Abstract
Adenylyl cyclases (ACs) are a family of critically important signaling molecules that are regulated by multiple pathways. Adenylyl cyclase 8 (AC8) is a Ca2+ stimulated isoform that displays a selective regulation by capacitative Ca2+ entry (CCE), the process whereby the entry of Ca2+ into cells is triggered by the emptying of intracellular stores. This selectivity was believed to be achieved through the localization of AC8 in lipid raft microdomains, along with components of the CCE apparatus. In the present study, we show that an intact leucine zipper motif is required for the efficient N-linked glycosylation of AC8, and that this N-linked glycosylation is important to target AC8 into lipid rafts. Disruption of the leucine zipper by site-directed mutagenesis results in the elimination of N-glycosylated forms and their exclusion from lipid rafts. Mutants of AC8 that cannot be N-glycosylated are not demonstrably associated with rafts, although they can still be regulated by CCE; however, raft integrity is required for the regulation of these mutants. These findings suggest that raft localized proteins in addition to AC8 are needed to mediate its regulation by CCE.
Keywords: lipid rafts, leucine zipper, capacitative Ca2+ entry, N-glycosylation
adenosine 3′,5′-cyclic monophosphate (cAMP), produced by nine mammalian membrane-bound adenylyl cyclases ACs (AC1-9) (49), mediates the actions of many hormones and neurotransmitters on a wide array of cellular processes. Adenylyl cyclase type 8 (AC8) typifies the membrane-bound enzymes in that it is susceptible to regulation by a number of stimulatory and inhibitory factors, which underpins its role in homeostatic integration (55). AC8 is primarily expressed in the brain; it is very prominent in the hippocampus and cerebellum (21, 32), and it is stimulated by Ca2+ acting through calmodulin (3).
Remarkably, in nonexcitable cells, the regulation of AC8 by cytosolic Ca2+ ([Ca2+]i) is orchestrated by a selective dependence on capacitative Ca2+ entry (CCE) (13), the process whereby Ca2+ enters the cell in response to the depletion of internal Ca2+ stores, either directly by phospholipase C-linked agonists or passively, by pharmacological inhibition of endoplasmic reticulum (ER) reuptake pumps (39). Other means of elevating [Ca2+]i, such as release from internal Ca2+ stores, or non-capacitative entry mechanisms such as those triggered by 1-oleyl-2-acetyl-sn-glycerol (OAG) and arachidonic acid, are ineffective at stimulating AC8 activity (13, 14, 30, 44). This selectivity of AC8 for CCE, over other means of elevating [Ca2+]i, has been proposed to reflect a close apposition with CCE channels (13, 19).
The cellular basis for the dependence of AC8 on CCE is still uncertain, but it is believed to at least partly reflect a colocalization of AC8 with CCE entry sites within specialized microdomains of the plasma membrane (PM), the lipid rafts (13, 48). Lipid rafts are variously considered to be static or dynamic domains of the membrane that are enriched in cholesterol and glycosphingolipids. Lipid rafts can form invaginations of the PM caused by constrictions organized by polymers of the protein caveolin, which are referred to as “caveolae” (52, 53). Rafts and caveolae, either passively or actively, accumulate a subset of membrane proteins (24, 41, 46). However, it must be acknowledged that a universal consensus of precisely how this concentration of signaling molecules is achieved, or even its regulatory significance, is not agreed (7, 20, 26, 28, 33). Candidate CCE components such as stromal interaction molecule 1 (STIM1) and transient receptor potential channels, as well as Ca2+-sensitive ACs, receptors, and G proteins, have been detected in lipid rafts following biochemical separation of membrane extracts (7, 22, 29, 38, 48). The localization of Ca2+-sensitive ACs in rafts as an essential component of their regulation by CCE, is suggested since the disruption of rafts by cholesterol extraction with methyl-β-cyclodextrin (MβCD) ablates the regulation of AC8 by CCE (15, 48), without affecting the CCE process. Thus an essential part of understanding the regulation of AC seems to rely on understanding its trafficking and organization in the PM. Because ACs are complex molecules, of over 1,200 amino acids, comprising two repeated cassettes of six-transmembrane spanning domains interspersed with two separated catalytic interfaces, this topic has received little attention. The first paper on this subject showed that the two six-transmembrane domains must associate to traffic the molecule to the PM, and in so doing they bring the two catalytic domains into functional apposition (19). Subsequently, the cytoplasmic domains of both AC5 (a Ca2+-inhibitable species) and AC8 were revealed to be critical for targeting to rafts (8). However, nothing else is known regarding the mechanisms through which ACs are targeted to specific cellular compartments.
In the present study, the trafficking and assembly of AC8 into raft domains of the PM has been addressed, with particular regard to the role of oligomerization and N-glycosylation. A leucine zipper motif is seen to play a key role in preventing mistargeting of the enzyme; a multistep process is revealed including a central role for N-linked glycosylation. Furthermore, the necessity for the integrity of lipid rafts for the regulation by CCE of AC8 is clarified.
EXPERIMENTAL PROCEDURES
Cell culture materials, protein-G-Sepharose 4B, tunicamycin, MβCD, cholesterol, and sphingomyelinase from Staphylococcus aureus were from Sigma (St. Louis, MO). Protein molecular weight standards and acrylamide/bisacrylamide 37.5:1 solution were from Bio-Rad (Hercules, CA). Restriction enzymes, DNA T4 ligase, and calf intestinal phosphatase were from New England Biolabs (Ipswich, MA). All radiochemicals were from GE Healthcare (Amersham, UK). The rabbit polyclonal anti-AC8 antibody was a kind gift of Dr. J. J. Cali. Anti-caveolin rabbit polyclonal antibody was from BD (610060). Anti-β-adaptin rabbit polyclonal antibody was from Santa Cruz (H-300, sc-10762). Thapsigargin (TG) and 2-aminoethoxydiphenyl borate (2-APB) were from Calbiochem. Oligonucleotides were from Sigma-Genosys. All other chemicals, where not indicated, were from Sigma.
Cell culture.
Human embryonic kidney (HEK)-293 cells were grown as previously described (8).
Transient and stable transfections.
Cells were transfected at ∼50% confluence by the calcium phosphate method (5). Typically, each 10-cm dish was transfected with 1 μg of DNA in 500 μl of CaCl2/HBS mix for 6 h. For full expression of all AC8 species, cells were analyzed not earlier that 72 h after transfection. Stable cell lines were generated by maintaining cells in media containing 400 μg/ml geneticin. Genes of interest were inserted into the pcDNA3.0 vector.
