Abstract
Myoglobin is an oxygen storage molecule that is selectively expressed in cardiac and slow-twitch skeletal muscles that have a high oxygen demand. Numerous studies have implicated hypoxia in the regulation of myoglobin expression as an adaptive response to hypoxic stress. However, the details of this relationship remain undefined. In the present study, adult mice exposed to 10% oxygen for periods up to 3 wk exhibited increased myoglobin expression only in the working heart, whereas myoglobin was either diminished or unchanged in skeletal muscle groups. In vitro and in vivo studies revealed that hypoxia in the presence or absence of exercise-induced stimuli reprograms calcium signaling and modulates myoglobin gene expression. Hypoxia alone significantly altered calcium influx in response to cell depolarization or depletion of endoplasmic reticulum calcium stores, which inhibited the expression of myoglobin. In contrast, our whole animal and transcriptional studies indicate that hypoxia in combination with exercise enhanced the release of calcium from the sarcoplasmic reticulum via the ryanodine receptors triggered by caffeine, which increased the translocation of nuclear factor of activated T-cells into the nucleus to transcriptionally activate myoglobin expression. The present study unveils a previously unrecognized mechanism where the hypoxia-mediated regulation of calcium transients from different intracellular pools modulates myoglobin gene expression. In addition, we observed that changes in myoglobin expression, in response to hypoxia, are not dependent on hypoxia-inducible factor-1 or changes in skeletal muscle fiber type. These studies enhance our understanding of hypoxia-mediated gene regulation and will have broad applications for the treatment of myopathic diseases.
Keywords: nuclear factor of activated T cells, calcineurin, skeletal muscle
myoglobin is a cytoplasmic hemoprotein that is abundantly expressed in heart and oxidative skeletal myofibers. Elegant studies using physiological, biochemical, and spectroscopic analyses support an important role for myoglobin in facilitated oxygen transport, as a reservoir for oxygen and as a scavenger of reactive oxygen species in the mammalian heart and skeletal muscle (6, 16, 20, 34, 35, 39). Detailed transcriptional analyses have been undertaken to define upstream activation motifs including a CCAC box, A/T element, nuclear factor of activated T cells (NFAT) response element, and E boxes that are necessary for muscle-specific transcription of the myoglobin gene (4, 5, 21, 22). Following differentiation, myoglobin expression is coordinately regulated by neural and muscular activities that stimulate calcium signaling within the cell. Stimuli that enhance intracellular calcium levels increase activity and gene expression of calcineurin, a Ca2+/calmodulin-dependent serine phosphatase (13, 47, 48). Upon activation, calcineurin dephosphorylates the transcription factor NFAT, which translocates to the nucleus and combinatorially interacts with other transcription factors to regulate myoglobin gene expression. These results support the coordinated role of transcription factors to promote myoglobin expression during differentiation, development, and hypertrophy of striated muscle.
Previous studies have shown a role for myoglobin in facilitated oxygen transport, in oxygen storage, or as a scavenger of nitric oxide within the myocytes (17). Mice lacking myoglobin are viable but mount important cellular and molecular adaptations to offset the loss of myoglobin, including the induction of the hypoxic gene program [i.e., hypoxia-inducible factor (HIF)-1, HIF-2, VEGF, etc.] to augment the delivery of oxygen to the myocytes (17, 20, 35). These adaptations serve to decrease the diffusion distance for oxygen in order for the mice to maintain normal function. In addition, mammals adapted for either breath-hold diving such as seals or those living at high altitude have more than a tenfold induction of myoglobin in their skeletal muscle (30, 31). While chronic hypoxia stimulates a spectrum of adaptations (the promotion of angiogenesis, erythropoiesis, cellular proliferation, vascular remodeling, and glycolytic metabolism) that are mediated by the transcriptional regulator HIF-1, previous studies have not addressed whether myoglobin is a direct downstream target of HIF-1 in striated muscle. These previous results suggest that myoglobin would be responsive to hypoxic conditions although the regulatory mechanisms governing myoglobin transcription under hypoxic conditions remain undefined.
While myoglobin has been extensively examined, the mechanisms of its induction in response to hypoxia are unknown. Studies that evaluated the interaction between hypoxia and myoglobin have yielded conflicting results (14, 26, 43). As outlined previously, selected animal and human studies suggest that myoglobin expression is induced in response to hypoxia. For example, Reynafarje's (38) 1962 study on skeletal muscle adaptation to high altitude reported a significant increase in myoglobin concentrations in the skeletal muscles of high altitude natives as compared with their sea level controls (43, 45). However, other studies of animals and humans exposed to various degrees of hypoxia have shown no change or a decrease in myoglobin levels (3, 44). This discrepancy may be a result of methodological issues because all of the studies that showed no change in myoglobin levels were undertaken in animals or humans that were exposed only to hypoxia in the absence of other inducing stimuli. Studies that showed an induction of myoglobin were performed in animals exposed to hypoxia in combination with a secondary stimulus such as cold acclimation, physical activity, or exercise training (26–28).
The purpose of the present study was to determine the effects of hypoxia on the transcriptional regulation and expression of myoglobin in both cell culture and whole animal studies. Our results demonstrate that hypoxia alone does not stimulate the induction of myoglobin expression. Rather, hypoxia differentially regulates the intracellular pools of calcium. Hypoxia significantly alters intracellular calcium signaling through the inhibition of calcium influx via the L-type and store-operated calcium (SOC) channels. This altered calcium signaling specifically inhibited the expression of myoglobin. However, hypoxia in combination with stimuli that enhanced the calcineurin/NFAT pathway (such as exercise) significantly increased the translocation of NFAT into the nucleus to promote the transcription of myoglobin. To our knowledge, this is the first study that mechanistically defines hypoxia-mediated regulation of the amplitude and duration of calcium transients from the different intracellular pools to significantly enhance the transcriptional activation of gene expression.
MATERIALS AND METHODS
Plasmid constructs.
The luciferase reporter plasmid was constructed by inserting the 2-kb upstream fragment of the human myoglobin gene into the commercially available pGL3 plasmid (Promega) (15). A second myoglobin luciferase plasmid was generated where putative NFAT binding sites within the myoglobin promoters were disrupted using a PCR-based mutagenesis procedure, as previously described (52). The specific nucleotide sequence modifications included myoglobin promoter (−690) AGGAAATA to GTCGACTA and (−232, reverse strand) TGGAAAGA to CTCGAGGA.
Tissue culture, cell transfection, and reporter gene assays.
For the majority of studies, C2C12 myoblasts were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 20% fetal bovine serum and antibiotics (100 U/ml of penicillin and 100 μg/ml of streptomycin) under normoxic conditions. Myotube formation was induced by switching confluent cells to differentiation media [DMEM supplemented with 2% heat-inactivated horse serum, 10 μg/ml insulin (Gibco-BRL) and 10 μg/ml transferrin (Gibco-BRL)]. For the calcium-free studies, mature myotubes were incubated in calcium-free media (140 mM NaCl, 2.8 mM KCl, 4 mM MgCl2, 10 mM glucose, 10 mM HEPES, and 10 mM EGTA). Twenty-four hours after differentiation, cells were cultured under normoxic (21% O2 and 5% CO2) or hypoxic (1% O2 and 5% CO2) conditions. For transient transfection assays, C2C12 cells were plated 12 h before transfection in six-well tissue culture dishes at ∼1.0 × 105 cells per well, and transfected. Each well was cotransfected with a promoter-reporter plasmid (0.5 μg), an expression plasmid that uses the cytomegalovirus (CMV) promoter to force expression of a constitutively active form of calcineurin, myocyte enhancer factor-2 C (Mef2c), HIF-1, or an empty vector (pCI-NEO; 0.5 μg), along with a CMV-lacZ plasmid (0.5 μg) as an internal control for transfection efficiency. Myotubes were harvested 96 h and 120 h after transfection. Luciferase and β-galactosidase assays of whole cell extracts were performed as previously described (15, 52).
