to elevate O2•− in Nox4 knockdown CMVEC, Nox4 is the catalytic isoform responsible for NADPH oxidase activation by the cytokine. Bilirubin (1 μM) and CORM-A1 (50 μM) completely blocked the NADPH oxidase response to TNF-α stimulation (Fig. 11), indicating that the Nox4 NADPH oxidase enzyme complex and/or NADPH oxidase-derived O2•− are the targets for antioxidant and antiapoptotic effects of the HO products during inflammation.
Fig. 11.
Effects of BR and a CO-releasing molecule (CORM-A1) on TNF-α-induced NADPH oxidase-derived O2•− production in CMVEC. Fractioned confluent quiescent CMVEC were treated with TNF-α (15 ng/ml, 1 h) in the absence or presence of BR (1 μM), CORM (50 μM), Apo (500 μM), and DPI (5 μM). NADPH oxidase activity was determined as O2•− production from NADPH (100 μM) measured by enhanced lucigenin luminescence, was normalized to the protein amount, and is expressed as percentage of the baseline control. Values are means ± SE. *P < 0.05 compared with control values. †P < 0.05 compared with TNF-α alone.
DISCUSSION
Cerebral vascular endothelial cells respond to proinflammatory cytokine TNF-α by oxidative stress and apoptosis. Constitutive HO-2 is an important antioxidant enzyme that is cytoprotective against TNF-α-induced damage of brain endothelial cells. We report major novel findings on the mechanism of apoptosis caused by the proinflammatory cytokine in cerebral vascular endothelial cells: 1) O2•− anions are major mediators of TNF-α-induced apoptosis; 2) Nox4 NADPH oxidase is highly expressed, constitutively active, and contributes to basal O2•− production; 3) Nox4 functions as a major source of proapoptotic O2•− that is rapidly activated by TNF-α; 4) Nox4 is colocalized with antioxidant HO-2 in the perinuclear compartment of the cell; and 5) the products of HO-2-catalyzed reaction of heme degradation, CO and bilirubin, block inflammation-induced apoptosis by decreasing the amount of O2•− produced by the Nox4 NADPH oxidase.
The proinflammatory cytokine TNF-α, a mediator of inflammatory brain disease, causes oxidative stress and apoptosis of cerebral vascular endothelial cells from newborn pig and adult mice (5) that may lead to cerebral vascular dysfunction. O2•− anions are primary contributors to TNF-α-induced oxidative stress in cerebral vascular endothelium. Our new data in cerebral vascular endothelial cells show that cell-permeable O2•−-scavenging enzyme PEG-SOD promotes endothelial survival, indicating that oxidative stress is a trigger of TNF-α-induced apoptosis. To determine the cellular sources of O2•− that are activated by TNF-α in cerebral vascular endothelial cells, we used pharmacological and molecular approaches. Inhibitors of NADPH oxidase and, to a lesser extent, complex I and II inhibitors of the mitochondrial electron transport chain reduced TNF-α-evoked O2•− production and inhibited key events of apoptosis (caspase-3 activation, DNA fragmentation, and loss of cell contacts). Conversely, inhibition of two other ROS-generating enzymes, NO synthase and xanthine oxidase, did not affect oxidative stress or apoptosis in TNF-α-stimulated cells. These data indicate that NADPH oxidase is a major source of proapoptotic O2•− produced in brain endothelial cells in response to inflammatory stimulation.
