Abstract
Monocarboxylate transporter (MCT) 4 is a heteromeric proton-coupled lactate transporter that is noncovalently linked to the extracellular matrix metalloproteinase inducer CD147 and is typically expressed in glycolytic tissues. There is increasing evidence to suggest that ion transporters are part of macromolecular complexes involved in regulating β1-integrin adhesion and cell movement. In the present study we examined whether MCTs play a role in cell migration through their interaction with β1-integrin. Using reciprocal coimmunoprecipitation assays, we found that β1-integrin selectively associated with MCT4 in ARPE-19 and MDCK cells, two epithelial cell lines that express both MCT1 and MCT4. In polarized monolayers of ARPE-19 cells, MCT4 and β1-integrin colocalized to the basolateral membrane, while both proteins were found in the leading edge lamellapodia of migrating cells. In scratch-wound assays, MCT4 knockdown slowed migration and increased focal adhesion size. In contrast, silencing MCT1 did not alter the rate of cell migration or focal adhesion size. Taken together, our findings suggest that the specific interaction of MCT4 with β1-integrin may regulate cell migration through modulation of focal adhesions.
Keywords: MCT4, CD147
monocarboxylate transporter 4 (MCT4) is a member of the SLC16 family of solute transporters and is primarily expressed in tissues that rely on glycolysis for their energy production (15, 17, 21, 57). MCT4 is a low-affinity, high-capacity symporter that catalyzes proton-coupled efflux of lactate across the cell membrane and is expressed at high levels in embryonic tissues, skeletal muscle, neural retina, and metastatic cancer cell lines (19, 25, 37, 52). Like other solute transporters, MCT4 has 12 membrane-spanning domains and forms a heteromeric complex with a highly glycosylated type I glycoprotein, CD147 (30). The catalytic (MCT4) and accessory (CD147) subunits of this heteromeric lactate transporter are assembled into a complex in the endoplasmic reticulum, and in the absence of either subunit, the other subunit is retained in the endoplasmic reticulum and is likely targeted for degradation (14, 19). CD147, which was first identified as an extracellular matrix metalloproteinase inducer, also interacts with CD98 (10, 55), CD44 (49), syndecan-1 (35), γ-secretase (58, 59), shrew-1 (41), and β1-integrin (3, 10, 11). The association of CD147 with proteins involved in lactate and amino acid transport, cell attachment, motility, and proliferation suggest that the heteromeric lactate transporter is a component of a larger macromolecular complex at the plasma membrane that influences numerous cellular processes.
Recent work has demonstrated a role for both CD147 and β1-integrin during cell migration in processes such as metastasis and wound healing. For example, increased expression of CD147 correlates with enhanced metastatic potential (4, 32, 40, 42, 56), while silencing CD147 in hepatic cancer, ovarian cancer, and lymphoma cell lines inhibited cell migration (8, 9, 60). CD147 plays a role in corneal epithelium wound healing as well, where increased expression and redistribution of CD147 and increased induction of matrix metalloproteinase 2 were observed following injury (18). Similarly, β1-integrin is required for both cancer cell migration and migration of the retinal pigment epithelium (RPE) following injury (1, 20, 22, 51). Interestingly, work from our lab has demonstrated a role for MCT4 in cell migration in a metastatic breast cancer cell line. Specifically, silencing MCT4 in MDA-MB-231 cells resulted in a significant reduction of cell invasion (19). The functional similarity of CD147, MCT4, and β1-integrin during cell migration, as well as the reports of CD147/MCT4 and CD147/β1-integrin complexes, suggests that these proteins could be forming a “supercomplex” that modulates cell adhesion and motility.
The RPE forms the outer blood-retinal barrier and performs functions critical to maintaining the health and function of the photoreceptor cells. Under normal conditions, the RPE is nonproliferative and nonmigratory; however, following an ocular trauma, such as rhegmatogenous retinal detachment, RPE cells can engage in an inappropriate or uncontrolled wound healing response termed proliferative vitreoretinopathy (PVR) (7, 27, 29). Following injury, RPE and glial cells begin to migrate along both the front and back surfaces of the retina and proliferate, forming PVR membranes, which can lead to further retinal detachment and loss of vision (7, 28). The early formation of PVR membranes is largely due to RPE cell migration, which occurs after these cells undergo an epithelial-to-mesenchymal transformation (EMT). This cellular event is also seen in various cancers, where tumor cells undergo EMT as they become more metastatic (24, 31).
The purpose of these studies was to examine whether MCTs play a role in RPE cell migration during wound healing. Using the human RPE cell line ARPE-19, we found that MCT4 and β1-integrin form a complex in the basolateral membrane of polarized cells and colocalized at the leading edge of migrating cells. Additionally, we found that silencing of MCT4 resulted in larger focal adhesions and slowed cell migration, indicating a role for the lactate transporter in migration of the RPE.
MATERIALS AND METHODS
Reagents and cell culture.
Cell culture reagents were purchased from Mediatech (Herndon, VA). Reagents used for SDS-PAGE were obtained from Invitrogen (Carlsbad, CA). All small interfering RNAs (siRNAs) used were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). ARPE-19 and MDCK cells were cultured as previously described (14, 37).
Antibodies.
MCT1 and MCT4 antibodies were generated for our laboratory by Zymed as previously described (36). The α3-integrin antibody was generated as previously described (33). Additional antibodies used in these studies were α5-integrin (sc-10729; Santa Cruz Biotechnology), paxillin (610619; BD Biosciences, Franklin Lakes, NJ), and β-actin (A5441; Sigma). Additionally, anti-human β1-integrin (MAB20797Z, Chemicon, Temecula, CA) was used for immunoblotting. Anti-human β1-integrin antibody (AIIB2) [developed by Werb et al. (54) and obtained by Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242] was used for both immunoprecipitation and immunofluorescence labeling. Alexa-Fluor-tagged secondary antibodies were purchased from Molecular Probes (Eugene, OR). Horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Bio-Rad Laboratories (Hercules, CA) and Molecular Probes.
Immunoblot analysis.
