Abstract
Selectins facilitate metastasis and tumor cell arrest in the microvasculature by mediating binding of selectin-expressing host cells to ligands on tumor cells. We recently identified CD44 variant isoforms as functional P-, but not E-/L-, selectin ligands on colon carcinoma cells. Furthermore, a ∼180-kDa sialofucosylated glycoprotein(s) mediated selectin binding in CD44-knockdown cells. Using immunoaffinity chromatography and tandem mass spectrometry, we identify podocalyxin-like protein (PCLP) as an alternative selectin ligand. Blot rolling and cell-free flow-based adhesion assays disclose that PCLP on LS174T colon carcinoma cells possesses E-/L-, but not P-, selectin binding activity. The selectin-binding determinants on LS174T PCLP are non-MECA-79-reactive sialofucosylated structures displayed on O-linked glycans, distinct from the MECA-79-reactive O-glycans on PCLP expressed by high endothelial venules, which is an L-selectin ligand. PCLP on CD44-knockdown LS174T cells exhibits higher HECA-452 immunoreactivity than PCLP on wild-type cells, suggesting that PCLP functions as an alternative acceptor for selectin-binding glycans. The enhanced expression of HECA-452 reactivity on PCLP from CD44-knockdown cells correlates with the increased avidity of PCLP for E- but not L-selectin. The novel finding that PCLP is an E-/L-selectin ligand on carcinoma cells offers a unifying perspective on the apparent enhanced metastatic potential associated with tumor cell PCLP overexpression and the role of selectins in metastasis.
Keywords: adhesion, metastasis, colon carcinoma cells, fluid shear
selectins play a pivotal role in the hematogenous dissemination of tumor cells in the vasculature by mediating specific interactions between selectin-expressing host cells and selectin ligands on tumor cells. The selectins (E-, L-, and P-selectin) constitute a family of structurally related transmembrane glycoproteins that mainly recognize specific glycoconjugates on apposing cell surfaces (28, 34, 49, 54). A variety of tumor cells, including colon carcinoma, express sialofucosylated molecules that can be recognized by selectins (16, 17, 38, 52). Enhanced expression of sialylated, fucosylated oligosaccharides such as sialyl Lewis x (sLex) or sialyl Lewis a (sLea) on the tumor cell surface correlates with poor prognosis because of tumor progression and metastatic spread (27, 42, 43). Along these lines, the metastatic potential of colon carcinoma cell lines is diminished by preincubating carcinoma cells with anti-sLea antibodies (48).
Endothelial E-selectin has been shown to support metastatic spread in vivo (6, 39). Similarly, a direct role for P- and L-selectin in the metastatic process has been disclosed by the pronounced inhibition of metastasis in P- and/or L-selectin-deficient mice (P-sel-null, L-sel-null, P-/L-sel-double null) compared with wild-type controls in a colon carcinoma cell model (4, 5). It is believed that E-selectin expressed on the surface of activated endothelial cells mediates tethering of malignant cells, thereby facilitating their extravasation from the vasculature and the seeding of metastatic foci (9). Platelets, by adhering to tumor cells via a P-selectin-dependent mechanism (3, 40, 41), provide a protective shield, which masks tumor cells from immunological and physiological stresses in the bloodstream and facilitate their lodging to the pulmonary vasculature (45). However, an additional role for P-selectin expressed on activated endothelial cells, which could potentially bind carcinoma cells and mediate their extravasation from the bloodstream, cannot be ruled out (4, 32). On the other hand, the contribution of L-selectin to cancer metastasis is less developed. It is thought that tumor cells can form multicellular complexes with platelets (via P-selectin) and leukocytes [via an L-selectin-dependent mechanism (24, 26)], which can then arrest in the microvasculature of distant organs and eventually extravasate and establish metastatic colonies. Alternatively, it has been reported that leukocyte L-selectin can enhance metastasis by interacting with endothelial L-selectin ligands induced adjacent to established intravascular murine colon carcinoma cell emboli which lack L-selectin ligands on their surfaces (35).
Podocalyxin-like protein (PCLP) is a cell surface adhesion molecule that is a member of the CD34 subfamily of sialomucins. Its expression by podocytes is thought to maintain the integrity of the filtration slits in the foot processes in the kidney glomerulus via its anti-adhesive properties due to a high net negative charge (30). PCLP is also expressed in high endothelial venules (HEVs) and by a subset of developing but not mature hematopoetic stem cells (31). More recently, it has been demonstrated that PCLP is expressed by hepatic carcinomas (21), blasts of acute leukemia (29), and stem cells of testicular tumors (10) and is highly overexpressed in a subset of invasive breast carcinomas (50) and malignant astrocytic tumors (20), suggesting that this molecule may be useful as a molecular marker specific for metastatic progression. However, the function of PCLP in tumor cells and a mechanistic interpretation for its role in metastasis remains obscure.
Using immunoaffinity chromatography in conjunction with tandem mass spectrometry, we identified PCLP as a potential selectin ligand on LS174T colon carcinoma cells. Through blot rolling assays and cell-free flow-based adhesion experiments, we validated that PCLP expressed by LS174T colon carcinoma cells possesses E-/L-, but not P-, selectin-binding activity. Moreover, the selectin-binding determinants on PCLP from LS174T cells are non-MECA-79-reactive sialofucosylated structures displayed on O-, but not N-, linked glycans, distinct from the MECA-79-reactive O-glycans on PCLP expressed by HEVs. PCLP expressed by CD44-knockdown LS174T cells exhibits higher sialofucosylation (HECA-452 immunoreactivity) than PCLP on wild-type cells, suggesting that PCLP functions as an alternative acceptor for selectin-binding glycans. The enhanced expression of HECA-452-reactive epitopes on PCLP from CD44-knockdown cells correlates with the increased avidity of PCLP for E- but not L-selectin. Our finding that sialofucosylated O-linked PCLP participates in colon carcinoma cell interactions with host cells via an E- and L-selectin-dependent mechanism provides potential insights into the overexpression of this molecule on metastatic tumor cells.
MATERIALS AND METHODS
Adhesion molecules, antibodies, and reagents.
The chimeric form of E- and P-selectin-IgG Fc (E-selectin; P-selectin) consisting of the lectin, epidermal growth factor, and consensus repeat domains for human E-selectin and P-selectin, respectively, linked to each arm of human IgG1, were generous gift of Wyeth External Research (Cambridge, MA) (51). L-selectin-IgG Fc (L-selectin) was purchased from R&D Systems (Minneapolis, MN). Anti-PCLP monoclonal antibody (mAb) 3D3 was from Santa Cruz Biotechonology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC)-conjugated anti-PCLP antibody 53D11 and isotype control were from MBL International (Woburn, MA). Alkaline phosphatase (AP)- and horseradish peroxidase (HRP)-conjugated antimouse IgG and AP-conjugated anti-rat IgM were from Southern Biotech (Birmingham, Alabama). All other unlabeled and phycoerythrin- or FITC-conjugated antibodies were from BD Biosciences Pharmingen (San Jose, CA) unless otherwise specified. All other reagents were from Sigma (St. Louis, MO) unless otherwise stated.
