Abstract
Caveolin-1 (Cav-1) regulates agonist-induced Ca2+ entry in endothelial cells; however, how Cav-1 regulates this process is poorly understood. Here, we describe that Cav-1 scaffold domain (NH2-terminal residues 82–101; CSD) interacts with transient receptor potential canonical channel 1 (TRPC1) and inositol 1,4,5-trisphosphate receptor 3 (IP3R3) to regulate Ca2+ entry. We have shown previously that the TRPC1 COOH-terminal residues 781-789 bind to CSD. In the present study, we show that the TRPC1 COOH-terminal residues 781-789 truncated (TRPC1-CΔ781-789) mutant expression abolished Ca2+ store release-induced Ca2+ influx in human dermal microvascular endothelial cell line (HMEC) and human embryonic kidney (HEK-293) cells. To understand the basis of loss of Ca2+ influx, we determined TRPC1 binding to IP3R3. We observed that the wild-type (WT)-TRPC1 but not TRPC1-CΔ781-789 effectively interacted with IP3R3. Similarly, WT-TRPC1 interacted with Cav-1, whereas TRPC1-CΔ781-789 binding to Cav-1 was markedly suppressed. We also assessed the direct binding of Cav-1 with TRPC1 and observed that the WT-Cav-1 but not the Cav-1ΔCSD effectively interacted with TRPC1. Since the interaction between TRPC1 and Cav-1ΔCSD was reduced, we measured Ca2+ store release-induced Ca2+ influx in Cav-1ΔCSD-transfected cells. Surprisingly, Cav-1ΔCSD expression showed a gain-of-function in Ca2+ entry in HMEC and HEK-293 cells. We observed a similar gain-of-function in Ca2+ entry when Cav-1ΔCSD was expressed in lung endothelial cells of Cav-1 knockout mice. Immunoprecipitation results revealed that WT-Cav-1 but not Cav-1ΔCSD interacted with IP3R3. Furthermore, we observed using confocal imaging the colocalization of IP3R3 with WT-Cav-1 but not with Cav-1ΔCSD on Ca2+ store release in endothelial cells. These findings suggest that CSD interacts with TRPC1 and IP3R3 and thereby regulates Ca2+ store release-induced Ca2+ entry in endothelial cells.
Keywords: transient receptor potential channel 1; inositol 1,4,5-trisphosphate receptor; caveolin-1 knockout mice
mediators such as thrombin and histamine activate endothelial responses by triggering Ca2+-sensitive signaling pathways (38). Ligation of endothelial cell surface protease-activated receptor-1 by thrombin caused a rapid and transient increase in cytosolic Ca2+ concentration ([Ca2+]i) due to release of stored Ca2+ and subsequent Ca2+ entry induced by store depletion (33, 34, 39). Plasma membrane cation channels in endothelial cells, known as store-operated cation channels (SOC), mediate the calcium entry (24). Mammalian homologues of transient receptor potential (TRP) gene family of channels are shown to be essential for store-operated Ca2+ entry (SOCE) in endothelial cells (24, 38). TRP genes encode a family of proteins with six transmembrane helices that are divided into seven subfamilies: TRPC (canonical or classical), TRPV (vaniloid related), TRPM (melastatin related), TRPA (ankyrin related), TRPML (mucolipin related), TRPP (polycystin related), and TRPN (no mechanoreceptor potential C) (22, 30). Members of the TRPC subfamily contain 700 to 1,000 amino acids, and seven isoforms (TRPC1 to 7) are expressed in mammalian cells. Mammalian TRPCs are grouped into four subfamilies. One group consists of TRPC1, TRPC4, and TRPC5. Their activation is dependent on Ca2+ store depletion and they have high Ca2+ selectivity as assessed by their sensitivity to La3+ (24). TRPC4 and TRPC5 are activated by G protein-coupled receptors and receptor tyrosine kinases coupled to phospholipase C. TRPC1 is closely related to TRPC4 and TRPC5; although it forms SOCs, it is a less selective Ca2+ channel. TRPC3, TRPC6, and TRPC7 form store-independent nonselective cation channels activated by diacylglycerol (6); however, a store-dependent activation mechanism has been described for human TRPC3 (6). TRPC2 is believed to be a pseudogene in humans, and its function is unclear (22).
We (25–27) and others (3) have shown that the TRPC1 isoform, prominently expressed in human vascular endothelial cells, is essential for SOCE. TRPC1 is localized within cholesterol-rich plasma membrane invaginations termed caveolae (17) that are coated with the 22-kDa protein caveolin-1 (Cav-1). Studies showed that Ca2+ influx occurred in caveolar microdomains in response to Ca2+ depletion of endoplasmic reticulum (ER) store in endothelial cells (10, 11, 13). Furthermore, studies have shown that the binding of Cav-1 with both the NH2 and COOH termini of TRPC1 was necessary for the caveolar distribution of TRPC1 (2). Patel et al. (29) have recently shown that the increased Cav-1 expression in smooth muscle cells from patients with idiopathic pulmonary artery hypertension was associated with enhanced Ca2+ entry in response to Ca2+ store depletion, indicating that increased Cav-1 expression contributes to Ca2+ influx in smooth muscle cells. Cav-1 knockout (Cav-1−/−) mouse studies showed that loss of Cav-1 expression in endothelial cells abrogated Ca2+ entry due to Ca2+ store depletion (23). Moreover, reexpression of wild-type Cav-1 (WT-Cav-1) in Cav-1−/− endothelial cells rescued Ca2+ entry (23), indicating the role of Cav-1 in regulating Ca2+ entry in endothelial cells.
The Cav-1 scaffold domain (CSD), located in Cav-1 residues between 82 and 101, binds multiple signaling molecules including endothelial nitric oxide (NO) synthase (eNOS), Src-like kinases, Ha-Ras, and heterotrimeric G proteins (15, 28, 36). Binding of these proteins to CSD in some cases negatively regulates their activity as in the case of eNOS (19). Bucci et al. (4) observed inhibition of acetylcholine-induced NO production and vasodilation in bovine aortic endothelial cells by a membrane-permeable CSD peptide. Acute vascular inflammation (4) and progression of lung fibrosis (40) in mice were also prevented by systemic administration of the CSD peptide. We have shown that CSD peptide markedly reduced ER Ca2+ store depletion as well as store depletion-activated Ca2+ entry in response to thrombin in endothelial cells (14). We also observed the interaction of CSD with TRPC1 COOH-terminal residues 781-789 (14). In the present study, we show that CSD interacts with inositol 1,4,5-trisphosphate receptor 3 (IP3R3) and TRPC1 to regulate the Ca2+ store release-induced Ca2+ entry in endothelial cells.
MATERIALS AND METHODS
Materials.
Human α-thrombin was obtained from Enzyme Research Laboratories (South Bend, IN). Endothelial growth medium (EGM-2) was obtained from Cambrex Bio Science (Walkersville, MD). Hanks' balanced salt solution (HBSS) and trypsin were from Invitrogen (Carlsbad, CA). Fetal bovine serum (FBS) was from Hyclone (Logan, UT). Fura-2 AM was purchased from Molecular Probes (Eugene, OR). Anti-Myc monoclonal antibody (mAb), anti-Cav-1 polyclonal, and anti-TRPC1 polyclonal were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-IP3R3 mAb was from BD Biosciences Pharmingen. Anti-Myc polyclonal antibody (pAb) was from Lab Vision (Fremont, CA), and anti-IP3R3 pAb was obtained from Novus Biologicals (Littleton, CO).
Reverse transcription-PCR.
Total RNA from human dermal microvascular endothelial cell line (HMEC) and human embryonic kidney (HEK-293) cells was isolated using TRIzol reagent. RT was performed using oligo(dT) primers and superscript RT (Invitrogen) following the manufacturer's instructions. Human IP3Rs and GAPDH were amplified using the following primer sets: IP3R1 (forward, 5′-GGTTTCATT TGCAAGTTAA TAAAG-3′ and reverse, 5′-AATGCTTTCATGGAACACTCGGTC-3′); IP3R2 (forward, 5′-CTTCATAGTCCTGGTGAAAGT-3′ and reverse, 5′-CTTCCTTTGTTCTGTCATCTG-3′), IP3R3 (forward, 5′-AGTGAGAAGCAGAAGAAGG-3′ and reverse, 5′-CATCCGGGGGAACCAGTC-3′), and GAPDH (forward, 5′-TATCGTGGAAGGACTCATGACC-3′ and reverse, 5′-TACATGGCAACTGTG AGGGG-3′). RT product (2 μl) was amplified in a 50-μl volume containing 100 pmol primers and 2.5 units Taq DNA polymerase. Reaction conditions were as follows: 95°C for 30 s, 55°C for 30 s, 72°C for 1 min for 35 cycles, and then 72°C for 7 min. PCR products were resolved using 1.2% agarose gel and identified by ethidium bromide staining.