Cells were washed with PBS and lysed with ice-cold lysis buffer (25 mM HEPES buffer, pH 7.4, 150 mM NaCl, 5 mM MgCl2, and 1% Triton X-100) containing protease inhibitors (Complete Mini, Roche, Indianapolis, IN) for 30 min and were then centrifuged at 14,000 g at 4°C for 30 min. The protein concentration of the cleared lysates was determined using BCA reagent (Pierce, Rockford, IL). Lysates were diluted in 2× LDS sample buffer (Invitrogen), and equal amounts of protein were loaded onto 4–12% NuPAGE Bis-Tris gels (Invitrogen). Separated proteins were transferred electrophoretically from gels to Immobilon-P membranes (Millipore, Bedford, MA). Membranes were incubated for 1 h at room temperature in blocking buffer (20 mM Tris, 137 mM NaCl, pH 7.5, and 5% dry skim milk), followed by 1 h incubation with primary antibodies and 30 min incubation with HRP-conjugated secondary antibodies diluted 1:5,000. Blots were probed with the following antibodies: CD147 (goat, 1:500), MCT1 (rabbit, 1:1,000), MCT4 (rabbit, 1:1,000), α3-integrin (rabbit, 1:1,000), α5-integrin (goat, 1:500), and β1-integrin (mouse, 1:1,000). β-Actin (mouse, 1:50,000) was used as a loading control. Reactive bands were visualized with enhanced chemiluminescent Western blotting detection reagents (GE Healthcare Bio-Sciences, Piscataway, NJ).
Immunoprecipitation.
Cells were washed two times with PBS and lysed with ice-cold lysis buffer (25 mM HEPES buffer, pH 7.4, 150 mM NaCl, 5 mM MgCl2, and 1% CHAPS detergent) containing protease inhibitors (Complete Mini) for 30 min. The lysate was clarified by centrifugation at 14,000 g for 30 min. An aliquot of the supernatant was removed for protein analysis, and the remaining supernatant (300–500 μg in 400 μl) was incubated with 3–5 μl of MCT1, MCT4, or β1-integrin antibodies overnight with end-over rotation at 4°C. The following day, 20 μl of Immunopure Immobilized protein A/G Plus beads (Pierce) were added and the samples were incubated for 2–4 h with end-over rotation at 4°C. Cell lysates were incubated with beads only (no antibody added) as a control. The beads were washed and pelleted by low-speed centrifugation. The supernatants were discarded, and the beads were resuspended in lysis buffer (this process was repeated three times). Bound proteins were eluted from the beads with 2× LDS sample buffer, and proteins were analyzed by SDS-PAGE and immunoblotting. Densitometry measurements were performed using AlphaEaseFC software and the Spot Denso tool (version 4.0.0, Alpha Innotech, San Leandro, CA). All blot images were measured in the linear range. Spot Denso output data were analyzed using Microsoft Excel, and immunoprecipitated fractions were expressed as a percentage of the input fraction (% input ± SE). Immunoprecipitation experiments were performed at least three times for each condition. Similar results were obtained in all experiments.
Immunofluorescence.
Preparation of ARPE-19 sections was performed as previously described (37). ARPE-19 cells used for wounding assays were cultured on two-well glass or Permanox slides or in 35-mm dishes. Cells were fixed in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) in PBS for 5 min at room temperature, and then 25 min on ice. Cells were washed in PBS, permeabilized with methanol for 3 min at −20°C, blocked using 5% BSA in PBS with 0.1% Tween 20 (PBST), and incubated with primary antibody overnight at 4°C. The next day, cells were washed with PBST and incubated in secondary antibody for 30 min, washed, and mounted with Gelvatol. Antibodies were used at the following dilutions: MCT1 (rabbit, 1:100); MCT4 (rabbit, 1:500); β1-integrin (DSHB, rat, 1:100-1:250); paxillin (mouse, 1:250-1:500); Alexa-Fluor 488 (rat and mouse, 1:500); Alexa-Fluor 555 (rabbit, 1:500). It should be noted that labeling with MCT1 antibody results in nonspecific nuclear staining. This staining was nonspecific because when MCT1 was silenced with siRNA, all cell-cell and plasma membrane staining was lost and nuclear staining persisted (Fig. 4, B and D). Images were obtained on a laser scanning confocal microscope (Zeiss LSM510) with a ×63 oil objective. Images shown in Fig. 4 were prepared from projections (2 slices) of Z-Stack images taken at ×63 magnification (large panels) or ×63 magnification with a ×2 zoom (insets). Images shown in Fig. 5 are single slices taken at ×63 magnification (Fig. 5, A, C, and E) and ×63 magnification with a ×4 zoom (Fig. 5, B, D, and F). Images were processed with LSM510 software and Adobe Photoshop 7.0 (San Jose, CA). Adjustments were made to brightness and contrast only. For area measurements, 135–230 adhesions were counted over five to seven cells per treatment condition. Focal adhesion area was measured using ImageJ software and the polygon and measure tools. Focal adhesions were grouped by size and are expressed as a percentage of total adhesions. Data are representative of two individual experiments with duplicate samples in each experiment. Similar results were obtained in all experiments.
PCR reactions.
Total RNA was extracted from cells using Qiagen RNeasy Mini kits according to the manufacturer's protocol. cDNA was synthesized from 4 μg total RNA by SuperScript III reverse transcriptase (Invitrogen) with oligo(dT) primers. PCR was performed with 1 μl cDNA with the primers and conditions described in Table S1 (supplemental material for this article is available online at the American Journal of Physiology: Cell Physiology website) and Fisher Taq polymerase.
siRNA-mediated silencing of MCTs and CD147.
Silencing of MCT1, MCT4, and CD147 expression was performed following the manufacturer's protocols. Before transfection, cells were grown to 70–80% confluence in 12-well plates. Cells were transfected with siRNA pools specific for hCD147 (accession no. NM_001728), hMCT1 (accession no. NM_003051), or hMCT4 (accession no. NM_004207) using the DharmaFECT 2 transfection reagent (Dharmacon). As an additional control, cells were mock transfected using transfection reagent alone. All cells were harvested 48 h posttransfection for RNA and 72 h posttransfection for immunoblot analysis or immunofluorescence. For wounding assays, cells were scratch-wounded at 48 h posttransfection, and wounds were analyzed at 72 h posttransfection. All siRNA experiments were performed in triplicate and were repeated at least three times. Similar results were obtained in all experiments.