Transgenic mice.
Transgenic mice were generated as previously described (34, 42). Adult male mice (2–4 mo old) from the two highest expressing reporter lines and wild-type littermates were used in this study. All experiments with animals were reviewed and approved by the Institutional Animal Care and Research Advisory Committee.
Hypoxic exposure.
A Plexiglas chamber (87 × 42 × 45 cm) was engineered to maintain a constant hypoxic environment of 10% O2 by infusing a gas mixture of 10% O2 and 90% N2 into the chamber at a rate of 1 l/min (34). It was continuously monitored for oxygen and carbon dioxide concentrations, temperature, and humidity. Within the hypoxic chamber, mice were individually housed in 15 × 32-cm cages and were maintained in a 12:12-h light-dark cycle. To ensure that the mice were exposed to a significant hypoxic stimulus, we measured the hematocrit levels of these mice using conventional methods.
Electrical pacing of the mouse hindlimb muscles.
Miniature neuromuscular stimulators were purchased from Dr. Jonathan Jarvis at the University of Liverpool, Liverpool, United Kingdom (jcj@liverpool.ac.uk) to undertake the nerve pacing studies in adult mice. The stimulators were implanted subcutaneously under the skin of anesthetized mice. The lead was tunneled and applied to the sciatic nerve. After the animals recovered from anesthesia (minimum 24 h after surgery), the circuit was activated by flashes of light transmitted through the skin to deliver supramaximal pulses of 0.2-ms duration at a frequency of 10 Hz.
β-Galactosidase staining.
Dissected muscles were transversely bisected and either frozen in liquid nitrogen for protein analysis or fixed in 0.2% glutaraldehyde in phosphate-buffered saline on ice for 30–45 min. The fixed samples were washed following fixation and X-gal stained (5 mM ferrocyanide, 5 mM ferricyanide, 2 mM MgCl2, 1 mg/ml X-gal, 0.01% sodium deoxycholate, and 0.02% Nonidet P-40) overnight at 37°C. Tissues were paraffin processed, sectioned, and imaged using a Leitz Laborlux-S microsope.
Western blot analysis and biochemical assays.
Western blot analysis was performed according to a previously published protocol (50). Rabbit anti-Mb serum (1:3,000; DAKO) and mouse anti-β-galactosidase serum (1:5,000; Promega) were used as the primary antiserum, which was detected using a horseradish peroxidase-conjugated secondary antiserum. Mouse monoclonal α-tubulin serum (1:3,000; Sigma) was used as a standard to ensure equal protein loading of the lanes. Band intensity was quantitated using a computerized digital analysis program and normalized to α-tubulin expression (Scion Image 1.62c).
Ca2+ imaging.
Cells were grown on glass coverslips and differentiated over 5 days as previously described (42). Cells were then loaded with 10 μM fura-2-AM (Molecular Probes, Eugene, OR) for 20 min. The coverslips with fura-2-loaded cells attached to them were assembled into a perfusion chamber and continuously perfused at a rate of 10 ml/min while a constant chamber volume of 200 μl was maintained. Application of agonists was made by inclusion in the perfusate. Loaded cells were illuminated by alternating 340/380 nm light delivered every 1–2 s by a DG-4 Argon exciter (Sutter Instruments, Novato, CA), and fluorescence images were captured at an emission of 510 nm with a Photometrics Cool SNAP HQ CCD camera (Roper Scientific, Tucson, AZ) based on a Nikon TE2000 fluorescent microscope. All experiments were performed in triplicate with at least 10 myotubes per experiment. Myoblasts were transfected with the NFATc1 cDNA cloned into pYFP-N1 (Invitrogen, Carlsbad, CA) to generate a yellow fluorescent protein (YFP) fusion protein. Myotubes were imaged using a filter selective for the YFP excitation (484/15× filters; Chroma, Rockingham, Vermont) and emission (HQ535/30, Chroma). Time-lapse images were acquired using Metamorph software every 25 s following exposure to agonist-evoked Ca2+ signals. For quantification, regions of interest were examined and the background was subtracted. Six independent experiments were performed with and without simultaneous fura-2 imaging. Results depict one representative experiment with fluorescence quantification for 24 nuclei.
RESULTS
Induction of myoglobin in response to hypoxia is mediated by the calcineurin/NFAT pathway in differentiated myotubes.
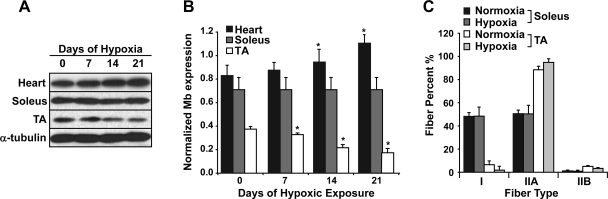
To examine the response of myoglobin gene expression to low oxygen, we exposed adult male C57BL/6 mice to chronic hypoxia (10% oxygen) for selected time periods (0, 7, 14 and 21 days). These studies were undertaken using an approved institutional animal protocol and a Plexiglas environmental chamber as previously described (34). The mice that were exposed to 10% oxygen appeared sedentary compared with those mice maintained under normoxic conditions. Western blot analysis revealed that hypoxia exerted differential effects on myoglobin expression (Fig. 1). We observed a significant induction (35.3 ± 0.4%; P < 0.05, ANOVA; n = 4) of myoglobin expression in the working heart following 21 days of chronic hypoxia (compared with normoxic controls). In contrast, a decrease in myoglobin expression (53.3 ± 0.1%; P < 0.05, ANOVA; n = 4) was observed in the nonworking, mixed fiber type tibialis anterior (TA) muscle in response to chronic hypoxia for a 3-wk period (0.17 ± 0.06 normalized myoglobin expression in the hypoxic TA vs. 0.36 ± 0.03 normalized myoglobin expression in the normoxic TA; P = 0.002, ANOVA; n = 4) (Fig. 1, A and B) (34). In addition, no change in myoglobin expression was observed in the weight-bearing slow-twitch soleus muscle (0.69 ± 0.03 normalized myoglobin expression in hypoxic soleus vs. 0.69 ± 0.002 normalized myoglobin expression in normoxic soleus; n = 4). These changes in myoglobin expression were not associated with changes in fiber type distribution between normoxic and hypoxic animals (Fig. 1C; n = 3 for each group). These results support the notion that a secondary stimulus in combination with hypoxia is necessary to maintain or increase myoglobin protein levels in cardiac and skeletal muscles. We predicted that this stimulus was related to contractile activity because myoglobin expression was induced in the working heart, unchanged in slow-twitch muscles, and reduced in sedentary muscles of mice exposed to chronic hypoxia.
Fig. 1.