Expression constructs.
Myc-tagged human TRPC1 (wild-type Myc-TRPC1) construct was prepared as described previously (26). Myc-tagged TRPC1 COOH-terminal residues 781-789-deleted (Myc-TRPC1-CΔ781-789) construct was prepared using a two-step PCR method. The wild-type Myc-TRPC1 (WT-Myc-TRPC1) cDNA was used as a template for PCR. In the first PCR amplification, the primers pCMV-Myc-F-longer (5′-TAGAGGATCCGGTACTAGAGGAACTGAAAAACCAG-3′) and hTRPC1-dCSD-R (5′-ATTTCTTGGATAGCCAAGTAAATCCCTTATTTC-3′) were used. In the second PCR amplification, the primers hTRPC1-dCSD-F (5′-GATCTTGGCTATCCAAGAAATTAAGCGGCCGC-3′) and pCMV-Myc-1640-R (5′-CATTAATGCAGCTGGCACGACAGGTTTCCCGACTGG-3′) were used to delete the CSD residues. The underlined primers indicate the overlapping region that flanked the deleted CSD residues. The first PCR product was analyzed by gel, purified, and used as overlapping DNA templates for the second PCR step using primers pCMV-Myc-F-longer and pCMV-Myc-1640-R. The final PCR product was digested with 5′-SalI and 3′-NotI and ligated into pCMV-Myc mammalian expression vector (Clontech) also digested at the same sites. Cav-1 wild-type (WT-Cav-1) cDNA in pcDNA3.1 expression was made as described previously (20). Two-step PCR method was also used to prepare CSD (residues 82–101)-deleted mutant (Cav-1ΔCSD). In the first PCR step, two separate reactions with primer pairs 5Cav1-KpnI (5′-CTAGGTACCCAGCATGTCTGGGGGCAAAT-3′[bold italics-KpnI]) and Cav1-d82–101-R (5′-GGGCAGACAGCAAAAAACTGTGTGTCCCTTC-3′ [bold DNA base pairs indicate the deleted CSD region]), or Cav1-d82-101-F (5′-AGGGACACACAGTTTTTTGCTGTCTGCCCTC-3′) and Sp6-R (5′-ATTTAGGTGACACTATAGAA-3′) were used to delete the CSD residues (amino acids 82-101). The resulting PCR products were analyzed by gel, purified, and used as overlapping DNA templates for the second PCR step using primers 5Cav1-KpnI and Sp6-R. The final PCR product was digested with 5′-KpnI and 3′-XbaI and ligated into pcDNA3 mammalian expression vector also digested at the same sites. The expression constructs were verified by DNA sequencing.
Cell culture.
HMEC cells were grown in MCDB-131 supplemented with 10% FBS, 2 mM l-glutamine, and 1 μg/ml hydrocortisone as described previously (26). HEK-293 cells were grown in DMEM supplemented with 10% FBS.
Mouse lung endothelial cells.
WT and Cav-1−/− mice were obtained from The Jackson Laboratories (9). Use of animals for this study was approved by the University of Illinois Animal Care and Use Committee. Lung endothelial cells from WT and Cav-1−/− mice were isolated as described previously using anti-platelet endothelial cell adhesion molecule (PECAM)-1 mAb (39). After isolation, the cells were placed in culture and were immortalized by infection with a retrovirus expressing the polyoma middle T antigen (23, 31). At 24 h after infection, cells were again purified with anti-PECAM-1 mAb (39). The affinity-purified cells were grown in MCDB-131 medium supplemented with 10% FBS, 10 ng/ml human EGF, and 1 μg/ml hydrocortisone. Immortalized endothelial cells were characterized by their cobblestone morphology and 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate-labeled acetylated LDL (Dil-Ac-LDL) uptake. We observed that >95% of the cell population were positive for Dil-Ac-LDL uptake. The immortalized lung endothelial cells passaged between 4 and 8 were used for experiments.
cDNA transfection.
Myc-TRPC1 and Cav-1 expression constructs were transfected into the HMEC, HEK-293 cells, or Cav-1−/− mouse lung endothelial cells (MLEC) using Superfect reagent (Qiagen) in serum-free medium according to the manufacturer's instructions. Cells grown to 80% confluence were transfected with either 2 μg/ml Myc-tagged-TRPC1 construct or 0.5 μg/ml Cav-1 construct. In some experiments, Myc-tagged-TRPC1 construct (2 μg/ml) and Cav-1 construct (0.5 μg/ml) were transfected together. DNA/Superfect reagent mixture was incubated with cells for 4 h in serum-free medium, and then complete growth medium was added. At 72 h after transfection, cells were used for experiments. We determined the transfection efficiency by transfecting green fluorescent protein (GFP) expression construct into HMEC and HEK-293 cells. We observed that 48 h after transfection, ∼60% of HMEC and 80% of HEK-293 cells were positive for GFP. In the case of Cav-1−/− MLEC, we observed that ∼50% cells were positive for GFP expression 72 h after transfection.
Cytosolic Ca2+ measurement.
Thrombin-induced increase in cytosolic Ca2+ concentration ([Ca2+]i) was measured using the Ca2+-sensitive fluorescent dye Fura-2 AM (14, 39). Cells were grown to confluence on gelatin-coated glass coverslips and were then washed two times with serum-free medium and incubated for 2 h at 37°C in culture medium containing 1% FBS. Cells were washed once and loaded with 3 μM Fura-2 AM for 30 min. After the loading, cells were washed with HBSS and then imaged using an Attoflor Ratio Vision digital fluorescence microscopy system (Atto Instruments, Rockville, MD) equipped with a Zeiss Axiovert S 100 inverted microscope (Zeiss, Thornwood, NY) and F-Fluar ×40, 1.3 numerical aperture oil immersion objective. Regions of interest in individual cells were marked and excited at 334 and 380 nm with emission at 520 nm at 5-s intervals. In each experiment, 40–50 cells were selected to measure change in [Ca2+]i as described previously (14, 39).
Immunoprecipitation.
Cells grown to ∼80% confluence were transfected (as described above) with Myc-tagged TRPC1 and Cav-1 expression constructs. At 48 h after transfection, cells were washed three times with phosphate-buffered saline and lysed in lysis buffer (50 mM Tris·HCl, pH 7.5, containing 150 mM NaCl, 1 mM EDTA, 0.25% sodium deoxycholate, 1.0% Nonidet P-40, 0.1% SDS, 1 mM Na3VO4, 1 mM NaF, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 2 μg/ml aprotinin, and 44 μg/ml PMSF) for 30 min at 4°C. Six-hundred micrograms (for Myc-TRPC-1, IP3R3) or 300 μg (Cav-1) protein from lysates was subjected to immunoprecipitation. Insoluble material was removed by centrifugation (13,000 g for 15 min) before overnight immunoprecipitation with 1 μg/ml antibody (as indicated) at 4°C. Protein A/G-agarose beads were added to each sample and incubated for 1 h at 4°C. Immunoprecipitates were gently washed three times with wash buffer (Tris-buffered saline containing 0.05% Triton X-100, 1 mM Na3VO4, 1 mM NaF, 2 μg/ml leupeptin, 2 μg/ml pepstatin A, 2 μg/ml aprotinin, and 44 μg/ml PMSF). Immunoprecipitated proteins were resolved on SDS-PAGE and immunoblotted with the appropriate antibodies.
Immunoblot.
Endothelial cell lysates or immunoprecipitates were resolved by SDS-PAGE on a 4–12% gradient or 10% separating gel under reducing conditions and transferred to Duralose membrane. Membranes were blocked with 5% dry milk in 10 mM Tris·HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20 for 1 h. Membranes were incubated with indicated primary antibody (diluted in blocking buffer) overnight. All primary antibodies were used at the dilution of 1:500 except for the anti-Cav-1 pAb, which were used at 1:2,000. Following three washes, membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit or mouse antibody. Protein bands were detected by the enhanced chemiluminescence method.