NADPH oxidase, a multisubunit enzymatic complex that generates O2•− from molecular oxygen and NADPH, is represented by distinct membrane-spanning catalytic subunits (large subunits Nox1–Nox5 in conjunction with the small subunit gp22phox) that are activated via spatial interaction with the cytosolic regulatory factors (gp47phox, gp67phox, and small GTPase Rac1/2). NADPH oxidase was originally described in phagocytes (gp91phox/Nox2), where it generates oxidative bursts involved in bacterial killing. Although the mechanism of activation of phagocytic Nox2 NADPH oxidase, dormant during the resting state, is well characterized, much less is known about the expression and regulation of other members of the Nox family (8, 29). We found that in cerebral vascular endothelial cells, Nox4 is highly expressed along with the small anchoring subunit p22phox and the regulatory subunit p47phox. Other investigators also detected Nox4 in cerebral arterioles (33, 36). In confluent cerebral vascular endothelial cells, Nox4, p22phox, and p47phox are localized to the perinuclear compartment of the cell, presumably, in the endoplasmic reticulum. This is in agreement with the observations in human vascular endothelial cells (9, 51). Nox4 localization is independent of F-actin stress fibers that are localized to the periphery of brain endothelial cells. In contrast, Nox4 in differentiated aortic smooth muscle cells is colocalized with actin (11). We did not find Nox4 immunofluorescence in the nuclei of confluent CMVEC in primary cultures, although there are reports on nuclear localization of Nox4 in subcultured human umbilical vein endothelial cells (HUVEC; 28) and in dedifferentiated aortic smooth muscle cells at late passages (11). Little is known on Nox4 functions in endothelial cells. In HUVECs, Nox4 regulates cell-cell adhesion, motility, and proliferation (12, 51).
The functional significance of endothelial Nox4 in cerebrovascular inflammatory disease is not known. Our data, for the first time, show that Nox4 is the major source of evoked O2•− that triggers apoptosis in cerebral vascular endothelial cells exposed to TNF-α. TNF-α rapidly (in ≤1 h) stimulated NADPH oxidase, indicating that new protein synthesis does not account for the enzyme activity. To investigate the functional significance of Nox4 in CMVEC, we downregulated Nox4 protein by gene-specific siRNA. Nox4 knockdown reduced basal NADPH oxidase activity by 40%, indicating that Nox4 is constitutively active during resting conditions in CMVEC. In addition to Nox4, expression of Nox1 and Nox2 in cerebral arteries has been reported by other investigators (27, 33, 36). We found that Nox2 expressed in cerebral vascular endothelium is dormant in unstimulated cells and does not contribute to O2•− production under resting conditions. Importantly, knockdown of Nox4 completely blocked O2•− responses to TNF-α, indicating that Nox4 is the only NADPH oxidase isoform in cerebral vascular endothelium that is activated by the inflammatory stimulation. Conversely, Nox2 knockdown had only limited effect on the burst of oxidative stress evoked by the cytokine. Furthermore, TNF-α did not cause apoptosis in Nox4 siRNA knockdown cells. Overall, these data demonstrate that Nox4 knockdown prevented acute deleterious effects of TNF-α and conferred resistance of brain endothelial cells to inflammation-induced injury. These observations support the concept that Nox4 is activated by TNF-α in cerebral vascular endothelium and functions as the major source of O2•− that causes apoptosis and endothelial injury during inflammatory cerebral vascular disease.
We report that Nox4 is constitutively active in resting brain endothelial cells and is rapidly activated by TNF-α via a posttranslational mechanism. NADPH oxidase activation by a variety of (patho)physiological factors, such as inflammatory cytokines, angiotensin II, histamine, and thrombin, has been described in phagocytes and other cell types (10, 21–24, 29, 31, 39, 49). Mechanisms that regulate activity of phagocytic Nox2-based NADPH oxidase include phosphorylation and translocation of regulatory cytosolic subunits (p47phox and p67phox) to the membrane catalytic complex Nox2-gp22phox and activation of small GTPase Rac1/2 (22, 23, 29). However, little is known on regulation of other members of the Nox family (23, 29, 39). In contrast to Nox2-based NADPH oxidase, Nox4 is constitutively active (32, 39), as supported by our data in brain endothelial cells. NADPH oxidase activation by TNF-α involves interaction of catalytic and regulatory (p47phox) subunits (18, 23, 29, 31). Conversely, Martyn et al. (32) reported that Nox4 activity does not require cytosolic subunits p47phox, p67phox, or Rac1. Our data in brain endothelial cells show that Rac1 inhibitor did not reduce Nox4-mediated apoptosis in response to TNF-α, supporting the notion that Rac1 activity is not essential in the mechanism of the Nox4 activation.