Myoglobin expression and fiber type diversity in mice exposed to chronic hypoxic conditions. A: Western blot analysis of myoglobin expression revealed an induction of myoglobin expression in the heart in response to chronic hypoxia (10% oxygen for 0, 7, 14, and 21 days). In contrast, myoglobin expression was decreased in the resting or quiescent tibialis anterior (TA) and did not change in the weight-bearing soleus skeletal muscles. The α-tubulin expression demonstrates equal loading of all the samples. B: quantification of the myoglobin protein expression as measured by Western blot analysis in the heart, soleus, and TA muscles (n = 3 for each). Values were normalized to α-tubulin expression. C: quantification of the fiber type population in the soleus and TA muscles harvested from mice that were exposed to 3 wk of hypoxia. Note that there is no significant change in fiber type that would account for the decrease in myoglobin expression observed in the sedentary TA muscles after 3 wk of hypoxic exposure. *Significant difference between hypoxic and normoxic conditions (n = 3, P < 0.05).
Previous studies support a role for myoglobin either in facilitated oxygen transport or as a store of oxygen for heart and oxidative skeletal myofibers (6, 16, 20, 34, 35, 39). These studies suggested that myoglobin functioned in part to alleviate hypoxic stress in the myocyte. Therefore, to determine the interaction between hypoxia and myoglobin, we examined the upstream fragment of the myoglobin gene for evolutionary conserved hypoxia response elements (HRE; 5′-GACGTGCT-3′) that would serve as binding sites for the HIF-1 complex (29, 33). Analysis of existing mouse, human, and rat genomic databases of the upstream fragment of the myoglobin gene for evolutionary conserved HREs failed to identify binding sites for the HIF-1 complex in the 10-kb upstream fragment of the myoglobin gene in these species (data not shown). The absence of HREs in the upstream region was further supported by our transcriptional analysis of the myoglobin promoter.
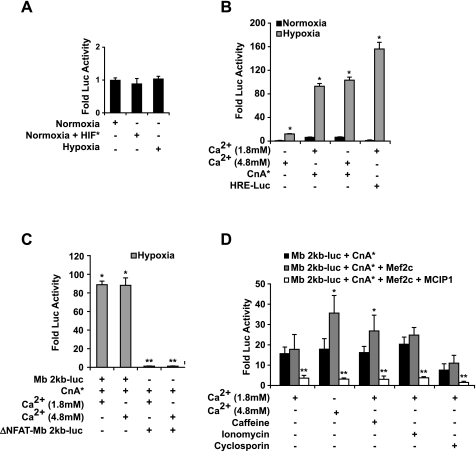
Using transgenic technologies, we established that the sequences contained within the 2-kb proximal 5′-flanking region of the myoglobin gene were sufficient to direct expression in the heart and oxidative skeletal myofibers (4, 5, 15). C2C12 myogenic cells were transfected with the myoglobin 2-kb promoter (Mb 2kb-luc) alone or in combination with constitutively active HIF-1α (HIF*) and/or a constitutively active form of calcineurin (CnA*). Following the differentiation of the transfected C2C12 myoblasts (day 5 following differentiation), we observed no differences in hypoxic (1% O2) or HIF-1-mediated transactivation of the myoglobin promoter compared with normoxic conditions (21% O2) (Fig. 2A, n = 6). In agreement with our previous studies, we observed that activated calcineurin stimulated myoglobin gene expression in normoxic myotubes (Fig. 2B; 8.5 ± 0.8 fold induction, n = 6) (13, 50). To verify that myotubes were exposed to hypoxic conditions, we transfected a hypoxia responsive reporter construct (HRE-Luc) and observed more than a 130-fold induction of reporter gene activity by hypoxia compared with normoxic conditions (Fig. 2B; 130 ± 10, n = 6). The results from the transcriptional assays further support the hypothesis that myoglobin is not a direct downstream target of HIF-1.
Fig. 2.
Effect of hypoxia on myoglobin transcriptional activity in C2C12 myotubes. A: C2C12 myoblasts were transfected with the 2-kb myoglobin promoter (Mb 2kb) linked to a luciferase reporter plasmid (pGL3) in the presence or absence of activated calcineurin (CnA*) or activated hypoxia-inducible factor (HIF*). The effect of hypoxia and/or activated HIF on the 2-kb myoglobin promoter in 5-day differentiated C2C12 myotubes demonstrated that hypoxia alone did not promote myoglobin transcriptional activity in C2C12 myotubes compared with normoxic conditions. B: effect of hypoxia and activated calcineurin and/or calcium stimulation on the 2-kb myoglobin promoter in 5-day differentiated C2C12 myotubes. Exposure to increased supplemental calcium and/or cotransfection with activated calcineurin resulted in increased (10 ± 0.05-fold to 110 ± 10-fold; P < 0.005, n = 12) myoglobin transcriptional activity compared with normoxic conditions. *Significant difference between hypoxic and normoxic conditions (P < 0.005, n = 12). HRE, hypoxia response element. C: inhibition of nuclear factor of activated T cell (NFAT) binding to the myoglobin promoter abolished the effects of hypoxia and calcium stimulation on myoglobin transcriptional activity. C2C12 cells were transfected with either the 2-kb myoglobin promoter or a 2-kb myoglobin promoter with mutated NFAT binding sites (ΔNFAT-Mb 2kb-luc). Following transfection of either of these promoter constructs, the myotubes were exposed to supplemental or extracellular calcium and/or cotransfected with activated calcineurin. After 4 days of differentiation under hypoxic conditions, the wild-type myoglobin promoter had a significant increase in myoglobin transcriptional activity in the presence of the dual stimulation of hypoxia and enhanced calcium signaling (i.e., activated calcineurin). However, removing the calcium sensitivity of the myoglobin promoter by mutating its NFAT binding sites completely abolished the hypoxic response (n = 6 for each experiment). *Significant difference between hypoxic and normoxic conditions (P < 0.05). **Significant reduction from hypoxic stimulated Mb 2kb-luc (P < 0.05). D: inhibition of calcium signaling attenuated the hypoxic response of the myoglobin promoter. Calcium signaling was inhibited in C2C12 myotubes by either forced expression of modulatory calcineurin interacting protein 1 (MCIP1) or cyclosporin administration, which abolished the increase in myoglobin transcriptional activity observed with calcium stimulation and hypoxia. *Statistically different from Mb 2kb-luc + activated calcineurin (n = 6, P < 0.05). **Statistically different from Mb 2kb-luc + activated calcineurin and Mb 2kb-luc + activated calcineurin + myocyte enhancer factor-2 C (Mef2c) (n = 6, P < 0.05).
In contrast to HIF-1, the myoglobin promoter activity was significantly induced in myotubes exposed to chronic hypoxia and either supplemental, extracellular calcium (3 mM supplemental calcium) (10 ± 0.05-fold, P = 0.005, ANOVA; n = 12) and/or activated calcineurin (110 ± 10-fold, P = 0.005, ANOVA; n = 12) (Fig. 2B). Extracellular calcium concentrations were selected on the basis of previously published data indicating that an additional 3 mM calcium supplemented to the growth or differentiation media elicited the maximal intracellular response with minimal cytotoxic effects (23). In myotubes, the hypoxic induction of myoglobin promoter activity was mediated in a calcineurin-dependent manner (and implicated NFAT transcription factors as the downstream effectors). These results suggested that the calcineurin/NFAT pathway was important for both the normoxic and hypoxic regulation of myoglobin.
Hypoxia-mediated induction of myoglobin requires a secondary stimulus that is mediated by the calcineurin/NFAT pathway.