Cell culture.
The human colorectal carcinoma cell line LS174T was obtained from the American Type Culture Collection (Manassas, VA) and cultured in the recommended medium. Before cell lysis, LS174T carcinoma cells were detached from culture flasks using Enzyme Free Cell Dissociation Media (15 min at 37°C; Chemicon, Phillipsburg, NJ) (44). For flow-based adhesion assays, LS174T cells were harvested by mild trypsinization (0.25% trypsin/EDTA for 5 min at 37°C) and subsequently incubated (107 cells/ml) at 37°C for 2 h to allow regeneration of surface glycoproteins (7, 38, 41). Chinese hamster ovary (CHO) cells, stably transfected with cDNA encoding full-length E- or with the phosphatidylinositol glycan-linked extracellular domain of P-selectin, were kindly donated by Affymax (Palo Alto, CA), and processed as previously described (16, 44). Cell lines were routinely checked and confirmed to be negative for mycoplasma infection (24).
Purification and mass spectrometry analysis of 180-kDa, HECA-452-reactive LS174T protein.
The putative selectin ligand corresponding to the HECA-452-reactive 180-kDa protein was isolated by performing affinity chromatography on the whole cell lysate of CD44-knockdown LS174T cells using KappaLock-agarose supports (Invitrogen, Carlsbad, CA) cross-linked with bis(sulfosuccinimidyl)suberate (Pierce Biotechnology, Rockford, IL) to HECA-452 mAb (53). Eluted proteins were then separated by SDS-PAGE and stained in gel with ProQ Emerald 300 glycoprotein stain (Invitrogen), which only binds to carbohydrate groups at glycosylation sites, thereby leaving the polypeptide core intact (53). The stained band at 180 kDa was then excised, and trypsin-digested gel fragments were submitted for analysis by nanoflow HPLC interfaced to electrospray ionization tandem mass spectrometry (HPLC-MS/MS) using a ThermoFinnigan LTQ mass spectrometer (53). The MS data were searched against all taxonomies in the NCBI nonredundant protein database with a 95% significance threshold (P < 0.05) using Mascot (Matrix Science) and with a P < 0.01 confidence using the BioWorks 3.3 software featuring the SEQUEST algorithm (ThermoFinnigan).
SDS-PAGE and Western blotting.
Whole cell lysate or immunopurified PCLP was diluted with reducing sample buffer and separated using 4–20% SDS-PAGE gels (Bio-Rad Laboratories, Hercules, CA) (44). Resolved proteins were transferred to Sequi-blot or Immun-blot polyvinylidene difluoride (PVDF) and blocked with StartingBlock (Pierce Biotechnology) for 15 min. Immunoblots were stained with HECA-452, MECA-79, or anti-PCLP (3D3) mAbs and rinsed with TBS-0.1% Tween 20. Subsequently, blots were incubated with appropriate AP- or HRP-conjugated secondary antibodies. Western Blue AP substrate (Promega, Madison, WI) and SuperSignal West Pico Chemiluminescent Substrate (Pierce Biotechnology) were used to develop the AP- and HRP-conjugated antibody-stained immunoblots, respectively.
Colon carcinoma cell lysis and immunoprecipitation of PCLP.
Whole cell lysate was prepared by membrane disruption using 2% Nonidet P-40 followed by differential centrifugation (17, 44, 53). PCLP was immunoprecipitated from colon carcinoma cell lysate with an anti-PCLP mAb, 3D3, using recombinant Protein G agarose beads (Invitrogen) (17, 44, 53).
Blot rolling assay.
Blots of immunopurified PCLP from wild-type or CD44-knockdown LS174T whole cell lysate were stained with anti-PCLP (3D3) or HECA-452 mAbs and rendered translucent by immersion in 90% Dulbecco's modified medium-PBS (D-PBS)-10% glycerol (15). The blots were placed under a parallel-plate flow chamber, and human peripheral blood lymphocytes or CHO transfectants, resuspended at 5 × 106 cells/ml in 90% D-PBS-10% glycerol, were perfused at the shear stress of 0.5 dyn/cm2 (17, 44, 53). Molecular weight markers were used as guides to aid placement of the flow chamber over stained bands of interest. The number of interacting cells per lane was averaged over ×10 fields of view (0.55 mm2 each) for 5 min within each stained region. Nonspecific adhesion was assessed by perfusing 5 mM EDTA in the flow medium.
Preparation of PCLP-coated microspheres.
Immunoprecipitated PCLP from wild-type or CD44-knockdown LS174T whole cell lysate was diluted to desired concentrations with binding buffer (0.2 M carbonate/bicarbonate buffer, pH 9.2) and incubated with 10 μm polystyrene microspheres (2.5 × 107 microspheres/ml; Polysciences, Warrington, PA) overnight at 4°C with constant rotation (17, 44, 53). Microspheres were washed two times with D-PBS and subsequently blocked with D-PBS-1% BSA for 30 min at room temperature. Microspheres were resuspended (2 × 106 microspheres/ml) in D-PBS-0.1% BSA for use in flow cytometric and flow chamber assays. Site densities of PCLP-coated microspheres were determined by flow cytometry (17, 44, 53).
Enzymatic treatments.
To remove terminal sialic acid residues, wild-type LS174T PCLP-coated microspheres were incubated with 0.1 U/ml Vibrio cholerae sialidase (Roche Molecular Biochemicals) for 90 min at 37°C (17, 44). In select experiments, PCLP-coated microsphere suspensions (5 × 106 microspheres/ml) were incubated for 2 h at 37°C with 120 μg/ml of osialoglycoprotein endopeptidase (OSGE; Accurate Chemical & Scientific, Westbury, NY) to specifically cleave glycoproteins with O-glycosylation on serine and threonine residues (18). Site densities of PCLP adsorbed on microspheres following enzymatic treatments were determined by flow cytometry before use in flow-based adhesion assays.
Inhibitor treatments.
Before metabolic inhibitor studies, LS174T cell suspensions (107 cells/ml) were pretreated with 0.1 U/ml V. cholerae sialidase for 60 min at 37°C to remove terminal sialic acid residues and ensure de novo synthesis of newly generated HECA-452-reactive carbohydrate structures (17, 44). Complete removal of sialylated structures was confirmed via flow cytometry using the mAb HECA-452 that recognizes sialic acid-bearing epitopes. Subsequently, LS174T cells were cultured for 48 h at 37°C in medium containing either 2 mM benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (benzyl-GalNAc) to inhibit O-linked glycosylation (17, 44) or 1 mM deoxymannojirimycin (DMJ) to disrupt N-linked processing (17, 44); D-PBS diluting was used for control untreated cells.
Flow cytometry.
PCLP and HECA-452 site densities on microspheres were quantified by single-color immunofluorescence and flow cytometry (FACSCalibur; BD Biosciences) using FITC-conjugated anti-PCLP (53D11) or HECA-452 mAbs. Similarly, PCLP expression by colon carcinoma cells was monitored by using the FITC-conjugated anti-PCLP antibody 53D11. Background levels were determined by incubating cell or microsphere suspensions with properly matched FITC-conjugated isotype control antibodies (17, 44).