Confocal imaging.
Confocal imaging was performed to identify the localization of Cav-1 and IP3R3 in WT-Cav-1 or Cav-1ΔCSD expressed in Cav-1−/− MLEC. Cells grown on glass coverslips were incubated with 2% FBS containing medium for 2 h, and then were exposed to thrombin for 5 min. Cells were then washed three times, fixed with 4% paraformaldehyde in HBSS for 30 min at room temperature, and blocked with 5% goat serum in HBSS containing 0.1% Triton X-100 (blocking buffer) for 30 min at room temperature. The primary antibody labeling was performed overnight at 4°C with anti-Cav-1 mAb (1 μg/ml) and anti-IP3R3 pAb diluted (1:500) in blocking buffer. After being washed two times, cells were incubated with Alexa 488-labeled goat anti-mouse and Alexa-546-labeled goat anti-rabbit secondary Ab in blocking buffer for 60 min. Images were acquired with Zeiss LSM 510 confocal microscope (20). Colocalization analysis was performed using Zeiss software (42). The calculations are based on Pearson's correlation coefficient (Rr), which describes the degree of overlap between two different signals. The two coefficients Cred and Cgreen are proportional to the amount of fluorescence of the colocalizing object in each region of interest, relative to the total amount of fluorescence in that region. Their values range from zero and one, and a value of zero means that there is no colocalization while a value of one means that there is a complete colocalization. The background correction was performed manually using the threshold values.
Statistical analysis.
Statistical comparison among groups was calculated using the two-tailed Student's t-test. Experimental values are reported as means ± SE. P < 0.05 was considered statistically significant.
RESULTS
Expression of TRPC1 COOH-terminal CSD-binding domain deletion prevents thrombin-induced Ca2+ influx.
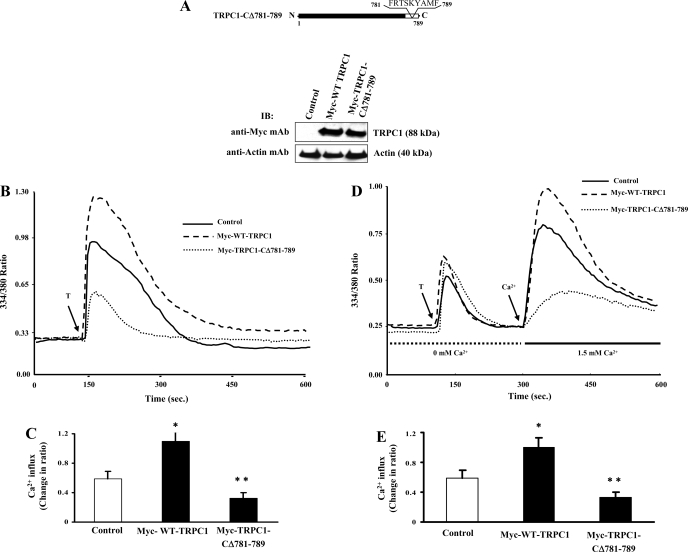
We ectopically expressed Myc-tagged TRPC1 (Myc-WT-TRPC1) and Myc-tagged TRPC1 COOH-terminal residues 781-789-deleted (Myc-TRPC1-CΔ781-789) constructs in HMEC and measured thrombin-induced changes in intracellular Ca2+. Expression levels of Myc-WT-TRPC1 and Myc-TRPC1-CΔ781-789 are shown in Fig. 1A. Thrombin-induced increase in intracellular Ca2+ ([Ca2+]i) in cells expressing Myc-WT-TRPC1 was significantly increased compared with control cells (Fig. 1, B and C). Expression of Myc-TRPC1-CΔ781-789 in HMEC, however, markedly reduced thrombin-induced increase in [Ca2+]i (Fig. 1, B and C). We next investigated whether the inhibitory effect was due to store Ca2+ release or Ca2+ influx. In the absence of external Ca2+, thrombin produced a similar initial transient increases in [Ca2+]i in cells expressing either Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 (Fig. 1D); however, Ca2+ store depletion-activated Ca2+ influx was markedly reduced in Myc-TRPC1-CΔ781-789-expressing cells compared with either Myc-WT-TRPC1-expressing cells or control cells (Fig. 1, D and E). Furthermore, we observed a similar inhibition of thapsigargin (TG)-induced Ca2+ influx in Myc-TRPC1-CΔ781-789-expressing HMEC compared with control or Myc-WT-TRPC1-expressing HMEC (data not shown).
Fig. 1.
Expression of transient receptor potential canonical channel 1 (TRPC1) COOH-terminal 781-789-truncated mutant prevents Ca2+ store depletion-activated Ca2+ influx in endothelial cells. A: human dermal microvascular endothelial cell line (HMEC) cells were transfected with the Myc-wild-type (WT)-TRPC1 or Myc-TRPC1-CΔ781-789 constructs. At 48 h after transfection, cells were lysed and immunoblotted (IB) with anti-Myc-mAb to determine TRPC1 protein expression. The membrane was stripped and reprobed using anti-β-actin mAb (bottom). Schematic of the TRPC1 COOH-terminal residues 781-789 deletion mutant construct is shown. B: HMEC grown on glass coverslips were transfected with either Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 expression constructs (see details in materials and methods). At 48 h after transfection, cells were loaded with Fura-2 AM and placed in Ca2+ and Mg2+ containing HBSS and stimulated with thrombin (50 nM) to measure increase in intracellular Ca2+. Arrow indicates time at which cells were stimulated with thrombin (T). Experiments were repeated at least 4 times. A representative profile is shown. C: peak change in fluorescence ratio (334/380) in control and Myc-WT-TRPC1 or Myc-WT-TRPC1 and Myc-TRPC1-CΔ781-789-expressing cells was compared. *P < 0.05 significantly different from control; **P < 0.001 significantly different from Myc-WT-TRPC1. D: thrombin-induced Ca2+ store depletion-mediated Ca2+ influx was measured. At 48 h after transfection, cells were loaded with Fura-2 AM and placed in Ca2+ and Mg2+-free HBSS and stimulated with thrombin (50 nM) to measure Ca2+ store depletion. After return of cytosolic Ca2+ concentration ([Ca2+]i) to baseline levels, 1.5 mM CaCl2 was applied to the extracellular medium to induce Ca2+ influx. Arrow indicates the time at which cells were stimulated with thrombin or Ca2+ was added. Experiments were repeated at least 4 times. A representative profile is shown. E: change in peak fluorescence ratio (334/380) for the Ca2+ influx in control and Myc-WT-TRPC1- or Myc-TRPC1-CΔ781-789-expressing cells was compared. *P < 0.05 significantly different from control; **P < 0.001 significantly different from Myc-WT-TRPC1.
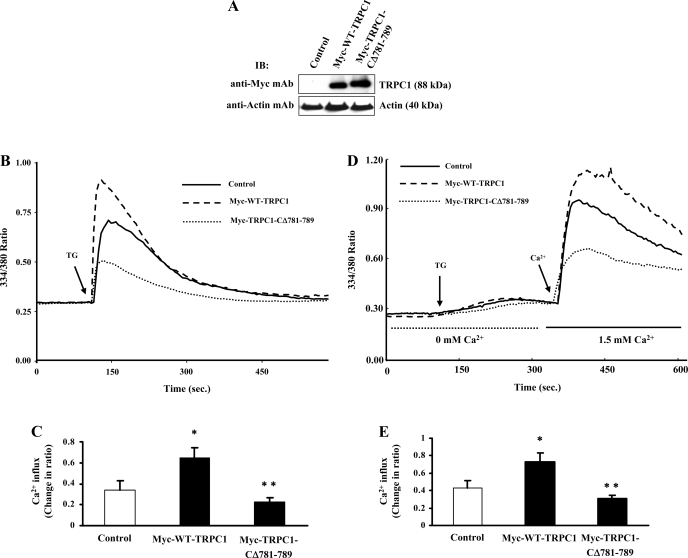
We also transfected the TRPC1 constructs in HEK-293 cells and measured TG-induced increase in [Ca2+]i. Expression levels of Myc-WT-TRPC1 and Myc-TRPC1-CΔ781-789 are shown in Fig. 2A. TG-induced increase in [Ca2+]i was significantly reduced in cells expressing Myc-TRPC1-CΔ781-789 compared with Myc-WT-TRPC1 (Fig. 2, B and C). Next we measured TG-induced Ca2+ influx in HEK-293 cells transfected with either Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 constructs. TG-induced Ca2+ influx was markedly suppressed in TRPC1-CΔ781-789-transfected cells compared with Myc-WT-TRPC1-transfected or control cells (Fig. 2, D and E). Thus, TRPC1 COOH-terminal residues 781-789 are required for Ca2+ store release-induced Ca2+ entry in endothelial cells.
