Abstract
The cytotoxic T cell (CT) GalNAc transferase, or Galgt2, is a UDP-GalNAc:β1,4-N-acetylgalactosaminyltransferase that is localized to the neuromuscular synapse in adult skeletal muscle, where it creates the synaptic CT carbohydrate antigen {GalNAcβ1,4[NeuAc(orGc)α2, 3]Galβ1,4GlcNAcβ-}. Overexpression of Galgt2 in the skeletal muscles of transgenic mice inhibits the development of muscular dystrophy in mdx mice, a model for Duchenne muscular dystrophy. Here, we provide physiological evidence as to how Galgt2 may inhibit the development of muscle pathology in mdx animals. Both Galgt2 transgenic wild-type and mdx skeletal muscles showed a marked improvement in normalized isometric force during repetitive eccentric contractions relative to nontransgenic littermates, even using a paradigm where nontransgenic muscles had force reductions of 95% or more. Muscles from Galgt2 transgenic mice, however, showed a significant decrement in normalized specific force and in hindlimb and forelimb grip strength at some ages. Overexpression of Galgt2 in muscles of young adult mdx mice, where Galgt2 has no effect on muscle size, also caused a significant decrease in force drop during eccentric contractions and increased normalized specific force. A comparison of Galgt2 and microdystrophin overexpression using a therapeutically relevant intravascular gene delivery protocol showed Galgt2 was as effective as microdystrophin at preventing loss of force during eccentric contractions. These experiments provide a mechanism to explain why Galgt2 overexpression inhibits muscular dystrophy in mdx muscles. That overexpression also prevents loss of force in nondystrophic muscles suggests that Galgt2 is a therapeutic target with broad potential applications.
Keywords: contraction, extensor digitorum longus, gene therapy, muscular dystrophy
duchenne muscular dystrophy (DMD) is a severe, X-linked myopathy resulting from mutations in the dystrophin gene (Dmd) that cause loss of dystrophin protein expression (19, 23). Consequently, the sarcolemmal membrane of skeletal myofibers is prone to damage during use, ultimately resulting in muscle wasting (1). In skeletal muscle, dystrophin provides an essential linkage between the actin cytoskeleton, to which it binds directly, and the extracellular matrix (ECM) surrounding each myofiber (12, 13). Dystrophin interacts with the ECM via its binding to the transmembrane proteins of the dystrophin-associated glycoprotein complex (DAG), particularly dystroglycan (12, 29). In the absence of dystrophin, many DAG proteins fail to be anchored properly in the sarcolemmal membrane, thereby inhibiting DAG-ECM interactions (31). Similar types of muscle pathology develop in the mdx mouse, where a mutation in the Dmd gene leads to loss of dystrophin protein expression in most skeletal myofibers (9, 46). Because the mdx mouse demonstrates some of the same muscle pathology found in DMD, it has become the preferred animal model for testing of therapeutic approaches to this disease (1).
One of the most robust tests of muscle damage is the response to eccentric contraction paradigms. Forced lengthening during stimulation typically causes muscle fiber damage of mdx myofibers with greater loss of force on subsequent stimulations compared with wild-type (WT) muscles (18, 43). WT muscles, however, also lose force in such paradigms (18, 43). Measurements of force drop during eccentric contractions, therefore, provide a window not only into the damage process that ultimately results in loss of muscle strength in DMD, but also damage that occurs in nondystrophic muscle. Importantly, mdx muscles that overexpress proteins, such as utrophin, which are known to compensate for loss of dystrophin as assessed by muscle pathology (9, 39, 48), show significantly reduced force drop during repetitive eccentric contractions, offering protection in the range of that found in nondystrophic muscles exposed to the same physiological stress (48).
Here we investigate the physiological response of mdx muscle made to transgenically overexpress Galgt2, a gene that, like utrophin, can inhibit the development of muscle pathology in mdx animals (35). Galgt2, also called the cytotoxic T cell (CT) GalNAc transferase in mice (47) and the Sda GalNAc transferase in humans (32), encodes a type II Golgi transmembrane UDP-GalNAc:β1,4-N-acetylgalactosaminyltransferase that is known to glycosylate a small number of glycoproteins and at least one glycolipid (22, 25, 26, 32, 37, 47, 53). In skeletal muscle, Galgt2 protein is highly concentrated in regions containing neuromuscular junctions (20, 53), where the CT carbohydrate, the carbohydrate it synthesizes, is also confined (30). Transgenic overexpression of Galgt2 in nonsynaptic regions of skeletal myofibers causes the ectopic expression of the CT carbohydrate, where it is found on α-dystroglycan (35, 53) and one as yet unidentified glycolipid (55). Galgt2 transgenic muscles also display ectopic expression of normally synaptic binding partners for dystroglycan, including laminin-α4, laminin-α5, and utrophin (35, 53). Thus, Galgt2 can alter the expression of a number of synaptic proteins that are homologous to those lost in forms of muscular dystrophy. Moreover, overexpression of Galgt2 in the skeletal muscles of mdx mice inhibits muscular dystrophy (35). Inhibition of muscular dystrophy continues for at least 18 mo in Galgt2 transgenic mdx animals, almost the entire life span of a normal mouse (54). In Galgt2 transgenic mice, the Galgt2 transgene is expressed from embryonic (E) time points (E13.5) onward, and muscles from these animals are reduced in size relative to nontransgenic littermates (53). In contrast, postnatal overexpression of Galgt2 in mdx muscle using adeno-associated virus (AAV) does not alter muscle growth, yet still inhibits the development of muscular dystrophy (54). Recent studies in dyW/dyW mice, a mouse model of laminin-α2-deficient congenital muscular dystrophy (MDC1A), show that Galgt2 overexpression also inhibits muscular dystrophy in this animal model (55). Thus, Galgt2 overexpression can inhibit muscular dystrophy in two very different models of muscular dystrophy, a finding that suggests its mechanism of action may be more broadly applicable than other approaches such as gene replacement. Here we show that overexpression of Galgt2 mediated by a recombinant adeno-associated vector confers protection against loss of muscle force during eccentric contractions, not only in mdx muscles but also in WT muscles, and that it is as effective as gene replacement with microdystrophin (17), a shortened version of the dystrophin protein that is able to be packaged into AAV gene therapy vectors that are currently preferred for translational gene therapy research (41, 42). Thus, Galgt2 overexpression may have therapeutic impact in many types of muscle injury.
EXPERIMENTAL PROCEDURES
Mice.
Galgt2 transgenic mice and Galgt2 transgenic mdx mice, both of which express full-length mouse Galgt2 cDNA specifically in skeletal muscles due to expression driven by the human skeletal α-actin promoter (34), have been described by us previously (35, 53). The mdx mice and C57Bl/10 (WT) controls were derived from the same litters as Galgt2 transgenic animals. Mice were bred and cared for in a barrier facility, and all animal care and experiments were done under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Nationwide Children's Hospital and at The Ohio State University.
Normalized specific force and eccentric contraction measurements.
Mice were anesthetized with urethane via intraperitoneal injection (300 mg/1 ml in 0.9% NaCl). The extensor digitorum longus (EDL) muscles of both legs were carefully dissected from tendon to tendon, and placed in Krebs-Henseleit (K-H) buffer containing (in mM) 137 NaCl, 5 KCl, 1.2 NaH2PO4, 1.2 MgSO4, 20 NaHCO3, 0.25 CaCl2, and 10 glucose. Twenty millimolar 2,3-butanedione monoxime (BDM) was added to the dissection buffer to prevent cutting injury (21, 33). Exposure to BDM for a short time has been shown to be reversible (21). Muscles were mounted using 6.0 silk. Each tendon was tied with three simple knots at the very end to avoid damaging the muscle. The muscle was attached onto minuten-pins through the second knot. Using this attachment method, we eliminated end-compliance of the preparation attachment, thus allowing the transfer of all imposed length changes directly onto the muscle. The muscles were attached while completely buckled (slack length) in the K-H solution, without BDM, and now containing 2.0 mM CaCl2 at 30°C. The muscle was attached at one end to a force transducer and at the other end to a linear servomotor (KG2 and MOT1, respectively; Scientific Instruments, Heidelberg, Germany). Two parallel platinum electrodes, placed 2–3 mm away from the muscle longitudinally, provided electrical stimulation. The bath was superfused with the K-H solution to keep temperature at 30°C at a flow rate of five times the bath volume per minute to ensure proper oxygenation and removal of waste products.
Once the muscle had stabilized in the bath for 10 min, without stimulation, resting tension of the muscle was set to the length where twitch contractions were optimal, typically 10 mN (42). This length was termed Lo. After 5 min, a tetanic contraction was elicited by applying a 500-ms tetanus at 150 Hz, using 1-ms individual pulse duration. Length of the muscle was adjusted until resting tension was again 10 mN if required. After another 5 min of rest, an eccentric contraction protocol was performed, as previously described in detail by the group of Liu and coworkers (27) and as adapted by us previously (42). For experiments with transgenic mice [those comparing WT Galgt2 transgenic wild-type (Galgt2WT), mdx, and Galgt2 transgenic mdx (Galgt2mdx) skeletal muscles], a 700-ms tetanus was applied, where in the last 200 ms the muscle was stretched by 10% (200-ms linear stretch at 0.5 Lo/s). After the tetanus ended at time (t) = 700 ms, the muscle length was returned (for 200 ms at −0.5 Lo/s). This eccentric contraction was repeated 10 times, with 2 min of rest between successive contractions. Using this method and protocol, because of the noncompliance of the attachment method, the entire stretch of the muscle preparation is transferred onto the actual sarcomeres. As a result, the 10% stretch using our attachment method caused a more robust damage than in some previous studies, as indicated by a >50% loss within the first few contractions in WT muscle. Although this robust damage narrowed the gap between mdx and WT muscles, it did provide a larger window for improvement to be observed between Galgt2 transgenic and nontransgenic muscles. After the 10 eccentric contractions, the muscle was weighed and prepared for histological analysis.