Construction of mutants of AC8.
A PCR-based site-directed mutagenesis approach was used to generate the four AC8 leucine-zipper mutants [L439A (L/A1); L432A, L439A (L/A2); L432A, L439A, L446A (L/A3); L432A, L439A, L446A, L453A (L/A4)] as well as the N-glycosylation mutants of AC8 (N814Q/N818Q; N814Q/N818Q/ N885E). PCR was conducted in the MiniCycler (MJ Research) using the fusion high-fidelity polymerase kit (FINNZYMES) according to manufacturers' instructions. For tagging with enhanced green fluorescent protein (EGFP), the AC8 constructs were subcloned into COOH-terminal EGFP vector (Clontech) as described previously (19). Rat AC8 sequence corresponds to the entry P40146 of UniProtKB/Swiss-Prot database.
Preparation of crude membranes.
HEK-293 cells were harvested by detaching with phosphate-buffered saline (PBS) containing 1 mM EDTA and centrifuged at 195 g for 5 min at +4°C. The cell pellet was resuspended in 1 ml of hypotonic lysis buffer (10 mM Tris·HCl pH 7.4, 1 mM EGTA, and 1 mM EDTA, supplemented with Sigma protease inhibitors 1:500, 1 mM benzamidine and 1 mM PMSF), equilibrated for 10 min on ice, and then homogenized with 50 strokes of a tight-fitting glass Dounce homogenizer. Nuclei and unbroken cells were removed by centrifugation at 195 g for 2 min at +4°C. The supernatant was removed, sonicated (3 times, 15 s each, power set at 2, using a Sonic Dismembrator 60, Fisher Scientific), and centrifuged at 13,200 g for 20 min at +4°C (Sorvall Biofuge Fresco). The supernatant was discarded, the membrane pellet washed with 1 ml PBS, centrifuged again, and used as crude membranes for subsequent applications. For in vitro adenylyl cyclase assays, the sonication step was omitted.
N-glycosidase F treatment.
Crude membranes were denatured by resuspension in 1% SDS and boiling for 3 min. Denatured crude membranes (25 μg) were incubated with 2 units of N-glycosidase F (Roche) or the same volume of 50% glycerol (controls) in 100 mM sodium phosphate pH 7.2, 0.5% NP-40, 10 mM EDTA, 1% β-mercaptoethanol, and 1 mM PMSF for 1 h at 37°C. After adding 6× Laemmli buffer and boiling for 5 min, samples were loaded on 7% polyacrylamide gels for electrophoresis (SDS-PAGE) and immunoblotting analysis.
Immunoblotting analysis.
Crude membranes were resuspended in 1% SDS. Equal volumes were diluted in 6× Laemmli buffer, boiled for 5 min, and stored at −20°C for immunoblotting analysis, except for detection of AC8 where samples resuspended in Laemmli buffer were not boiled but warmed up at 37°C for 10 min to limit aggregation between AC8 molecules. Extracted proteins were separated by SDS-PAGE with 7% or 12% acrylamide concentration (Mini-Protean 3 apparatus, Bio-Rad) and transferred (300 mA, 90 min, +4°C) onto 0.2-μm supported nitrocellulose membranes (Bio-Rad) using a Mini Trans-Blot apparatus (Bio-Rad). Transfer of proteins was checked by staining of bound proteins with Ponceau-S (0.1% in 5% acetic acid). Nonspecific binding sites were blocked by incubation for 1 h at room temperature in 5% nonfat dried milk in PBS (pH 7.4)-0.1% Tween 20 (PBS-T). Blots were incubated with primary antibodies overnight at 4°C at appropriate dilutions in 1% nonfat dried milk-PBS-T + 0.02% sodium azide. After washings in PBS-T, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit (Pierce) or goat anti-mouse (Promega) secondary antibodies (1:10,000–1:20,000 dilution) in 1% nonfat dried milk-PBS-T with no sodium azide, for 45 min at room temperature. After three washings in PBS-T, the immunoreactive bands were visualized using a chemiluminescence detection system (ECL Plus, GE Healthcare) according to the manufacturer's instructions, and exposure to films (Hyperfilm ECL, GE Healthcare). All experiments were repeated at least three times. For quantification of optical densities of bands, digital images of films were acquired at a 1,200-dpi resolution through a transparency scanner (Heidelberg Linoscan 1450) using the VueScan software, and optical densities were obtained with ImageJ software.
Coimmunoprecipitation.
HEK-293 cells grown in 10-cm dishes were transiently transfected with 0.75 μg of each DNA [pcDNA3.0-AC8-hemagglutinin (HA), pcDNA3.0-AC8-FLAG or pcDNA3.0] on their own or in combination. Crude membranes, prepared as indicated above (except that sonication was avoided and DNA strands were sheared by incubation with 1 U/ml of DNase I, GE Healthcare), were resuspended in 1 ml of immunoprecipitation buffer (50 mM Tris·HCl pH 7.5, 150 mM NaCl, and 2 mM EDTA) containing 0.6% (vol/vol) C12E9 (Sigma) and protease inhibitors, and rotated at +4°C for 1 h to solubilize AC8. Samples were split in half and precleared with 20 μl of 50% slurry Protein-G-Sepharose 4B (Sigma). Small aliquots were taken for determination of protein concentration and immunoblotting (inputs). Precleared samples were immunoprecipitated with 2 μl of either anti-HA (mouse monoclonal, clone HA-7, Sigma) or anti-FLAG M2 (mouse monoclonal, Stratagene) antibodies (overnight at +4°C) and 50 μl of Protein-G-Sepharose 4B (2 h at +4°C). Bound proteins were eluted by adding to beads the same volume of 2× Laemmli buffer, incubated at 37°C for 30 min, and loaded on gels for immunoblotting.
Preparation of lipid rafts by detergent-free method and flotation by isopycnic centrifugation.