To define the role of NFAT transcription factors in the hypoxic induction of myoglobin promoter activity, we mutated the NFAT regulatory elements in the myoglobin 2-kb promoter (*NFAT-Mb) by site-directed mutagenesis (52). As we previously demonstrated (Fig. 2B), we observed a significant increase (89 ± 8-fold over normoxic myotubes; P = 0.005, ANOVA; n = 6) in luciferase activity in myotubes exposed to hypoxic conditions following cotransfection of the Mb 2kb-luc with activated calcineurin and supplemental extracellular calcium (Fig. 2C). However, mutation of the functional NFAT-binding sites in the myoglobin promoter completely abolished the hypoxic induction of the reporter transgene, confirming that the secondary stimulus involved in the hypoxic induction of myoglobin was mediated by the calcineurin/NFAT signaling pathway (Fig. 2C).
We next assessed the role of Mef2c, a transcription factor that functions as a downstream transducer of calcium signaling. Unlike calcineurin, Mef2c alone or in the presence of supplemental, extracellular calcium minimally transactivated (2.5 ± 0.35-fold, n = 6) the myoglobin gene in mature myotubes exposed to either normoxic or hypoxic conditions (data not shown). However, Mef2c in combination with activated calcineurin and supplemental, extracellular calcium resulted in a synergistic increase in the transcription of the myoglobin gene (Fig. 2D, 37.3 ± 8.2-fold, P = 0.05, ANOVA; n = 6). These results indicated that NFAT and Mef2c proteins are downstream effectors of calcium signaling and function in combination to drive myoglobin promoter activity.
Additional support for the hypoxic induction of NFAT-dependent transactivation of the myoglobin promoter was provided by the use of pharmacological and endogenous inhibitors of calcineurin. Under hypoxic conditions, a marked reduction (86.2 ± 1.9%, n = 6) in myoglobin promoter activity was observed in myotubes that were either incubated with cyclosporine A or cotransfected with an expression construct containing modulatory calcineurin interacting protein (MCIP1) (Fig. 2, C and D). The results of both experiments (mutagenesis and MCIP1 studies) further supported the essential role of calcineurin/NFAT pathway as the secondary stimulus necessary for a hypoxic response.
Hypoxia modulates the mobilization of distinct pools of calcium in the myotube to regulate myoglobin expression.
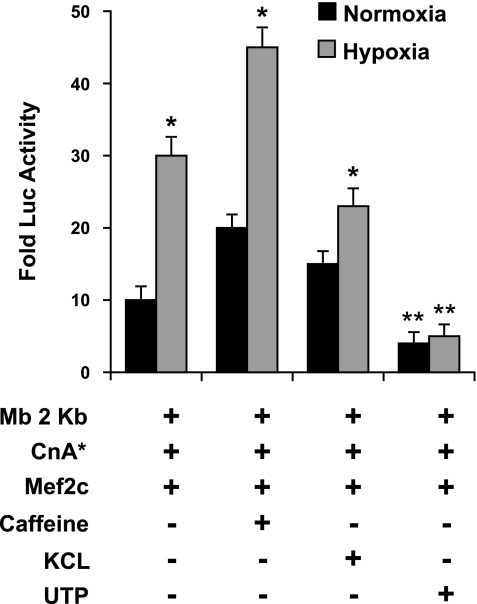
To further examine the hypothesis that hypoxia regulates myoglobin expression through the calcium/calcineurin/NFAT signaling pathway, we designed experiments to define the pools of calcium that were essential for the hypoxic induction of calcineurin activity. C2C12 cells were differentiated for 5 days under normoxic conditions and then exposed to different agents to release distinct intracellular pools of calcium under either normoxic or hypoxic conditions. Increasing cytoplasmic Ca2+ by activation of L-type channels by KCl (80 mM) depolarization or by activation of the ryanodine receptors (RyR) with caffeine (20 mM) significantly increased the transcription of the myoglobin promoter under normoxic conditions (n = 6, P = 0.02, ANOVA), which reproduced up to 73% of the hypoxic induction of myoglobin under normoxic conditions (Fig. 3). In addition, mobilization of the RyR pool by caffeine in combination with hypoxia showed an additional (45%) increase in myoglobin transcription. Remarkably, the stimulation of the inositol 1,4,5-trisphosphate (IP3) receptor (IP3R) calcium release channels in the endoplasmic reticulum (ER) with uridine 5′-triphosphate (UTP; 100 μM) significantly inhibited the transcriptional response of myoglobin under both normoxic and hypoxic conditions (n = 6, P = 0.02 ANOVA) (Fig. 3). Hence, increasing intracellular Ca2+ by the mobilization of distinct pools of calcium (RyR, L-type channel, and IP3R) revealed that the source of calcium is critical in stimulation of myoglobin transcription with Ca2+ release from the RyR pool or Ca2+ influx through L-type Ca2+ channels resulted in increased transcription, while Ca2+ release through the IP3 receptors and the associated store-dependent Ca2+ influx served to inhibit its transcription.
Fig. 3.
Release of calcium from the ryanodine receptors (RyR) of the sarcoplasmic reticulum is essential for the hypoxic induction of myoglobin. Pharmaceutical stimulation of the different intracellular calcium pools [caffeine→RyR, KCl→L-type channel, and uridine 5′-triphosphate (UTP)→inositol 1,4,5-trisphosphate (IP3) receptors] indicated that the hypoxic induction in myoglobin was dependent on calcium release specifically by the ryanodine receptors of the sarcoplasmic reticulum. Stimulation of normoxic 4-day myotubes with caffeine or KCl recapitulated ∼75% or 60% of the hypoxic stimulation, respectively. In contrast, stimulation of the IP3 receptor pool of calcium from the endoplasmic reticulum with UTP inhibited the transcription of myoglobin (n = 6, P = 0.02). *Significant difference between normoxic and hypoxic conditions. **Significant difference between UTP stimulation and all other conditions.
Hypoxia exerts dual control over sarcoplasmic reticulum Ca2+ release.
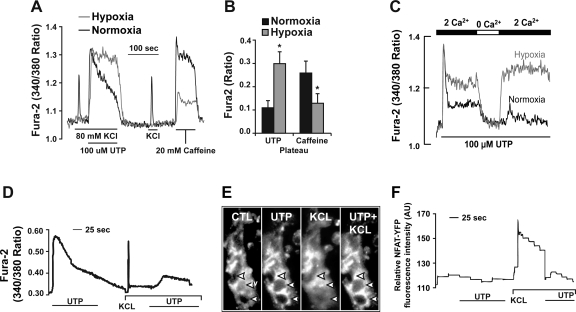
Collectively, our results supported the notion that hypoxia and exercise (as part of an adaptive response) stimulated the calcineurin signaling pathway to up-regulate myoglobin expression in striated muscle. To establish a mechanistic basis for these observations, we examined Ca2+ transients from normoxic and hypoxic myotubes stimulated with RyR and IP3R agonists using fura-2-based imaging. Hypoxia alone significantly inhibited the influx of calcium through the L-type calcium channels (Fig. 4A). We observed an increased duration of the Ca2+ signal in hypoxic myotubes, compared with normoxic myotubes, exposed to agents that released the IP3R Ca2+ pool (UTP; Fig. 4, A and B). In contrast, agents that release the sarcoplasmic reticulum (SR) Ca2+ pool (caffeine) via the RyR led to a dramatic decrease in the amplitude of the Ca2+ transient without affecting the overall duration of the signal (Fig. 4A). In addition, the inhibition of the KCl response was observed as early as 18 h after hypoxic exposure (data not shown). Experiments with calcium-free media abolished the UTP-mediated increase in steady-state cytoplasmic Ca2+ under hypoxic conditions, which indicate that the hypoxic induction of the elevated UTP-mediated Ca2+ response is due to increased Ca2+ influx (Fig. 4C). In summary, our results indicate that hypoxia eliminated the response to membrane depolarization, by inhibition of Ca2+ influx through the L-type Ca2+ channels. At the same time, hypoxia appears to increase Ca2+ influx by SOCs since the initial Ca2+ peak is the same between normoxic and hypoxic conditions and only the sustained response is prolonged. These alterations in Ca2+ dynamics indicated that hypoxia alone preferentially induced Ca2+ influx activated by IP3R-mediated Ca2+ released while inhibiting excitation-contraction coupling in the myotube.