Flow-based adhesion assays.
To simulate the physiological shear environment of the vasculature, PCLP-coated microspheres suspended in D-PBS-0.1% BSA were perfused over immobilized IgG- or E-, L-, or P-selectin-coated dishes at prescribed wall shear stresses using a parallel-plate flow chamber (250 μm channel depth, 5.0 mm channel width) (7, 41). The extent of adhesion was quantified by perfusing cells/microspheres at 1 × 106/ml and enumerating the total number of tethering events in a single ×10 field of view during a 2-min period. Average rolling velocities were computed as the distance traveled by the centroid of the translating cell/microsphere divided by the time interval at the given wall shear stress (7, 8, 41). A minimum of 30 cells was tracked for each condition. In select experiments, PCLP-coated microspheres were perfused over substrates with 5 mM EDTA in the flow medium.
Statistical analysis.
Data are expressed as means ± SE for at least 3 independent experiments. Statistical significance of differences between means was determined by ANOVA. If means were shown to be significantly different (P < 0.05), multiple comparisons were performed by the Tukey test.
RESULTS
Sialofucosylated PCLP expressed by LS174T colon carcinoma cells is an E-/L-, but not P-, selectin ligand.
We recently reported that glycosylated variant isoforms of the widely distributed cell surface CD44 protein (CD44v) are major functional P-selectin ligands and serve as auxiliary E-/L-selectin ligands on colon carcinoma cells (44). Moreover, blot rolling assays revealed the presence of alternative sialofucosylated glycoprotein(s) with an apparent molecular mass of ∼170–180 kDa, which can mediate selectin binding in CD44-knockdown LS174T colon carcinoma cells (44). To identify and characterize the putative selectin ligand(s), we performed immunoaffinity chromatography to purify the ∼180-kDa sialofucosylated glycoprotein from whole cell lysates of CD44-knockdown LS174T cells using KappaLock-agarose supports cross-linked with a HECA-452 mAb (Fig. 1). Eluted proteins were separated by SDS-PAGE and stained in gel using ProQ Emerald 300 glycoprotein stain, which fluorescently labels periodate-oxidized glycans while leaving the polypeptide backbone intact. Alternatively, the proteins were transferred to PVDF membranes and immunoblotted using HECA-452 to confirm the retention of the putative glycoprotein targets throughout purification. This purification process retained the ∼180-kDa HECA-452-reactive band in both stained gels and Western blots (Fig. 2A). This band was then excised and submitted for HPLC-MS/MS analysis of trypsin-digested fragments. Bioinformatics analysis of the MS data revealed peptide fragment matches for PCLP (podocalyxin-like isoform 1 precursor, accession no. NP_001018121; podocalyxin-like isoform 2 precursor, accession no. NP_005388).
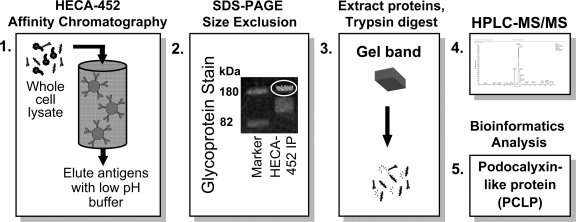
Fig. 1.
Schematic diagram of the process of HECA-452 affinity chromatography, in-gel glycoprotein staining of eluted samples, HPLC-tandem mass spectrometry, and bioinformatics analysis of trypsin-digested specimens used to identify podocalyxin-like protein as a 180-kDa sialofucosylated glycoprotein selectin ligand band in whole cell lysates of CD44-knockdown LS174T cells. 1: HECA-452-reactive proteins were isolated from CD44-knockdown LS174T cell lysate by immunoaffinity chromatography wherein lysate was incubated with HECA-452 monoclonal antibody (mAb) that was immobilized and cross-linked via bis(sulfosuccinimidyl)suberate (BS3) on KappaLock-agarose supports. HECA-452-reactive molecules were eluted using a low-pH elution buffer subsequent to extensive washing. 2: Proteins were then resolved by SDS-PAGE and stained in gel using ProQ Emerald 300 glycoprotein stain. The fluorescently labeled band at 180 kDa was then excised from the gel. 3 and 4: Proteins were extracted and trypsin digested (3) and then subjected to HPLC interfaced to electrospray ionization tandem mass spectrometry (HPLC-MS/MS) (4). 5: Bioinformatics analysis of the MS data identified podocalyxin-like protein in the sample.
Fig. 2.
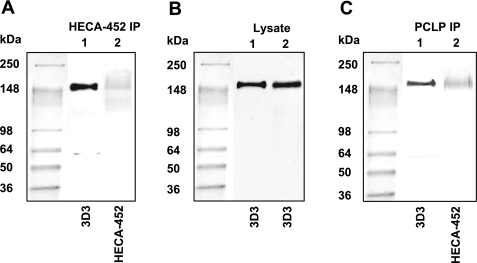
A: Western blots of HECA-452 immunoaffinity product from CD44-knockdown LS174T colon carcinoma cell lysate stained with the anti-podocalyxin-like protein (PCLP) 3D3 (lane 1) or HECA-452 (lane 2) mAbs. Western blots reveal that PCLP is a 180-kDa molecule recovered after HECA-452 immunoaffinity chromatography of CD44-knockdown LS174T whole cell lysate. B: Western blots of whole cell lysate from wild-type (lane 1) and CD44-knockdown (lane 2) LS174T colon carcinoma cells stained with the anti-PCLP mAb 3D3. C: Western blots of immunoprecipitated PCLP from CD44-knockdown LS174T whole cell lysate stained with the anti-PCLP (3D3) (lane 1) or HECA-452 (lane 2) mAbs demonstrating that PCLP is a 180-kDa sialofucosylated glycoprotein.
We next performed a series of experiments to validate that PCLP is indeed a selectin ligand in LS174T colon carcinoma cells. As a first step, Western blots of HECA-452 immunoaffinity product from CD44-knockdown LS174T cell lysate stained with either an anti-PCLP mAb, 3D3, or HECA-452 mAb revealed that PCLP is an ∼180-kDa protein recovered by affinity chromatography (Fig. 2A). Immunoblot analysis using an anti-PCLP mAb, 3D3, also disclosed the presence of PCLP with an apparent molecular mass of ∼180 kDa in whole cell lysates from both wild-type and CD44-knockdown LS174T colon carcinoma cells (Fig. 2B).
Using immunopurified PCLP from wild-type (data not shown) and CD44-knockdown LS174T cell lysates (Fig. 2C) blotted with HECA-452 mAb, we demonstrated that PCLP expressed by these cells is sialofucosylated. Using a blot rolling assay, we next evaluated the ability of immunopurified PCLP to support selectin-dependent adhesion in shear flow. To this end, E- and P-selectin-expressing CHO cells as well as L-selectin-expressing human peripheral blood lymphocytes were perfused over the SDS-PAGE resolved immunopurified PCLP protein band from CD44-knockdown LS174T cells under physiologically relevant levels of shear stress. This assay revealed that E- and L-, but not P-, selectin-expressing cells tethered appreciably over the ∼180-kDa region (Table 1), which corresponds to sialofucosylated PCLP, thereby suggesting that PCLP possesses E-/L-, but not P-, selectin ligand activity.