A cell pellet from, typically, three 10-cm dishes was resuspended in 1.34 ml of 0.5 M sodium carbonate, pH 11.5, with protease inhibitors, then sonicated with three 30-s bursts. The homogenate was adjusted to 40% sucrose by adding 2.06 ml of 60% sucrose in MBS (25 mM MES, pH 6.4, 150 mM NaCl, and 250 mM sodium carbonate), placed under a 5–30% discontinuous sucrose gradient, and centrifuged at 34,000 rpm for 15–18 h at +4°C in a Beckman SW-41Ti rotor. Nine fractions (1.24 ml each) were harvested from the top of the tube (keeping aliquots to determine the sucrose concentration), mixed with 9 volumes of MBS, and centrifuged at 40,000 rpm for 1 h at +4°C (Beckman SW-41Ti rotor). Supernatants were discarded, and membrane pellets were resuspended in an adequate volume (100–150 μl) of 1% SDS. Thirty microliters were saved for protein quantification (bicinchoninic acid method, BCA1–1KT, Sigma).
Measurement of intracellular cAMP accumulation.
cAMP accumulation in intact cells was measured according to the method of Evans et al. (12), with modifications. Transfected HEK-293 cells were grown on 24-well plates. Cells were incubated in MEM (2 h, 37°C) with 2-[3H]adenine (1.5 μCi/well) to label the ATP pool and then washed and incubated (10 min at 30°C) in nominally Ca2+-free Krebs buffer (900 μl/well) supplemented with 0.1% bovine serum albumin. Experiments were carried out at 30°C in the presence of 3-isobutyl-1-methylxanthine (IBMX, 100 μM) and EGTA (100 μM), which were preincubated with the cells for 10 min and 6 min, respectively, before a 1-min assay. To trigger CCE, cells were incubated with the Ca2+-ATPase inhibitor, TG (100 nM), for 4 min to empty intracellular Ca2+ stores before the addition of 100 μl of assay solution (Krebs buffer, 10 μM forskolin, and CaCl2 at the indicated concentrations), and cAMP accumulation was monitored over a period of 1 min, terminating the reaction by the addition of ice-cold 5% (wt/vol) trichloroacetic acid. Where required, cells were preincubated with 2-APB (100 μM) or DMSO (1%) for 10 min before the 1-min assay. Subsequent steps were performed as described previously (30). Accumulation of cAMP is expressed as the percentage of conversion of [3H]ATP into [3H]cAMP and shown as means ± SE of triplicate determinations of three separate experiments.
Measurement of in vitro AC activity.
AC activity was measured in vitro as described previously (47). In brief, crude (unsonicated) membrane preparations were incubated (20 min, 30°C) in the presence of 12 mM phosphocreatine, 1.4 mM MgCl2, 40 μM GTP, 100 μM cAMP, 100 μM ATP, 25 U/ml creatine kinase, 70 mM Tris·HCl pH 7.4, 500 μM IBMX, 1 μM calmodulin, 1 μCi of α-[32P]ATP, 10 μM forskolin, and Ca2+. Free Ca2+ concentrations were established using an EGTA-buffering system. Final assay mixture concentrations of Ca2+ (in the presence of 200 μM EGTA) are shown in figure legends. Reactions were terminated, and the [32P]cAMP formed was quantified using [3H]cAMP added as a recovery marker. Data points are presented as the average ± SE of three independent experiments performed in triplicate.
Disruption of lipid rafts by cholesterol depletion with MβCD.
Cell culture medium was removed and cells were washed twice with PBS and incubated for 1 h at 37°C with or without 10 mM MβCD in medium without serum.
Cholesterol replenishment with cholesterol-MβCD complexes.
Cholesterol-MβCD complexes were formed (as described in Ref. 25) by the addition of cholesterol (6 mg dissolved in 80 μl of isopropanol:chloroform, 2:1) to a stirred MβCD solution (200 mg in 2.2 ml H2O) kept at 80°C, until clear (cholesterol is 6.8 mM). The complexes were then added to serum-free cell culture medium to a final concentration of 0.2 mM cholesterol and 2 mM MβCD, for 45 min at 37°C.
Disruption of lipid rafts by sphingomyelinase treatment.
Cells were exposed to cell culture medium without serum containing 400 mU/ml of sphingomyelinase, at 37°C for 90 min.
Confocal imaging.
Cells expressing fluorophore-tagged proteins (EGFP-AC8 and EGFP-triple N-glycosylation mutants of AC8) were plated onto glass-bottom dishes (WillcoWells) coated with poly-l-lysine. The cells were visualized on a Leica SP5 confocal microscope running LAS AF 1.6.0 software using a ×63 plan apochromat 1.4 numerical aperture oil immersion objective (Leica Microsystems). To stain the PM, cells were incubated in 5 μg/ml CellMask Deep Red PM stain (Invitrogen) in Krebs buffer for 5 min at 37°C. For visualization of the EGFP-tagged proteins, a 488-nm laser line of an Argon laser was used, with an emission bandwidth of 500–550 nm. For imaging using CellMask Deep Red PM stain, a 633-nm laser line of a HeNe laser was used with an emission bandwidth of 650–700 nm.
Measurement of [Ca2+]i.
[Ca2+]i was measured in populations of HEK-293 cells using Fura-2/AM (2 μM) as Ca2+-indicator. A Perkin-Elmer LS50B spectrofluorimeter was used to measure fluorescence emission ratios at 340/380 nm (F340/F380), which were converted to Ca2+ concentrations by applying the Grynkiewicz equation (17) as described previously (30). Briefly, cells loaded with 2 μM Fura-2/AM for 40 min were washed and resuspended in nominally Ca2+-free Krebs buffer and used for [Ca2+]i measurements. Where appropriate, cells were pretreated with or without 100 μM 2-APB for 10 min before the addition of 2 mM extracellular Ca2+. EGTA (0.1 mM) and TG (100 nM) were added 6 and 4 min, respectively, before extracellular Ca2+ was added.
RESULTS
Multiple forms of AC8 are seen in HEK-293 cells.
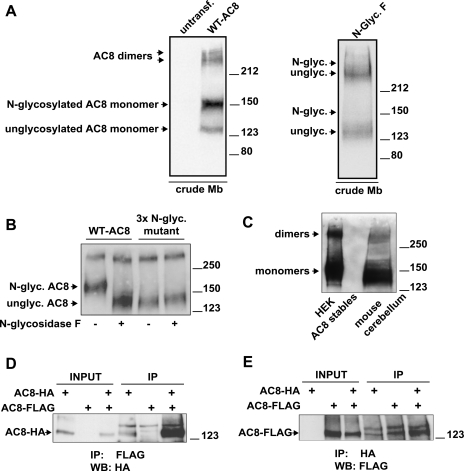
Upon heterologous expression in HEK-293 cells, AC8 can be detected by immunoblotting in crude membranes using a highly specific rabbit polyclonal antibody raised against the COOH terminus of AC8 (3) (Fig. 1). No AC8 signal is detected with the same antibody on crude membranes from untransfected cells, indicating that the antibody does not cross-react with any endogenous AC isoform in HEK-293 cells (Fig. 1). Multiple molecular species of AC8 are visible following immunoblotting analysis. The AC8 species running above the 212-kDa molecular mass marker are compatible with dimers or oligomers of AC8 that resist the denaturing conditions imposed by the resuspension of the crude membrane preparations in 1% SDS-containing buffer and the SDS-PAGE process. The finding of more than one molecular species of AC8 raises the question of whether all of these proteins are targeted to the same cellular compartment and/or represent functional AC8.