Wounding assays.
Scratch-wounding assays were performed on ARPE-19 cells in a 12-well plate (for protein and quantification), 35-mm dish, or two-well Permanox slides (for immunofluorescence). Two hours before wounding, cells were treated with the DNA polymerase inhibitor aphidicolin (2 μg/ml; Invitrogen) to block cell proliferation. After culture medium was removed, a wound was made in the center of ARPE-19 cultures by scratching with a 1-ml pipette tip. The cultures were washed twice with PBS to remove any debris resulting from wounding. Media were replaced and cells were incubated overnight at 37°C in a 5% CO2 atmosphere. For immunolocalization studies, cells were fixed 24 h postwounding as described. For analysis of wound closure, wounded cells were imaged on an inverted microscope with a ×4 objective at 0 h and 25 h postwounding. Following imaging at the 25-h time point, cells were harvested for protein as described. Wound area at each time point was measured using the polygon and measure tools in Zeiss LSM510 imaging software. Wound closure was expressed as percentage of the original wound area ± SE. Significance was determined using a one-way ANOVA test.
RESULTS
β1-Integrin preferentially interacts with MCT4.
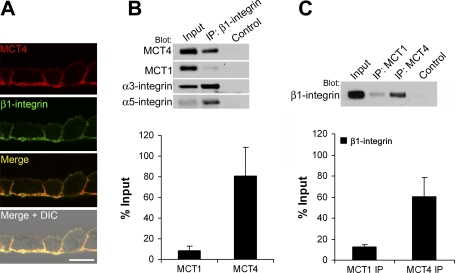
While studies have shown that CD147 associates with β1-integrin, none of these studies addressed whether MCTs were also present in the complex. In polarized monolayers of ARPE-19 cells, β1-integrin was restricted to the basolateral membrane where it colocalized with MCT4 (Fig. 1A). Since CD147 expression is not restricted to regions of β1-integrin expression, we hypothesized that the interaction between CD147 and β1-integrin is mediated by specific MCT isoforms. To test this hypothesis, reciprocal coimmunoprecipitation experiments were performed (Fig. 1A). β1-Integrin was immunoprecipitated from detergent-soluble lysates of ARPE-19 cells, and blots were probed with antibodies specific for MCT1, MCT4, α3-integrin, and α5-integrin. We found that MCT4 was more abundant in the immunoprecipitated pellet than MCT1 (Fig. 1B, top). Specifically, when expressed as a ratio of the input, ∼80% of MCT4 coimmunoprecipitated with β1-integrin, while only ∼8% of MCT1 was found to associate (Fig. 1B, bottom). As expected, both α3-integrin and α5-integrin coimmunoprecipitated with β1-integrin (Fig. 1B). In reciprocal immunoprecipitation experiments, more β1-integrin coimmunoprecipitated with MCT4 than with MCT1 (Fig. 1C). Similarly, in MDCK cells (where both MCT1 and MCT4 are expressed on the basolateral surface), β1-integrin preferentially coimmunoprecipitated with MCT4 (data not shown). These results, therefore, demonstrate that the interaction between MCTs and β1-integrin is isoform specific.
Fig. 1.
Monocarboxylate transporter (MCT) 4 associates with β1-integrin in ARPE-19 cells. A: immunofluorescence microscopy of frozen sections of ARPE-19 cells labeled with MCT4 and β1-integrin antibodies. Bar = 10 μm. DIC, differential interference contrast. B and C: the association of MCT1 and MCT4 with β1-integrin was studied by reciprocal coimmunoprecipitation of ARPE-19 lysates and immunoblotting. B: ARPE-19 detergent-soluble lysates were immunoprecipitated (IP) with β1-integrin antibody and blotted with MCT4, MCT1, α3- and α5-integrin antibodies. Relative amounts of MCT1 and MCT4 coimmunoprecipitated with β1-integrin were expressed as a percentage of the input fraction ± SE (bottom) C: ARPE-19 lysates were immunoprecipitated with MCT4 and MCT1 antibodies and probed with β1-integrin antibody. Levels of association were determined as a percentage of the input fraction ± SE (bottom). Ten micrograms of total protein were loaded in “input” lanes; 20 μl of pellet fraction were loaded in “IP” and “control” lanes. Control, beads + lysate.
Silencing MCTs or CD147 does not impact integrin expression.
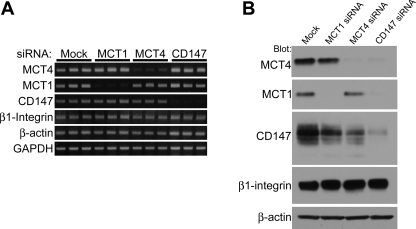
Since β1-integrin associates with MCT4/CD147 heterodimers, we next wanted to examine whether silencing MCTs or CD147 altered β1-integrin expression. ARPE-19 cells were transfected with siRNAs specific for MCT1, MCT4, and CD147. Semiquantitative RT-PCR analysis showed that silencing with MCT1, MCT4, or CD147 specific siRNAs reduced the abundance of only the target RNA (Fig. 2A). Additionally, silencing MCTs or CD147 had no effect on the levels of β1-integrin mRNA (Fig. 2A). These results indicate that silencing of MCTs or CD147 does not affect the expression of each other or β1-integrin at the mRNA level.
Fig. 2.
Silencing MCTs/CD147 in ARPE-19 cells does not impact integrin expression. A: RT-PCR was performed on cells to determine the effectiveness of small interfering RNA (siRNA)-mediated silencing of MCT1, MCT4, or CD147 and if there were changes in expression of nontarget mRNAs. Cells were harvested for total RNA preparation 48 h posttransfection. PCR results show that MCT1, MCT4, and CD147 siRNAs efficiently silenced their respective targets. No change in mRNA levels of β1-integrin was observed. GAPDH and β-actin served as loading controls. B: cells were harvested for protein analysis 72 h posttransfection. Silencing MCT1 and MCT4 resulted in decreased expression of CD147, while silencing of CD147 resulted in decreased expression of MCT1 and MCT4. β1-Integrin expression was not affected by silencing MCT1, MCT4, or CD147. β-Actin was used as a loading control. Ten micrograms of total protein were loaded per lane.