For gene therapy experiments, the muscle was stabilized in the bath for 10 min, without stimulation. Resting tension of the muscle was then set to the length where twitch contractions were optimal, typically 10 mN (42). This length was termed Lo. After 5 min, a tetanic contraction was elicited by applying a 500-ms tetanus at 150 Hz, using 1-ms individual pulse duration. Length of the muscle was adjusted until resting tension was again 10 mN if required. Following a 10-min rest period, muscles were subjected to an eccentric contraction protocol consisting of a series of 10 isometric 700-ms tetani, at 2-min intervals, with a 5% lengthening of the muscles (0.25 fiber length per second for duration of 200 ms) when maximal force has developed at 500 ms. After the tetanus ended (at t = 700 ms), the muscle was brought back to initial length (at the same speed as the stretch), allowing for full relaxation to the initial length. This was a less intense paradigm than was used for transgenic experiments, which allowed us to more clearly tease out differences between mdx and WT control muscles. After the 10 eccentric contractions, the muscle was weighed and prepared for histological analysis.
For comparative purposes, all force measurements are expressed per unit cross-sectional area (CSA; normalized isometric force or tension: mN/mm2). Because Galgt2 transgenic EDL muscles contained significantly longer myofibers than control WT or mdx muscles, it was necessary to normalize these experiments to the CSA of myofibers as opposed to the CSA of the muscle as a whole, and this was done much as previously described (5). This involved dividing the muscle wet mass by the product of the myofiber length and the myofiber density. Myofiber length/muscle length measures were done by snap freezing dissected EDL muscles, after stretching to their optimal length, in liquid nitrogen-cooled isopentane. EDL muscles were then mounted longitudinally and serially sectioned on a cryostat and stained with hematoxylin and eosin. Optimal sections including the belly of the muscle were counted (at least 200 myofibers per section and at least 6 sections per muscle) and measured relative to the length of the muscle. To calculate myofiber density, serial cross sections of similarly dissected and frozen EDL muscles were cut on a cryostat at 8-μm thickness and stained with hematoxylin and eosin. The area of all myofibers in each section (at least 5 sections per muscle) was measured relative to the area of the entire muscle section. Because the fractional density of the muscle composed of myofibers did not vary between groups, all muscle lengths were multiplied by the myofiber density value of Mendez and Keys (31a) (1.06 g/cm3) (5). For gene therapy experiments, where postnatal transgene expression did not affect myofiber number or length, CSA of the entire muscle was used to normalize force measurements. Here, CSA was calculated using the following equation: CSA = (muscle mass in g)/[(optimal fiber length in cm) × (muscle density in g/cm3)], where muscle density is again 1.06 g/cm3.
Grip strength.
When mice were 2 mo or 5 mo old, grip strength measurements for forelimbs and hindlimbs were tested daily by using a grip strength meter (Columbus Instruments). Each measurement taken was the average of 8–16 grip strength measures over an interval of 5 min for both hindlimbs or both forelimbs. All mice were trained to do the procedure for several days before data were recorded. Measurements were taken daily and averaged of the course of 14 days for each time point. Each daily session consisted of at least five tests per limb per animal. Total body weight was measured after each grip strength test. The individual strength was indicated as limb strength (g) over body weight (g). For each mouse, the measurements during the 2-wk test period were averaged. An unpaired Students t-test was used for statistical analysis of significance between genotypes for the 2-mo or 5-mo age conditions. Males and females were assessed separately because of their different muscle and total body weights.
Procion orange dye uptake.
At the conclusion of the eccentric contraction paradigm, muscles were removed and bathed in K-H buffer containing 1% procion orange dye for 1 h at 25°C. Muscles were washed and snap frozen in liquid nitrogen-cooled isopentane, after which they were serially sectioned (in cross section) at 8 μm on a cryostat. Sections were taken and counted from the midpoint of the belly of the muscle for 200 μm toward the rostal or caudal tip, as previously described (55), in a series of cuts of 10-μm thickness. Dye uptake into the cytoplasm of myofibers was determined across the entire cross-sectional area of the EDL for each section, and the average percentage of positive myofibers was averaged from all sections, as before (48).
Production and purification of AAV vectors.
Serotype 8-like recombinant (r)AAV vectors (rh.74), herein called rAAV8, were produced by a modified cross-packaging approach using an adenovirus-free, triple-plasmid DNA transfection (CaPO4 precipitation) method in human embryonic kidney 293 cells (38). The expression cassette is derived from plasmid pCMVβ (Clontech) and contains the cytomegalovirus (CMV) immediate early promoter/enhancer (791 bp), the Galgt2 transgene, and a polyadenylation signal from Simian Virus 40 (SV40). Additional Galgt2 and microdystrophin vector constructs were made where the CMV promoter/enhancer was replaced by the muscle creatine kinase (MCK) promoter (44). All expression cassettes are flanked by AAV2 inverted terminal repeats. The novel rAAV8-like serotype (rh.74) was isolated at TRI-NCH from a Rhesus macaque and shares 93% amino acid identity with AAV8 and is most similar to a related clade E virus rh.10 described by Gao and colleagues (99% amino acid identity) (14). Vector was produced by cotransfecting with rh.74 and adenovirus helper plasmids as previously described (49). Plasmid rep2-caprh.74 encodes the wild-type AAV2 rep gene and rh.74 cap gene, and the adenovirus helper plasmid (pAdhelper) expresses the adenovirus type 5 E2A, E4ORF6, and VA I/II RNA genes that are required for high-titer rAAV production. Vectors were purified from clarified 293 cell lysates by sequential iodixanol gradient purification and anion-exchange column chromatography using a linear NaCl salt gradient as previously described (6). Vector genome titers were measured using quantitative real-time PCR-based detection with a specific primer/probe set and used the Prism 7500 Taqman detector system (PE Applied Biosystems) as previously described (6). Vector stock titers ranged between 1–5 × 1012 vg/ml.
AAV-mediated gene delivery into mdx skeletal muscle.
Two groups of experiments were performed to overexpress the Galgt2 gene in postnatal mdx muscle, one with the mouse cDNA and one with the human cDNA. For postnatal overexpression of mouse Galgt2 in mdx mice, one dose of 1 × 1012 vg rAAV8-CMV-Galgt2(mouse) was injected via the femoral artery. rh.74 is an AAV8-like viral vector, and this family of vectors are especially good at crossing the vascular barrier when delivered into the bloodstream (42). Briefly, mice were sedated and anesthetized with a ketamine and xylazine cocktail (100 mg/kg and 10 mg/kg, respectively; ip). In addition, all animals received a preoperative dose of buprenorphine (0.1 mg/kg; sq). The left groin was shaved and prepared with 95% EtOH and Povidine solution, and the animal was secured onto a warming blanket (37°C) that overlaid the stage of a dissecting microscope. The femoral bundle was exposed with a single scalpel (no. 11 blade) incision (0.25–0.5 cm) and blunt dissected to reveal an isolated femoral artery and vein. A 2-0 braided silk tourniquet was then loosely placed around the limb at the level of the incision, over the isolated vessels, such that when tightened, blood flow in and out of the leg was occluded. The femoral artery was cannulated with a customized heat pulled polypropylene 10 (PE-10) catheter following placement of an introducer hole with a 33-gauge needle. The arterial catheter was flushed with sterile normal saline, 100 μl vol. The tourniquet was applied, and the volume of virus (100 μl) was administered with slow gentle pressure over the specified time. With the volume of virus instilled and the tourniquet secure, a dwell time of 10 min was allowed. Normal saline (100 μl) was administered to the arterial catheter as a flush. The arterial catheter was removed and the tourniquet was taken down. Bleeding was controlled with direct pressure. The wound was flushed with sterile normal saline and closed with a single 5-0 silk suture. The animals were allowed to recover on a 37°C warmer and, once ambulatory, were returned to their cages. In each instance, an equivalent volume of injection buffer was injected into the contralateral limb, and contralateral limb muscles were used for comparison to assess the effect of the transgene. For this first experiment, rAAV8-CMV-Galgt2(mouse) was injected into 4-wk-old mdx animals, and these animals were analyzed 6 wk after infection.
In the second experiment, we compared overexpression of the human Galgt2 cDNA(32) and a cDNA encoding human microdystrophin (17). Both transgenes were subcloned into AAV packaging vector (rh.74) that contained the same muscle-specific promoter, MCK (45). Packaged AAV vectors were purified as before (42) and titered to show equivalent packaging of each transgene and also equivalent infectivity of purified, packaged, AAV particles. 1 × 1011 vg rAAV8-MCK-Galgt2(human) or rAAV8-MCK-microdystrophin(human) were delivered using the protocol described previously (42). rAAV8-MCK-Galgt2(human) or rAAV8-MCK-microdystrophin(human) was injected into 5-wk-old mdx animals, and these animals were analyzed 12 wk after vector administration. Contralateral mdx limbs receiving vector dilutant alone in an identical manner or muscles of untreated WT (C57Bl/10) mice served as controls.