Fig. 1.
Adenyl cyclase type 8 (AC8) exists as multiple species, is N-glycosylated, and dimerizes. A: crude membrane (Mb) preparations from untransfected or transiently transfected human embryonic kidney (HEK)-293 cells were solubilized, and proteins (25 μg) were resolved by 7% acrylamide SDS-PAGE and probed for AC8 by immunoblotting. For the treatments with N-glycosidase F, crude membranes were treated as described in experimental procedures. B: crude membranes prepared from HEK-293 cells stably expressing wild-type (WT) AC8 or a triple N-glycosylation mutant of AC8 were treated or not with N-glycosidase F. Proteins (1.2 μg) were resolved by 8% acrylamide SDS-PAGE and analyzed for AC8 by immunoblotting. C: detection of endogenous AC8. Proteins (250 μg) from crude mouse cerebellar membranes were loaded on 7% acrylamide gel (SDS-PAGE), alongside 0.4 μg of proteins from HEK-293 AC8 stables as a positive control, and analyzed for expression of AC8 by immunoblotting. D and E: coimmunoprecipitation of COOH-terminally hemagglutinin (HA)- or FLAG-tagged AC8 molecules (AC8-HA and AC8-FLAG), transiently expressed alone or in combination. Each immunoprecipitation was performed on 0.5–1 mg of precleared crude membrane proteins. The immunoprecipitated proteins were eluted from the beads and analyzed by 7% acrylamide SDS-PAGE and immunoblotting with the indicated antibodies (at 1:5,000 dilution). Inputs were 5% of the precleared crude membrane proteins used for immunoprecipitation. IP, immunoprecipitation; WB, immunoblotting.
That AC8 can exist as dimers/oligomers accords with previous findings, which suggested that ACs form higher-order complexes (18). AC5 and AC6 appear to form heterodimers, since NH2-terminally myc-tagged AC5 can coimmunoprecipitate NH2-terminally FLAG-tagged AC6 when coexpressed, and vice versa (1, 10). Moreover, AC5 has been shown to heterodimerize with AC2 (34). The regulatory significance of AC dimer formation was further underlined when Chen-Goodspeed and colleagues (4) demonstrated that dimer formation is required for AC5 regulation by the α-subunit of Gs.
Two further species of AC8 appear at approximately 150 and 125 kDa (Fig. 1), which are compatible with N-glycosylated and nonglycosylated monomeric AC8, respectively. It has previously been established that AC8 is N-glycosylated and its primary structure has several putative N-glycosylation sites (2) although these have never been directly addressed. Exposure of crude HEK-293 membranes expressing AC8 to N-glycosidase F (which removes N-linked glycans from proteins) affected the migration of both the N-glycosylated monomers and dimers of AC8 (Fig. 1A); the band migrating at ∼150 kDa effectively disappeared, and the band at 125 kDa became more pronounced. These findings strongly suggest that the band migrating at ∼150 kDa represents an N-glycosylated monomer of AC8, while the band migrating at ∼125 kDa corresponds to a unglycosylated monomer.
Since even after N-glycosidase F treatment the AC8 band was still somewhat diffuse, we considered the possibility that some core glycosylation resisted the enzymatic treatment. To address this possibility, we treated some membranes from cells expressing AC8 triple N-glycosylation mutant (see Fig. 4 for details) with N-glycosidase F. The product of this treatment appears identical to the deglycosylated band of AC8 (Fig. 1B). Consequently, we conclude that the removal of glycans is complete. (In trying to reconcile the rather diffuse N-glycosidase F-treated lower-molecular-weight band with the sharper lower-molecular-weight band in controls, it may be worth noting that the former band is a deglycosylated mature protein product, whereas the latter is probably an immature unglycosylated protein.)
To exclude the possibility that apparent dimers were a consequence of heterologous overexpression, we asked whether dimers would be observed in an endogenous setting. Crude membranes were prepared from mouse cerebellum, a rich source of AC8 (4) and compared with a positive control (crude membranes from HEK-293 cells stably expressing AC8). AC8 was detected by SDS-PAGE and immunoblotting. Higher-molecular-weight forms were observed in both cases (Fig. 1C).
To prove that AC8 forms dimers, HA- and FLAG-tagged versions of AC8 were constructed. These proteins were coexpressed in HEK-293 cells and subjected to immunoprecipitation with either anti-FLAG-or anti-HA-antibodies and detection with anti-HA or anti-FLAG antibodies on Western blots (Fig. 1, D and E). These data clearly show mutual association of the differently tagged forms and therefore the formation of AC8 dimers.
These experiments showed that multiple species of AC8 molecules are expressed. We endeavored to characterize the relative importance of these species of AC8 in terms of both the targeting of AC8 to microdomains of the PM and the regulation of AC8 by CCE.
Disruption of the leucine zipper motif of AC8 leads to loss of activity and impairs N-glycosylation.