Table 1.
E-, P-, and L-selectin-dependent adhesion to SDS-PAGE resolved immunopurified PCLP protein band under fluid flow
| Blot Condition | CHO-E (E-selectin) | CHO-P (P-selectin) | Lymphocyte (L-selectin) |
|---|---|---|---|
| Immunopurified PCLP | +++++ | – | *** |
CHO-E, Chinese hamster ovary (CHO) cells stably transfected with cDNA encoding full-length E-selectin; CHO-P, CHO cells stably transfected with cDNA encoding full-length P-selectin. CHO E- or P-selectin-expressing CHO cells or L-selectin-expressing peripheral blood lymphocytes were perfused at 0.5 dyn/cm2 over SDS-PAGE immunoblots of immunopurified podocalyxin-like protein (PCLP) from whole cell lysate of CD44-knockdown LS174T colon carcinoma cells. The number of interacting cells/mm2 was quantified over five fields of view surrounding the 180-kDa region that marked the center of the PCLP band. Each “+” represents 50 stationary cells/mm2, whereas an asterisk (*) represents 50 transiently tethered/rolling cells/mm2. –, indicates no adhesion.
PCLP serves as an alternative glycosylation acceptor on colon carcinoma cells.
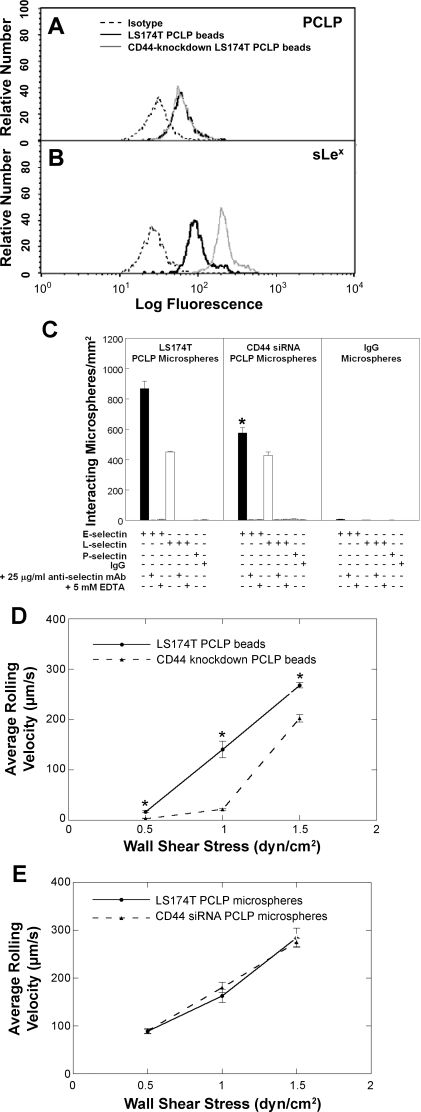
We next used a cell-free flow-based adhesion assay (17, 44, 53) to validate that PCLP is an E-/L-, but not P-, selectin ligand and to compare the adhesion of microbeads coated with PCLP immunoprecipitated from wild-type vs. CD44-knockdown LS174T colon carcinoma cells to purified selectin substrates under flow. This technique allows quantitative comparisons of PCLP-mediated adhesion to selectin substrates at prescribed PCLP and selectin site densities in shear flow (17, 44, 53). By coating microbeads with equivalent levels of PCLP from each cell type (Fig. 3A), we found that PCLP on CD44-knockdown LS174T cells exhibit higher HECA-452 immunoreactivity than PCLP on wild-type LS174T cells (Fig. 3B), thereby suggesting that PCLP serves as an alternative glycosylation acceptor on colon carcinoma cells. Because sialofucosylated structures are pivotal to selectin binding function (8, 49, 54), we hypothesized that this difference in biochemical reactivity would directly impact the biophysics of selectin-PCLP interactions. We tested this hypothesis by perfusing PCLP-coated microbeads from each cell type over selectin substrates at a wall shear stress of 1 dyn/cm2. As expected from blot rolling assays, microbeads coated with PCLP from either cell type were capable of tethering and rolling over E- and L-, but not P-, selectin, albeit with varying efficiencies (Fig. 3C). The specificity of PCLP-selectin binding in these assays was disclosed through the use of nonspecific IgG-bearing microbeads and by preincubating the selectin-coated dishes with the respective function-blocking anti-selectin mAb before the perfusion of PCLP-coated microbeads. As an additional control, EDTA (5 mM) was added to the perfusion medium in select experiments. In all three control experiments, no microbeads tethered to the selectin substrates in shear flow. It is noteworthy that the extent of interaction of CD44-knockdown LS174T PCLP-decorated microbeads with E-selectin was significantly less than that of microbeads coated with PCLP from wild-type LS174T cells (Fig. 3C). This difference is ascribed to the lower average rolling velocities of the former microbeads [and thus lower number of microspheres entering the field of observation (41)] relative to wild-type LS174T PCLP-coated microspheres over a wide range of wall shear stresses (Fig. 3D). On the other hand, no difference was noted in either the extent of microbead binding (Fig. 3C) or the average rolling velocities (Fig. 3E) of microbeads coated with PCLP from either cell type over L-selectin.
Fig. 3.
A–B: representative flow cytometric histograms of PCLP (A) and sLex (B) levels on microbeads coated with PCLP immunopurified from wild-type (black line) and CD44-knockdown (gray line) LS174T cells. Microspheres were stained with the fluorescein isothiocyanate (FITC)-conjugated anti-PCLP mAb 53D11 (A), FITC-conjugated HECA-452 mAb (B), or FITC-conjugated isotype control antibody (dashed lines). C: extent of adhesion of microbeads (106/ml) coated with PCLP immunopurified from wild-type or CD44-knockdown LS174T cells over 10 μg/ml E-(filled bars), L-(open bars), or P-selectin at a wall shear stress level of 1 dyn/cm2 for 2 min. Data represent means ± SE. *P < 0.05 with respect to microbeads coated with PCLP immunopurified from wild-type LS174T cells. D and E: average rolling velocities of microspheres (106/ml) coated with PCLP immunopurified from wild-type or CD44-knockdown LS174T cells over 10 μg/ml E-selectin (D) or L-selectin (E) over a range of shear stresses. Data represent means ± SE. *P < 0.05 with respect to microbeads coated with PCLP immunopurified from wild-type LS174T cells.
The selectin-binding determinants of PCLP are displayed on sialofucosylated O-linked glycans.