Protein levels of β1-integrin were also examined in ARPE-19 cells silenced as described above with MCT1, MCT4, or CD147 siRNAs. Western blot analysis revealed that silencing either MCT1 or MCT4 in ARPE-19 cells resulted in decreased expression of their accessory subunit, CD147 (Fig. 2B). Likewise, silencing CD147 resulted in loss of both MCT1 and MCT4 protein expression. In contrast, silencing MCT1, MCT4, or CD147 had no effect on β1-integrin expression, indicating that expression of this integrin is independent of its association with the MCT4/CD147 lactate transporter (Fig. 2B).
Silencing MCT4 results in slowed cell migration.
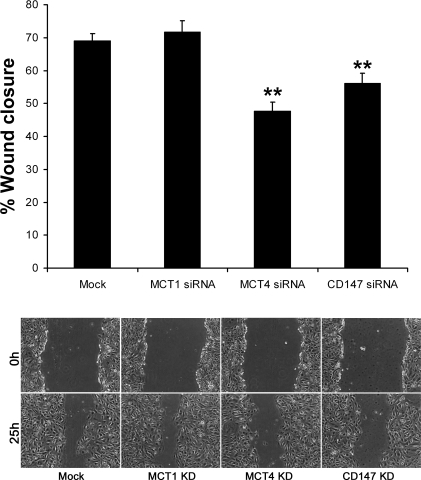
Previous studies with cancer cells demonstrated a role for MCT4 and CD147 in cell migration. In these studies, we examined whether MCT4 also plays a role in epithelial wound healing. Scratch-wound assays were performed on mock-transfected ARPE-19 cells and on cells transfected with MCT1, MCT4, or CD147 siRNAs. By 25-h postwounding, the wounds were 69% closed in mock-transfected ARPE-19 cells (Fig. 3). Similar results were observed when cells were transfected with MCT1-siRNA (72% closure), indicating that this transporter had no effect on the rate of wound closure. However, when ARPE-19 cells were transfected with MCT4 or CD147 siRNA, wound closure was reduced to 48% or 56%, respectively (Fig. 3).
Fig. 3.
Silencing MCT4 slows cell migration. ARPE-19 cells were wounded using a 1-ml micropipette tip 48 h following transfection with MCT1, MCT4, or CD147 siRNAs. Wounds were measured at 0 h and 25 h postwounding. Top: silencing MCT4 reduced wound closure to 48% as compared with mock-transfected control (69% closure). Silencing CD147 had a similar effect on wound closure (56% closure). Loss of MCT1 expression did not alter cell migration (72% closure), as compared with mock-transfected controls. Bars represent SE. **P < 0.0001 as determined by one-way ANOVA. Bottom: images depicting wounds at 0 h and 25 h postwounding following transfection with MCT1, MCT4, or CD147 siRNAs. Mock, untransfected control; KD, knockdown.
MCT4 colocalizes with β1-integrin at the leading edge of migrating epithelial cells but is not required for its trafficking.
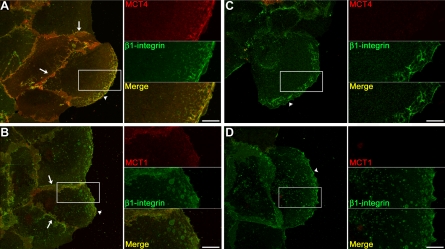
It has been clearly demonstrated that integrin receptors are critical for cell migration and are often enriched in the leading edge of migrating cells. We have observed here that MCT4 and β1-integrin form a complex in ARPE-19 cells and that MCT4 appears to play a role in cell migration. Therefore, we next examined whether this complex was found at the leading edge of cells, where it could aid in cell migration. ARPE-19 cells were scratch-wounded as described, and protein localization to the leading edge was observed by immunofluorescence confocal microscopy. Following scratch-wounding, we found that MCT4 and β1-integrin colocalize at high levels in the lamellapodia of cells at the wound edge (Fig. 4A, arrowhead and inset) as well as at the lateral cell borders (Fig. 4A, arrows). MCT1 also colocalized with β1-integrin at moderate levels in both the lateral cell borders and the leading edge (Fig. 4B, arrows and arrowhead, respectively). However, since silencing MCT1 did not affect cell migration, we hypothesize that MCT1 does not impact β1-integrin-mediated attachment or motility.
Fig. 4.
MCT4 colocalizes with β1-integrin at the leading edge of migrating ARPE-19 cells. A: wounded ARPE-19 cell monolayers were fixed 24 h postwounding and immunolabeled with MCT4 and β1-integrin antibodies. Immunoconfocal micrographs show that MCT4 (red) and β1-integrin (green) colocalized (yellow, see merge) at the lateral cell borders and in the leading edge of migrating cells. B: moderate levels of MCT1 (red) colocalized with β1-integrin (green) at lateral cell borders (yellow, see merge) and at the leading edge of wounded cells (see merge and inset). C and D: silencing of MCT4 (C) or MCT1 (D) did not alter the distribution of β1-integrin (green) to the leading edge. Smaller panels in A–D denote higher magnification of boxed area in larger image. Arrows denote cell-cell borders and arrowheads denote the leading edge. Bar = 10 μm.
Since MCTs and their accessory subunit CD147 are dependent on one another for their trafficking to the plasma membrane, we next wanted to determine whether β1-integrin also relies on MCT4 for its localization to the leading edge. ARPE-19 cells were transfected with siRNAs specific for MCT4 or MCT1 and were then fixed for immunofluorescence to visualize the distribution of β1-integrin. We found that when MCT4 was silenced, β1-integrin was still present in the lamellapodia of migrating cells (Fig. 4C, arrowhead and inset). Similar results were observed when cells were treated with MCT1 siRNA (Fig. 4D, arrowhead and inset).
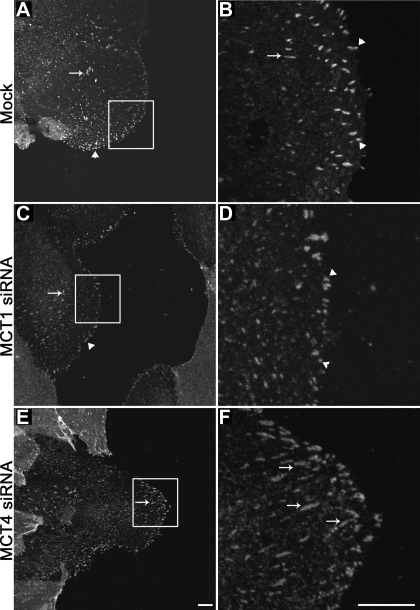
Loss of MCT4 impacts focal adhesion size.