Fig. 2.
Inhibition of Ca2+ store depletion-activated Ca2+ influx in TRPC1-CΔ781-789-expressing human embryonic kidney (HEK)-293 cells. A: HEK-293 cells were transfected with Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 expression constructs as described in materials and methods. At 48 h after transfection, cells were lysed and immunoblotted with anti-Myc mAb to determine TRPC1 protein expression. B: HEK-293 cells grown on glass coverslips were transfected with either Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 expression constructs. At 48 h after transfection, cells were loaded with Fura-2 AM and placed in Ca2+ and Mg2+ containing HBSS and used to measure thapsigargin (TG; 1 μM)-induced increase in [Ca2+]i. Experiments were repeated 4 times. C: change in peak fluorescence ratio (334/380) in control and Myc-WT-TRPC1- or Myc-TRPC1-CΔ781-789-expressing cells was compared. *P < 0.05, significantly different from control; **P < 0.001, significantly different from Myc-WT-TRPC1-expressing cells. D: TG-induced Ca2+ store depletion-mediated Ca2+ influx was measured as described in Fig. 1B. Experiments were repeated 4 times. E: change in peak fluorescence ratio (334/380) for the Ca2+ entry in control and Myc-WT-TRPC1- or Myc-TRPC1-CΔ781-789-expressing cells was compared. *P < 0.05, significantly different from control and **P < 0.001, significantly different from Myc-WT-TRPC1-expressing cells.
TRPC1 COOH-terminal residues 781-789 bind to both IP3R3 and Cav-1.
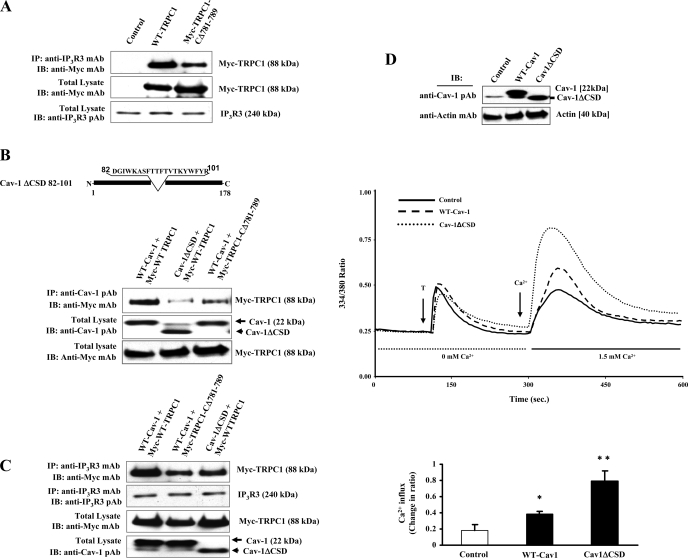
Previous studies have shown that IP3Rs binding to TRPC3 activates SOCE (41). In this study to address the mechanism of SOCE, we transfected TRPC1 expression constructs in HEK-293 cells and determined the association of TRPC1 with endogenous IP3R3 by coimmunoprecipitation (see details in materials and methods). HEK-293 cells were used since they express very low level of Cav-1 compared with endothelial cells. We transfected Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 expression constructs, and at 48 h after transfection, cells were lysed and immunoprecipitated using anti-IP3R3 mAb. Precipitated proteins were blotted with anti-Myc mAb to determine TRPC1 binding. We observed the binding of IP3R3 to Myc-WT-TRPC1 (Fig. 3A, top), whereas Myc-TRPC1-CΔ781-789 binding to IP3R3 was markedly reduced (Fig. 3A, top), showing that TRPC1 COOH-terminal residues 781-789 are essential for TRPC1 optimal binding to IP3R3.
Fig. 3.
A: caveolin-1 (Cav-1) scaffold domain (CSD)-binding motif deletion of TRPC1 COOH-terminal impairs TRPC1 binding with inositol 1,4,5-trisphosphate receptor 3 (IP3R3). HEK-293 cells were transfected with Myc-WT-TRPC1 or Myc-TRPC1-CΔ781-789 expression constructs (see details in materials and methods). At 48 h after transfection, cells were harvested and the proteins extracted were subjected to immunoprecipitation using anti-IP3R3 mAb. Immunoprecipitate was resolved in a SDS-PAGE and immunoblotted with anti-Myc mAb to determine TRPC1 binding (top). Total cell lysates were immunoblotted with anti-Myc mAb (middle) or anti-IP3R3 pAb (bottom). Results are representative of 3 independent experiments. B: Cav-1ΔCSD expression reduces TRPC1 binding to Cav-1. HEK-293 cells were cotransfected with different combinations of TRPC1 and Cav-1 expression constructs. At 48 h after transfection, cells were lysed and immunoprecipitated with anti-Cav-1 pAb and blotted with anti-Myc mAb (top). Total cell lysates were immunoblotted with anti-Cav-1 pAb (middle) and anti-Myc-mAb (bottom). The experiment was repeated 4 times, and results from representative experiments are shown. C: Cav-1ΔCSD expression reduces TRPC1 binding to IP3R3. HEK-293 cells were cotransfected with different combinations of TRPC1 and Cav-1 expression constructs. At 48 h after transfection, cells were lysed and immunoprecipitated with anti-IP3R3 mAb and blotted with anti-Myc mAb (top). Total cell lysates were immunoblotted with anti-IP3R3 pAb (second from top), anti-Myc-mAb (third from top), or anti-Cav-1 pAb (bottom). The experiment was repeated 3 times, and results from representative experiments are shown. D and E: Cav-1ΔCSD mutant expression augments agonist-induced Ca2+ store release-induced Ca2+ influx in HMEC and HEK-293 cells. D: HMEC were transfected with the WT-Cav-1 or Cav-1ΔCSD expression constructs. At 48 h after transfection, cells were lysed and immunoblotted with anti-Cav-1-pAb to determine Cav-1 protein expression. The membrane was then stripped and reprobed using anti-β-actin mAb (bottom). Next, HMEC transfected with either WT-Cav-1 or Cav-1ΔCSD expression constructs were used for thrombin-induced intracellular Ca2+ influx measurements (see details in materials and methods). At 48 h after transfection, cells were loaded with Fura-2 AM and placed in Ca2+ and Mg2+-free HBSS and stimulated with thrombin (50 nM) to measure Ca2+ store depletion. After return of [Ca2+]i to baseline levels, 1.5 mM CaCl2 was applied to the extracellular medium to induce Ca2+ influx. Arrow indicates the time at which cells were stimulated with thrombin or Ca2+ was added. Experiments were repeated at least 4 times. A representative profile is shown. Change in peak fluorescence ratio (334/380) for the Ca2+ influx in control and WT-Cav-1- or Cav-1ΔCSD-expressing cells was compared. *P < 0.05, significantly different from control; **P < 0.001, significant difference when compared with control cells. E: HEK-293 cells were transfected with WT-Cav-1 or Cav-1ΔCSD expression constructs as described in materials and methods. At 48 h after transfection, cells were lysed and immunoblotted with anti-Cav-1 pAb to determine Cav-1 protein expression. HEK-293 cells grown on glass coverslips were transfected with either WT-Cav-1 or Cav-1ΔCSD expression constructs. At 48 h after transfection, cells were loaded with Fura-2 AM and TG-induced Ca2+ store depletion-mediated Ca2+ influx was measured as described in Fig. 1B. Experiments were repeated at least 3 times. Change in peak fluorescence ratio (334/380) for Ca2+ influx in control and WT-Cav-1- or control and Cav-1ΔCSD expressing cells was compared. *P < 0.05, significantly different from control; **P < 0.001, significantly different from control cells. F: Cav-1ΔCSD mutant expression markedly increases Ca2+ store release-induced Ca2+ influx in Cav-1−/− mouse lung endothelial cells (MLEC). Thrombin-induced Ca2+ store release and Ca2+ influx in lung endothelial cells isolated from WT and Cav-1 null mice was measured (top left). Cav-1−/− MLEC were transfected with Cav-1 expression constructs (WT-Cav-1 or Cav-1ΔCSD). At 48 h after transfection, the expression levels of Cav-1 were determined by immunoblot (top right). Cav-1−/− MLEC grown on coverslips were transfected with WT-Cav-1 or Cav-1ΔCSD, and at 48 h after transfection, cells were loaded with Fura-2 AM and thrombin-induced (third from top) or TG-induced (second from top) Ca2+ store release and Ca2+ store release-mediated Ca2+ influx were measured. Experiments were repeated at least 4 times. Change in peak fluorescence ratio (334/380) for thrombin-induced Ca2+ influx in control and WT-Cav-1 or Cav-1ΔCSD expressing cells was compared (bottom). *P < 0.05, significantly different from control; **P < 0.001, significantly different from WT-Cav-1 expressed cells.