Fig. 4.
Hypoxia inhibits the Ca2+ influx through L-type and RyR Ca2+ channels while stimulating receptor-stimulated Ca2+ influx. A: C2C12 myotubes were maintained under either normoxic or hypoxic conditions for 5 days after differentiation. Fura-2-loaded C2C12 myotubes were acutely stimulated with 100 μM UTP, depolarized with 80 mM KCl, and then treated with 20 mM caffeine to activate the RyRs. Hypoxic exposure significantly inhibited the influx of calcium through the L-type calcium channels (n = 3, P = 0.002, paired t-test). We observed an increased duration of the Ca2+ signal in hypoxic myotubes, compared with normoxic myotubes, exposed to agents that released the IP3 receptor Ca2+ pool (UTP). B: mean differences in the area beneath the calcium signal between the normoxic and hypoxic conditions. *Significant difference between normoxic and hypoxic conditions (n = 3, P = 0.002, paired t-test). C: fura-2-loaded C2C12 cells were stimulated with 100 μM UTP in media containing 2 mM Ca2+ and were then exposed to Ca2+-free media and Ca2+-containing media to evaluate the store-mediated Ca2+ influx. D: trace displays the change in fura-2 fluorescence in C2C12 myotubes following stimulation with 100 μM UTP, then 80 mM KCl, and finally KCl with UTP. This trace represents the fluxes in fura-2 associated with NFAT translocation to the nucleus. E: representative micrographs showing the localization of yellow fluorescent protein (YFP)-NFAT in response to calcium changes (arrowheads indicate myonuclei). Under control (CTL) and UTP stimulation, NFAT was localized in the cytoplasm. In response to KCl stimulation, NFAT translocated into the myonuclei. F: representative trace measuring the change in the YFP fluorescence in the nuclei of fura-2-loaded C2C12 myotubes to track the translocation of YFP-tagged NFAT into or out of the nucleus in response to mobilization of the different calcium pools by KCl and UTP. Under both normoxic and hypoxic conditions, stimulation with KCl translocated NFAT into the nucleus, whereas UTP stimulation either translocated NFAT out of the nucleus or maintained its cytoplasmic localization. AU, arbitrary units.
Hypoxia alone preferentially stimulated the Ca2+ influx pathways that have been previously associated with inhibition of NFAT's translocation to the nucleus (Fig. 4, E and F) (42). Figure 4E illustrates the effects of specific calcium-evoked agonists on the translocation of NFAT-YFP fusion protein in mature normoxic myotubes. Under control conditions, NFAT-YFP was localized in the cytosol surrounding the nuclei. Upon stimulation with UTP, NFAT-YFP remained localized to the cytosol. However, following stimulation with either KCl or caffeine, NFAT-YFP translocated to the nucleus. Interestingly, the inhibitory effects of UTP stimulation were sufficient to remove NFAT from the nucleus even in the presence of KCl or caffeine stimulation (Fig. 4, E and F). These experiments in combination with our calcium flux studies demonstrate that hypoxia alone stimulates the release of calcium transients from the ER while inhibiting the influx and release of calcium associated with excitation-contraction coupling. These results defined the mechanism by which hypoxia as the only stimulus inhibits the expression of myoglobin in muscle.
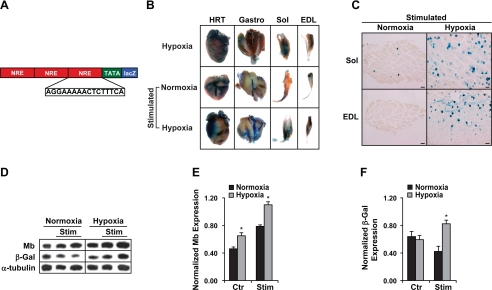
Hypoxia increased the sensitivity of the cell to calcium.
Previous studies have emphasized the limitations of the existing calcineurin enzymatic assays (46). An alternative but indirect measure of calcineurin activity was to examine NFAT transcriptional activity. We had previously generated transgenic NFAT indicator mice that had three copies of the NFAT regulatory element fused to the lacZ reporter gene (Fig. 5A) (42). We used these transgenic NFAT-lacZ indicator mice to examine NFAT activity. Hindlimbs of NFAT-lacZ indicator mice were electrically stimulated for 30 h under normoxic (21% O2) or hypoxic (10% O2) conditions. The hypoxic stimulated transgenic indicator mice had significantly greater calcineurin activity as indicated by increased β-galactosidase expression compared with their normoxic stimulated counterparts (Fig. 5, B and C). This increased calcineurin activity also resulted in significantly greater myoglobin (normalized myoglobin 0.79 ± 0.03 vs. 1.1 ± 0.05; normoxic vs. hypoxic, respectively; P < 0.05) and β-galactosidase (normalized β-galactosidase, 0.35 ± 0.02 vs. 0.83 ± 0.05; normoxic vs. hypoxic, respectively; P < 0.05) protein expression in the hypoxic stimulated animals (Fig. 5, D–F). These results established that under the same level of stimulation there was a significantly greater activation of NFAT under hypoxic as compared with normoxic conditions, which indicated a significantly greater calcineurin activity under hypoxic conditions.
Fig. 5.
Electrical stimulation in combination with hypoxia was a potent inducer of NFAT activity and myoglobin expression in vivo. A: schematic illustrating the transgene construct for the NFAT indicator mice. NFAT response elements (NRE) are linked in tandem to a basal promoter to direct lacZ reporter activity. The hindlimb muscles of transgenic NFAT indicator mice were electrically stimulated for 30 h (supramaximal pulses of 0.2-ms duration at a frequency of 10 Hz) using miniature neuromuscular stimulators implanted subcutaneously and attached to the sciatic nerve. B: whole-mount staining for β-galactosidase enzymatic activity in the heart (HRT) and skeletal muscles [gastrocnemius/plantaris (Gastro) complex, soleus (Sol), and extensor digitorum longus (EDL)] reveals increased expression in striated muscles of mice exposed to hypoxic conditions compared with normoxic conditions. All mice received electrical stimulation. C: transverse sections through the EDL and soleus showing the increased expression of β-galactosidase in the hypoxic stimulated muscles as compared with normoxic stimulated controls. D: Western blot analysis of myoglobin, β-galactosidase (β-Gal), and α-tubulin (loading control) expression from electrically stimulated tibialis anterior muscles (TA) of NFAT indicator mice after 30 h of neuromuscular stimulation. Mice were exposed to normoxic or hypoxic (10% oxygen) conditions. The electrically stimulated TA (stimulated) and nonstimulated (control) TA muscles were analyzed for protein expression. The results reveal a significant increase in the protein concentration of both myoglobin and β-galactosidase in hypoxic stimulated TA as compared with normoxic stimulated muscle. These results differ from the significant reduction in myoglobin protein concentration seen in the TA from unstimulated animals exposed to chronic hypoxia for up to 21 days. E: quantitation of the Western blot analysis of myoglobin expression from the respective animals (stimulated vs. control under normoxic and hypoxic conditions) normalized to the expression of α-tubulin. F: quantitation of the Western blot analysis of β-galactosidase expression from the respective animals (stimulated vs. control under normoxic and hypoxic conditions) normalized to the expression of α-tubulin. *Statistically different from normoxic values (n = 3, P < 0.05).