Mitochondrial electron chain complexes I and II also appear to contribute to a burst of proapoptotic O2•− generated by CMVEC exposed to TNF-α. Complex I and II inhibitors, rotenone and TTFA, inhibited O2•− production and apoptosis evoked by the cytokine, whereas CCCP, a respiratory uncoupling agent, had less effect. Surprisingly, CMVEC with knockdown Nox4 did not respond to TNF-α by increasing O2•− formation or apoptosis, demonstrating the absence of mitochondrial involvement. Recent reports indicate a signaling cross talk between NADPH oxidase- and mitochondria-derived ROS (30, 54). These observations support the concept that Nox4 is the primary target for TNF-α in promoting cell death, whereas the ROS responses of the mitochondrial complexes are secondary to Nox4 stimulation by the cytokine in cerebral vascular endothelium.
In cerebral vascular endothelium, constitutive HO-2 is a critical member of the antioxidant cell defense system (37). We have found that deletion of HO-2 gene or inhibition of HO activity aggravates the apoptotic effects of TNF-α in CMVEC (5). Because Nox4 NADPH oxidase is a major source of O2•− in TNF-α-stimulated cells, we investigated its relationships with antioxidant HO-2. We found that HO-2 and Nox4 are colocalized in the perinuclear area of the cytoplasm, thus providing a structural basis for functional association between the two enzymes. We investigated whether the antiapoptotic effects of the HO products CO and/or bilirubin are based on their abilities to block Nox4-derived O2•− anions in cerebral vascular endothelium. For the CO source, we used sodium boranocarbonate (CORM-A1) that provides spontaneous dose-dependent release of gaseous CO into the media (35, 57). In our experiments, TNF-α rapidly increased NADPH oxidase activity in the brain endothelial cells measured as O2•− production from the NADPH substrate. Nox4 appears to be the major isoform that accounts for TNF-α's stimulation of NADPH oxidase activity in cerebral vascular endothelial cells, because Nox4 knockdown completely abolished responses to the cytokine. CORM-A1 and bilirubin in physiologically relevant doses completely blocked Nox4-mediated O2•− responses to TNF-α. Taken together with close subcellular compartmentalization of HO-2 and Nox4, these data suggest that the HO-2 products can attenuate Nox4 activation in response to inflammatory stimulation.
What are the mechanisms by which CO and bilirubin reduce Nox4 NADPH oxidase activity? CO is known for its ability to bind to heme and, therefore, may regulate functions of heme-containing proteins. The large transmembrane Nox subunit contains two heme-binding domains that are critical for the enzyme activity (7, 8, 17). Therefore, we may suggest that NADPH oxidase activation by TNF-α can be reduced by CO binding to the Nox heme and/or decreasing heme availability. HO-1 overexpression reduced NADPH oxidase activity in phagocytes and vascular smooth muscle cells (12, 46). A CO-releasing molecule, CORM-2, inhibited NADPH oxidase activity in human airway smooth muscle (47). Bilirubin, on the other hand, is rapidly oxidized to biliverdin, and is recognized as a potent ROS scavenger (34, 44). The antioxidant potencies of bilirubin are enhanced by biliverdin reductase activity that provides recycling of biliverdin to bilirubin. Our findings may support the notion that CO inhibits Nox4 activation and subsequent ROS formation induced by TNF-α, whereas bilirubin eliminates Nox-derived ROS by scavenging.
In conclusion, we have demonstrated that Nox4 is an important component of the NADPH oxidase complex in porcine cerebral vascular endothelial cells. Furthermore, Nox4 is a major prooxidant that is rapidly activated by TNF-α and accounts for oxidative stress leading to apoptosis in cerebral vascular endothelium under inflammatory conditions. HO-2 is colocalized with Nox4, providing a structural basis for functional interaction between the two enzymes. HO-2, via production of CO and bilirubin, inhibitors of Nox4-derived ROS, provides antioxidant protection of cerebral vascular endothelium against TNF-α-induced apoptosis.
GRANTS
This research was supported by National Institute of Neurological Disorders and Stroke and by National Heart, Lung, and Blood Institute. S. Basuroy is supported by the Beginning-Grant-in-Aid from American Heart Association.