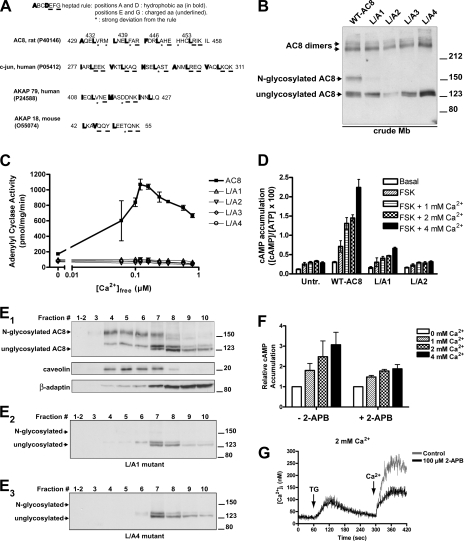
A striking feature of mammalian ACs is their possession of a leucine zipper motif, which is remarkably conserved among all AC isoforms (6) (Fig. 2A). The significance of this feature of ACs has never been addressed experimentally. Leucine zippers are protein-protein interaction motifs composed of a heptad repeat of amino acids (27, 54). The “ABCDEFG rule,” based on the structure of classical leucine zippers (for example, those of the c-Jun protein; Fig. 2A), holds that positions A and D of the heptad are occupied by hydrophobic amino acids (in bold in Fig. 2A), with leucines being particularly frequent in position D (54). Positions E and G, on the contrary, are occupied by charged amino acids (underlined in Fig. 2A). All of the above rules are met by the leucine zipper motif of c-Jun and other “classical” leucine zipper motifs (Fig. 2A). Modified leucine zipper motifs observe the above rules to a lesser degree. In Fig. 2A the reported modified leucine zipper motifs of A-kinase anchoring protein-79 (AKAP-79) and AKAP-18 are shown in which, despite a more lax adherence to those rules (for example, not all amino acids at position D are leucines), the mediation of the interaction with other leucine zipper partners is still preserved (23, 37). Fig. 2A shows the structure of the leucine zipper of AC8, which displays the classical leucine zipper motif.
Fig. 2.
Disruption of the leucine zipper motif of AC8 affects N-glycosylation, activity, and localization in plasma membrane microdomains. A: leucine zipper motifs of rat AC8 (aa 429–458), human c-Jun (aa 277–311), human A-kinase anchoring protein-79 (AKAP-79; aa 408–427), and mouse AKAP-18 (aa 42–55) are compared. Accession numbers for the UniprotKB/Swiss-Prot database are indicated. Bolded: amino acids that obey the rule of hydrophobic residues in positions A and D of the heptad of canonical leucine zippers. Underlined: amino acids that obey the rule of charged residues in positions E and G of the heptad. Starred: amino acids that disobey the rule for that position of the heptad of canonical leucine zippers. B: crude membranes from HEK-293 cells transiently transfected with WT AC8 or the indicated leucine to alanine (L/A) mutants of AC8 (L/A1 = L439A; L/A2 = L432A, L439A). Proteins (30 μg) were subjected to 7% polyacrylamide SDS-PAGE/immunoblotting. (The L/A2 lane strongly suggests lower expression of that construct relative to all others on this gel since all bands are reduced in intensity.) C: crude membranes prepared from HEK-293 cells transiently expressing WT AC8 or the indicated leucine zipper AC8 mutants (L/A1, L/A2, L/A3, or L/A4) were used to measure in vitro AC activity in the absence (basal) and presence of the indicated Ca2+ concentrations (see experimental procedures). D: cAMP accumulation was determined in intact HEK-293 cells transiently transfected with WT AC8 or the indicated leucine to alanine mutants of AC8. After replating in 24-well cluster plates, cells were pretreated with IBMX (100 μM) for 10 min, EGTA (100 μM) for 6 min, and thapsigargin (TG; 100 nM) for 4 min before a 1-min assay that included, or not, forskolin (FSK) (10 μM) and the indicated Ca2+ concentrations. Data, expressed as percentage of conversion of [3H]ATP into [3H]cAMP, are representative of three separate experiments performed in triplicate and are presented as means ± SE. E: fractionation of lipid raft and non-raft membranes with a detergent-free method of HEK-293 cells transiently transfected with either WT AC8 (E1), L/A1 (E2), or L/A4 mutant (E3). Ten equal fractions were taken from the top of the tube (fraction 1, top, lighter membrane fraction; fraction 10, bottom, heavier membrane fraction), diluted 1:10 in MBS (25 mM MES, pH 6.4, 150 mM NaCl, 250 mM sodium carbonate) and spun again for 1 h. The precipitated membrane pellet was resuspended in 1% SDS, and even volumes were loaded for SDS-PAGE; resolved proteins were immunoblotted with the indicated antibodies (AC8, caveolin, or β-adaptin). Sucrose and protein concentrations in each fraction were determined. F: in vivo cAMP accumulation was determined in intact HEK-293 cells transiently expressing AC8, in the presence of 2-aminoethoxydiphenyl borate (2-APB; 100 μM) or vehicle (DMSO, 1%), preincubated for 10 min before a 1-min assay with the indicated extracellular Ca2+ concentrations. G: cytosolic Ca2+ concentrations ([Ca2+]i)measured on HEK-293 cells preincubated for 10 min with DMSO (1%, control) or 2-APB (100 μM) (means ± SE of four independent experiments). Capacitative Ca2+ entry (CCE) was detected in the presence of 2 mM extracellular Ca2+. The arrows indicate the addition of TG and extracellular Ca2+.
Given the role of leucine zippers in protein-protein interactions and their conservation across AC isoforms, we considered the possibility that the leucine zipper motif of AC8 might play a role in oligomerization and/or protein-protein interaction and, thereby, influence its cellular targeting.
To reveal a possible role of leucine zippers in AC8, the motif was disrupted by site-directed mutagenesis. The critical leucines in position D of the heptad were progressively replaced with alanines (L/A mutants), up to a mutant in which all four leucines of the motif were replaced (L439A; L432A, L439A; L432A, L439A, L446A; L432A, L439A, L446A, L453A; these mutants for the sake of simplicity are also referred to as L/A1, L/A2, L/A3, and L/A4, respectively). To our surprise, the disruption of the leucine zipper motif did not significantly change the relative levels of AC8 dimers as seen by immunoblotting (Fig. 2B). This strongly suggests that the leucine zippers are not responsible for the dimer formation. However, there was a dramatic reduction in the levels of the N-glycosylated monomeric species of AC8 (the band running at 150 kDa), whereas the signal for the unglycosylated species increased (Fig. 2B). Given this dramatic consequence to mutations of the leucine zipper motif, we asked whether there were consequences for the activity of the AC8 in terms of regulation by CCE (in vivo) and Ca2+/calmodulin (in vitro). Whereas wild-type AC8 (WT-AC8) was robustly stimulated by CCE, all of the mutants were inactive, both in vitro (Fig. 2C) and in vivo (Fig. 2D). The dramatic effect of the leucine zipper mutations on both the N-linked glycosylation and activity of AC8 strongly implicated the importance of N-glycosylation for both the regulation and targeting of AC8, whereas the relevance of dimeric/aggregated forms to activity seems uncertain.