Analysis of transgene expression after rAAV gene delivery.
Tibialis anterior (TA) and EDL muscles were dissected from mdx-treated and contralateral control limbs. EDL muscles were subjected to eccentric contraction protocol before histological analysis. Muscles were then embedded in 7% gum tragacanth, snap frozen in liquid nitrogen-cooled isopentane, and sectioned on a cryostat at 12-μm thickness. For rAAV8-CMV-Galgt2(mouse)-injected muscles or rAAV8-MCK-Galgt2(human)- treated muscles, gene expression was analyzed by immunostaining for overexpression of the CT carbohydrate, the product of Galgt2 activity (47). Because this carbohydrate is normally confined to the neuromuscular synapse in adult muscles (30), overexpressing fibers were easily scored by their high levels of extrasynaptic CT carbohydrate expression along the muscle sarcolemmal membrane, as we had previously done (54, 55). For CT2 immunostaining, muscle sections were blocked for 30 min in PBS with 3% BSA and incubated with CT2 monoclonal antibody (hybridoma supernatant, neat) overnight at 4°C (7). Sections were washed three times using 1× PBS for 10 min each, followed by addition of secondary antibody (1:200, Cy2-conjugated goat anti-mouse IgM; Jackson Immunoresearch) for 30 min. Sections were washed (3 × 1× PBS, 10 min each) and mounted using Prolong Gold Antifade reagent (Invitrogen). For muscles infected with rAAV8-MCK-microdystrophin, gene expression was analyzed via immunostaining for expression of dystrophin protein, which is absent from almost all myofibers in mdx muscle (19). Muscle sections were incubated with the NH2-terminal Manex1a primary antibody (an anti-dystrophin monoclonal antibody from Developmental Studies Hybridoma Bank) at a dilution of 1:50 in blocking buffer (PBS, 10% goat serum, 0.1% Triton X-100) for 1 h at room temperature in a wet chamber. Sections were then washed with PBS three times, each for 20 min and reblocked. Visualization was achieved by incubation for 30 min at room temperature with an Alexa 488-conjugated IgG2a isotype-specific goat anti-mouse antibody at a 1:300 dilution (Molecular Probes). Sections were washed in PBS three times for 20 min and mounted with Vectashield mounting medium (Vector Laboratories). Fluorescence staining for microdystrophin and CT carbohydrate was visualized using a Zeiss Axioskop2 Plus microscope or Olympus BX61 microscope, and images were captured with a Zeiss AxioCam MRC5 camera or Hamamatsu ORCA-ER camera with Slidebook 4.4 software. The number of fibers with sarcolemmal dystrophin or CT2 staining was expressed as a percentage of total fibers (10 × image EDL − ∼500 fibers per muscle; 3–10 × images TA − ∼1,500 fibers per muscle). In all cases examined, the mock-infected contralateral muscle was also stained with the same antibodies, and no significant staining was identified (no more 0.2%-positive myofibers).
Muscle fiber typing.
Assessment of fast- and slow-twitch fiber types was done as we have previously described (16, 53). Briefly, muscles were mounted and snap frozen in liquid nitrogen-cooled isopentane. Sections were then incubated with ATP in barbital acetate buffers at pH 4.2, 4.6, or 9.4 using the cobalt chloride staining method (16). Fiber typing at pH 4.2 is shown in results, but all results were internally consistent and showed no significant change between each group analyzed.
Data analysis and statistics.
In vitro muscle contractile data were collected using custom written software in LabView (National Instruments, Austin, TX). From each eccentric contraction protocol, maximal tetanic tension was determined by taking the developed tension at time (t) = 500 ms (total tension minus resting tension at t = 0 ms). Force deterioration curves were constructed by normalizing each tetanic force to the one obtained from the first tetanic contraction. Eccentric contraction curves were compared using Random effects regression analysis for longitudinal data. Values for P < 0.05 were considered significant. Identical results and range of significance were found if the same data were analyzed by two-way ANOVA with repeated measures with Bonferroni pre- and post-hoc analysis. All data are expressed as means ± SE. For comparisons between groups for (e.g., for muscle weight, age, and strain), three-way ANOVA with repeated measures with pre- and post-hoc Bonferroni analysis was performed. For gene therapy experiments, percentage transgene expression data were analyzed using a Student's t-test between the two groups compared and are expressed as means ± SE (or as indicated). Grip strength differences were similarly analyzed. Significance level was set at P < 0.05.
RESULTS
Measurement of muscle length, muscle weight, muscle cross-sectional area, myofiber length, and myofiber density.
Because Galgt2 transgenic mice have impaired muscle growth (35, 53) and because the specific force of muscle contraction must be normalized to muscle size or weight, we began by measuring the muscle parameters of length, weight, and cross-sectional area (Table 1). The muscle we chose to study here was the extensor digitorum longus (EDL), a relatively thin hindlimb muscle for which a number of studies have shown physiological impairment in mdx mice (10, 36, 40, 48, 51). We began by comparing our four genotypes of transgenic and nontransgenic mice: wild type (WT), Galgt2 transgenic wild type [Galgt2WT, which overexpress the CT carbohydrate specifically in skeletal muscles (53)], mdx [a mouse model for Duchenne muscular dystrophy (1, 8)], and Galgt2 transgenic mdx [Galgt2mdx, which have greatly diminished muscular dystrophy due to Galgt2 transgene overexpression (35)]. Several age groups were also assessed: 3 wk old (3wk), 2 mo old (2mo), and 5 mo old (5mo). Comparing muscle length in groups with only one different variable (age; genotype-matched or genotype; age-matched), we found that WT 5mo were longer than WT 3wk, and that mdx 2mo and mdx 5mo were longer than mdx 3wk; however, all muscle lengths were within 30% of one another, regardless of age or genotype. When examining weight and cross-sectional area, however, we found profound differences, consistent with our previous studies showing embryonic Galgt2 transgene expression inhibits postnatal muscle growth both in wild type and mdx backgrounds (35, 53). As to weight at relative ages, WT 5mo was significantly heavier than the WT 3wk, and the mdx 5mo weighed more than the mdx 2mo, which in turn was heavier than the mdx 3wk. Thus, muscle weight increased with age, consistent with expected postnatal muscle growth. In addition, mdx 5mo was heavier than the WT 5mo, consistent with muscle hypertrophy that occurs in mdx mice as their muscular dystrophy becomes more advanced with age (8). As expected based on our previous studies (53), the Galgt2WT and Galgt2mdx muscles weighed significantly less than their respective nontransgenic strains, with Galgt2mdx slightly, but significantly, heavier than Galgt2WT for 5mo. These weight observations were similarly reflected in the cross-sectional area data, which is not surprising since the spread in muscle length varied far less than it did for muscle weight. Importantly, Galgt2WT and Galgt2mdx muscles at 2 mo and 5 mo, both with regard to weight and to cross-sectional area, were as great or were greater than WT and mdx muscles at 3 wk of age. For this reason, we also used 3-wk-old mdx and WT muscles as comparisons for some physiological measures. The forces generated by the muscles were in the same range as observed throughout the literature; WT muscles generated in excess of 200 mN/mm2, while mdx muscles generated between 80 and 170 mN/mm2 depending on age, indicating a normal viability of physiological muscle function.
Table 1.
Average muscle length, muscle weight, muscle cross-sectional area, myofiber/muscle length, myofiber density, and myofiber cross-sectional area were measured for the extensor digitorum longus muscle of wild-type, Galgt2 transgenic wild-type, mdx, and Galgt2 transgenic mdx mice at 3 wk, 2 mo, or 5 mo of age
| Condition | n | Age | Muscle Length, mm | Muscle Weight, mg | CSA, mm2 | Total Force, mN | Specific Force, mN/mm2 |
|---|---|---|---|---|---|---|---|
| WT | 2 | 3 wk | 9.5±0.5 | 4.1±0.1 | 0.41±0.01 | 94.2±14.4 | 229±29 |
| WT | 7 | 5 mo | 10.8±0.3* | 12.1±0.4* | 1.07±0.06* | 221±8* | 211±13 |
| Mdx | 6 | 3 wk | 10.4±0.4 | 4.2±0.4 | 0.38±0.04 | 45.8±9.2† | 115±20† |
| Mdx | 5 | 2 mo | 12.1±0.3* | 13.8±0.7* | 1.08±0.03* | 206±24* | 170±26* |
| Mdx | 4 | 5 mo | 11.4±0.8 | 19.8±1.0*† | 1.68±0.13* | 150±33† | 80±25*† |
| Galgt2mdx | 10 | 2 mo | 11.9±0.2 | 5.4±0.2‡ | 0.43±0.01‡ | 54.2±7.1‡ | 139±13‡ |
| Galgt2mdx | 5 | 5 mo | 11.6±0.3 | 6.0±0.4*‡ | 0.49±0.02*‡ | 48.2±6.8‡ | 98±18* |
| Galgt2WT | 3 | 2 mo | 12.5±0.3 | 4.7±0.6 | 0.35±0.04† | 31.6±9.9† | 86±18† |
| Galgt2WT | 8 | 5 mo | 10.8±0.2* | 4.9±0.3†‡ | 0.43±0.02†‡ | 35.0±7.8†‡ | 83±21‡ |
| Condition | n | Age | Myofiber/Muscle Length Ratio | Myofiber Density, area fraction | CSA Normalized, mm2 | Specific Force Normalized, mN/mm2 |
|---|---|---|---|---|---|---|
| WT | 7 | 5 mo | 0.45±0.04 | 0.98±0.02 | 2.34±0.13 | 96.1±5.8 |
| mdx | 6 | 2 mo | 0.45±0.04 | 0.97±0.02 | 2.40±0.07 | 76.5±11.0 |
| mdx | 4 | 5 mo | 0.45±0.06 | 0.99±0.03 | 2.98±0.24* | 44.8±13.0*† |
| Galgt2mdx | 10 | 2 mo | 0.70±0.06‡ | 0.99±0.02 | 0.64±0.03‡ | 93.3±8.6 |
| Galgt2mdx | 5 | 5 mo | 0.75±0.08‡ | 0.97±0.03 | 0.65±0.03‡ | 73.3±13.5‡ |
| Galgt2WT | 3 | 2 mo | 0.75±0.08 | 0.96±0.03 | 0.50±0.06† | 60.0±12.7‡ |
| Galgt2WT | 8 | 5 mo | 0.79±0.08‡ | 0.99±0.02 | 0.52±0.02†‡ | 69.1±17.4‡ |
Values are means ± SE. Total and specific force values are based on uncorrected cross-sectional area (CSA). Specific force normalized values, corrected for CSA, take into account myofiber-to-muscle length ratio.