DISCUSSION
While the transcriptional regulation of myoglobin has been extensively studied under normoxic conditions (4, 5, 7, 21, 22, 51), the mechanism regulating myoglobin under hypoxic conditions is unclear. A long-standing debate has centered on the relationship of hypoxia and myoglobin content of skeletal muscle. To address this controversy, we undertook the present study focused on myoglobin transcriptional regulation using in vitro and whole animal models. The principle conclusion of this study establishes that myoglobin is not directly stimulated by hypoxia alone. Rather, conditions of hypoxia in combination with stimuli that initiate specific calcium signaling pathways (i.e., calcineurin or exercise) become a powerful stimulus for the induction of myoglobin. In addition, this study defined the mechanism of how hypoxia altered regulation of depolarization-evoked and receptor-stimulated Ca2+ signals to significantly increase the expression of myoglobin in response to exercise or other stimuli that initiated the calcium/calcineurin pathway in muscle.
An important result of this study was that changes in myoglobin expression can occur without a concomitant change in fiber type. The skeletal muscles of our chronic hypoxic mice showed no change in fiber type population in either the soleus or TA muscles. In contrast, there was a significant reduction in myoglobin protein expression in the nonworking TA. Previous studies have used changes in myoglobin expression as an indication of fiber type transformation. While changes in fiber type and myoglobin share similar molecular pathways, the results of this study demonstrate that they can be regulated independently.
Chronic hypoxia stimulates a spectrum of adaptations that collectively promote viability and functional performance. Studies undertaken in rodents exposed to chronic hypoxia reveal changes that promote oxygen supply and utilization such as an increase in capillary density, a polycythemic response (i.e., increased hematocrit), and the preferential use of metabolic pathways that favor the use of carbohydrate rather than lipid substrates (20, 34, 35). The phenotypic plasticity of skeletal muscle in response to hypoxia includes a modest reduction in fiber diameter (i.e., decreased fiber cross-sectional area) with minimal changes in fiber type composition. In contrast to the mouse model, animals adapted to work under hypoxic conditions such as diving mammals or high altitude mammals and birds have adaptations that promote facilitated oxygen transport to the mitochondria and ultimately maintain an aerobic lipid-based metabolism. In general, these adaptations include an increased capillary density, increased myoglobin concentration, and increased volume density of mitochondria as compared with normoxic-adapted animals of comparable size (24, 30–32, 43, 45). These enhanced concentrations of myoglobin play an important role in maintaining the adequate delivery of oxygen to the mitochondria under hypoxic conditions. Furthermore, the induction of myoglobin in skeletal muscle allows hypoxic-adapted animals to maintain higher levels of oxygen consumption under hypoxic conditions as compared with those exposed to normoxic environments. These physiological and anatomical adaptations are thought to be orchestrated by molecular programs that include HIF-1 (40). In the present study, we demonstrated that the hypoxia-mediated transcriptional activation of myoglobin gene expression was a HIF-1-independent mechanism.
Myoglobin is a relatively small multifunctional hemoprotein that functions, in part, to facilitate oxygen transport from the erythrocyte to the mitochondria in striated muscle. Oxygen avidly binds to the heme residue located within the central pocket of myoglobin, which typically remains 40% to 70% saturated with oxygen (49). Consequently, myoglobin also functions as a store of oxygen for the heart and oxidative skeletal myofibers. An additional role for myoglobin includes the scavenging of nitric oxide and reactive oxygen species that are increased in the stressed or hypoxic myocyte. Mice lacking myoglobin have a number of cellular (decreased fiber diameter and increased capillary density) and molecular adaptations including the induction of the HIF-1 pathway, further emphasizing the critical role for myoglobin in the maintenance of oxygen homeostasis within the myocytes (18, 19, 34, 35).
A conclusion of the present study was that the regulatory mechanisms of myoglobin expression used similar transcriptional pathways under hypoxic and normoxic conditions. Following differentiation, changes in myoglobin levels were regulated by neural and muscular activities that stimulate calcium signaling within the cell under both hypoxic and normoxic conditions (11, 13). Under normoxic conditions, the role of Ca2+ in regulating the calcineurin/NFAT pathway and its subsequent effect on gene expression has been established (10–12, 36); yet the changes in calcium signaling and which NFAT isoforms are regulated or induced with hypoxia remained undefined in striated muscle (37). Increased calcineurin activity results in an induction and translocation of NFATs to the nucleus under normoxic conditions (36). The results of this study showed that the main difference between the two conditions was that hypoxia alone in the absence of any secondary stimuli, such as activated calcineurin, exercise, or electrical stimulation, significantly decreased the Ca2+ influx mediated by the activation of the L-type Ca2+ channels and prolonged the duration of Ca2+ released from the ER (Fig. 4). Both of these results inhibited the translocation of NFAT into the nucleus, thus inhibiting the transcription and expression of myoglobin (Fig. 2A). In contrast, hypoxia in combination with a secondary stimulus, such as activated calcineurin, exercise, or electrical stimulation, significantly enhanced the translocation of NFAT into the nucleus, which significantly increased the transcription and expression of the myoglobin gene even under the same levels of stimulation in the whole animal (Figs. 2, 3, and 5).
Hypoxia has been shown to modulate intracellular calcium levels in smooth muscle, cardiomyocytes, epithelial, neuronal and in nonexcitable cells such as astrocytes (1, 2, 9, 25, 41). However, the effect of hypoxia on the mobilization of distinct calcium pools in skeletal muscle is unclear. These published studies reported that chronic hypoxia as the lone stimulus increases the levels of cytosolic Ca2+ specifically by enhancing the release from the ER and potentiating Ca2+ influx via the L-type Ca2+ channel. Our results in unstimulated myotubes also showed decreased calcium influx through the L-type calcium channels and decreased response to caffeine, while stimulating Ca2+ influx by SOCs in myocytes. These alterations in Ca2+ flux significantly inhibited the expression of myoglobin. Previous studies have described a general inhibition of gene expression and protein synthesis in response to hypoxia (8, 40, 41). The results of the present study clearly defined the mechanism by which hypoxia inhibits gene expression. In myocytes, hypoxia stimulated Ca2+ influx by SOCs while decreasing its influx through the L-type channels, which specifically inhibited the Calcineurin/NFAT pathway and forced NFAT, a key transcription factor in the expression of myoglobin, out of the nucleus. In contrast to unstimulated cells exposed to hypoxic conditions, we found that cells or whole animals exposed to stimulation in combination with hypoxia preserved their normal flux of Ca2+. In summary, this study further established that hypoxia alone altered intracellular Ca2+ to inhibit protein expression. However, hypoxia in combination with stimulation preserved and enhanced the release of Ca2+ from the SR as evidenced by the increased transcription of myoglobin in cell culture (Figs. 2 and 3) and the significantly greater expression of NFAT in our transgenic mice (Fig. 5), which acted to augment the protein expression of adaptive genes (i.e., myoglobin).