Leucine zipper mutants of AC8 localize outside of lipid rafts.
Previous studies revealed the essential residence of AC8 in lipid rafts for regulation by CCE, so we considered the possibility that the AC8 leucine zipper mutants might be excluded from lipid rafts. Consequently, HEK-293 cells transiently expressing either WT-AC8 or leucine zipper mutants were lysed by resuspension in 0.5 M bicarbonate buffer, pH 11.5, and subsequent sonication; this procedure breaks the cellular membranes in small fragments, stripping them of peripheral membrane and cytosolic proteins, and forming sheets of membranes, while at the same time preserving the integrity of the lipid bilayer with its integral membrane proteins (16). The membrane fragments obtained were fractionated by isopycnic centrifugation on a discontinuous sucrose gradient to separate low-density lipid rafts/caveolae enriched in cholesterol and sphingolipids, from high-density membranes. Raft membranes can be enriched in caveolin (42), whereas non-raft membranes are enriched in β-adaptin, a subunit of the PM adaptor complex of clathrin-coated pits and vesicles (35). When lipid raft and non-raft membranes were separated in different fractions, the monomeric N-glycosylated species of AC8 clearly cosedimented with the raft marker, caveolin (Fig. 2E1). In contrast, the monomeric unglycosylated AC8 forms were enriched in non-raft membranes, along with β-adaptin. In contrast to WT-AC8, the monomers of the leucine zipper AC8 mutants were predominantly enriched in non-raft membranes (Fig. 2, E2 and E3). The observations that the leucine zipper mutants appear no longer to be N-glycosylated and are excluded from lipid rafts suggest that N-glycosylation is responsible for raft-targeting and thereby the susceptibility to regulation by CCE.
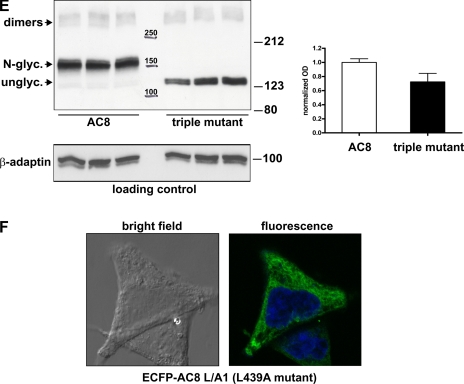
To examine further the cellular distribution of an exemplar leucine zipper mutant (L/A1), we placed a fluorescent enhanced cyan fluorescent protein (ECFP) tag upstream of the NH2 terminus. This construct, when examined by fluorescence confocal microscopy, is excluded from the PM and localizes intracellularly in a reticular pattern closely resembling ER staining (Fig. 4F).
In our in vivo experiments, the design is such that stores are passively depleted with the SERCA inhibitor, TG, which triggers Ca2+ entry through CCE, upon the addition of extracellular CaCl2 (40). However, to confirm that the effects were specifically mediated by Ca2+ entry through CCE channels and not by other means of Ca2+ entry, we assessed in vivo cAMP accumulation and intracellular Ca2+ concentration in the presence or absence of 2-APB, a specific inhibitor of CCE (9). The experiments demonstrated a 60% inhibition of Ca2+ entry in the presence of 2-APB (Fig. 2G, black trace), as compared with DMSO control (gray trace). When these conditions were applied to the effect of CCE on AC8, the 2-APB-induced reduction in Ca2+ entry was associated with a 60% inhibition of CCE-stimulation of AC8 activity, which directly confirms the role of CCE currents in the stimulation of AC8 activity (Fig. 2F).
Pharmacological inhibition of N-glycosylation of AC8 does not inhibit its activity.
To address directly whether N-glycosylation influences the raft-targeting and regulation of AC8, pharmacological tools were employed to inhibit the N-glycosylation of AC8.
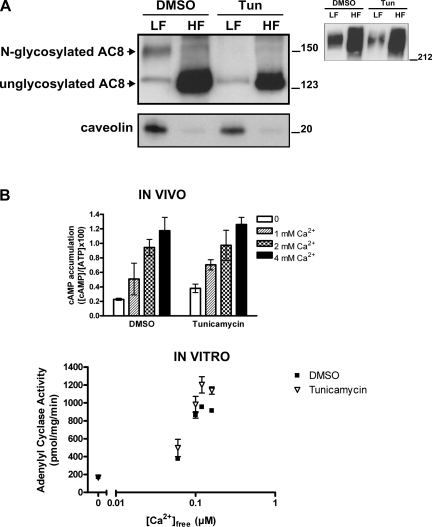
HEK-293 cells stably expressing AC8 were treated in vivo with drugs that interfere at different steps of the N-glycosylating pathway (11), and then membranes were fractionated into raft and non-raft fractions. Tunicamycin blocks the transfer of N-acetyl-glucosamine-1-phosphate (GlcNAc-1-P) from UDP-GlcNAc (UDP-N-acetylglucosamine) to dolichol phosphate in the first step of glycoprotein synthesis and therefore completely prevents the synthesis of all N-linked glycoproteins (43, 50). Treatment with tunicamycin in vivo modified the subsequent migration properties of AC8 on SDS gels (Fig. 3A) analogous to the in vitro removal of N-linked glycans using N-glycosidase F (compare with Fig. 1). Upon tunicamycin treatment, the majority of AC8 migrates at 125 kDa (Fig. 3A), which is compatible with the unglycosylated form of AC8 obtained with N-glycosidase F, rather than the N-glycosylated 150 kDa species (cf. Fig. 1). There is no discernible change in the high-molecular-weight species upon treatment with tunicamycin (Fig. 3A, inset).
Fig. 3.
Pharmacological inhibition of N-glycosylation of AC8 impairs localization of AC8 into lipid rafts but does not inhibit activity. A: HEK-293 cells transiently expressing AC8 were treated with 0.02% DMSO or 1 μg/ml tunicamycin (1.2 μM, Tun) for 48 h. Lipid rafts and non-raft membranes were prepared by a detergent-free method. After collection of 10 fractions, fractions 3, 4, and 5 (LF, light fractions) and fractions 7, 8, and 9 (HF, heavy fractions) were pooled, diluted in MBS, and centrifuged to pellet membranes, which were then analyzed by SDS-PAGE/immunoblotting for AC8 or caveolin (13 and 1.5 μg of proteins, respectively). Inset: immunoblotting of high-molecular-weight species of AC8. B, top: in vivo cAMP accumulation in HEK-293 cells transiently expressing AC8, exposed to drugs as in A. The CCE response was determined in the absence or presence of 1, 2, or 4 mM Ca2+. Bottom: HEK-293 cells transiently expressing AC8 were treated as in A. Crude membranes (4 μg) were used to measure in vitro AC activity in the absence (basal) and in the presence of the indicated Ca2+ concentrations. Data are presented as means ± SE of three independent experiments.