Significant difference (P < 0.05) based on age (identical strain, compared with younger mice).
Significant difference (P < 0.05) based on wild-type (WT) vs. mdx.
Significant difference (P < 0.05) based on Galgt2 overexpression.
However, we identified a potential confounding variable in using cross-sectional area of the muscle as a normalization factor for isometric force measurements of the transgenic strains; despite the relatively similar length of Galgt2 transgenic and nontransgenic muscles, the myofibers within Galgt2WT and Galgt2mdx muscles were considerably longer than their nontransgenic counterparts (Table 1). Myofiber length-to-muscle length ratios for WT and mdx EDL muscles were on the order of 0.45, consistent with previous studies (5), while the same ratio for Galgt2 transgenic muscles ranged from 0.7 to 0.79, a ratio more consistent with measures previously reported in the soleus (5). This finding is consistent with our previous studies showing increased total length of some muscles in Galgt2WT animals (53). The density of the EDL muscle consisting of myofibers, however, was not significantly changed among groups. Thus, to normalize force measurements between groups, we calculated CSA as referenced to myofiber length for each genotype [muscle wet mass/average myofiber length × myofiber density (1.06 g/cm3)]. As before, mdx muscles had increased CSA compared with WT at 5 mo, and again all Galgt2 transgenic muscles had decreased CSA relative to nontransgenic controls.
Measurement of normalized specific force.
Next, we assessed specific force obtained from the tetanic contractures. In Table 1, we present the specific developed force, corrected for CSA, as previously described by Brooks and Faulkner (5). As expected based on previously published studies (2, 9, 10, 40), mdx muscle exhibited reduced specific force when compared with wild-type muscle; 2-mo-old and 5-mo-old mdx muscles had reduced specific force compared with 5-mo-old wild-type muscle, with a greater (and significant) difference observed at 5 mo. It is important to note that such differences have not been found between WT and mdx muscles in other studies (52), and that such differences may reflect the relatively large potential differences in muscle pathology that can occur in young mdx muscles (8). For example, the weight of our mdx muscles at 5 mo was significantly increased relative to WT 5mo and mdx 2mo, and this may reflect the fact that these muscles were more severely dystrophic than mdx muscles used in some other studies. No significant difference was found between Galgt2mdx and mdx muscles at either 2 mo or 5 mo of age, but the trend was toward increased strength, while Galgt2WT 2mo and 5mo muscles were significantly reduced compared with WT 5mo. Thus, Galgt2 transgenic mdx muscles were as strong as their nontransgenic mdx counterparts, while Galgt2 transgenic WT muscles were 30–40% weaker than their WT counterparts. The peak and mean stresses the muscles underwent during the eccentric contraction showed the same finding. The motor pulling at the stimulated muscle caused an increase in total stress on the muscle (peaking at t = 700 ms), which, after normalization to cross-fiber section area, was not significantly different between Galgt2 transgenic and nontransgenic mdx, while these stresses in the Galgt2 transgenic WT muscles were 15–20% weaker than nontransgenic WT counterparts.
Measurement of forelimb and hindlimb grip strength.
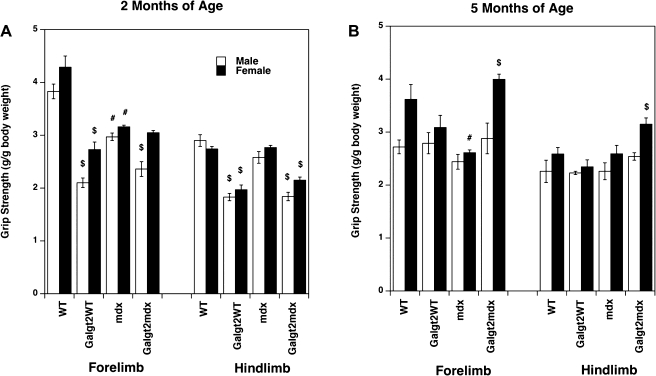
Because we had observed a change in normalized specific force in some Galgt2WT muscles as compared with WT, we chose to also measure grip strength for hindlimb and for forelimb muscles at 2 mo (Fig. 1A) and 5 mo (Fig. 1B) of age, much as we had done previously (15). Both the hindlimb and forelimb grip strength of Galgt2WT and Galgt2mdx mice was lower than strengths in their nontransgenic, stain-, and age-matched mice at 2 mo of age (with the exception of female forelimbs in Galgt2mdx vs. mdx, which were not significantly different). In addition, both male and female mdx mice were significantly weaker than WT; reduction in grip strength resulting from Galgt2 transgene expression in 2-mo-old mice ranged from 21% (male forelimb; Galgt2mdx vs. mdx) to 45% (male forelimb; Galgt2 vs. WT). None of these differences, however, were significant at 5 mo of age; in fact, both forelimb and hindlimb grip strength of Galgt2mdx females was significantly increased relative to mdx at this age. Thus, as with specific force measures in the EDL, grip strength at 5 mo of age was not significantly different between Galgt2 transgenic mdx and mdx muscles. These two measures, however, need not match exactly, because limb strength is a composite of multiple muscles, while specific force measures in Table 1 represent only the EDL.
Fig. 1.
Grip strength measurements in GalNAc transferase (Galgt2) transgenic and nontransgenic wild-type (WT) and mdx mice. Hindlimb and forelimb grip strength was measured in WT, Galgt2 transgenic WT (Galgt2WT), mdx, and Galgt2 transgenic mdx (Galgt2mdx) mice at 2 mo (2mo; A) and 5 mo (5mo; B) of age. Errors are SE for n = 7 (WT 2mo, male), 12 (WT 2mo, female), 18 (Galgt2WT 2mo, male), 10 (Galgt2WT, female), 10 (mdx 2mo, male), 17 (mdx 2mo, female), 8 (Galgt2mdx 2mo, male), 15 (Galgt2mdx 2mo, female), 4 (WT 5mo, male), 8 (WT 5mo, female), 6 (Galgt2WT 5mo, male), 9 (Galgt2WT 5mo, female), 4 (mdx 5mo, male), 8 (mdx 5mo, female), 4 (Galgt2mdx 5mo, male), or 8 (Galgt2mdx 5mo, female) animals. In otherwise identical mice (strain, treatment, or age): *significant difference (P < 0.05) based on age compared with younger mice; #significant difference (P < 0.05) based on WT vs. mdx; $significant difference (P < 0.05) based on Galgt2 overexpression.
Measurement of force drop during eccentric contractions.
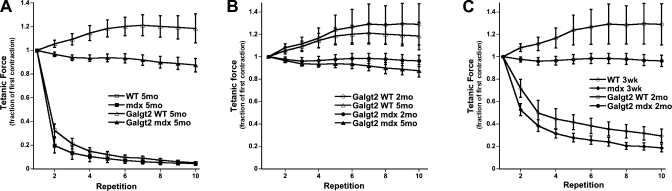
To test whether Galgt2 overexpression could reduce the impact of eccentric contraction injury, we subjected the muscles to 10 repeats of lengthening contractions. In Fig. 2A, all four genotypes are plotted for the age of 5 mo. When normalized to the initial contraction, mdx and WT muscles progressively lost tetanic force, with a percent force drop of over 90% for both genotypes by the 5th contraction and 95% by the 10th contraction. WT muscle, however, was better protected against this mechanical insult than mdx muscle, a result that is consistent with numerous previous studies (2, 9, 18, 40, 42). That these muscles decayed in strength so quickly is the result of the 10% stretch paradigm used, which is more significant than many other studies. We chose this more severe regimen to unmask the full effects of Galgt2 transgene expression because, even using this paradigm, both Galgt2 transgenic wild-type and mdx muscles were virtually completely protected against eccentric contraction injury (Fig. 2A); only a very small decay was found in the Galgt2mdx muscles (<10% force drop by the fifth contraction), and there was no decay at all, on average, in the Galgt2WT group. This finding was confirmed in younger mice of these two groups (Fig. 2B). At 2 mo of age, both of these groups exhibit the same behavior. The general trend is that in these younger mice, the protection was even slightly better, although the 2-mo-old and 5-mo-old groups were not significantly different in response to eccentric contraction-induced injury. Statistical analysis did, however, reveal that in the 2-mo-old Galgt2mdx muscles, the decay was no longer significant. We cannot explain why Galgt2WT measures trended upward with successive stimulations, but the significance of the difference in force drop between transgenic and nontransgenic muscles was unaffected by this.