The results of this study indicated that even under hypoxic conditions, NFAT was found to be the critical downstream mediator of the increased calcineurin activity. In the present study, several lines of evidence were provided to demonstrate that the calcineurin/NFAT signaling pathway was amplified in response to hypoxia and a secondary stimulus. Using transcriptional assays and the myoglobin promoter, we demonstrated that calcineurin inhibition, with either cyclosporine or the endogenous calcineurin inhibitor MCIP1, or site-directed mutagenesis of the NFAT regulatory elements within the myoglobin promoter blocks the hypoxic-mediated induction of gene expression. Each of these strategies verified the importance of the calcineurin/NFAT pathway for the transcriptional regulation of myoglobin in response to hypoxia. In addition, we provided evidence that the mechanism for hypoxic augmentation of the calcineurin/NFAT pathway during stimulation was dependent on alterations in intracellular Ca2+ transients. This combination appeared to have augmented the duration of Ca2+ transients released specifically from the SR as evidenced by the significant increase in calcineurin activity (Fig. 5). These studies were extended into the whole animal as we used a novel transgenic NFAT indicator mouse to evaluate NFAT activity in response to both electrical stimulation and hypoxia. Collectively, these data support a mechanistic model that requires motor nerve stimulation in combination with hypoxia for the induction of myoglobin gene expression in muscle.
Our data established that the calcineurin/NFAT pathway was induced in the working hypoxic skeletal muscle and was a potent transcriptional regulator of myoglobin gene expression. Future studies are needed to further examine which NFAT isoforms are regulated or induced in response to hypoxic conditions in skeletal muscle. These data supported a mechanistic cytoprotective role in striated muscle for the calcineurin/NFAT pathway as it may promote oxygen delivery and/or minimize oxidative stress under hypoxic or stressful conditions through the regulation of myoglobin expression. In addition, this was the first study to define the hypoxic-mediated mechanisms in striated muscle that altered the Ca2+ signal evoked by mobilization of distinct cellular Ca2+ pools to significantly enhance the transcriptional response of a specific adaptive gene. In this fashion, the muscle maintains oxygen homeostasis and facilitates functional performance. Future studies will be necessary to further examine the molecular hypoxic response in skeletal muscle using genetic mouse models including the myoglobin-deficient mouse to further define hypoxia-induced pathways that regulate transcriptional activity within the myocyte.
GRANTS
We acknowledge funding support from the National Institutes of Health (National Heart, Lung, and Blood Institute Grant HL-63788).
Acknowledgments
We thank Drs. B. A. Rothermel and A. M. Masino for helpful discussions throughout the course of these studies. We also thank Dr. J. Richardson and the pathology core for assistance with the histological analyses. The HIF-1 overexpression plasmid was donated by Dr. Ralph Shohet.
Present addresses: S. B. Kanatous, Department of Biology, Colorado State University, Fort Collins, CO 80523-1878; C. M. Martin and D. J. Garry, Lillehei Heart Institute, University of Minnesota, Minneapolis, MN 55455.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aley PK, Murray HJ, Boyle JP, Pearson HA, Peers C. Hypoxia stimulates Ca2+ release from intracellular stores in astrocytes via cyclic ADP ribose-mediated activation of ryanodine receptors. Cell Calcium 39: 95–100, 2006. [DOI] [PubMed] [Google Scholar]
- 2.Aley PK, Porter KE, Boyle JP, Kemp PJ, Peers C. Hypoxic modulation of Ca2+ signaling in human venous endothelial cells. Multiple roles for reactive oxygen species. J Biol Chem 280: 13349–13354, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong RB, Essen-Gustavsson B, Hoppeler H, Jones JH, Kayar SR, Laughlin MH, Lindholm A, Longworth KE, Taylor CR, Weibel ER. O2 delivery at V̇o2 max and oxidative capacity in muscles of standardbred horses. J Appl Physiol 73: 2274–2282, 1992. [DOI] [PubMed] [Google Scholar]
- 4.Bassel-Duby R, Grohe CM, Jessen ME, Parsons WJ, Richardson JA, Chao R, Grayson J, Ring WS, Williams RS. Sequence elements required for transcriptional activity of the human myoglobin promoter in intact myocardium. Circ Res 73: 360–366, 1993. [DOI] [PubMed] [Google Scholar]
- 5.Bassel-Duby R, Hernandez MD, Gonzalez MA, Krueger JK, Williams RS. A 40-kilodalton protein binds specifically to an upstream sequence element essential for muscle-specific transcription of the human myoglobin promoter. Mol Cell Biol 12: 5024–5032, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunori M Nitric oxide moves myoglobin centre stage. Trends Biochem Sci 26: 209–210, 2001. [DOI] [PubMed] [Google Scholar]
- 7.Brunori M Nitric oxide, cytochrome-c oxidase and myoglobin. Trends Biochem Sci 26: 21–23, 2001. [DOI] [PubMed] [Google Scholar]
- 8.Callinan L, McCarthy TV, Maulet Y, Mackrill JJ. Atypical L-type channels are down-regulated in hypoxia. Biochem Soc Trans 33: 1137–1139, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Lu XY, Li J, Fu JD, Zhou ZN, Yang HT. Intermittent hypoxia protects cardiomyocytes against ischemia-reperfusion injury-induced alterations in Ca2+ homeostasis and contraction via the sarcoplasmic reticulum and Na+/Ca2+ exchange mechanisms. Am J Physiol Cell Physiol 290: C1221–C1229, 2006. [DOI] [PubMed] [Google Scholar]
- 10.Chin ER Role of Ca2+/calmodulin-dependent kinases in skeletal muscle plasticity. J Appl Physiol 99: 414–423, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Chin ER The role of calcium and calcium/calmodulin-dependent kinases in skeletal muscle plasticity and mitochondrial biogenesis. Proc Nutr Soc 63: 279–286, 2004. [DOI] [PubMed] [Google Scholar]
- 12.Chin ER, Grange RW, Viau F, Simard AR, Humphries C, Shelton J, Bassel-Duby R, Williams RS, Michel RN. Alterations in slow-twitch muscle phenotype in transgenic mice overexpressing the Ca2+ buffering protein parvalbumin. J Physiol 547: 649–663, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, Wu H, Zhu W, Bassel-Duby R, Williams RS. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev 12: 2499–2509, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clanton TL, Klawitter PF. Invited review: adaptive responses of skeletal muscle to intermittent hypoxia: the known and the unknown. J Appl Physiol 90: 2476–2487, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Devlin BH, Wefald FC, Kraus WE, Bernard TS, Williams RS. Identification of a muscle-specific enhancer within the 5′-flanking region of the human myoglobin gene. J Biol Chem 264: 13896–13901, 1989. [PubMed] [Google Scholar]
- 16.Flogel U, Godecke A, Klotz LO, Schrader J. Role of myoglobin in the antioxidant defense of the heart. FASEB J 18: 1156–1158, 2004. [DOI] [PubMed] [Google Scholar]