Acknowledgments
We are deeply grateful to Hector Knight from Tyco-Mallincrodt Medical (Petten, Holland) for the generous gift of CORM-A1. The authors thank Danny Morse for helping with preparation of the figures.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Abraham NG, Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev 60: 79–127, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Abramov AY, Jacobson J, Wientjes F, Hothersall J, Canevari L, Duchen MR. Expression and modulation of an NADPH oxidase in mammalian astrocytes. J Neurosci 40: 9176–9184, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ago T, Kitazono T, Ooboshi H, Iyama T, Han YH, Takada J, Wakisaka M, Ibayashi S, Utsumi H, Iida M. Nox4 as the major catalytic component of an endothelial NAD(P)H oxidase. Circulation 109: 227–233, 2004. [DOI] [PubMed] [Google Scholar]
- 4.Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke 28: 1233–1244, 1997. [DOI] [PubMed] [Google Scholar]
- 5.Basuroy S, Bhattacharya S, Tcheranova D, Qu Y, Regan RF, Leffler CW, Parfenova H. HO-2 provides endogenous protection against oxidative stress and apoptosis caused by TNF-α in cerebral vascular endothelial cells. Am J Physiol Cell Physiol 291: C897–C908, 2006. [DOI] [PubMed] [Google Scholar]
- 6.Bruce AJ, Boling W, Kindy MS, Peschon J, Kraemer PJ, Carpenter MK, Holtsberg FW, Masson MP. Altered neuronal and microglial responses to excitotoxic and ischemic brain injury in mice lacking TNF receptors. Nat Med 7: 788–794, 1996. [DOI] [PubMed] [Google Scholar]
- 7.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24: 471–478, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Cave AC, Brewer AC, Narayanapanicker A, Ray R, Grieve DJ, Walker S, Shah AM. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal 8: 691–728, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chen K, Kirber MT, Xioa H, Yang Y, Keaney JF. Regulation of ROS signal transduction by NADP oxidase 4 localization. J Cell Biol 181: 1129–1139, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheranov SY, Jaggar JH. TNF-α dilates cerebral arteries via NAD(P)H oxidase-dependent Ca2+ spark activation. Am J Physiol Cell Physiol 290: C964–C971, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clempus RE, Sorescu D, Dikalova AE, Pounkova L, Jo P, Sorescu GP, Schmidt HH, Lassegue B, Griendling KK. Nox4 is required for maintenance of the differentiated vascular smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol 27: 42–48, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Datla SR, Dusting GJ, Mori TA, Taylor CJ, Croft KD, Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension 50: 636–642, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Datla SR, Peshavariya H, Dusting GJ, Mahadev K, Goldstein BJ, Jiang F. Important role of Nox4 type NADPH oxidase in angiogenic responses in human microvascular endothelial cells in vitro. Arterioscler Thromb Vasc Biol 27: 2319–2324, 2007. [DOI] [PubMed] [Google Scholar]
- 14.DeLeo FR, Quinn MT. Assembly of the phagocyte NADPH oxidase: molecular interaction of oxidase proteins. J Leukoc Biol 6: 677–961, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Didion SP, Faraci FM. Angiotensin II produces superoxide-mediated impairment of endothelial function in cerebral arterioles. Stroke 34: 2038–2042, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Gao Y, Dickerson JB, Guo F, Zheng J, Zheng Y. Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc Natl Acad Sci USA 101: 7618–7623, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geiszt M NADPH oxidases: new kids on the block. Cardiovasc Res 71: 289–299, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Gertzberg N, Neumann P, Rizzo V, Johnson A. NAD(P)H oxidase mediates the endothelial barrier dysfunction induced by TNF-α. Am J Physiol Lung Cell Mol Physiol 286: L37–L48, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Glass MJ, Huang J, Oselkin M, Tarsitano MJ, Wang G, Iadecola C, Pickel VM. Subcellular localization of nicotinamide adenine dinucleotide phosphate oxidase subunits in neurons and astroglia of the rat medial nucleus tractus solitarius: relationship with tyrosine hydroxylase immunoreactive neurons. Neuroscience 143: 547–564, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Görlach A, Brandes RP, Nguyen K, Amidi M, Dehghani F, Busse R. A gp91phox containing NADPH oxidase selectively expressed in endothelial cells is a major source of oxygen radical generation in the arterial wall. Circ Res 87: 26–32, 2000. [DOI] [PubMed] [Google Scholar]