Fig. 2.
Galgt2 transgenic WT and mdx muscles are protected from loss of force during eccentric contractions. A: 5-mo-old WT, Galgt2WT, mdx, and Galgt2mdx muscles were compared for force drop during repetitive eccentric contractions. Galgt2WT and Galgt2mdx were both significantly changed relative to either WT or mdx. B: 2-mo-old and 5-mo-old Galgt2WT and Galgt2mdx muscles were compared for force drop during eccentric contractions. C: 2-mo-old Galgt2WT and Galgt2mdx muscles were compared with 3-wk-old (3wk) WT and mdx muscles. The 3-wk-old WT and mdx muscles are as small or smaller in cross-sectional area than 2-mo-old Galgt2WT and Galgt2mdx muscles. P < 0.001 for Galgt2WT 5mo or Galgt2mdx 5mo compared with either WT 5mo or mdx 5mo (in A) and for Galgt2WT 2mo or Galgt2mdx 2mo compared with either WT 3wk or mdx 3wk (in C). Errors are SE for n = 7 (WT 5mo), 8 (Galgt2WT 5mo), 4 (mdx 5mo), 5 (Galgt2mdx 5mo), 3 (Galgt2WT 2mo), 10 (Galgt2mdx 2mo), 2 (WT 3wk), and 6 (mdx 3wk) muscles.
Because tetanic contractions are very ATP costly, proper oxygenation of the muscle is warranted for optimal function. From our size measurements we observed all the Galgt2 transgenic muscles were significantly smaller in diameter compared with age-matched nontransgenic WT and mdx littermates. To make sure that the size of the muscle was not a prominent determinant of function, due to potential improper oxygenation, we repeated the eccentric contraction experiments on a cohort of WT and mdx mice only 3 wk old (Fig. 2C). These 3wk WT and mdx muscles were as small, or smaller than, 2 mo and 5 mo Galgt2WT and Galgt2mdx muscles (Table 1). Three-week-old mice (WT and mdx) showed a lesser decay response than their respective older (5mo) strain-matched littermates (Fig. 2C vs. Fig. 2A). Also, the difference between WT and mdx was more pronounced; 3wk WT muscles exhibited significantly less decay than 3wk mdx muscles (∼60% force drop for WT vs. 70% for mdx by the fifth contraction). Still, both 2mo Galgt2 transgenic muscle groups (Galgt2WT and Galgt2mdx) clearly performed much better than nontransgenic 3-wk-old WT or mdx muscles, despite the fact that they were now of equal size. These data show that it is Galgt2 overexpression, rather than a simple reduction in muscle size, that led to the prevention of eccentric contraction induced injury.
Three general conclusions are apparent from these experiments shown in Fig. 2. First, overall statistical analysis revealed that in each age comparison (6 comparisons in total) between otherwise identical animals, the younger mice perform better (i.e., show increased protection) versus older ones. Second, in each WT versus mdx comparison (3 comparisons total) in otherwise identical animals, the WT mice perform better (i.e., show increased protection) versus mdx. Last, and most important, in Galgt2 overexpression comparisons (3 comparisons total) in otherwise identical animals, the Galgt2 transgenic mice perform better (i.e., show increased protection) versus nontransgenic ones.
Measurement of procion orange dye uptake.
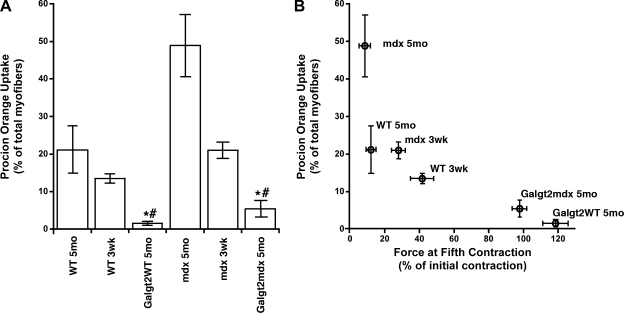
While loss of force during eccentric contractions is suggestive of injury, this need not reflect direct damage to the sarcolemmal membrane. As dystrophin and the dystrophin-glycoprotein complex are thought to provide stability to the sarcolemmal membrane (1, 11), we also wished to assess sarcolemmal membrane integrity more specifically. To do this, we performed procion orange dye uptake measurements on EDL muscles after the eccentric contraction protocol (Fig. 3A). The greatest percentage of myofibers with procion orange dye was mdx 5mo, where almost 50% of myofibers, on average, were positive. WT 5mo muscles showed dye uptake in >20% of myofibers. In both instances, 3wk muscles of these genotypes also showed significant, but reduced, levels of dye uptake compared with strain-matched 5mo muscles. Galgt2WT 5mo muscles had significantly fewer positive myofibers than 3wk or 5mo WT muscles, and Galgt2mdx 5mo muscles had significantly fewer positive myofibers than mdx 3wk or 5mo muscles. We correlated the physiology with the histology by comparing the remaining relative force after the fifth eccentric contraction, a point at which the majority of loss of force was evident, to the procion orange uptake (Fig. 3B). We observed a tight relationship between loss of force and procion orange uptake over a wide range of data. Thus, Galgt2 transgenic muscles were significantly protected from damage to the sarcolemmal membrane in both WT and mdx backgrounds, which correlated with improved function.
Fig. 3.
Galgt2 overexpression protects myofibers from membrane damage. A: extensor digitorum longus (EDL) muscles from WT, Galgt2WT, mdx, and Galgt2mdx mice were incubated with procion orange after eccentric contraction paradigm, and the percentage of myofibers taking up dye was measured. Muscles were from animals at 5 mo or 3 wk of age. Errors are SE for n = 2 (WT 3wk), 7 (WT 5mo), 8 (Galgt2WT 5mo), 4 (mdx 5mo), 6 (mdx 3wk), or 5 (Galgt2mdx 5mo) muscles. *,#Significant difference (P < 0.05) vs. WT 3 wk and Galgt2WT 5 mo or between mdx 3wk and Galgt2mdx 5 mo. B: correlation between loss of tetanic force after 5 contractions and procion orange uptake. Errors are SE for n as in A.
Measurement of normalized specific force and force drop during eccentric contractions with postnatal transgene overexpression.
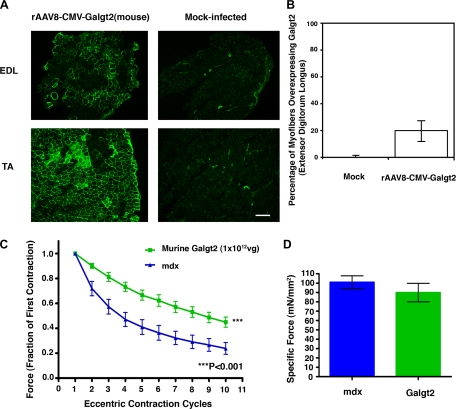
Because some of resistance to loss of force during eccentric contractions in Galgt2 transgenic animals could theoretically be attributed to their smaller muscle size and/or weaker specific force, we also assessed the effects of transgene overexpression in postnatal mdx muscles, where we had previously shown overexpression does not alter myofiber size or length (54, 55). We did two experiments. In the first, we treated mdx muscles with rAAV8-CMV-Galgt2(mouse), a recombinant adeno-associated virus (AAV) vector that we had previously shown can be used to inhibit the development of muscular dystrophy in mdx muscle (54, 55). The AAV8 viral serotype is particularly good at delivering transgenes to skeletal myofibers from the blood due to its ability to cross the vascular barrier (42, 50). AAV was packaged with a transgene that contained the cytomegalovirus (CMV) promoter, which allows robust transgene expression from many tissues, and the mouse Galgt2 cDNA, which is the same cDNA present in the Galgt2 transgenic animals (53).
The mdx mice were treated with 1 × 1012 vg of rAAV8-CMV-Galgt2(mouse) by injection into the femoral artery, as previously described (42). Assessment of Galgt2 overexpression was done by immunostaining with CT2 (Fig. 4A), a monoclonal antibody that recognizes the CT carbohydrate (7, 30), which is the enzymatic product of Galgt2 activity (47). Since this carbohydrate is normally confined only to the neuromuscular junction in skeletal muscle (and also in capillaries) (30), transgene overexpression was easily scored by the appearance of extrasynaptic sarcolemmal staining for the CT carbohydrate (54, 55). No myofibers showed overexpression in control (mock-infected muscles), although staining of some large blood vessels and capillaries, where the carbohydrate is normally expressed (30), could be seen, and these served as positive controls for the immunostaining protocol (Fig. 4A). This approach achieved transgene overexpression in 20 ± 10% of myofibers, a highly significant (P < 0.001) increase compared with mock-infected muscles (Fig. 4B). In general, infection of the TA muscle was greater here than for the EDL (e.g., Fig. 4A). Analysis of eccentric contractions in rAAV8-CMV-Galgt2-treated mdx muscles showed a significant inhibition of force drop during eccentric contractions when compared with mock-infected contralateral control muscles (Fig. 4C). These control muscles were subjected to an identical vascular delivery procedure, but they received injection buffer alone; tetanic force for rAAV8-CMV-Galgt2-treated muscles was, on average, reduced by 56 ± 4% by the 10th eccentric contraction, while mock-treated muscles were reduced by 76 ± 5%. Analyzing both data sets as a whole using random effects regression analysis for longitudinal data showed that Galgt2 overexpression led to a highly significant resistance to injury (P < 0.001). Similar levels of significance were observed if analysis was done using 2-way ANOVA between entire eccentric contraction data sets with Bonferroni pre- and post-hoc analysis. Because muscles were tested for gene expression after the eccentric contraction protocol, they were weighed wet, and the increase in perceived mass may generally reduce the apparent specific force. However, all muscles were treated the same, and no significant change in specific force was found between treated and untreated muscles; treated muscles had a specific force of 90 ± 10 mN/mm2 compared with 100 ± 7 mN/mm2 for mock-treated control (P > 0.05) (Fig. 4D).