It has recently been shown that intracellular pH can affect focal adhesion assembly and disassembly. Therefore, we next wanted to examine whether the slowed cell migration observed in MCT4 silenced cells was a result of alterations in focal adhesions at the leading edge. ARPE-19 cells were scratch-wounded after silencing of either MCT1 or MCT4 and were then labeled with paxillin to visualize focal adhesions. In mock-treated cells, we observed punctate paxillin labeling, indicative of focal adhesions at the leading edge (Fig. 5, A and B). These adhesions were small in size and persisted across the extent of the leading lamellapodium in migrating cells (Fig. 5, A and B, arrowheads). A few larger focal adhesions were also observed in the cell body (Fig. 5, A and B, arrow). Silencing of MCT1 yielded similar results to those seen in mock-treated cells (Fig. 5, C and D). However, when MCT4 was silenced, we noted larger focal adhesions across the leading edge, as well as in the cell body (Fig. 5, E and F, arrows). Overall, we found an increased proportion of large (>8 μm2) focal adhesions in MCT4-silenced cells (9.39%) compared with mock- or MCT1-silenced cells (0% and 1.48%, respectively; Table 1). This striking alteration of focal adhesion size could, therefore, provide an explanation for the decreased cell migration observed in cells in which MCT4 has been silenced.
Fig. 5.
Focal adhesions are increased in size in MCT4 siRNA-transfected cells. ARPE-19 cell cultures were fixed and labeled with paxillin antibody 24 h after wounding. Shown are immunoconfocal micrographs of mock-transfected cells (A and B), MCT1 siRNA-transfected cells (C and D), and MCT4 siRNA-transfected cells (E and F). Note that when MCT4 was silenced there was an increase in size of the adhesion plaques. Arrows denote large focal adhesions and arrowheads denote small focal adhesions. B, D, and F, higher-magnification images of the boxed region shown in A, C, and E. Bar = 10 μm.
Table 1.
Focal adhesion area after siRNA-mediated silencing of MCTs
| 0–1.99 μm2 | 2–4.99 μm2 | 5–7.99 μm2 | >8 μm2 | |
|---|---|---|---|---|
| Mock | 73.39 | 25.32 | 1.29 | 0.00 |
| MCT1 siRNA | 56.29 | 35.56 | 6.67 | 1.48 |
| MCT4 siRNA | 36.62 | 39.44 | 14.55 | 9.39 |
Values are % total adhesions; n, total number of focal adhesions measured. Mock, n = 233; monocarboxylate transporter (MCT) 1 small interfering (si)RNA, n = 135; MCT4 siRNA, n = 213.
DISCUSSION
Embryonic development, wound healing, and metastasis are characterized by increased glycolysis and cell migration. In the present study, we found that cell metabolism and cell migration may be linked through the structural and functional interaction between β1-integrin and the heteromeric lactate transporter MCT4/CD147. Over the past decade, a number of studies reported an association between CD147 and β1-integrin, as well as other transmembrane proteins such as the amino acid transporter CD98 (3, 6, 10, 11, 55). It was suggested that this supercomplex could mediate a variety of cellular functions, including cell signaling, cell-cell adhesion, and cell spreading. One of the first studies demonstrating an interaction between CD147 and β1-integrin was performed in the HT1080 fibrosarcoma cell line, identifying CD147 as one of the proteins that coimmunoprecipitated with α3β1-integrin (3). The amino acid transporter, CD98, was also found in a complex with both β1-integrin and CD147 (6, 10, 55). Thus, it was suggested that CD98 binding to β1-integrin could enhance integrin affinity for its matrix substrates by activating the phosphatidylinositol 3-kinase signaling pathway (6). Other investigators have described the interactions among CD98/β1-integrin/CD147 as a “sensory complex” that activated signaling pathways to regulate cell physiology and function (10). Recent work examining the function of CD147 in the CD147/β1-integrin complex suggested that CD147 was able to impact cytoskeletal architecture (by inducing cell spreading) via an integrin-dependent mechanism (11).
One element lacking in all of these studies was consideration of the role of the obligate binding partner of CD147—MCT1, MCT3, or MCT4. As our lab has previously demonstrated, MCT1, MCT3, and MCT4 are codependent on CD147 for their maturation and trafficking to the plasma membrane (14, 19). The current studies sought to examine whether MCTs are also in a complex with CD147 and β1-integrin. Importantly, we found that β1-integrin preferentially binds to MCT4, even in cells in which MCT1 and MCT4 are polarized to the basolateral membrane. This is the first study to demonstrate an association between MCTs and β1-integrin, and it opens the door to new understanding of how components of this supercomplex could impact cellular function.
Previous work has shown that MCTs (specifically, MCT1, MCT3, or MCT4) and CD147 assemble as a heterodimer in the endoplasmic reticulum and are codependent on one another for their trafficking to the plasma membrane. However, it has not been determined whether this complex is also required for trafficking of β1-integrin. In these studies, we found that while silencing of subunits of the MCT/CD147 complex resulted in reduced expression of the other subunit at the protein level, mRNA levels were not affected. This indicates that association of MCTs and CD147 occurs either translationally or posttranslationally. In support of this hypothesis, previous work from our lab demonstrated that MCT1 and MCT3, the isoforms expressed in RPE in vivo, were not expressed in the RPE of CD147-knockout mice (36). Similar results were observed in MCT3-knockout mice (12). In vitro, we found that silencing MCT4 in the breast cancer cell line, MDA-MB-231, resulted in retention of CD147 in the endoplasmic reticulum (19). In contrast, we found that β1-integrin expression was unaffected after silencing either component of the MCT/CD147 transporter. Additionally, we found that β1-integrin remains present at the leading edge of migrating cells, even after silencing MCT1 or MCT4. These findings indicate that β1-integrin is not dependent on either of these components for its trafficking to the plasma membrane.