To explore the impact of N-glycosylation on the functionality of AC8, cells expressing AC8, treated with tunicamycin, were tested in in vivo cAMP accumulation assays to explore their regulation by CCE, or in in vitro AC assays (Fig. 3B). Tunicamycin had no significant effect on the regulation of AC8 by Ca2+ in vivo or in vitro (Fig. 3B).
When lipid raft and non-raft fractions were separated, the N-glycosylated population of AC8 clearly appeared in the caveolin-containing light fractions, but this band was absent following tunicamycin treatment (Fig. 3A). Given that following tunicamycin treatment, little of the AC8 appears in the light fractions, an increase in the more nonglycosylated AC8 might have been expected in the heavy fractions. The fact that this is not the case indicates that some of the AC8 must have been degraded. This is not surprising given that glycoproteins require carbohydrates for enhanced stability (11). Nevertheless, AC8 is still fully active following tunicamycin treatment, indicating that, despite any degradation, sufficient levels of AC8 remained to generate a maximal response to Ca2+, both in vivo and in vitro (Fig. 3B).
More surprising was the fact that cells treated with tunicamycin were still responsive to CCE, given that the localization of AC8 in lipid rafts was considered essential for regulation by CCE. Nevertheless, we were open to the possibility that a small fraction of N-glycosylated AC8 remained in the lipid rafts following tunicamycin treatment, since drug treatment might not have been fully effective at precluding N-glycosylation of already synthesized AC8.
N-glycosylation-defective mutants of AC8 are localized at the plasma membrane.
Pharmacological inhibitors of N-glycosylation affect all cellular glycoproteins. The results observed in Fig. 3 may therefore not necessarily reflect the specific deglycosylation of AC8, but also side effects of the deglycosylation of other proteins. As mentioned previously, it is also not possible to be convinced that glycosylation inhibitors are fully effective at precluding the glycosylation of all of the AC8. A more direct strategy therefore was adopted of generating mutants of AC8 that could not be N-glycosylated and testing their responsiveness to CCE in vivo. Such mutants ensure that none of the AC8 expressed is N-glycosylated, and the generation of these mutants circumvents the issues arising from the use of pharmacological inhibitors of N-glycosylation.
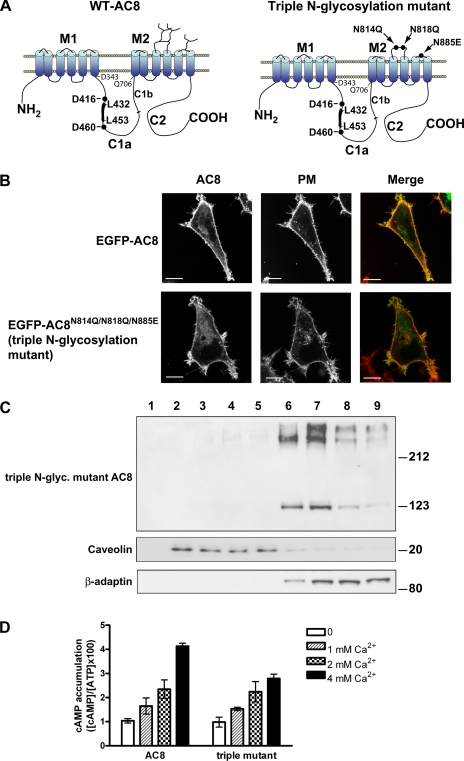
Three different asparagines are predicted to be N-glycosylated on AC8, N814, N818, and N885 (2). These N-glycosylation sites are localized on the second and third extracellular loop of the second transmembrane cassette of AC8 and were mutated to glutamine (asparagines 814 and 818) and glutamic acid (asparagine 885) (AC8N814Q/N818Q/N885E, the “triple N-glycosylation mutant”) (Fig. 4A). Replacement of asparagines with glutamine or glutamic acid was chosen because of similarities in the chemical properties (asparagine and glutamine are both amides) and in amino acid side chain lengths.
Fig. 4.
An N-glycosylation-defective mutant of AC8 is localized in non-raft membranes but is still stimulated by CCE. A: schematic showing the predicted topology of AC8 with its potential sites of N-glycosylation in the extracellular loops 5 and 6, located on the second transmembrane cassette. Those sites were mutated by site-directed mutagenesis into glutamines (asparagines 814 and 818) and glutamic acid (asparagine 885). In the C1a loop, the position of the leucine zipper motif is highlighted, with the two leucines at the beginning and at the end of the motif. Two black circles highlight the two aspartate residues involved in the coordination of Mg2+ in the catalytic site. B: HEK-293 cells transiently transfected with enhanced green fluorescent protein (EGFP)-tagged versions of WT or the triple N-glycosylation mutant of AC8 were plated onto glass-bottom dishes coated with poly-l-lysine, stained with 5 μg/ml CellMask Deep Red plasma membrane (PM) stain, and imaged by confocal imaging. C: lipid rafts and bulk non-raft membranes were prepared from HEK-293 cells transiently expressing the triple N-glycosylation-defective mutant of AC8 (AC8N814Q/N818Q/N855E). Even volumes of fractionated membranes were subjected to SDS-PAGE/immunoblotting for the detection of the indicated proteins. D: response to CCE of HEK-293 cells transiently transfected with WT AC8 or the triple N-glycosylation-defective mutant of AC8 (AC8N814Q/N818Q/N855E) in the presence of the indicated concentrations of Ca2+. Data represent at least three separate experiments. E: relative quantification of the expression of WT-AC8 vs. the triple N-glycosylation mutant AC8. Crude membranes were prepared from HEK-293 cells transiently expressing WT-AC8 or the triple N-glycosylation mutant of AC8. Twenty-five micrograms of each crude membrane preparation were loaded on 7% acrylamide gels, in triplicate, for SDS-PAGE and immunoblotting for AC8 or β-adaptin. Optical densities (OD) (see experimental procedures) for each lane were normalized to an internal loading control (β-adaptin) and used to calculate means ± SD. ODs were expressed relatively to the WT-AC8 values. F: enhanced cyan fluorescent protein (ECFP)-tagged AC8 L/A1 mutant expressed in HEK-293 cells was imaged by confocal microscopy. Fluorescence image is an overlay of CFP and 4′,6-diamidino-2-phenylindole.