Fig. 4.
Postnatal Galgt2 overexpression protects mdx muscle from loss of force during eccentric contractions. A: cytotoxic T cell (CT) carbohydrate immunostaining (with the CT2 monoclonal antibody) in the EDL and tibialis anterior (TA) muscle shows overexpression of the CT carbohydrate in a subset of myofibers resulting from Galgt2 transgene overexpression after serotype 8-like recombinant adeno-associated virus (AAV) vector (rAAV8)-CMV-Galgt2(mouse) treatment via the femoral artery 6 wk postinfection. Mock-treated muscle was stained in an identical manner, and exposures are time matched. CT2 staining of large blood vessels occurs normally in these sections. Bar is 200 μm. B: quantification of the average percentage of myofibers overexpressing Galgt2 in rAAV8-CMV-Galgt2-infected mdx muscles. C: mdx muscles infected (via the femoral artery) with 1 × 1012 vg of rAAV8-CMV-Galgt2(mouse) were compared with mock-infected contralateral mdx EDL muscles for force drop during repetitive eccentric contractions. rAAV8-CMV-Galgt2 treatment (green) significantly protected against loss of force compared with mock-treated (contralateral) muscles (blue) (P < 0.001). D: measurement of normalized specific force in rAAV8-CMV-Galgt2-infected and mock-infected contralateral mdx EDL muscles show no significant change. Errors are SE for n = 6 muscles per condition.
For the second gene therapy experiment, we used a protocol developed by us that is more relevant to translational medicine (42). Because we wish to ultimately move any effective gene therapy to clinical trials, we generated a cDNA of the human Galgt2 gene and subcloned it into a viral packaging vector behind a muscle-specific promoter (human MCK). This vector, rAAV8-MCK-Galgt2(human), allows expression of the human cDNA only in skeletal muscle. Additionally, intravascular delivery of the vector allows the transgene to be overexpressed in myofibers throughout the hindlimb, including the EDL, TA, and gastrocnemius (not shown). As the gastrocnemius muscle is relatively spared even in older Duchenne patients with severe disease, such a protocol could allow gene therapy trials to proceed in this patient population. In this experiment, we also compared the effects of rAAV8-MCK-Galgt2(human) to rAAV8-MCK-Micro-dys(human), a transgenic viral vector that expresses microdystrophin using the same MCK promoter. This vector was used as a control to compare the effects of Galgt2 to a protein already known to ameliorate muscle damage during eccentric contractions in mdx muscle (17, 27). The final changes to make this experiment more translationally relevant were to use a 1 × 1011 vg dose of AAV vector, a dose that is scalable from mice to humans, and a duration time before analysis of 3 mo. The 3-mo treatment in mice meets previous Food and Drug Administration expectations for demonstration of functional efficacy for preclinical gene therapy studies.
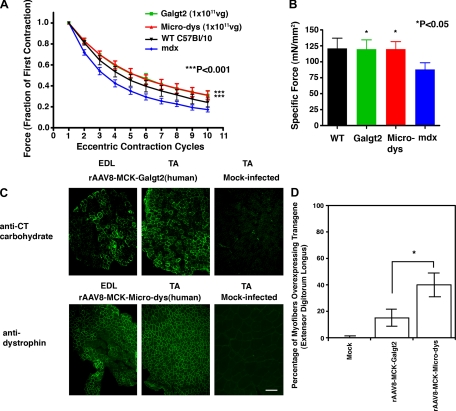
We used a vascular delivery protocol developed in our Center (42). There were four conditions: mdx muscles treated with 1 × 1011 vg rAAV8-MCK-Galgt2(human), mdx muscles treated with 1 × 1011 vg rAAV8-MCK-Micro-dys(human), mdx contralateral muscles treated with injection buffer, and untreated wild-type muscles. Wild-type muscles were not subjected to the limb perfusion protocol so that they might serve as the best-case scenario for a normal muscle response. Five-week-old mdx mice were treated using this gene therapy protocol, and mice were analyzed 12 wk after treatment. To tease out differences between mdx and wild type, we subjected muscles to a 5% lengthening paradigm for these experiments. Using this protocol, mdx muscles were significantly worse than wild type, showing a force drop of 83 ± 2% by the 10th contraction compared with 76 ± 4% for wild type (P < 0.001) (Fig. 5A). Both rAAV8-MCK-Galgt2 and rAAV8-MCK-Micro-dys improved force during eccentric contractions, each averaging a force drop of 69 ± 4% by the 10th contraction (Fig. 5A). This level of force drop was equal to (or slightly above) measures for wild type. Changes were highly significant for both transgenes when compared with mock-treated mdx (P < 0.001 for both). Equally important, Galgt2 overexpression in postnatal mdx muscle, unlike embryonic overexpression in Galgt2 transgenic mice, led to an increase in maximal specific force; rAAV8-MCK-Galgt2 and rAAV8-MCK-Micro-dys increased maximal specific force to 120 ± 14 mN/mm2 and 120 ± 12 mN/mm2, respectively, from 88 ± 11 mN/mm2 for mock-treated mdx controls (P < 0.05 for both), and this was equivalent to the specific force measured in wild-type muscles (121 ± 16 mN/mm2), where no treatment was given (Fig. 5B). As with the transgenic muscles, where limb grip strength was relatively improved at 5 mo compared with 2 mo (Fig. 3), it may be that the longer time of Galgt2 overexpression in this experiment (12 wk) is the reason for the more significant change in specific force (Fig. 5B) compared with the shorter (6 wk) experiment with the mouse Galgt2 gene (Fig. 4D).
Fig. 5.
rAAV8-muscle creatine kinase (MCK)-Galgt2-treated mdx muscles are protected from loss of force during eccentric contractions and have increased normalized specific force equivalent to rAAV8-MCK-microdystrophin-treated mdx muscles. A: mdx muscles infected with 1 × 1011 vg of rAAV8-MCK-Galgt2(human) (green) or with 1 × 1011 vg of rAAV8-MCK-Micro-dys(human) (red) were compared with mock-infected contralateral mdx EDL muscles (blue) and WT (WT C57Bl/10) EDL muscles (black) for force drop during repetitive eccentric contractions at 12 wk postinfection. Both rAAV8-MCK-Galgt2 (Galgt2) and rAAV8-MCK-microdystrophin (Micro-dys) treatment significantly protected against loss of force compared with mock-treated mdx muscles (P < 0.001 for either vs. mdx). B: treatment with either rAAV8-MCK-Galgt2 or rAAV8-MCK-Micro-dys significantly increased normalized specific force relative to mock-treated mdx muscles (P < 0.05 for either vs. mdx). Errors are SE for n = 10 (rAAV8-MCK-microdystrophin, Micro-dys), 12 (rAAV8-MCK-Galgt2, Galgt2), 21 (WT, C57Bl/10), or 25 (mdx) muscles per condition. C: CT carbohydrate immunostaining in the EDL and TA shows overexpression of the CT carbohydrate in a subset of mdx myofibers resulting from Galgt2 transgene overexpression after rAAV8-MCK-Galgt2(human) treatment via the femoral artery. Mock-infected muscle was stained in an identical manner and exposures are time matched. Dystrophin protein immunostaining in the EDL and TA shows overexpression in a subset of mdx myofibers resulting from microdystrophin overexpression after rAAV8-MCK-Micro-dys(human) treatment via the femoral artery. Mock-infected muscle was stained in an identical manner and exposures are time matched. Bar is 200 μm. D: quantification of the average percentage of myofibers overexpressing Galgt2 in rAAV8-MCK-Galgt2(human)- or rAAV8-MCK-Micro-dys(human)-infected muscles. Microdystrophin was expressed in significantly more myofibers than Galgt2 (P < 0.05).
In analysis of transgene overexpression, it was clear that even though both treatments yielded similar levels of change in eccentric contractions and specific force, rAAV8-MCK-Galgt2 led to overexpression of transgene in half the number of myofibers that rAAV8-MCK-Micro-dys did (Fig. 5C); CT carbohydrate overexpression occurred, on average, in only 15 ± 6% of rAAV8-MCK-Galgt2-treated mdx myofibers, while microdystrophin overexpression occurred in 40 ± 9% of rAAV8-MCK-Micro-dys-treated myofibers [P < 0.05, for Galgt2 vs. microdystrophin, n = 10 (microdystrophin)–12 (Galgt2)] (Fig. 5D). As with the previous experiment, our vascular delivery approach was more successful in transducing the tibialis anterior than the extensor digitorum longus (Fig. 5C). While we cannot explain the altered levels of gene expression for the two vectors, a differential immune response to the different human transgenic proteins in the mouse is one possibility. Regardless, these data suggest that Galgt2 can accomplish the same therapeutic benefit as microdystrophin even when expressed in half as many myofibers. Importantly, Galgt2 is a naturally occurring gene and protein in DMD patients and would therefore not be expected to stimulate an immune response in humans (as microdystrophin may indeed do).