Extracellular lactate has been reported to upregulate the synthesis of two extracellular matrix proteins that enhance migration: hyaluronan and type I collagen (50, 51). Typically, a healing wound has a hyaluronan-rich stroma, which can lead to rearrangement of the extracellular matrix, making it more permissive for cell migration (16, 44, 53). It has been observed that addition of exogenous lactate to cells results in increased random motility (16). The presence of MCT4 at the leading edge could decrease such random motility by mediating polarized lactate and H+ efflux. Furthermore, silencing MCT4 could reduce lactate efflux at the leading edge of the cells, leading to decreased synthesis of collagen and hyaluronan needed for movement. Since CD147 is cotrafficked with MCT4 to the plasma membrane, silencing MCT4 could also result in a reduction of matrix metalloproteinase activity, thereby preventing the matrix remodeling needed for directed cell migration (34). While the effect of silencing MCT4 in ARPE-19 cells does not result in complete inhibition of migration, these results do suggest that this transporter has at least a partial role in mediating this process. It is likely that the MCT4/CD147/β1-integrin supercomplex acts in concert with other protein complexes to facilitate efficient, directed cell migration.
It has been well established that cells rely on ionic transporters and exchangers to modulate both intracellular and extracellular pH (23, 26, 38, 48). Much of this work has focused on a specific class of acid-base transporters, the sodium-proton exchangers (NHE). NHE-1 was shown to play a major role in cell migration by modulating intracellular and extracellular pH, as well as in the maintenance of cellular homeostasis and volume (5, 13, 45, 47). Interestingly, recent work has indicated a role for NHE1 in modulating integrin-mediated attachment to the matrix (45, 46, 47). Specifically, it was observed that the strength of cell attachment differed between cells cultured under either acidic (pH 6.6) or alkaline (pH 7.5) conditions. Acidic pH conditions resulted in stronger cell attachments and alkaline pH produced weaker attachments (45). Likewise, this group also showed that in melanoma cells, integrin-mediated motility was most efficient at pH 6.8 (46). An additional study published recently demonstrated a role for pH modulation in mediating intracellular attachments to the cytoskeleton. It was shown that an acidic intracellular environment resulted in stronger adhesion of talin to the actin cytoskeleton, whereas a more alkaline intracellular environment weakened the binding between talin and actin (43). These alterations in binding thus resulted in slowed cell migration, due to tighter adhesion of the cell to its matrix. We found that ARPE-19 cells express little NHE-1 (data not shown). Therefore, other acid-base transporters must be involved in regulating pH in these cells. The MCTs are prime candidates for this role since they are proton-coupled lactate symporters and are abundantly expressed in ARPE-19 cells.
The dynamic assembly and disassembly of focal adhesions is crucial for efficient cell migration. For example, actively migrating cells typically exhibit small, nascent focal adhesions (2, 39) that allow for their rapid assembly/disassembly, which is required for cell motility. Conversely, larger focal adhesions exert more resistive forces to migration by anchoring cells more strongly to their matrix substrate (2). Recently, a role for ion translocation in modulating focal adhesion assembly and disassembly has been reported. Specifically, mutation of the ion translocation domain of NHE-1 resulted in larger focal adhesions as compared with wild type (13, 43). These studies led us to examine whether silencing MCT4 could result in a similar phenotype, thus explaining the slowed cell migration observed following MCT4 knockdown in ARPE-19 cells. We did, in fact, observe larger focal adhesions at the leading edge of ARPE-19 cells following MCT4 silencing, as compared with mock- or MCT1 siRNA-transfected cells. Silencing MCT4 in ARPE-19 cells would be expected to disrupt H+ efflux at the leading edge required to enhance cell migration following wounding. Additionally, the alteration in intracellular pH in MCT4-silenced cells could result in tighter binding of actin-binding proteins to the cytoskeleton, thereby forming larger, more mature focal adhesions.
In summary, our results suggest that MCT4 plays a multifaceted role in ARPE-19 cell migration. During epithelial wound healing, the leading edge of migrating cells tends to be glycolytic, producing excess lactate in this region that, if not removed, could decrease intracellular pH. However, expression of MCT4 at the leading edge could relieve the cell of the intracellular acid load, allowing glycolysis to continue uninterrupted. Current discussion in the literature suggests that tight regulation of both extracellular and intracellular pH is necessary for cell migration. Thus, the efflux of lactate via MCT4 could serve to stabilize integrin-mediated attachment and modulate assembly/disassembly of focal adhesions at the leading edge, thereby enhancing directed cell migration. Taken together, our findings support a role for MCT4 in pathologies characterized by aberrant cell migration, including PVR and metastatic cancer.
GRANTS
This work was supported by a National Institutes of Health (NIH) grant (EY-012042, to N. J. Philp). S. M. Gallagher is supported by a National Institute on Alcohol Abuse and Alcoholism training grant (AA-07463). J. J. Castorino is supported by an NIH post-doctoral training grant (P32-ES-07282).