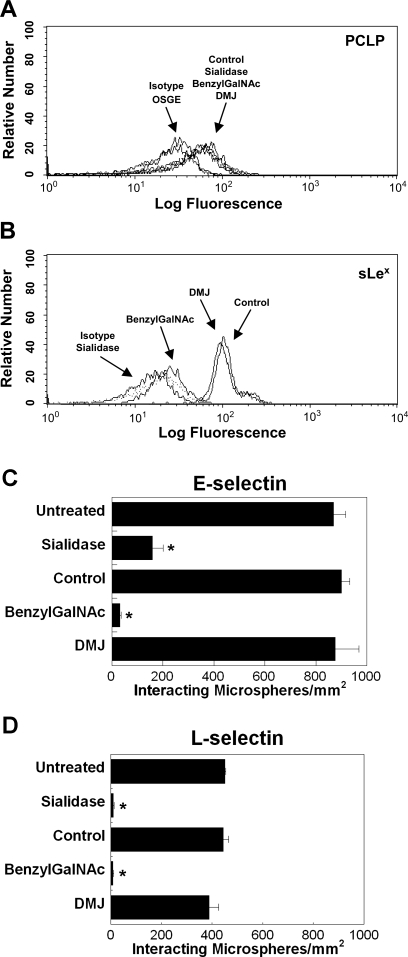
To characterize the structural linkage-bearing selectin-binding determinants on PCLP, we treated PCLP-coated microbeads with highly selective enzymes that cleave specific carbohydrate moieties from the PCLP glycoprotein. Treatment of wild-type LS174T PCLP-coated microbeads with sialidase eliminated HECA-452 reactivity (Fig. 4 B) without affecting the PCLP site density on the bead surface (Fig. 4A). This intervention nearly abolished microbead adhesion to L-selectin and reduced binding to E-selectin by ∼80% (Fig. 4C). It is noteworthy that sialidase treatment converted the remaining interactions between PCLP-bearing microbeads and E-selectin from stable rolling to swift tethers.
Fig. 4.
A and B: site densities of PCLP (A) and sialyl Lewis x (sLex; B) on wild-type LS174T PCLP-bearing polystyrene microbeads, pretreated with enzymes or metabolic inhibitors, determined by flow cytometry. Wild-type LS174T PCLP-absorbed microspheres were treated with Vibrio cholerae sialidase (0.1 U/ml) or osialoglycoprotein endopeptidase (OSGE, 120 μg/ml). In other experiments, microbeads were coated with PCLP immunoprecipitated from wild-type LS174T whole cell lysate pretreated with deoxymannojirimycin (DMJ) or benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (benzyl-GalNAc). Site densities of adsorbed PCLP were quantified using the FITC-conjugated anti-PCLP mAb 53D11. Site densities of sLex epitopes on untreated (control) and treated wild-type LS174T PCLP-coated microbeads were determined by flow cytometry using a HECA-452 mAb. C and D: extent of adhesion of wild-type LS174T PCLP-coated polystyrene microbeads pretreated with sialidase or microbeads coated with PCLP immunopurified from the whole cell lysate of wild-type LS174T cells cultured in benzyl-GalNAc or DMJ-containing media to 10 μg/ml E-selectin (C) or L-selectin (D) at a wall shear stress level of 1 dyn/cm2 for 2 min. Data represent means ± SE. *P < 0.05 by ANOVA.
To assess the potential contribution of N-linked glycans to PCLP-selectin interactions, microbeads were generated using PCLP immunopurified from LS174T cells cultured for 48 h in medium containing DMJ (1 mM) to disrupt N-linked processing (17, 44). This treatment did not alter the PCLP site density on the bead surface (Fig. 4A), the HECA-452 reactivity (Fig. 4B), or the extent of bead tethering to E- and L-selectin substrates under flow (Fig. 4, C and D).
To determine the potential role of O-glycans in PCLP-selectin binding, microbeads were prepared using PCLP immunoprecipitated from colon carcinoma cells cultured for 48 h in medium containing 2 mM benzyl-GalNAc to inhibit O-linked glycosylation (17, 44). Site densities of adsorbed PCLP were verified by flow cytometry to be similar to those of untreated controls (Fig. 4A). However, benzyl-GalNAc treatment eliminated HECA-452-reactive epitopes from LS174T PCLP (Fig. 4B), suggesting that the majority of sLex displayed on PCLP are O-linked glycans. Moreover, PCLP-coated microspheres from benzyl-GalNAc-treated LS174T cells bound minimally to E- and L-selectin in shear flow (Fig. 4D), suggesting that the selectin-binding determinants on PCLP from LS174T colon carcinoma cells are sialofucosylated structures displayed on O-linked glycans. The presence of a high level of O-linked glycans on the LS174T PCLP is further substantiated by the fact that enzymatic treatment of PCLP-bearing microbeads with OSGE completely eliminated PCLP detection on the bead surface by flow cytometry (Fig. 4A).
Prior work suggested that PCLP on HEVs is a MECA-79-reactive (sulfated glycan) L-selectin ligand (47). To determine whether PCLP on colon carcinoma cells is MECA-79-reactive, immunopurified PCLP from wild-type LS174T cells was resolved by SDS-PAGE and stained with MECA-79 via Western blotting. We found that, in contrast to PCLP expressed by HEVs as reported previously (47), PCLP expressed by LS174T colon carcinoma cells is not MECA-79-reactive (data not shown).
DISCUSSION
Because of a high net-negative charge resulting from extensive sialofucosylated O-glycosylation, the primary physiological function of PCLP is widely considered to be serving as an anti-adhesive molecule maintaining the foot podocyte function of the kidney (14). However, recent work describes the preferential expression of PCLP by aggressive and metastatic cancers compared with normal tissues (10, 20, 21, 50), raising the possibility of a role for this sialomucin in the metastatic process. Although PCLP's anti-adhesive properties have been regarded as the dominant contribution of this molecule to metastatic progression (50), the potential interaction between PCLP and selectins has been largely overlooked. E-selectin-dependent adhesion has been widely linked with metastatic spread in vivo (6, 23), whereas other reports reveal a role for P-selectin (4, 32) and L-selectin (5, 35) in the metastatic process. Moreover, the interaction of metastatic tumor cells with host cells such as endothelial cells (7, 8, 38), platelets (7, 25, 38, 41), and leukocytes (24, 26) via selectin-mediated adhesion has been clearly demonstrated. An important step in understanding the metastatic process and its prevention may be the elucidation of the tumor-expressed selectin ligands. Although different tumor cell types may display selectin-recognized sialofucosylated oligosaccharides such as sLex or sLea on different protein backbones, cataloging these ligands, especially those overexpressed by carcinomas, is of particular interest to better understand this family of molecules.
We sought to systematically assess the selectin-binding activity of sialofucosylated glycoproteins expressed by LS174T colon carcinoma cells by employing a functional assay (blot rolling) combined with a biochemical/bioinformatics approach involving immunoaffinity chromatography, SDS-PAGE analysis, and mass spectrometry. This approach identified PCLP as a putative selectin ligand expressed by these cells. Through blot rolling and cell-free flow-based adhesion assays, we verified PCLP to be a high-affinity E-/L-, but not P-, selectin ligand on wild-type and CD44-knockdown LS174T colon carcinoma cells under physiological flow conditions. This finding may offer a unifying perspective on the apparent metastatic potential associated with PCLP overexpression (10, 20, 21, 50) and the role of selectins in metastatic spread.