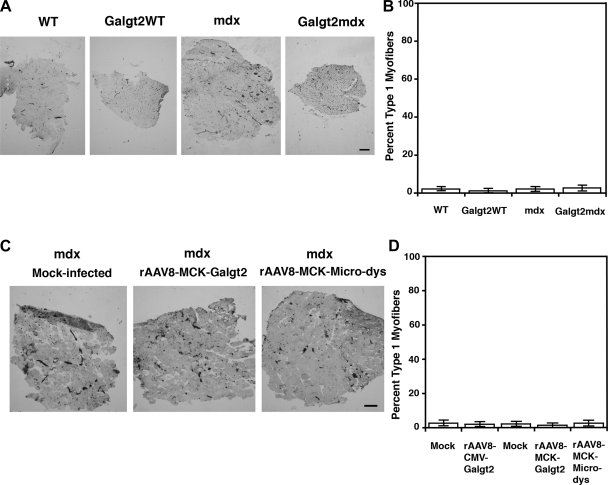
To ensure that changes were not due to altered fiber type composition, we measured the percentage of slow twitch (type 1) myofibers for all muscle groups using ATPase fiber type activity staining (Fig. 6). For experiments using Galgt2 transgenic mice, no significant change was identified in the percentage of myofibers that were type 1 (slow twitch) (Fig. 6, A and B). Because blood vessels and capillaries are also stained black with this method (Fig. 6A), Galgt2WT and Galgt2mdx muscles, which are far smaller in diameter, appear to have more staining; however, none of this was due to staining of myofibers but rather to an increased density of vessels per unit area. As with Galgt2 transgenic muscles, no change in fiber type composition was seen in rAAV8-MCK-Galgt2 mdx muscles compared with mock-infected mdx controls (Fig. 6, C and D).
Fig. 6.
Fiber type composition of EDL muscles in all experimental conditions. A: fiber type composition of the EDL muscle of WT, Galgt2WT, mdx, or Galgt2mdx 2-mo-old muscles was determined using ATPase immunohistochemical staining at pH 4.2 to define slow (type 1) fibers, which are black, and fast (type II) subtypes of myofibers, which are not stained. Blood vessels and capillaries are also stained black with this staining method. B: quantitation of fiber type composition showed no change between the four groups. C: fiber type composition of mdx muscles treated with rAAV8-CMV-Galgt2(mouse), rAAV8-MCK-Galgt2(human), rAAV8-MCK-microdystrophin(human), or appropriate mock-infected controls. D: quantitation of fiber type composition showed no change between the five groups. Bar is 200 μm. Errors are SE for n = 6 muscles per condition.
DISCUSSION
The experiments presented here demonstrate that Galgt2 overexpression can protect skeletal muscle from injury, both in dystrophic and nondystrophic animals; the level of force drop during eccentric contractions was insignificant in most instances for Galgt2 transgenic mouse skeletal muscles, mdx and wild-type muscles, even in eccentric contraction paradigms where nontransgenic muscles showed force drop of 95% or more. Similarly robust improvements were observed with regard to procion orange dye uptake. These experiments are consistent with our previous studies showing that Galgt2 overexpression prevents the development of muscle pathology associated with muscular dystrophy (35, 54, 55) and suggest that the absence of such pathology is the result of the protection of mdx myofibers from injury. Postnatal overexpression of the Galgt2 transgene using gene therapy methods also reduced force drop during eccentric contractions and could increase maximal specific force. These effects were at least on a par with results achieved with microdystrophin, a gene replacement strategy known to have such effects (17, 27). These data suggest that Galgt2 overexpression confers increased resistance to muscle damage, even in the absence of dystrophin.
A number of studies have shown improved protection in force drop measures during eccentric contraction in the mdx muscles, but to our knowledge so far none have shown these therapies to exceed wild-type controls, as we do here. The studies of Gillis, Davies, and colleagues on mdx myofibers overexpressing utrophin show that utrophin overexpression decreases force drop to levels approaching, but never exceeding, those found in wild-type muscle (48). Similarly, Chamberlain and colleagues have shown that overexpression of microdystrophin transgenes can partially rescue force drop in the EDL (27) and tibialis anterior (17) muscles of mdx mice; but again, these did not exceed wild type. Karpati and colleagues show similar results with full-length dystrophin (10). Likewise, treatment of mdx muscles with heregulin (24), myostatin inhibitors (3, 4), creatine (28), calcium channel blockers (51), or corticosteroids (56) all did not exceed wild-type measures. Thus, it is likely that altering glycosylation of the myofiber membrane via Galgt2 overexpression stabilizes the muscle membrane from injury in ways these other molecules cannot. Such a therapeutic approach could be useful, for example, in myopathies where dystrophin expression is normal. Both gene therapy and pharmacologic approaches to increase Galgt2 overexpression should be pursued to translate these findings into therapies that could protect skeletal muscles from injury.
GRANTS
This work was supported by National Institutes of Health Grants AR050202AR and AR049722 (to P. T. Martin), NHLBI083957 (to P. M. L. Janssen), and NS055958 (to J. R. Mendell); a collaborative grant from The Ohio State University College of Medicine and Nationwide Children's Hospital (to P. T. Martin and P. M. L. Janssen); and an Established Investigator Award from the American Heart Association (to P. M. L. Janssen).
Acknowledgments
The authors thank Julie Stephens of The Ohio State University Center for Biostatistics for technical support, and Anil Birdi, Benjamin Canan, and Erin Shaffer for technical assistance.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Blake DJ, Weir A, Newey SE, Davies KE. Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Bobet J, Mooney RF, Gordon T. Force and stiffness of old dystrophic (mdx) mouse skeletal muscles. Muscle Nerve 21: 536–539, 1998. [DOI] [PubMed] [Google Scholar]
- 3.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420: 418–421, 2002. [DOI] [PubMed] [Google Scholar]
- 4.Bogdanovich S, Perkins KJ, Krag TO, Whittemore LA, Khurana TS. Myostatin propeptide-mediated amelioration of dystrophic pathophysiology. FASEB J 19: 543–549, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Brooks SV, Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol 404: 71–82, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark KR, Liu X, McGrath JP, Johnson PR. Highly purified recombinant adeno-associated virus vectors are biologically active and free of detectable helper and wild-type viruses. Hum Gene Ther 10: 1031–1039, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Conzelmann A, Lefrancois L. Monoclonal antibodies specific for T cell-associated carbohydrate determinants react with human blood group antigens CAD and SDA. J Exp Med 167: 119–131, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deconinck N, Tinsley J, De Backer F, Fisher R, Kahn D, Phelps S, Davies K, Gillis JM. Expression of truncated utrophin leads to major functional improvements in dystrophin-deficient muscles of mice. Nat Med 3: 1216–1221, 1997. [DOI] [PubMed] [Google Scholar]
- 9.De la Porte S, Morin S, Koenig J. Characteristics of skeletal muscle in mdx mutant mice. Int Rev Cytol 191: 99–148, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Dudley RW, Lu Y, Gilbert R, Matecki S, Nalbantoglu J, Petrof BJ, Karpati G. Sustained improvement of muscle function one year after full-length dystrophin gene transfer into mdx mice by a gutted helper-dependent adenoviral vector. Hum Gene Ther 15: 145–156, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Ervasti JM Costameres: the Achilles' heel of Herculean muscle. J Biol Chem 278: 13591–13594, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Ervasti JM, Campbell KP. A role for the dystrophin-glycoprotein complex as a transmembrane linker between laminin and actin. J Cell Biol 122: 809–823, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ervasti JM, Campbell KP. Membrane organization of the dystrophin-glycoprotein complex. Cell 66: 1121–1131, 1991. [DOI] [PubMed] [Google Scholar]
- 14.Gao G, Vandenberghe LH, Alvira MR, Lu Y, Calcedo R, Zhou X, Wilson JM. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol 78: 6381–6388, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haidet AM, Rizo L, Handy C, Umapathi P, Eagle A, Shilling C, Boue D, Martin PT, Sahenk Z, Mendell JR, Kaspar BK. Long-term enhancement of skeletal muscle mass and strength by single gene administration of myostatin inhibitors. Proc Natl Acad Sci USA 105: 4318–4322, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamalainen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41: 733–743, 1993. [DOI] [PubMed] [Google Scholar]
- 17.Harper SQ, Hauser MA, DelloRusso C, Duan D, Crawford RW, Phelps SF, Harper HA, Robinson AS, Engelhardt JF, Brooks SV, Chamberlain JS. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat Med 8: 253–261, 2002. [DOI] [PubMed] [Google Scholar]
- 18.Head SI, Williams DA, Stephenson DG. Abnormalities in structure and function of limb skeletal muscle fibres of dystrophic mdx mice. Proc Biol Sci 248: 163–169, 1992. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman EP, Brown RH Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell 51: 919–928, 1987. [DOI] [PubMed] [Google Scholar]