Because Cav-1 mediated trafficking of TRPC1 to plasma membrane may be required for SOCE (17), we determined the interaction between Cav-1 and TRPC1 in HEK-293 cells by immunoprecipitation using anti-Cav-1 pAb. We observed binding of Cav-1 with TRPC1 in cells expressing both WT-Cav-1 and Myc-WT-TRPC1 (Fig. 3B, top). Expression of WT-Cav-1 and Myc-TRPC1-CΔ781-789 showed a significant reduction (>50%) in the binding of TRPC1 with Cav-1 (Fig. 3B, top) compared with the cells expressing Myc-WT-TRPC1 and WT-Cav-1. Also, coexpression of Cav-1ΔCSD with Myc-WT-TRPC1 resulted in >60% reduction in Cav-1 binding to Myc-WT-TRPC1 (Fig. 3B, top). In another set of experiments, we co-expressed different combinations of Cav-1 and TRPC1 constructs in HEK-293 cells and determined TRPC1 binding to endogenous IP3R3. In WT-Cav-1 expressing cells, we observed the effective binding of expressed Myc-WT-TRPC1 with IP3R3, whereas the expressed Myc-WT-TRPC1 binding to IP3R3 was significantly reduced but not abolished in Cav-1ΔCSD expressing cells (Fig. 3C, top). We made a similar observation when WT-Cav-1 and Myc-TRPC1-CΔ781-789 expressed together (Fig. 3C, top). These results suggest that interaction between IP3R3 and TRPC1 occurs in the absence of CSD.
CSD deletion mutant expression augments Ca2+ store release-induced Ca2+ influx.
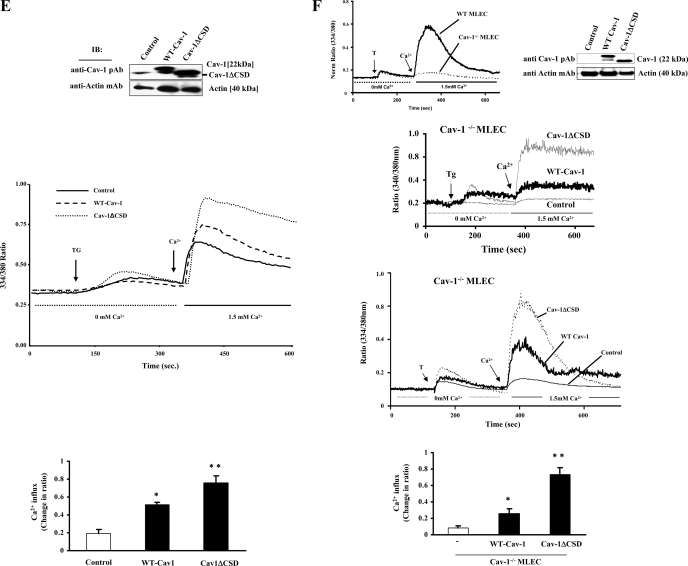
In our recent study we have shown a specific interaction between CSD and TRPC1 COOH-terminal residues (781-789) in endothelial cells (13). Furthermore, we showed that a cell-permeable CSD peptide or TRPC1 COOH-terminal peptide inhibited Ca2+ entry. To address the functional relevance of CSD, we measured Ca2+ store release-induced Ca2+ influx in WT-Cav-1 and Cav-1ΔCSD-expressing HMEC and HEK-293 cells. In the absence of external Ca2+, thrombin or TG produced a similar initial transient increases in [Ca2+]i in cells expressing either WT-Cav-1 or Cav-1ΔCSD (Fig. 3, D and E). Interestingly, with readdition of external Ca2+ after Ca2+ store release, there was an increase in the Ca2+ influx in cells expressed with WT-Cav-1, whereas cells expressed with Cav-1ΔCSD showed more than a twofold increase in store Ca2+ release-induced Ca2+ influx compared with control (Fig. 3, D and E), indicating a gain-of-function in the absence of CSD in both HMEC and HEK-293 cells.
CSD deletion mutant expression in Cav-1−/− MLEC augments Ca2+ store release-induced Ca2+ influx.
To further confirm the gain-of-function of Ca2+ entry in Cav-1ΔCSD expressing cells, we expressed WT-Cav-1 or Cav-1ΔCSD in Cav-1−/− MLEC and measured thrombin-induced Ca2+ entry. At 72 h after transfection, we determined the expression of WT-Cav-1 and Cav-1ΔCSD by immunoblotting the cell lysate. The results are shown in Fig. 3F (top). In vector alone or nontransfected Cav-1−/− MLEC, thrombin-induced Ca2+ store depletion as well as Ca2+ store depletion-induced Ca2+ entry were minimal when compared with WT-MLEC (Fig. 3F, top). In WT-Cav-1-transfected Cav-1−/− MLEC, thrombin-induced Ca2+ entry was increased more than threefold compared with nontransfected control cells (Fig. 3F, third from top). Interestingly, in Cav-1ΔCSD-transfected Cav-1−/− MLEC, thrombin-induced Ca2+ entry was more than twofold compared with WT-Cav-1-transfected cells (Fig. 3F, third from top). We also observed that TG-induced Ca2+ entry was increased more than twofold in Cav-1ΔCSD-expressing Cav-1−/− MLEC compared WT-Cav-1-expressing cells (Fig. 3E, second from top). These results are consistent with the results described above (Fig. 3, E and F) supporting the notion that Cav-1ΔCSD expression induces a gain-of-function in agonist-induced Ca2+ entry.
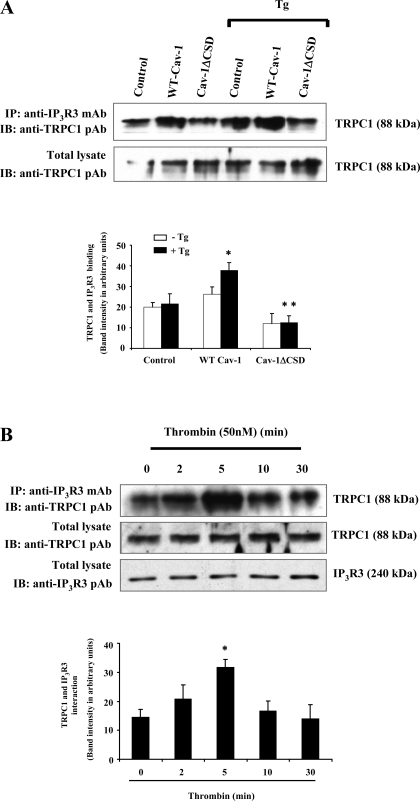
Cav-1ΔCSD expression inhibits Ca2+ store release-induced association of IP3R3 with TRPC1.
Next, we investigated whether store Ca2+ release influences the binding of TRPC1 with IP3R3 in HEK-293 cells. As shown in Fig. 4A, we observed a direct interaction of TRPC1 with IP3R3 by immunoprecipitation. Stimulation of WT-Cav-1-expressing cells with TG increased the association of TRPC1 with IP3R3 (Fig. 4A, top), whereas the increased association between IP3R3 and TRPC1 was not observed in cells expressing Cav-1ΔCSD (Fig. 4A), indicating that Ca2+ store release may be required for CSD-mediated IP3R3-Cav-1-TRPC1 complex formation. In addition, we determined the effect of thrombin-induced store Ca2+ release on the binding of endogenous TRPC1 with IP3R3 in HMEC. We observed that thrombin stimulation increased the association of TRPC1 with IP3R3 in a time-dependent manner (Fig. 4B, top), and the association returned to basal level 10 min after thrombin stimulation (Fig. 4B, top). These findings collectively suggest that store Ca2+ release signal mediates increased IP3R3 binding to TRPC1, which requires Cav-1 expression in endothelial cells.