Because sialofucosylated epitopes are important determinants of selectin-binding potential (25, 28, 49), we hypothesized that the difference in the site density of sLex decorating PCLP on wild-type vs. CD44-knockdown colon carcinoma cells would have a direct effect on the avidity of PCLP for selectins. In partial support of this hypothesis, PCLP immunopurified from CD44-knockdown LS174T cells has a higher avidity for E-, but not L-, selectin compared with PCLP from wild-type cells. The enhanced sialofucosylation of PCLP on CD44-knockdown cells may also explain the observed increase in E-selectin-dependent adhesion to the ∼180-kDa region previously observed in blot rolling assays (44). Because PCLP acts as an alternative carrier of selectin-binding glycans, it may play a role in E- and L-selectin-dependent tumor cell adhesion in cells lacking or depleted in CD44. As has been appropriately argued in the literature (54), distinctions must be drawn between structures that can bind to selectins under certain conditions in vitro and functional ligands that engage selectins under fluid dynamic conditions in vivo. Therefore, elucidating the functional role of PCLP in selectin-dependent adhesion by knocking down its expression on colon carcinoma cells warrants exploration.
PCLP expressed by HEVs has previously been demonstrated to mediate L-selectin-dependent adhesion via sialofucosylated epitopes displayed on O-linked glycans (47). It appears that PCLP expressed by LS174T colon carcinoma cells is heavily decorated with O-linked glycans given its sensitivity to OSGE treatment; moreover, the differential effects of benzyl-GalNAc (inhibition of O-glycosylation) vs. DMJ (disruption of N-linked processing) support the preferential role of O-linked glycans on PCLP in E-/L-selectin-dependent tumor cell adhesion. PCLP's emerging role as a sialomucin marker for metastatic carcinoma cells (10, 20, 21, 50) suggests that this molecule's ability to support selectin-mediated adhesion confers metastatic potential to PCLP-expressing carcinomas. Indeed, the pivotal role of mucin-like molecules in metastasis has been demonstrated in an in vivo study indicating that enzymatic removal of O-linked mucin-like glycoproteins from colon carcinoma cells results in marked reduction of experimental metastasis in a mouse model (4).
In contrast to HEV-expressed PCLP, LS174T colon carcinoma cells express a MECA-79-negative (presumably nonsulfated) glycoform of PCLP that nonetheless mediates interactions with E-/L-selectin. Although MECA-79 reactivity has been considered important for L-selectin-dependent adhesion (47), there exist notable exceptions. Previous reports demonstrated that sulfation is not required for L-selectin-dependent adhesion by the standard form of CD44 expressed by KG-1a hematopoietic cells (11, 46). Sulfation may therefore be one of many posttranslational modifications (including sialylation and fucosylation) that confers but is not requisite for selectin-dependent adhesion of glycoproteins, but whose regulation may control cell adhesive interactions. It has been proposed that dysregulation of posttranslational modifications, in particular altered glycotransferase activities, confers a metastatic phenotype to carcinomas (33). Hence, differences in the biochemistry of normal vs. malignant cell selectin ligands may provide unique insight into the regulation of this complex process. For instance, we recently demonstrated that the E-, L-, and P-selectin-binding determinants of CD44 variant isoforms expressed by LS174T colon carcinoma cells are sialofucosylated O-linked glycans, similar to those on P-selectin glycoprotein ligand-1 (PSGL-1). However, the standard form of CD44 expressed by hematopoetic progenitor cells can only interact with E- and L-selectin via sialofucosylated N-glycans (11, 46). It is thought that the splicing of variant exons into CD44 by tumor cells extends the membrane-proximal stem domain into a structure and adds additional sites for glycosylation, thereby transforming CD44v into a “mucin-like” sialofucosylated glycoprotein. CD44v can therefore function as a more efficient selectin ligand compared with the standard form of CD44 expressed by normal blood cells (17). Given the high affinity of CD44v for selectins and the selectins' pivotal role in metastasis, it is not surprising that CD44v overexpression is associated with increased metastatic potential (19, 22). Furthermore, CD24 binding to P-selectin has been reported to be completely vs. partially insensitive to sialidase treatment when expressed by neutrophils vs. malignant carcinomas (2). A table summarizing the biochemical differences in the recently identified selectin ligands expressed by normal vs. malignant cells is presented in Table 2. These characteristics may induce the adhesion of PCLP-expressing tumor cells to the vascular endothelium, activated platelets, and leukocytes and facilitate metastatic spread.
Table 2.
Biochemical and known selectin-binding characteristics of normal cell- versus malignant cell-expressed selectin ligands
| PSGL-1 | PCLP | CD44 | CD24 | ||||
|---|---|---|---|---|---|---|---|
| Cell type | Leukocytes, platelets | Colon | HEVs | Colon (CD44v) | HPC (CD44s) | Breast, small cell lung carcinoma | Neutrophils |
| Cell line | Primary | LS174T | Primary | LS174T, T84, Colo205 | KG1a | KS, SW2 | Primary |
| Mol wt, kDa | 240 | 180 | 160 | 150 | 98 | 45 | 45 |
| High-affinity selectin binding | E, L, P (18) | E, L | L (47) | E, L, P (17, 44) | E (11), L (13) | P (1, 2) | P (2) |
| Selectin binding glycan | O-linked (36, 37) | O-linked | O-linked (47) | O-linked (17, 44) | N-linked (11, 13) | O-linked (2) | O-linked (2) |
| GPI linked | No | No | No | No | No | Yes | Yes |
| Sialidase sensitive | E (55) (L, P: ND) | E*, L | L (47) | E†, L, P (17, 44) | E (12) (L: ND) | Partially (2) | No (2) |
| MECA-79 reactive | ND | No | Yes (47) | ND | No‡ (11) | ND | ND |
Nonbold, selectin ligands expressed in normal cell types; boldface, selectin ligands expressed by malignant cells. LS174T, T84, and Colo 205, colon carcinoma cell lines; KS, breast carcinoma cell line; SW2, small cell lung cancer cell line; KG-1a, hematopoetic progenitor cell line (HPC).
Sialidase-treated LS174T PCLP retained <20% tethering to E-selectin.
Sialidase-treated LS174T CD44 retained >60% tethering to E-selectin compared with the control.
Selectin-dependent adhesion was shown to be sulfation-independent by chlorate treatment. ND, no data.
In summary, we have identified that PCLP is a high-affinity E-/L-, but not P-, selectin ligand on LS174T colon carcinoma cells. In accord with previous studies reporting on the biochemical nature of PCLP on HEVs, which function as L-selectin ligands, PCLP on LS174T cells contains heavily O-linked glycans decorated with HECA-452-reactive carbohydrate residues. However, there are important differences in the MECA-79 reactivity of PCLP expressed by these different cell types, which may directly effect their adhesive interactions. The novel finding of PCLP-mediated adhesion of tumor cells to E-/L-selectin may explain the relationship between elevated expression levels of PCLP and increased metastatic potential. Our findings support further research to investigate PCLP as a potential therapeutic target to combat metastasis and contribute to the complexity of the possible functions for this ubiquitous adhesion molecule.