- 20.Hoyte K, Kang C, Martin PT. Definition of pre- and postsynaptic forms of the CT carbohydrate antigen at the neuromuscular junction: ubiquitous expression of the CT antigens and the CT GalNAc transferase in mouse tissues. Brain Res Mol Brain Res 109: 146–160, 2002. [DOI] [PubMed] [Google Scholar]
- 21.Janssen PM, Hunter WC. Force, not sarcomere length, correlates with prolongation of isosarcometric contraction. Am J Physiol Heart Circ Physiol 269: H676–H685, 1995. [DOI] [PubMed] [Google Scholar]
- 22.Kawamura YI, Kawashima R, Fukunaga R, Hirai K, Toyama-Sorimachi N, Tokuhara M, Shimizu T, Dohi T. Introduction of Sd(a) carbohydrate antigen in gastrointestinal cancer cells eliminates selectin ligands and inhibits metastasis. Cancer Res 65: 6220–6227, 2005. [DOI] [PubMed] [Google Scholar]
- 23.Koenig M, Hoffman EP, Bertelson CJ, Monaco AP, Feener C, Kunkel LM. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 50: 509–517, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Krag TO, Bogdanovich S, Jensen CJ, Fischer MD, Hansen-Schwartz J, Javazon EH, Flake AW, Edvinsson L, Khurana TS. Heregulin ameliorates the dystrophic phenotype in mdx mice. Proc Natl Acad Sci USA 101: 13856–13860, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefrancois L, Bevan MJ. Functional modifications of cytotoxic T-lymphocyte T200 glycoprotein recognized by monoclonal antibodies. Nature 314: 449–452, 1985. [DOI] [PubMed] [Google Scholar]
- 26.Lefrancois L, Bevan MJ. Novel antigenic determinants of the T200 glycoprotein expressed preferentially by activated cytotoxic T lymphocytes. J Immunol 135: 374–383, 1985. [PubMed] [Google Scholar]
- 27.Liu M, Yue Y, Harper SQ, Grange RW, Chamberlain JS, Duan D. Adeno-associated virus-mediated microdystrophin expression protects young mdx muscle from contraction-induced injury. Mol Ther 11: 245–256, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Louis M, Raymackers JM, Debaix H, Lebacq J, Francaux M. Effect of creatine supplementation on skeletal muscle of mdx mice. Muscle Nerve 29: 687–692, 2004. [DOI] [PubMed] [Google Scholar]
- 29.Martin PT Dystroglycan glycosylation and its role in matrix binding in skeletal muscle. Glycobiology 13: 55R–66R, 2003. [DOI] [PubMed] [Google Scholar]
- 30.Martin PT, Scott LJ, Porter BE, Sanes JR. Distinct structures and functions of related pre- and postsynaptic carbohydrates at the mammalian neuromuscular junction. Mol Cell Neurosci 13: 105–118, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Matsumura K, Ervasti JM, Ohlendieck K, Kahl SD, Campbell KP. Association of dystrophin-related protein with dystrophin-associated proteins in mdx mouse muscle. Nature 360: 588–591, 1992. [DOI] [PubMed] [Google Scholar]
- 31a.Méndez J and Keys A. Density and composition of mammalian skeletal muscle. Metab Clin Exp 9: 184–199, 1960. [Google Scholar]
- 32.Montiel MD, Krzewinski-Recchi MA, Delannoy P, Harduin-Lepers A. Molecular cloning, gene organization and expression of the human UDP-GalNAc:Neu5Acalpha2-3Galbeta-R beta1,4-N-acetylgalactosaminyltransferase responsible for the biosynthesis of the blood group Sda/Cad antigen: evidence for an unusual extended cytoplasmic domain. Biochem J 373: 369–379, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR. Altered myocardial force-frequency relation in human heart failure. Circulation 85: 1743–1750, 1992. [DOI] [PubMed] [Google Scholar]
- 34.Muscat GE, Kedes L. Multiple 5′-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol 7: 4089–4099, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen HH, Jayasinha V, Xia B, Hoyte K, Martin PT. Overexpression of the cytotoxic T cell GalNAc transferase in skeletal muscle inhibits muscular dystrophy in mdx mice. Proc Natl Acad Sci USA 99: 5616–5621, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Odom GL, Gregorevic P, Chamberlain JS. Viral-mediated gene therapy for the muscular dystrophies: successes, limitations and recent advances. Biochim Biophys Acta 1772: 243–262, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parkhomovskiy N, Kammesheidt A, Martin PT. N-acetyllactosamine and the CT carbohydrate antigen mediate agrin-dependent activation of MuSK and acetylcholine receptor clustering in skeletal muscle. Mol Cell Neurosci 15: 380–397, 2000. [DOI] [PubMed] [Google Scholar]
- 38.Rabinowitz JE, Rolling F, Li C, Conrath H, Xiao W, Xiao X, Samulski RJ. Cross-packaging of a single adeno-associated virus (AAV) type 2 vector genome into multiple AAV serotypes enables transduction with broad specificity. J Virol 76: 791–801, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafael JA, Tinsley JM, Potter AC, Deconinck AE, Davies KE. Skeletal muscle-specific expression of a utrophin transgene rescues utrophin-dystrophin deficient mice. Nat Genet 19: 79–82, 1998. [DOI] [PubMed] [Google Scholar]
- 40.Raymackers JM, Debaix H, Colson-Van Schoor M, De Backer F, Tajeddine N, Schwaller B, Gailly P, Gillis JM. Consequence of parvalbumin deficiency in the mdx mouse: histological, biochemical and mechanical phenotype of a new double mutant. Neuromuscul Disord 13: 376–387, 2003. [DOI] [PubMed] [Google Scholar]
- 41.Rodino-Klapac LR, Chicoine LG, Kaspar BK, Mendell JR. Gene therapy for Duchenne muscular dystrophy: expectations and challenges. Arch Neurol 64: 1236–1241, 2007. [DOI] [PubMed] [Google Scholar]
- 42.Rodino-Klapac LR, Janssen PM, Montgomery CL, Coley BD, Chicoine LG, Clark KR, Mendell JR. A translational approach for limb vascular delivery of the micro-dystrophin gene without high volume or high pressure for treatment of Duchenne muscular dystrophy. J Transl Med 5: 45, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sacco P, Jones DA, Dick JR, Vrbova G. Contractile properties and susceptibility to exercise-induced damage of normal and mdx mouse tibialis anterior muscle. Clin Sci (Lond) 82: 227–236, 1992. [DOI] [PubMed] [Google Scholar]
- 44.Salva MZ, Himeda CL, Tai PW, Nishiuchi E, Gregorevic P, Allen JM, Finn EE, Nguyen QG, Blankinship MJ, Meuse L, Chamberlain JS, Hauschka SD. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol Ther 15: 320–329, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Scott JM, Li S, Harper SQ, Welikson R, Bourque D, DelloRusso C, Hauschka SD, Chamberlain JS. Viral vectors for gene transfer of micro-, mini-, or full-length dystrophin. Neuromuscul Disord 12, Suppl 1: S23–S29, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Sicinski P, Geng Y, Ryder-Cook AS, Barnard EA, Darlison MG, Barnard PJ. The molecular basis of muscular dystrophy in the mdx mouse: a point mutation. Science 244: 1578–1580, 1989. [DOI] [PubMed] [Google Scholar]
- 47.Smith PL, Lowe JB. Molecular cloning of a murine N-acetylgalactosamine transferase cDNA that determines expression of the T lymphocyte-specific CT oligosaccharide differentiation antigen. J Biol Chem 269: 15162–15171, 1994. [PubMed] [Google Scholar]
- 48.Tinsley J, Deconinck N, Fisher R, Kahn D, Phelps S, Gillis JM, Davies K. Expression of full-length utrophin prevents muscular dystrophy in mdx mice. Nat Med 4: 1441–1444, 1998. [DOI] [PubMed] [Google Scholar]
- 49.Wang C, Wang CM, Clark KR, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther 10: 1528–1534, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Wang Z, Zhu T, Qiao C, Zhou L, Wang B, Zhang J, Chen C, Li J, Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat Biotechnol 23: 321–328, 2005. [DOI] [PubMed] [Google Scholar]
- 51.Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord 16: 845–854, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Williams DA, Head SI, Lynch GS, Stephenson DG. Contractile properties of skinned muscle fibres from young and adult normal and dystrophic (mdx) mice. J Physiol 460: 51–67, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xia B, Hoyte K, Kammesheidt A, Deerinck T, Ellisman M, Martin PT. Overexpression of the CT GalNAc transferase in skeletal muscle alters myofiber growth, neuromuscular structure, and laminin expression. Dev Biol 242: 58–73, 2002. [DOI] [PubMed] [Google Scholar]
- 54.Xu R, Camboni M, Martin PT. Postnatal overexpression of the CT GalNAc transferase inhibits muscular dystrophy in mdx mice without altering muscle growth or neuromuscular development: evidence for a utrophin-independent mechanism. Neuromuscul Disord 17: 209–220, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu R, Chandrasekharan K, Yoon JH, Camboni M, Martin PT. Overexpression of the cytotoxic T cell (CT) carbohydrate inhibits muscular dystrophy in the dyW mouse model of congenital muscular dystrophy 1A. Am J Pathol 171: 181–199, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang L, Luo J, Petrof BJ. Corticosteroid therapy does not alter the threshold for contraction-induced injury in dystrophic (mdx) mouse diaphragm. Muscle Nerve 21: 394–397, 1998. [DOI] [PubMed] [Google Scholar]