- 17.Garry DJ, Kanatous SB, Mammen PP. Emerging roles for myoglobin in the heart. Trends Cardiovasc Med 13: 111–116, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Garry DJ, Meeson A, Yan Z, Williams RS. Life without myoglobin. Cell Mol Life Sci 57: 896–898, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garry DJ, Ordway GA, Lorenz JN, Radford NB, Chin ER, Grange RW, Bassel-Duby R, Williams RS. Mice without myoglobin. Nature 395: 905–908, 1998. [DOI] [PubMed] [Google Scholar]
- 20.Grange RW, Meeson A, Chin E, Lau KS, Stull JT, Shelton JM, Williams RS, Garry DJ. Functional and molecular adaptations in skeletal muscle of myoglobin-mutant mice. Am J Physiol Cell Physiol 281: C1487–C1494, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Grayson J, Bassel-Duby R, Williams RS. Collaborative interactions between MEF-2 and Sp1 in muscle-specific gene regulation. J Cell Biochem 70: 366–375, 1998. [PubMed] [Google Scholar]
- 22.Grayson J, Williams RS, Yu YT, Bassel-Duby R. Synergistic interactions between heterologous upstream activation elements and specific TATA sequences in a muscle-specific promoter. Mol Cell Biol 15: 1870–1878, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawke TJ, Allen DG, Lindinger MI. Paraxanthine, a caffeine metabolite, dose dependently increases [Ca2+]i in skeletal muscle. J Appl Physiol 89: 2312–2317, 2000. [DOI] [PubMed] [Google Scholar]
- 24.Hochachka PW Balancing conflicting metabolic demands of exercise and diving. Fed Proc 45: 2948–2952, 1986. [PubMed] [Google Scholar]
- 25.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev 17: 2205–2232, 2003. [DOI] [PubMed] [Google Scholar]
- 26.Hoppeler H, Desplanches D. Muscle structural modifications in hypoxia. Int J Sports Med 13, Suppl 1: S166–S168, 1992. [DOI] [PubMed] [Google Scholar]
- 27.Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol 204: 3133–3139, 2001. [DOI] [PubMed] [Google Scholar]
- 28.Howald H, Pette D, Simoneau JA, Uber A, Hoppeler H, Cerretelli P. Effect of chronic hypoxia on muscle enzyme activities. Int J Sports Med 11, Suppl 1: S10–S14, 1990. [DOI] [PubMed] [Google Scholar]
- 29.Jiang BH, Zheng JZ, Leung SW, Roe R, Semenza GL. Transactivation and inhibitory domains of hypoxia-inducible factor 1alpha. Modulation of transcriptional activity by oxygen tension. J Biol Chem 272: 19253–19260, 1997. [DOI] [PubMed] [Google Scholar]
- 30.Kanatous SB, Davis RW, Watson R, Polasek L, Williams TM, Mathieu-Costello O. Aerobic capacities in the skeletal muscles of Weddell seals: key to longer dive durations? J Exp Biol 205: 3601–3608, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Kanatous SB, DiMichele LV, Cowan DF, Davis RW. High aerobic capacities in the skeletal muscles of pinnipeds: adaptations to diving hypoxia. J Appl Physiol 86: 1247–1256, 1999. [DOI] [PubMed] [Google Scholar]
- 32.Kanatous SB, Elsner R, Mathieu-Costello O. Muscle capillary supply in harbor seals. J Appl Physiol 90: 1919–1926, 2001. [DOI] [PubMed] [Google Scholar]
- 33.Lee PJ, Jiang BH, Chin BY, Iyer NV, Alam J, Semenza GL, Choi AM. Hypoxia-inducible factor-1 mediates transcriptional activation of the heme oxygenase-1 gene in response to hypoxia. J Biol Chem 272: 5375–5381, 1997. [PubMed] [Google Scholar]
- 34.Mammen PP, Kanatous SB, Yuhanna IS, Shaul PW, Garry MG, Balaban RS, Garry DJ. Hypoxia-induced left ventricular dysfunction in myoglobin-deficient mice. Am J Physiol Heart Circ Physiol 285: H2132–H2141, 2003. [DOI] [PubMed] [Google Scholar]
- 35.Meeson AP, Radford N, Shelton JM, Mammen PP, DiMaio JM, Hutcheson K, Kong Y, Elterman J, Williams RS, Garry DJ. Adaptive mechanisms that preserve cardiac function in mice without myoglobin. Circ Res 88: 713–720, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Michel RN, Dunn SE, Chin ER. Calcineurin and skeletal muscle growth. Proc Nutr Soc 63: 341–349, 2004. [DOI] [PubMed] [Google Scholar]
- 37.Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respir Physiol Neurobiol 164: 277–281, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reynafarje B Myoglobin content and enzymatic activity of human skeletal muscle–their relation with the process of adaptation to high altitude. Tech Doc Rep SAMTDR USAF Sch Aerosp Med SAM-TDR-62–89: 8p, 1962. [DOI] [PubMed]
- 39.Schlieper G, Kim JH, Molojavyi A, Jacoby C, Laussmann T, Flogel U, Godecke A, Schrader J. Adaptation of the myoglobin knockout mouse to hypoxic stress. Am J Physiol Regul Integr Comp Physiol 286: R786–R792, 2004. [DOI] [PubMed] [Google Scholar]
- 40.Semenza GL HIF-1: mediator of physiological and pathophysiological responses to hypoxia. J Appl Physiol 88: 1474–1480, 2000. [DOI] [PubMed] [Google Scholar]
- 41.Seta KA, Yuan Y, Spicer Z, Lu G, Bedard J, Ferguson TK, Pathrose P, Cole-Strauss A, Kaufhold A, Millhorn DE. The role of calcium in hypoxia-induced signal transduction and gene expression. Cell Calcium 36: 331–340, 2004. [DOI] [PubMed] [Google Scholar]
- 42.Stiber JA, Tabatabaei N, Hawkins AF, Hawke T, Worley PF, Williams RS, Rosenberg P. Homer modulates NFAT-dependent signaling during muscle differentiation. Dev Biol 287: 213–224, 2005. [DOI] [PubMed] [Google Scholar]
- 43.Terrados N, Jansson E, Sylven C, Kaijser L. Is hypoxia a stimulus for synthesis of oxidative enzymes and myoglobin? J Appl Physiol 68: 2369–2372, 1990. [DOI] [PubMed] [Google Scholar]
- 44.Terrados N, Melichna J, Sylven C, Jansson E. Decrease in skeletal muscle myoglobin with intensive training in man. Acta Physiol Scand 128: 651–652, 1986. [DOI] [PubMed] [Google Scholar]
- 45.Widmer HR, Hoppeler H, Nevo E, Taylor CR, Weibel ER. Working underground: respiratory adaptations in the blind mole rat. Proc Natl Acad Sci USA 94: 2062–2067, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, Jones F, Kimball TR, Molkentin JD. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res 94: 110–118, 2004. [DOI] [PubMed] [Google Scholar]
- 47.Wilkins BJ, De Windt LJ, Bueno OF, Braz JC, Glascock BJ, Kimball TF, Molkentin JD. Targeted disruption of NFATc3, but not NFATc4, reveals an intrinsic defect in calcineurin-mediated cardiac hypertrophic growth. Mol Cell Biol 22: 7603–7613, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol 541: 1–8, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wittenberg JB, Wittenberg BA. Myoglobin-enhanced oxygen delivery to isolated cardiac mitochondria. J Exp Biol 210: 2082–2090, 2007. [DOI] [PubMed] [Google Scholar]
- 50.Wu H, Naya FJ, McKinsey TA, Mercer B, Shelton JM, Chin ER, Simard AR, Michel RN, Bassel-Duby R, Olson EN, Williams RS. MEF2 responds to multiple calcium-regulated signals in the control of skeletal muscle fiber type. EMBO J 19: 1963–1973, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yan Z, Serrano AL, Schiaffino S, Bassel-Duby R, Williams RS. Regulatory elements governing transcription in specialized myofiber subtypes. J Biol Chem 276: 17361–17366, 2001. [DOI] [PubMed] [Google Scholar]
- 52.Yang Q, Bassel-Duby R, Williams RS. Transient expression of a winged-helix protein, MNF-beta, during myogenesis. Mol Cell Biol 17: 5236–5243, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]