GRANTS
This work was supported by National Cancer Institute Grant RO1 CA-101135 and the National Science Foundation Graduate Research Fellowship.
Acknowledgments
We thank Drs. Robert Cole and Robert N. O'Meally who performed the peptide analysis and protein identification in the Mass Spectrometry and Proteomics Facility at Johns Hopkins School of Medicine with support from the Institute for Cell Engineering. We thank Wyeth External Research for the generous gifts of E- and P-selectin Fc IgG chimera protein and Affymax for the E- and P-selectin transfected CHO cells.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Aigner S, Ramos CL, Hafezi-Moghadam A, Lawrence MB, Friederichs J, Altevogt P, Ley K. CD24 mediates rolling of breast carcinoma cells on P-selectin. FASEB J 12: 1241–1251, 1998. [DOI] [PubMed] [Google Scholar]
- 2.Aigner S, Sthoeger ZM, Fogel M, Weber E, Zarn J, Ruppert M, Zeller Y, Vestweber D, Stahel R, Sammar M, Altevogt P. CD24, a mucin-type glycoprotein, is a ligand for P-selectin on human tumor cells. Blood 89: 3385–3395, 1997. [PubMed] [Google Scholar]
- 3.Alves CS, Burdick MM, Thomas SN, Pawar P, KK. The dual role of CD44 as a functional P-selectin and fibrin ligand in colon carcinoma cell adhesion. Am J Physiol Cell Physiol 294: C907–C916, 2008. [DOI] [PubMed] [Google Scholar]
- 4.Borsig L, Wong R, Feramisco J, Nadeau DR, Varki NM, Varki A. Heparin and cancer revisited: mechanistic connections involving platelets, P-selectin, carcinoma mucins, and tumor metastasis. Proc Natl Acad Sci USA 98: 3352–3357, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borsig L, Wong R, Hynes RO, Varki NM, Varki A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc Natl Acad Sci USA 99: 2193–2198, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodt P, Fallavollita L, Bresalier RS, Meterissian S, Norton CR, Wolitzky BA. Liver endothelial E-selectin mediates carcinoma cell adhesion and promotes liver metastasis. Int J Cancer 71: 612–619, 1997. [DOI] [PubMed] [Google Scholar]
- 7.Burdick MM, Konstantopoulos K. Platelet-induced enhancement of LS174T colon carcinoma and THP-1 monocytoid cell adhesion to vascular endothelium under flow. Am J Physiol Cell Physiol 287: C539–C547, 2004. [DOI] [PubMed] [Google Scholar]
- 8.Burdick MM, McCaffery JM, Kim YS, Bochner BS, Konstantopoulos K. Colon carcinoma cell glycolipids, integrins, and other glycoproteins mediate adhesion to HUVECs under flow. Am J Physiol Cell Physiol 284: C977–C987, 2003. [DOI] [PubMed] [Google Scholar]
- 9.Burdick MM, McCarty OJT, Jadhav S, Konstantopoulos K. Cell-cell interactions in inflammation and cancer metastasis. IEEE Eng Med Biol 20: 86–91, 2001. [DOI] [PubMed] [Google Scholar]
- 10.Casey G, Neville PJ, Liu X, Plummer SJ, Cicek MS, Krumroy LM, Curran AP, McGreevy MR, Catalona WJ, Klein EA, Witte JS. Podocalyxin variants and risk of prostate cancer and tumor aggressiveness. Hum Mol Genet 15: 735–741, 2006. [DOI] [PubMed] [Google Scholar]
- 11.Dimitroff CJ, Lee JY, Fuhlbrigge RC, Sackstein R. A distinct glycoform of CD44 is an L-selectin ligand on human hematopoietic cells. Proc Natl Acad Sci USA 97: 13841–13846, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major Eselectin ligand on human hematopoietic progenitor cells. J Cell Biol 153: 1277–1286, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimitroff CJ, Lee JY, Schor KS, Sandmaier BM, Sackstein R. Differential L-selectin binding activities of human hematopoietic cell L-selectin ligands, HCELL and PSGL-1. J Biol Chem 276: 47623–47631, 2001. [DOI] [PubMed] [Google Scholar]
- 14.Doyonnas R, Kershaw DB, Duhme C, Merkens H, Chelliah S, Graf T, McNagny KM. Anuria, omphalocele, and perinatal lethality in mice lacking the CD34-related protein podocalyxin. J Exp Med 194: 13–27, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuhlbrigge RC, King SL, Dimitroff C, Kupper TS, Sackstein R. Direct real-time observation of E- and P-selectin-mediated rolling on cutaneous lymphocyte-associated antigen immobilized on western blots. J Immunol 168: 5645–5651, 2002. [DOI] [PubMed] [Google Scholar]
- 16.Hanley WD, Burdick MM, Konstantopoulos K, Sackstein R. CD44 on LS174T colon carcinoma cells possesses E-selectin ligand activity. Cancer Res 65: 5812–5817, 2005. [DOI] [PubMed] [Google Scholar]
- 17.Hanley WD, Napier SL, Burdick MM, Schnaar RL, Sackstein R, Konstantopoulos K. Variant isoforms of CD44 are P- and L-selectin ligands on colon carcinoma cells. FASEB J 20: 337–339, 2006. [DOI] [PubMed] [Google Scholar]
- 18.Hanley WD, Wirtz D, Konstantopoulos K. Distinct kinetic and mechanical properties govern selectin-leukocyte interactions. J Cell Sci 117: 2503–2511, 2004. [DOI] [PubMed] [Google Scholar]
- 19.Harada N, Mizoi T, Kinouchi M, Hoshi K, Ishii S, Shiiba K, Sasaki I, Matsuno S. Introduction of antisense CD44S CDNA down-regulates expression of overall CD44 isoforms and inhibits tumor growth and metastasis in highly metastatic colon carcinoma cells. Int J Cancer 91: 67–75, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hayatsu N, Kaneko MK, Mishima K, Nishikawa R, Matsutani M, Price JE, Kato Y. Podocalyxin expression in malignant astrocytic tumors. Biochem Biophys Res Commun 374: 394–398, 2008. [DOI] [PubMed] [Google Scholar]
- 21.Heukamp LC, Fischer HP, Schirmacher P, Chen X, Breuhahn K, Nicolay C, Buttner R, Gutgemann I. Podocalyxin-like protein 1 expression in primary hepatic tumours and tumour-like lesions. Histopathology 49: 242–247, 2006. [DOI] [PubMed] [Google Scholar]
- 22.Hofmann M, Rudy W, Zoller M, Tolg C, Ponta H, Herrlich P, Gunthert U. CD44 splice variants confer metastatic behavior in rats: homologous sequences are expressed in human tumor cell lines. Cancer Res 51: 5292–5297, 1991. [PubMed] [Google Scholar]
- 23.Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res 216: 215–221, 1995. [DOI] [PubMed] [Google Scholar]
- 24.Jadhav S, Bochner BS, Konstantopoulos K. Hydrodynamic shear regulates the kinetics and receptor specificity of polymorphonuclear leukocyte: colon carcinoma cell adhesive interactions. J Immunol 167: 5986–5993, 2001. [DOI] [PubMed] [Google Scholar]
- 25.Jadhav S, Eggleton CD, Konstantopoulos K. Mathematical modeling of cell adhesion in shear flow pertinent to inflammation and cancer metastasis. Curr Pharm Des 13: 1511–1526, 2007. [DOI] [PubMed] [Google Scholar]