- 21.Griendling KK, Minieri CA, Ollerenshaw JD, Alexander RW. Angiotensin II stimulates NADH and NADPH oxidase activity in cultured vascular smooth muscle cells. Circ Res 74: 1141–1148, 1994. [DOI] [PubMed] [Google Scholar]
- 22.Griendling KK, Sorescy D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res 86: 494–501, 2000. [DOI] [PubMed] [Google Scholar]
- 23.Hordijk PL Regulation of NADPH oxidases: the role of Rac proteins. Circ Res 98: 453–462, 2006. [DOI] [PubMed] [Google Scholar]
- 24.Hu Q, Yu ZX, Ferrans VJ, Takeda K, Irani K, Ziegelstein RC. Critical role of NADPH oxidase-derived reactive oxygen species in generating Ca2+ oscillations in human aortic endothelial cells stimulated by histamine. J Biol Chem 277: 32546–32551, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation and function. Antioxd Redox Signal 8: 1583–1596, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38: 3000–3006, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kazama K, Anrather J, Zhou P, Girouard H, Frys K, Milner TA, Iadecola C. Angiotensin II impairs neurovascular coupling in neocortex through NADPH oxidase-derived radicals. Circ Res 10: 1019–1026, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Kuroda J, Nakagawa K, Yamasaki T, Nakamura K, Takeya R, Kuribayashi F, Imajoh-Ohmi S, Igarashi K, Shibata Y, Sueishi K, Sumimoto H. The superoxide-producing NAD(P)H oxidase Nox4 in the nucleus of human vascular endothelial cells. Genes Cells 10: 1139–1151, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med 43: 319–331, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SB, Bae IH, Bae YS, Um HD. Link between mitochondria and NADPH oxidase 1 isozyme for the sustained production of reactive oxygen species and cell death. J Biol Chem 281: 36228–36235, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Li JM, Fan LM, Christie MR, Shah AM. Acute tumor necrosis factor alpha signaling via NADPH oxidase in microvascular endothelial cells: role of p47phox phosphorylation and binding to TRAF4. Mol Cell Biol 25: 2320–2330, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cell Signal 18: 69–82, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Miller AA, Drummond GR, Sobey CG. Novel isoforms of NADPH-oxidase in cerebral vascular control. Pharmacol Ther 111: 928–948, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Minetti M, Mallozzi C, Di Stasi AM, Pietraforte D. Bilirubin is an effective antioxidant of peroxynitrite-mediated protein oxidation in human blood plasma. Arch Biochem Biophys 352: 165–174, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Motterlini R, Sawle P, Hammad J, Baines S, Alberto R, Foresti R, Green CJ. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J 19: 284–286, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Paravicini TM, Chrissobolis S, Drummond GR, Sobey CG. Increased NADPH-oxidase activity and Nox4 expression during chronic hypertension is associated with enhanced cerebral vasodilatation to NADPH in vivo. Stroke 35: 584–589, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Parfenova H, Leffler CW. Cerebroprotective functions of HO-2. Curr Pharm Des 14: 443–453, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park HS, Chun JN, Jung HY, Choi C, Bae YS. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc Res 3: 447–455, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Ray R, Shah AM. NADPH oxidase and endothelial cell function. Clin Sci (London) 109: 217–226, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev 86: 583–650, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Ryter SW, Kim HP, Hoetzel A, Park JW, Nakahira K, Wang X, Choi AM. Mechanisms of cell death in oxidative stress. Antioxid Redox Signal 9: 49–89, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Sawada N, Salomone S, Kim HH, Kwiatkowski DJ, Liao JK. Regulation of endothelial nitric oxide synthase and postnatal angiogenesis by Rac1. Circ Res 103: 360–368, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorescu D, Weiss D, Lassegue B, Clempus RE, Szocs K, Sorescu GP, Valppu L, Quinn MT, Lambeth JD, Vega JD, Taylor WR, Griendling KK. Superoxide production and expression of Nox family proteins in human atherosclerosis. Circulation 105: 1429–1435, 2002. [DOI] [PubMed] [Google Scholar]
- 44.Stocker R Antioxidant activities of bile pigments. Antioxid Redox Signal 6: 841–849, 2004. [DOI] [PubMed] [Google Scholar]
- 45.Szöcs K, Lassègue B, Sorescu D, Hilenski LL, Valppu L, Couse TL, Wilcox JN, Quinn MT, Lambeth JD, Griendling KK. Upregulation of Nox-based NAD(P)H oxidases in restenosis after carotid injury. Arterioscler Thromb Vasc Biol 22: 21–27, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Taille C, El-Benna J, Lanone S, Dang MC, Ogier-Denis E, Aubier M, Boczkowski J. Induction of heme oxygenase-1 inhibits NAD(P)H oxidase activity by down-regulating cytochrome b558 expression via the reduction of heme availability. J Biol Chem 279: 28681–28688, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Taille C, El-Benna J, Lanone S, Boczkowski J, Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem 280: 25350–25360, 2005. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T, Shimizu H, Morimatsu H, Inoue K, Akagi R, Morita K, Sassa S. Heme oxygenase-1: a fundamental guardian against oxidative tissue injuries in acute inflammation. Mini Rev Med Chem 7: 745–753, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Ushio-Fukai M Redox signaling in angiogenesis: role of NADPH oxidase. Cardiovasc Res 71: 226–235, 2006. [DOI] [PubMed] [Google Scholar]
- 50.Vallet P, Charnay Y, Steger K, Ogier-Denis E, Kovari E, Herrmann F, Michel JP, Szanto I. Neuronal expression of the NADPH oxidase NOX4, and its regulation in mouse experimental brain ischemia. Neuroscience 132: 233–238, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Van Buul JD, Fernandez-Borja M, Anthony EC, Hordijk PL. Expression and localization of NOX2 and NOX4 in primary human endothelial cells. Antioxid Redox Signal 7: 308–317, 2005. [DOI] [PubMed] [Google Scholar]
- 52.Vezzani A, Balosso S, Ravizza T. The role of cytokines in the pathophysiology of epilepsy. Brain Behav Immun 22: 797–803, 2008. [DOI] [PubMed] [Google Scholar]
- 53.Wang X, Wang Y, Kim HP, Nakahira K, Ryter SW, Choi AK. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem 282: 1718–1726, 2007. [DOI] [PubMed] [Google Scholar]
- 54.Wenzel P, Mollnau H, Oelze M, Schulz E, Wickramanayake JMD, Müller J, Schuhmacher S, Hortmann M, Baldus S, Gori T, Brandes RP, Münzel T, Daiber A. First evidence for a crosstalk between mitochondrial and NADPH oxidase-derived reactive oxygen species in nitroglycerin-triggered vascular dysfunction. Antiox Redox Signal 10: 1435–1448, 2008. [DOI] [PubMed] [Google Scholar]
- 55.Xi Q, Cheranov SY, Jaggar JH. Mitochondria-derived reactive oxygen species dilate cerebral arteries by activating Ca2+ sparks. Circ Res 97: 354–62, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao L, Pimentel DR, Wang J, Singh K, Colucci WS, Sawyer DB. Role of reactive oxygen species and NAD(P)H oxidase in α1-adrenoceptor signaling in adult rat cardiac myocytes. Am J Physiol Cell Physiol 282: C926–C934, 2002. [DOI] [PubMed] [Google Scholar]
- 57.Zimmermann A, Leffler CW, Tcheranova D, Fedinec AL, Parfenova H. Cerebroprotective effects of the CO-releasing molecule CORM-A1 against seizure-induced neonatal vascular injury. Am J Physiol Heart Circ Physiol 293: H2501–H2507, 2007. [DOI] [PubMed] [Google Scholar]