Fig. 4.
A: TG-induced Ca2+ store depletion enhances the interaction between IP3R3 and TRPC1. HEK-293 cells transfected with WT-Cav-1 or Cav-1ΔCSD were treated with or without 1 μM TG for 5 min at 37°C. Untransfected cells with or without TG treatment served as controls. After TG treatment, cells were washed and extracted proteins were immunoprecipitated using anti-IP3R3 mAb. The precipitated proteins were immunoblotted with anti-TRPC1 pAb (top). The total cell lysates were also immunoblotted with anti-TRPC1 pAb (bottom). A representative blot is shown. Immunoprecipitated protein bands were quantified by densitometry and are expressed in arbitrary units. Results are means ± SE from 4 experiments. *P < 0.05, significantly different from the control; **P < 0.001, significantly different compared with WT-Cav-1-expressed cells. B: thrombin-induced Ca2+ store depletion enhances the association of TRPC1 and IP3R3 in endothelial cells. HMEC stimulated with thrombin (50 nM) for different time intervals were lysed and immunoprecipitated with anti-IP3R3 mAb and immunoblotted with anti-TRPC1 pAb (top). Total cell lysates were immunoblotted with anti-TRPC1 pAb (middle) or anti-IP3R3 pAb (bottom). A representative blot is shown from 4 different experiments. Immunoprecipitated protein bands were quantified as in A. Results are means ± SE from 4 experiments. *P < 0.05, significantly different from the control (not stimulated with thrombin).
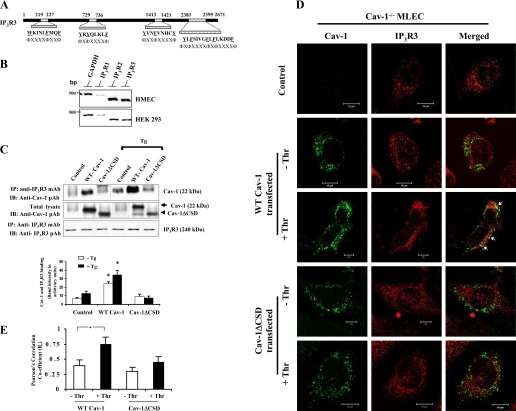
CSD directly interacts with IP3R3.
CSD is known to interact with aromatic amino acids containing motifs ΦΧΦΧΧΧΧΦ and ΦΧΧΧΧΦΧΧΦ, where Φ is an aromatic residue (tryptophan, phenylalanine, or tyrosine) (5). We have shown that TRPC1 COOH terminus contains similar motif where CSD binds (14). Therefore, we addressed whether CSD directly binds to IP3R3. Interestingly, analysis of human IP3R3 sequence revealed the presence of putative CSD-binding motifs in both the NH2 and COOH termini (Fig. 5A). Next we determined the expression pattern of IP3R isoforms in HMEC and HEK-293 cells by RT-PCR (21). Both HMEC and HEK-293 cells expressed IP3R3 (Fig. 5B). We transfected HEK-293 cells with Cav-1 expression constructs and determined Cav-1 binding to IP3R3 by immunoprecipitation using anti-IP3R3 mAb and by immunoblotting with anti-Cav-1 pAb. We observed IP3R3 binding with Cav-1 in WT-Cav-1-expressing cells (Fig. 5C, top). In contrast to WT-Cav-1 expression, anti-IP3R3 mAb failed to precipitate Cav-1 from Cav-1ΔCSD-expressing cells (Fig. 5C, top), indicating that CSD is required for Cav-1 binding to IP3R3. We also determined the effect of Ca2+ store release on the interaction between Cav-1 and IP3R3. TG stimulation enhanced the binding of Cav-1 with IP3R3 when compared with cells with no treatment (Fig. 5C, top), whereas cells expressing Cav-1ΔCSD failed to interact with IP3R3 (Fig. 5C, top).
Fig. 5.
IP3R3 interacts with Cav-1 through CSD. A: CSD-binding motifs in human IP3R3. Both the NH2 and COOH termini contain CSD-binding motifs. B: expression profile of the IP3R types in HMEC and HEK-293 cells. RT-PCR was performed to determine the expression of IP3R types (see details in materials and methods). C: HEK-293 cells transfected with WT-Cav-1 or Cav-1ΔCSD expression constructs (see details in materials and methods) were treated with TG (1 μM) for 5 min at 37°C. After TG treatment, cells were lysed and the proteins were immunoprecipitated with anti-IP3R3 mAb. Cells that were not treated with TG served as controls. The precipitated proteins were immunoblotted with anti-Cav-1 pAb (top) and anti-IP3R3 pAb (bottom). Total cell lysates were immunoblotted with anti-Cav-1 pAb (middle). The TG-induced Ca2+ store depletion enhanced the binding between IP3R3 and Cav-1; however, this binding was not observed in Cav-1ΔCSD-expressing cells. The experiment was repeated 4 times, and the immunoprecipitated protein bands were quantified by densitometry. The plot shown represents means ± SE; *P < 0.05, significantly different from control. D: confocal images show the colocalization of Cav-1 with IP3R3 in control, WT-Cav-1, or Cav-1ΔCSD-expressed Cav-1−/− MLEC treated without or with 50 nM thrombin (Thr) for 5 min. Following thrombin treatment, cells were fixed and stained with anti-Cav-1 mAb (green) and anti-IP3R3 pAb (red) to visualize the localization of Cav-1 and IP3R3. This experiment was repeated 3 times, and the results are from a representative experiment. Bar, 10 μm. Arrows represent the colocalization (yellow) of Cav-1 with IP3R3. E: thrombin-induced colocalization of WT-Cav-1 or Cav-1ΔCSD with IP3R3 was quantified (see details in materials and methods). An average of five examined areas (regions of interest) were collected and plotted. *P < 0.05, significantly different from control (not stimulated with thrombin).
The above results indicate that the Ca2+ store release activates the association of Cav-1 and IP3R3, which requires CSD. We also used confocal imaging to address whether thrombin-induced Ca2+ store release mediates the interaction of Cav-1 and IP3R3 in Cav-1−/−MLEC expressed with WT-Cav-1 or Cav-1ΔCSD. In WT-Cav-1-expressed cells, we observed a minimal colocalization of Cav-1 and IP3R3 (Fig. 5, D and E). Thrombin stimulation significantly increased the colocalization of Cav-1 with IP3R3 in WT-Cav-1-expressing cells (Fig. 5, D and E). In contrast to WT-Cav-1-expressed cells, thrombin-induced colocalization of Cav-1 (i.e., Cav-1ΔCSD) with IP3R3 was not observed in Cav-1ΔCSD mutant-expressed cells (Fig. 5, D and E). These imaging data along with the immunoprecipitation results demonstrate that the agonist-induced Ca2+ store release may signal the interaction of Cav-1 with IP3R3 via CSD in endothelial cells.
DISCUSSION
In vascular endothelial cells, the release of internal Ca2+ stores activates extracellular Ca2+ entry that signals responses as varied as increased endothelial permeability to inflammatory gene expression (38). We have shown that expression of TRPC1 is essential for SOCE in human vascular endothelial cells (25–27). Studies also showed that the caveolar microdomain of endothelial cells organizes and compartmentalizes the SOCE channel complex (2, 23) and that this process involves interaction between the CSD and the TRPC1 COOH-terminal sequence 781-789 (14). However, regulation of SOCE by Cav-1 remains incompletely understood. In the present study, we showed that the expression of CSD-binding domain deletion mutant of TRPC1 (TRPC1-CΔ781-789) produced a dominant suppressive effect on thrombin- and thapsigargin-induced Ca2+ entry and also suppressed the interaction between TRPC1 and IP3R3. All of the TRPC homologues contain six transmembrane domains, and the cation-permeable pore region is located between transmembrane helices 5 and 6 (16). TRPC channels assemble into homo- and heterotetrameric structures to form the putative SOCE channel complex (1, 8). We observed that thrombin-induced Ca2+ entry but not the Ca2+ store release was markedly suppressed by expression of Myc-TRPC1-CΔ781-789 in endothelial cells. A similar observation was made following TG-induced Ca2+ entry in Myc-TRPC1-CΔ781-789 -expressing HEK-293 cells, demonstrating that TRPC1 COOH terminus CSD-binding sequence contributes to the assembly of functional SOCE channel complex.