- 26.Jadhav S, Konstantopoulos K. Fluid shear- and time-dependent modulation of molecular interactions between polymorphonuclear leukocytes and colon carcinomas. Am J Physiol Cell Physiol 283: C1133–C1143, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N. Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 95: 377–384, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kansas GS Selectins and their ligands: current concepts and controversies. Blood 88: 3259–3287, 1996. [PubMed] [Google Scholar]
- 29.Kelley TWHD, McNagny KM, Roskelley CD, Hsi ED. Podocalyxin: a marker of blasts in acute leukemia. Am J Clin Pathol 124: 134–142, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Kerjaschki D Epitopes and radicals: early events in glomerular injury in membranous nephropathy. Exp Nephrol 3: 1–8, 1995. [PubMed] [Google Scholar]
- 31.Kerosuo LJE, Alitalo R, Gylling M, Kerjaschki D, Miettinen A. Podocalyxin in human haematopoietic cells. Br J Haematol 124: 809–818, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci USA 95: 9325–9330, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YJ, Varki A. Perspectives on the significance of altered glycosylation of glycoproteins in cancer. Glycoconj J 14: 569–576, 1997. [DOI] [PubMed] [Google Scholar]
- 34.Konstantopoulos K, Kukreti S, McIntire LV. Biomechanics of cell interactions in shear fields. Adv Drug Deliv Rev 33: 141–164, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Laubli H, Stevenson JL, Varki A, Varki NM, Borsig L. L-selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res 66: 1536–1542, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Leppanen A, Yago T, Otto VI, McEver RP, Cummings RD. Model glycosulfopeptides from P-selectin glycoprotein ligand-1 require tyrosine sulfation and a core 2-branched O-glycan to bind to L-selectin. J Biol Chem 278: 26391–26400, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Li F, Wilkins PP, Crawley S, Weinstein J, Cummings RD, McEver RP. Posttranslational modifications of recombinant P-selectin glycoprotein ligand-1 required for binding to P- and E-selectin. J Biol Chem 271: 3255–3264, 1996. [PubMed] [Google Scholar]
- 38.Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucintype glycoproteins. Cancer Res 55: 4425–4431, 1995. [PubMed] [Google Scholar]
- 39.Mannori G, Santoro D, Carter L, Corless C, Nelson RM, Bevilacqua MP. Inhibition of colon carcinoma cell lung colony formation by a soluble form of E-selectin. Am J Pathol 151: 233–243, 1997. [PMC free article] [PubMed] [Google Scholar]
- 40.McCarty OJT, Jadhav S, Burdick MM, Bell WR, Konstantopoulos K. Fluid shear regulates the kinetics and molecular mechanisms of activation-dependent platelet binding to colon carcinoma cells. Biophys J 83: 836–848, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McCarty OJT, Mousa SA, Bray PF, Konstantopoulos K. Immobilized platelets support human colon carcinoma cell tethering, rolling and firm adhesion under dynamic flow conditions. Blood 96: 1789–1797, 2000. [PubMed] [Google Scholar]
- 42.Nakamori S, Kameyama M, Imaoka S, Furukawa H, Ishikawa O, Sasaki Y, Kabuto T, Iwanaga T, Matsushita Y, Irimura T. Increased expression of sialyl Lewisx antigen correlates with poor survival in patients with colorectal carcinoma: clinicopathological and immunohistochemical study. Cancer Res 53: 3632–3637, 1993. [PubMed] [Google Scholar]
- 43.Nakayama T, Watanabe M, Katsumata T, Teramoto T, Kitajima M. Expression of sialyl Lewis(a) as a new prognostic factor for patients with advanced colorectal carcinoma. Cancer 75: 2051–2056, 1995. [DOI] [PubMed] [Google Scholar]
- 44.Napier SL, Healy ZR, Schnaar RL, Konstantopoulos K. Selectin ligand expression regulates the initial vascular interactions of colon carcinoma cells: the roles of CD44V and alternative sialofucosylated selectin ligands. J Biol Chem 282: 3433–3441, 2007. [DOI] [PubMed] [Google Scholar]
- 45.Nieswandt B, Hafner M, Echtenacher B, Mannel DN. Lysis of tumor cells by natutal killer cells in mice is impeded by platelets. Cancer Res 59: 1295–1300, 1999. [PubMed] [Google Scholar]
- 46.Sackstein R, Dimitroff CJ. A hematopoietic cell L-selectin ligand that is distinct from PSGL-1 and displays N-glycan-dependent binding activity. Blood 96: 2765–2774, 2000. [PubMed] [Google Scholar]
- 47.Sassetti C, Tangemann K, Singer MS, Kershaw DB, Rosen SD. Identification of podocalyxin-like protein as a high endothelial venule ligand for L-selectin: parallels to CD34. J Exp Med 187: 1965–1975, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sato M, Narita T, Kimura N, Zenita K, Hashimoto T, Manabe T, Kannagi R. The association of sialyl Lewis(a) antigen with the metastatic potential of human colon cancer cells. Anticancer Res 17: 3505–3511, 1997. [PubMed] [Google Scholar]
- 49.Simon SI, Green CE. Molecular mechanics and dynamics of leukocyte recruitment during inflammation. Annu Rev Biomed Eng 7: 151–185, 2005. [DOI] [PubMed] [Google Scholar]
- 50.Somasiri A, Nielsen JS, Makretsov N, McCoy ML, Prentice L, Gilks CB, Chia SK, Gelmon KA, Kershaw DB, Huntsman DG, McNagny KM, Roskelley CD. Overexpression of the anti-adhesin podocalyxin is an independent predictor of breast cancer progression. Cancer Res 64: 5068–5073 2004. [DOI] [PubMed] [Google Scholar]
- 51.Somers WS, Tang J, Shaw GD, Camphausen RT. Insights into the molecular basis of leukocyte tethering and rolling revealed by structures of P- and E-selectin bound to SLe(X) and PSGL-1. Cell 103: 467–479, 2000. [DOI] [PubMed] [Google Scholar]
- 52.Stone JP, Wagner DD. P-selectin mediates adhesion of platelets to neuroblastoma and small cell lung cancer. J Clin Invest 92: 804–813, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem 283: 15647–15655, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Varki A Selectin ligands: will the real ones please stand up? J Clin Invest 100: S31–35, 1997. [PubMed] [Google Scholar]
- 55.Zou X, Shinde Patil VR, Dagia NM, Smith LA, Wargo MJ, Interliggi KA, Lloyd CM, Tees DF, Walcheck B, Lawrence MB, Goetz DJ. PSGL-1 derived from human neutrophils is a high-efficiency ligand for endothelium-expressed E-selectin under flow. Am J Physiol Cell Physiol 289: C415–C424, 2005. [DOI] [PubMed] [Google Scholar]