Supplementary Material
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Ahmed N, Riley C, Rice G, Quinn M. Role of integrin receptors for fibronectin, collagen and laminin in the regulation of ovarian carcinoma functions in response to a matrix microenvironment. Clin Exp Metastasis 22: 391–402, 2005. [DOI] [PubMed] [Google Scholar]
- 2.Beningo KA, Dembo M, Kaverina I, Small JV, Wang YL. Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J Cell Biol 153: 881–888, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berditchevski F, Chang S, Bodorova J, Hemler ME. Generation of monoclonal antibodies to integrin-associated proteins. Evidence that alpha3beta1 complexes with EMMPRIN/basigin/OX47/M6. J Biol Chem 272: 29174–29180, 1997. [DOI] [PubMed] [Google Scholar]
- 4.Biswas C, Zhang Y, DeCastro R, Guo H, Nakamura T, Kataoka H, Nabeshima K. The human tumor cell-derived collagenase stimulatory factor (renamed EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res 55: 434–439, 1995. [PubMed] [Google Scholar]
- 5.Bourguignon LY, Singleton PA, Diedrich F, Stern R, Gilad E. CD44 interaction with Na+-H+ exchanger (NHE1) creates acidic microenvironments leading to hyaluronidase-2 and cathepsin B activation and breast tumor cell invasion. J Biol Chem 279: 26991–27007, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Cai S, Bulus N, Fonseca-Siesser PM, Chen D, Hanks SK, Pozzi A, Zent R. CD98 modulates integrin beta1 function in polarized epithelial cells. J Cell Sci 118: 889–899, 2005. [DOI] [PubMed] [Google Scholar]
- 7.Charteris DG, Sethi CS, Lewis GP, Fisher SK. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye 16: 369–374, 2002. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Lin J, Kanekura T, Su J, Lin W, Xie H, Wu Y, Li J, Chen M, Chang J. A small interfering CD147-targeting RNA inhibited the proliferation, invasiveness, and metastatic activity of malignant melanoma. Cancer Res 66: 11323–11330, 2006. [DOI] [PubMed] [Google Scholar]
- 9.Chen X, Su J, Chang J, Kanekura T, Li J, Kuang YH, Peng S, Yang F, Lu H, Zhang JL. Inhibition of CD147 gene expression via RNA interference reduces tumor cell proliferation, activation, adhesion, and migration activity in the human Jurkat T-lymphoma cell line. Cancer Invest 26: 689–697, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Cho JY, Fox DA, Horejsi V, Sagawa K, Skubitz KM, Katz DR, Chain B. The functional interactions between CD98, beta1-integrins, and CD147 in the induction of U937 homotypic aggregation. Blood 98: 374–382, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Curtin KD, Meinertzhagen IA, Wyman RJ. Basigin (EMMPRIN/CD147) interacts with integrin to affect cellular architecture. J Cell Sci 118: 2649–2660, 2005. [DOI] [PubMed] [Google Scholar]
- 12.Daniele LL, Sauer B, Gallagher SM, Pugh EN Jr, Philp NJ. Altered visual function in monocarboxylate transporter 3 (Slc16a8) knockout mice. Am J Physiol Cell Physiol 295: C451–C457, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol 159: 1087–1096, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deora AA, Philp N, Hu J, Bok D, Rodriguez-Boulan E. Mechanisms regulating tissue-specific polarity of monocarboxylate transporters and their chaperone CD147 in kidney and retinal epithelia. Proc Natl Acad Sci USA 102: 16245–16250, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimmer KS, Friedrich B, Lang F, Deitmer JW, Broer S. The low-affinity monocarboxylate transporter MCT4 is adapted to the export of lactate in highly glycolytic cells. Biochem J 350: 219–227, 2000. [PMC free article] [PubMed] [Google Scholar]
- 16.Edward M, Gillan C, Micha D, Tammi RH. Tumour regulation of fibroblast hyaluronan expression: a mechanism to facilitate tumour growth and invasion. Carcinogenesis 26: 1215–1223, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Enerson BE, Drewes LR. Molecular features, regulation, and function of monocarboxylate transporters: implications for drug delivery. J Pharm Sci 92: 1531–1544, 2003. [DOI] [PubMed] [Google Scholar]
- 18.Gabison EE, Mourah S, Steinfels E, Yan L, Hoang-Xuan T, Watsky MA, De Wever B, Calvo F, Mauviel A, Menashi S. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: role in epithelio-stromal interactions and matrix metalloproteinase induction. Am J Pathol 166: 209–219, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gallagher SM, Castorino JJ, Wang D, Philp NJ. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res 67: 4182–4189, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Giannelli G, Bergamini C, Fransvea E, Marinosci F, Quaranta V, Antonaci S. Human hepatocellular carcinoma (HCC) cells require both alpha3beta1-integrin and matrix metalloproteinases activity for migration and invasion. Lab Invest 81: 613–627, 2001. [DOI] [PubMed] [Google Scholar]
- 21.Halestrap AP, Meredith D. The SLC16 gene family-from monocarboxylate transporters (MCTs) to aromatic amino acid transporters and beyond. Pflügers Arch 447: 619–628, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Hergott GJ, Nagai H, Kalnins VI. Inhibition of retinal pigment epithelial cell migration and proliferation with monoclonal antibodies against the beta 1-integrin subunit during wound healing in organ culture. Invest Ophthalmol Vis Sci 34: 2761–2768, 1993. [PubMed] [Google Scholar]
- 23.Hertz L, Dienel GA. Lactate transport and transporters: general principles and functional roles in brain cells. J Neurosci Res 79: 11–18, 2005. [DOI] [PubMed] [Google Scholar]
- 24.Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol 17: 548–558, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Juel C, Halestrap AP. Lactate transport in skeletal muscle - role and regulation of the monocarboxylate transporter. J Physiol 517: 633–642, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanaan A, Douglas RM, Alper SL, Boron WF, Haddad GG. Effect of chronic elevated carbon dioxide on the expression of acid-base transporters in the neonatal and adult mouse. Am J Physiol Regul Integr Comp Physiol 293: R1294–R1302, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Kim IK, Arroyo JG. Mechanisms in proliferative vitreoretinopathy. Ophthalmol Clin North Am 15: 81–86, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Kim JW, Dang CV. Multifaceted roles of glycolytic enzymes. Trends Biochem Sci 30: 142–150, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Kirchhof B Strategies to influence PVR development. Graefes Arch Clin Exp Ophthalmol 242: 699–703, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Kirk P, Wilson MC, Heddle C, Brown MH, Barclay AN, Halestrap AP. CD147 is tightly associated with lactate transporters MCT1 and MCT4 and facilitates their cell surface expression. EMBO J 19: 3896–3904, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, He DL, Ning L, Shen SL, Li L, Li X. Hypoxia-inducible factor-1alpha induces the epithelial-mesenchymal transition of human prostate cancer cells. Chin Med J (Engl) 119: 713–718, 2006. [PubMed] [Google Scholar]