TRPC homologues have been shown to interact with IP3Rs to activate SOCE in HEK-293 cells (37, 41) and in human submandibular gland cells (17). In the absence of Cav-1, glutathione S-transferase fusion protein pull-down assays and expression studies using HEK-293 cells showed effective binding of IP3R3 with the COOH terminus of TRPC3, TRPC6, and TRPC7 (37), but not with TRPC1, TRPC2, TRPC4, and TRPC5 (37). The IP3R3-binding domain on the COOH terminus of TRPC channels also interacts with calmodulin in a Ca2+-dependent manner to regulate SOCE (36, 37, 41). Lockwich et al. (17) have shown that TRPC1 coimmunoprecipitated with IP3R3 and Cav-1 in human submandibular gland cells, but they did not define the molecular mechanisms involved in the association of TRPC1 with Cav-1 and IP3R3. Moreover, the binding between IP3R3 and TRPC channels has been investigated mainly using cell types such as HEK-293 cells in which Cav-1 expression level is negligible compared with endothelial cells. Furthermore, genetic deletion of Cav-1 expression in endothelial cells leads to the loss of Ca2+ store depletion-activated Ca2+ entry (23). In addition, TRPC channels have been shown to associate with IP3Rs in Cav-1-expressing cells (2, 17) but not in Cav-1-null endothelial cells (23), suggesting that Cav-1 is required for the assembly of the SOCE channel complex in endothelial cells. However, the role of CSD in the mechanism of assembly and function of the SOCE complex is unclear. To understand the role of CSD in the mechanism of SOCE channel assembly and function, we determined the direct binding of TRPC1 with Cav-1. We showed that the interaction between Cav-1 and TRPC1 and deletion of either CSD or CSD-binding motif on the TRPC1 COOH terminus markedly reduced the Cav-1-TRPC1 interaction. It is possible, however, that the TRPC1 NH2 terminus can also bind with the sites on Cav-1 other than CSD (2, 32). We cannot rule out this possibility in the present study. In previous studies, we showed that cell-permeable synthetic CSD peptide or TRPC1 COOH-terminal peptide inhibited both the Ca2+ store depletion and Ca2+ entry in endothelial cells (14). An explanation for this finding based on the present observations is that the inhibitory effect could be the result of competition between the synthetic peptides and the interacting sites on endogenous Cav-1, TRPC1, and IP3R3.
Since Cav-1 is expressed abundantly in endothelial cells and it is crucial for vascular function, we addressed whether CSD deletion influences the agonist-induced Ca2+ entry by expressing Cav-1ΔCSD in HMEC and HEK-293 cells. Surprisingly, we observed a gain-of-function in Ca2+ store release-induced Ca2+ influx in Cav-1ΔCSD-expressing cells. We made a similar observation by expressing Cav-1ΔCSD in Cav-1-null MLEC. These findings collectively suggest that CSD is required for optimal agonist-induced Ca2+ entry in endothelial cells.
Previous studies have implicated that Ca2+ store release enhances the association between the ER membrane-localized IP3Rs and the plasma membrane-localized TRPC channels (11, 12). Furthermore, Isshiki and Anderson (11) have proposed a model in that agonist-induced Ca2+ store release brings both the ER and caveolae in close proximity to enable these organelle-associated molecules to interact. In support of this model, we observed an increased association between IP3R3 and TRPC1 on agonist-induced Ca2+ store release. Expression of Cav-1ΔCSD but not the WT-Cav-1 markedly reduced the Ca2+ store release-induced association between IP3R3 and TRPC1. To understand the basis of Cav-1-mediated association between IP3R3 and TRPC1, we measured the direct interaction between Cav-1 and IP3R3. The deduced IP3R3 sequence revealed the presence of CSD-binding motifs in both the NH2 and COOH termini. As predicted, we observed the interaction between WT-Cav-1 and IP3R3, whereas Cav-1ΔCSD failed to interact with IP3R3, suggesting that Cav-1 interacts directly with IP3R3 via CSD. Furthermore, we observed by confocal imaging the colocalization between IP3R3 and WT-Cav-1 but not between Cav-1ΔCSD and IP3R3 on Ca2+ store release. These results further demonstrate that CSD plays a crucial role in mediating the interaction between ER-associated IP3R3 and caveolae-associated TRPC1 to regulate agonist-induced Ca2+ influx.
We observed an enhanced Ca2+ store release-induced Ca2+ influx in Cav-1ΔCSD-expressing cells. This finding could be explained on the basis of CSD interaction with IP3R3. Both the NH2 and COOH termini of TRPC1 interact with Cav-1 (2, 32). CSD specifically bind to the TRPC1 COOH-terminal residues (781-789). We have shown that in the absence of CSD, the interaction between TRPC1 and Cav-1 is reduced but not abolished. However, CSD is required for Cav-1-IP3R3 interaction. In unstimulated cells, TRPC1 COOH terminus (CSD-binding motif) is associated with Cav-1 in caveolae, but not with IP3R3. Agonist-induced Ca2+ store release signal brings the ER and the caveolae in close proximity to mediate the association between TRPC1 COOH terminus and IP3R3 to cause Ca2+ entry (11, 37, 41). The Ca2+ entry could be reversed by the increase in Ca2+ concentration due to Ca2+ influx and also by CSD binding to IP3R3.
CSD is known to interact with many signal transduction proteins in endothelial cells (28). In many cases, CSD binding negatively regulates the function of the interacting protein. The best studied example is eNOS (17). So far, it is unclear how multiple signal transduction proteins interact with CSD. However, studies have raised the possibility that oligomerization of Cav-1, which is known to occur at the plasma membrane, may allow multiple signal transduction proteins to bind to Cav-1 (7, 18). Therefore, it is possible that additional signaling molecules such as kinases and phosphatases may transiently bind to the oligomerized Cav-1 scaffold domain to associate with channel complex to regulate the Ca2+ influx (28). Thus, the findings reported in this study suggest that Cav-1 scaffold domain interacts with TRPC1 and IP3R3 and thereby regulates Ca2+ store release-induced Ca2+ entry in endothelial cells.