- 32.Marieb EA, Zoltan-Jones A, Li R, Misra S, Ghatak S, Cao J, Zucker S, Toole BP. Emmprin promotes anchorage-independent growth in human mammary carcinoma cells by stimulating hyaluronan production. Cancer Res 64: 1229–1232, 2004. [DOI] [PubMed] [Google Scholar]
- 33.Menko AS, Philip NJ. Beta 1-integrins in epithelial tissues: a unique distribution in the lens. Exp Cell Res 218: 516–521, 1995. [DOI] [PubMed] [Google Scholar]
- 34.Nabeshima K, Iwasaki H, Koga K, Hojo H, Suzumiya J, Kikuchi M. Emmprin (basigin/CD147): matrix metalloproteinase modulator and multifunctional cell recognition molecule that plays a critical role in cancer progression. Pathol Int 56: 359–367, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Pakula R, Melchior A, Denys A, Vanpouille C, Mazurier J, Allain F. Syndecan-1/CD147 association is essential for cyclophilin B-induced activation of p44/42 mitogen-activated protein kinases and promotion of cell adhesion and chemotaxis. Glycobiology 17: 492–503, 2007. [DOI] [PubMed] [Google Scholar]
- 36.Philp NJ, Ochrietor JD, Rudoy C, Muramatsu T, Linser PJ. Loss of MCT1, MCT3, and MCT4 expression in the retinal pigment epithelium and neural retina of the 5A11/basigin-null mouse. Invest Ophthalmol Vis Sci 44: 1305–1311, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Philp NJ, Wang D, Yoon H, Hjelmeland LM. Polarized expression of monocarboxylate transporters in human retinal pigment epithelium and ARPE-19 cells. Invest Ophthalmol Vis Sci 44: 1716–1721, 2003. [DOI] [PubMed] [Google Scholar]
- 38.Pinheiro C, Longatto-Filho A, Scapulatempo C, Ferreira L, Martins S, Pellerin L, Rodrigues M, Alves VA, Schmitt F, Baltazar F. Increased expression of monocarboxylate transporters 1, 2, and 4 in colorectal carcinomas. Virchows Arch 452: 139–146, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Pullar CE, Grahn JC, Liu W, Isseroff RR. Beta2-adrenergic receptor activation delays wound healing. FASEB J 20: 76–86, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Riethdorf S, Reimers N, Assmann V, Kornfeld JW, Terracciano L, Sauter G, Pantel K. High incidence of EMMPRIN expression in human tumors. Int J Cancer 119: 1800–1810, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Schreiner A, Ruonala M, Jakob V, Suthaus J, Boles E, Wouters F, Starzinski-Powitz A. Junction protein shrew-1 influences cell invasion and interacts with invasion-promoting protein CD147. Mol Biol Cell 18: 1272–1281, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sienel W, Polzer B, Elshawi K, Lindner M, Morresi-Hauf A, Vay C, Eder F, Passlick B, Klein CA. Cellular localization of EMMPRIN predicts prognosis of patients with operable lung adenocarcinoma independent from MMP-2 and MMP-9. Mod Pathol 21: 1130–1138, 2008. [DOI] [PubMed] [Google Scholar]
- 43.Srivastava J, Barreiro G, Groscurth S, Gingras AR, Goult BT, Critchley DR, Kelly MJ, Jacobson MP, Barber DL. Structural model and functional significance of pH-dependent talin-actin binding for focal adhesion remodeling. Proc Natl Acad Sci USA 105: 14436–14441, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stern R, Shuster S, Neudecker BA, Formby B. Lactate stimulates fibroblast expression of hyaluronan and CD44: the Warburg effect revisited. Exp Cell Res 276: 24–31, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Stock C, Gassner B, Hauck CR, Arnold H, Mally S, Eble JA, Dieterich P, Schwab A. Migration of human melanoma cells depends on extracellular pH and Na+/H+ exchange. J Physiol 567: 225–238, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stock C, Mueller M, Kraehling H, Mally S, Noel J, Eder C, Schwab A. pH nanoenvironment at the surface of single melanoma cells. Cell Physiol Biochem 20: 679–686, 2007. [DOI] [PubMed] [Google Scholar]
- 47.Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf) 187: 149–157, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev 26: 299–310, 2007. [DOI] [PubMed] [Google Scholar]
- 49.Toole BP, Slomiany MG. Hyaluronan: a constitutive regulator of chemoresistance and malignancy in cancer cells. Semin Cancer Biol 18: 244–250, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trabold O, Wagner S, Wicke C, Scheuenstuhl H, Hussain MZ, Rosen N, Seremetiev A, Becker HD, Hunt TK. Lactate and oxygen constitute a fundamental regulatory mechanism in wound healing. Wound Repair Regen 11: 504–509, 2003. [DOI] [PubMed] [Google Scholar]
- 51.Tsuji T Physiological and pathological roles of alpha3beta1-integrin. J Membr Biol 200: 115–132, 2004. [DOI] [PubMed] [Google Scholar]
- 52.Ullah MS, Davies AJ, Halestrap AP. The plasma membrane lactate transporter MCT4, but not MCT1, is up-regulated by hypoxia through a HIF-1alpha-dependent mechanism. J Biol Chem 281: 9030–9037, 2006. [DOI] [PubMed] [Google Scholar]
- 53.Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 14: 267–274, 2004. [DOI] [PubMed] [Google Scholar]
- 54.Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol 109: 877–889, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu D, Hemler ME. Metabolic activation-related CD147-CD98 complex. Mol Cell Proteomics 4: 1061–1071, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yan L, Zucker S, Toole BP. Roles of the multifunctional glycoprotein, emmprin (basigin; CD147), in tumour progression. Thromb Haemost 93: 199–204, 2005. [DOI] [PubMed] [Google Scholar]
- 57.Yoon H, Fanelli A, Grollman EF, Philp NJ. Identification of a unique monocarboxylate transporter (MCT3) in retinal pigment epithelium. Biochem Biophys Res Commun 234: 90–94, 1997. [DOI] [PubMed] [Google Scholar]
- 58.Zhou S, Zhou H, Walian PJ, Jap BK. The discovery and role of CD147 as a subunit of gamma-secretase complex. Drug News Perspect 19: 133–138, 2006. [DOI] [PubMed] [Google Scholar]
- 59.Zhou S, Zhou H, Walian PJ, Jap BK. CD147 is a regulatory subunit of the gamma-secretase complex in Alzheimer's disease amyloid beta-peptide production. Proc Natl Acad Sci USA 102: 7499–7504, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zou W, Yang H, Hou X, Zhang W, Chen B, Xin X. Inhibition of CD147 gene expression via RNA interference reduces tumor cell invasion, tumorigenicity and increases chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett 248: 211–218, 2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.