GRANTS
This work was supported by National Institutes of Health Grants GM-058531 and P01-HL-077806.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Alfonso S, Benito O, Alicia S, Angélica Z, Patricia G, Diana K, Luis V. Regulation of the cellular localization and function of human transient receptor potential channel 1 by other members of the TRPC family. Cell Calcium 43: 375–387, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Brazer SW, Singh BB, Liu X, Swaim W, Ambudkar IS. Caveolin-1 contributes to assembly of store-operated Ca2+ influx channels by regulating plasma membrane localization of TRPC1. J Biol Chem 278: 27208–27215, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brough GH, Wu S, Cioffi D, Moore TM, Li M, Dean N, Stevens T. Contribution of endogenously expressed Trp1 to a Ca2+-selective, store-operated Ca2+ entry pathway. FASEB J 15: 1727–1738, 2001. [PubMed] [Google Scholar]
- 4.Bucci M, Gratton JP, Rudic RD, Acevedo L, Roviezzo F, Cirino G, Sessa WC. In vivo delivery of the caveolin-1 scaffold domain inhibits nitric oxide synthesis and reduces inflammation. Nat Med 6: 1362–1367, 2000. [DOI] [PubMed] [Google Scholar]
- 5.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffold domain. Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem 272: 6525–6533, 1997. [DOI] [PubMed] [Google Scholar]
- 6.Dietrich A, Mederos Y, Schnitzler M, Emmel J, Kalwa H, Hofmann T, Gudermann T. N-linked protein glycosylation is a major determinant for basal TRPC3 and TRPC6 channel activity. J Biol Chem 278: 47842–47852, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez I, Ying Y, Albanesi J, Anderson RGW. Mechanism of caveolin filament assembly. Proc Natl Acad Sci USA 99: 11193–11198, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann T, Schaefer M, Schultz G, Gudermann T. Subunit composition of mammalian transient receptor potential channels in living cells. Proc Natl Acad Sci USA 99: 7461–7466, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu G, Ye RD, Dinauer MC, Malik AB, Minshall RD. Neutrophil caveolin-1 expression contributes to mechanism of lung inflammation and injury. Am J Physiol Lung Cell Mol Physiol 294: L178–L186, 2008. [DOI] [PubMed] [Google Scholar]
- 10.Isshiki M, Ando J, Korenaga R, Kogo H, Fujimoto T, Fujita T, Kamiya A. Endothelial Ca2+ waves preferentially originate at specific loci in caveolin-rich cell edges. Proc Natl Acad Sci USA 95: 5009–5014, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Isshiki M, Anderson RGW. Function of caveolae in Ca2+ entry and Ca2+-dependent signal transduction. Traffic 4: 717–723, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Isshiki M, Anderson RGW. Calcium signal transduction from caveolae. Cell Calcium 26: 201–8, 1999. [DOI] [PubMed] [Google Scholar]
- 13.Isshiki M, Ying YS, Fujita T, Anderson RGW. A molecular sensor detects signal transduction from caveolae in living cells. J Biol Chem 277: 43389–43398, 2002. [DOI] [PubMed] [Google Scholar]
- 14.Kwiatek AM, Minshall RD, Cool DR, Skidgel RA, Malik AB, Tiruppathi C. Caveolin-1 regulates store-operated Ca2+ influx by binding of it scaffold domain to transient receptor potential channel-1 in endothelial cells. Mol Pharmacol 70: 1174–83, 2006. [DOI] [PubMed] [Google Scholar]
- 15.Levin AM, Murase K, Jackson PJ, Flinspach ML, Poulos TL, Weiss GA. Double barrel shotgun scanning of the caveolin-1 scaffolding domain. ACS Chem Biol 2: 493–500, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu X, Singh BB, Ambudkar IS. TRPC1 is required for functional store-operated Ca2+ channels. Role of acidic amino acid residues in the S5–S6 region. J Biol Chem 278: 11337–11343, 2003. [DOI] [PubMed] [Google Scholar]
- 17.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffold lipid raft domains. J Biol Chem 275: 11934–11942, 2000. [DOI] [PubMed] [Google Scholar]
- 18.Machleidt T, Li WP, Liu P, Anderson RGW. Multiple domains in caveolin-1 control its intracellular traffic. J Cell Biol 148: 17–28, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem 272: 15583–15586, 1997. [DOI] [PubMed] [Google Scholar]
- 20.Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via G(i)-coupled Src kinase signaling pathway. J Cell Biol 150: 1057–1070, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miyakawa T, Maeda A, Yamazawa T, Hirose K, Kurosaki T, Iino M. Encoding of Ca2+ signals by differential expression of IP3 receptor subtypes. EMBO J 18: 1303–1308, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montell C, Birnbaumer L, Flockerzi V. The TRP channels, a remarkably functional family. Cell 108: 595–598, 2002. [DOI] [PubMed] [Google Scholar]
- 23.Murata T, Lin MI, Stan RV, Bauer PM, Yu J, Sessa WC. Genetic evidence supporting caveolae microdomain regulation of calcium entry in endothelial cells. J Biol Chem 282: 16631–16643, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Nilius B, Droogmans G. Ion channels and their functional role in vascular endothelium. Physiol Rev 81: 1415–1459, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Paria BC, Bair AM, Xue J, Yu Y, Malik AB, Tiruppathi C. Ca2+ influx induced by protease-activated receptor-1 activates a feed-forward mechanism of TRPC1 expression via nuclear factor-kappa B activation in endothelial cells. J Biol Chem 281: 20715–20727, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Paria BC, Malik AB, Kwiatek AM, Rahman A, May MJ, Ghosh S, Tiruppathi C. Tumor necrosis factor-alpha induces nuclear factor-kappa B-dependent TRPC1 expression in endothelial cells. J Biol Chem 278: 37195–37203, 2003. [DOI] [PubMed] [Google Scholar]
- 27.Paria BC, Vogel SM, Ahmmed GU, Alamgir S, Shroff J, Malik AB, Tiruppathi C. Tumor necrosis factor-alpha-induced TRPC1 expression amplifies store-operated Ca2+ influx and endothelial permeability. Am J Physiol Lung Cell Mol Physiol 287: L1303–L1313, 2004. [DOI] [PubMed] [Google Scholar]
- 28.Patel HH, Murray F, Insel PA. Caveolae as organizers of pharmacologically relevant signal transduction molecules. Annu Rev Pharmacol Toxicol 48: 359–391, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel HH, Zhang S, Murray F, Suda RY, Head BP, Yokoyama U, Swaney JS, Niesman IR, Schermuly RT, Pullamseti SS, Thistlethwaite PA, Miyanohara A, Farquhar MG, Yuan JX, Insel PA. Increased smooth muscle cell expression of caveolin-1 and caveolae contribute to the pathophysiology of idiopathic pulmonary arterial hypertension. FASEB J 21: 2970–2979, 2007. [DOI] [PubMed] [Google Scholar]
- 30.Pedersen SF, Owsianik G, Nilius B. BTRP channels: an overview. Cell Calcium 38: 233–252, 2005. [DOI] [PubMed] [Google Scholar]
- 31.Primo L, Roca C, Ferrandi C, Lanfrancone L, Bussolino F. Human endothelial cells expressing polyoma middle T induce tumors. Oncogene 19: 3632–3641, 2000. [DOI] [PubMed] [Google Scholar]
- 32.Remillard CV, Yuan JX. Transient receptor potential channels and caveolin-1: good friends in tight spaces. Mol Pharmacol 70: 1151–1154, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Sandoval R, Malik AB, Minshall RD, Kouklis P, Ellis CA, Tiruppathi C. Ca2+ signalling and PKC α activate increased endothelial permeability by disassembly of VE-cadherin junctions. J Physiol (Lond) 533: 433–445, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sandoval R, Malik AB, Naqvi T, Mehta D, Tiruppathi C. Requirement for Ca2+ signaling in the mechanism of thrombin-induced increase in endothelial permeability. Am J Physiol Lung Cell Mol Physiol 280: L239–L247, 2001. [DOI] [PubMed] [Google Scholar]
- 35.Schlegel A, Lisanti MP. Caveolae and their coat proteins, the caveolins: from electron microscopic novelty to biological launching pad. J Cell Physiol 186: 329–337, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Singh BB, Liu X, Tang J, Zhu MX, Ambudkar IS. Calmodulin regulates Ca2+-dependent feedback inhibition of store-operated Ca2+ influx by interaction with a site in the C terminus of TrpC1. Mol Cell 9: 739–750, 2002. [DOI] [PubMed] [Google Scholar]
- 37.Tang J, Lin Y, Zhang Z, Tikunova S, Birnbaumer L, Zhu MX. Identification of common binding sites for calmodulin and inositol 1,4,5-trisphosphate receptors on the carboxy termini of Trp channels. J Biol Chem 276: 21303–21310, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tiruppathi C, Ahmmed GU, Vogel SM, Malik AB. Ca2+ signaling, TRP channels, and endothelial permeability. Microcirculation 39: 173–185, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Tiruppathi C, Freichel M, Vogel SM, Paria BC, Mehta D, Flockerzi V, Malik AB. Impairment of store-operated Ca2+ entry in TRPC4(−/−) mice interferes with increase in lung microvascular permeability. Circ Res 91: 70–76, 2002. [DOI] [PubMed] [Google Scholar]
- 40.Tourkina E, Richard M, Gooz P, Bonner M, Pannu J, Harley R, Bernatchez PN, Sessa WC, Silver RM, Hoffman S. Antifibrotic properties of caveolin-1 scaffold domain in vitro and in vivo. Am J Physiol Lung Cell Mol Physiol 294: L843–L861, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Tang J, Tikunova S, Johnson JD, Chen Z, Qin N, Dietrich A, Stefani E, Birnbaumer L, Zhu MX. Activation of Trp3 by inositol 1,4,5-trisphosphate receptors through displacement of inhibitory calmodulin from a common binding domain. Proc Natl Acad Sci USA 98: 3168–3173, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zinchunk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem 40: 101–111, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]