By EGFP-tagging these proteins (the WT and the mutant AC8) at the NH2 terminus, the consequences for their cellular targeting could also be addressed. Confocal analysis of live HEK-293 cells expressing EGFP-AC8 showed predominant PM targeting (Fig. 4B). PM targeting was verified by the substantial overlap in signals from cells where the PM had been labeled with CellMask Deep Red PM stain (Fig. 4B). EGFP-AC8N814Q/N818Q/N885E was also targeted to the PM, with most cells showing substantial overlap with the PM marker (Fig. 4B). The distribution of the triple AC8 N-glycosylation-deficient mutant and WT AC8 appeared similar, which suggests that the N-glycosylation sites on the 5th and 6th extracellular loops do not determine PM targeting. Similar results were obtained with a double N-glycosylation-deficient mutant of AC8 (EGFP-AC8N814Q/N818Q; data not shown).
N-glycosylation-defective mutants of AC8 are localized outside of lipid rafts.
Despite not being important for the gross targeting of AC8 to the PM, our data with the leucine zipper mutants of AC8 and the pharmacological inhibitors of N-glycosylation lead us to speculate that N-glycosylation is important for targeting AC8 into lipid rafts. The membrane distribution of AC8N814Q/N818Q/N885E was therefore assessed (Fig. 4C). The triple mutant, AC8N814Q/N818Q/N855E, was concentrated in non-raft membranes, as shown by cofractionation with the β-adaptin marker, with almost no overlap with the caveolar marker. The high-molecular-weight species (dimers) were also localized outside of rafts (Fig. 4C). Similar results were obtained with the “double N-glycosylation-deficient mutant of AC8” (AC8N814Q/N818Q; data not shown).
N-glycosylation-defective mutants of AC8 are active and respond fully to CCE.
To assess whether the N-glycosylation-deficient mutant is regulated by CCE, in vivo cAMP accumulation experiments were performed in HEK-293 cells transiently expressing WT AC8 or the triple N-glycosylation mutant (AC8N814Q/N818Q/N855E)(Fig. 4D). These experiments revealed that the triple N-glycosylation-defective mutant of AC8 is fully responsive to CCE. Differences in maximal activities between the products of the transfected constructs can be attributed to modest differences in the levels of expression of the transfected enzymes. [A representative immunoblot is presented, where crude membranes prepared from the relevant HEK-293 cells were loaded in triplicate. Films were scanned, and the optical densities of lanes were measured with ImageJ software and expressed as a function of the loading marker, β-adaptin. Approximately 75% as much of the N-glycosylation mutant is expressed as the WT AC8 (Fig. 4E)]. The NH2-terminally EGFP-tagged versions of WT AC8 and AC8N814Q/N818Q/N855E are also fully responsive to CCE (data not shown), which shows that the EGFP tag has no artifactual effects on their regulation.
The functional analysis of the N-glycosylation-defective mutant of AC8 reinforces the data obtained with the pharmacological inhibitors, i.e., that N-glycosylation of AC8 is not necessary for the regulation of its enzymatic activities (Figs. 3B and 4D). Following tunicamycin treatment, the amount of AC8 in rafts was dramatically reduced (Fig. 3A) and the N-glycosylation-defective mutant of AC8 is mainly, if not exclusively, concentrated outside of rafts (Fig. 4C). These data strongly suggest that N-glycosylation is a key element for the targeting of AC8 to lipid rafts. However, the fact that the AC8 N-glycosylation-defective mutant can respond to CCE, despite its localization in non-raft membranes, was quite surprising, given the current view of the stimulation of AC8 by CCE, which envisages colocalization of AC8 and CCE channels in the same lipid raft microdomain.
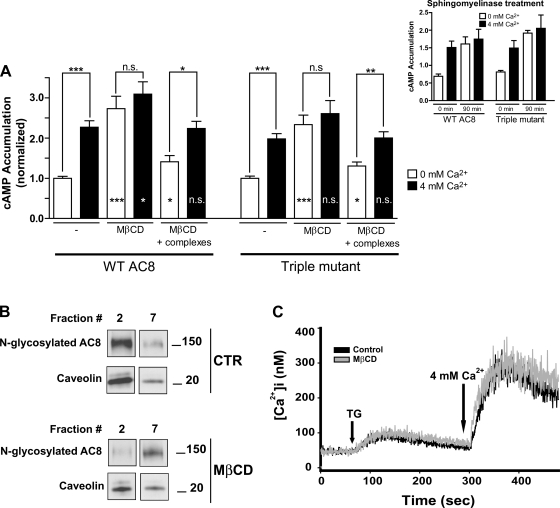
The cholesterol-depleting agent MβCD is widely employed to disrupt lipid rafts in cultured cells, because the bulky and hydrophobic cholesterol molecules of the membrane are extracted by the cavity of the cyclodextrin ring (36). The residence of AC8 in lipid rafts was previously believed to be essential for its regulation by CCE, since disruption of rafts by MβCD caused the loss of CCE responsiveness of AC8 (48). Thus, in the light of our new findings, we compared the consequences for the regulation by CCE of wild-type and N-glycosylation mutant AC8 following MβCD treatment. As expected, disruption of lipid rafts with MβCD fully ablated the regulation of WT AC8 by CCE (Fig. 5A). Disruption of lipid rafts by MβCD also results in a dramatic reduction of the levels of monomeric AC8 in lipid rafts, accompanied by an increase of monomeric AC8 in non-raft membranes (cf. fraction 2 vs. 7 in Fig. 5B). Strikingly, however, the regulation of the triple N-glycosylation mutant by CCE was also ablated by MβCD treatment (Fig. 5A). This latter result is quite surprising, given that the N-glycosylation-deficient mutant does not demonstrably reside in lipid rafts (Fig. 4C). To address the possibility that MβCD was exerting nonspecific effects unrelated to its extraction of cholesterol, cells were also treated with MβCD/cholesterol complexes to restore cholesterol levels to MβCD-treated cells. This latter treatment substantially restored sensitivity to CCE of both the WT and triple glycosylation mutant (cf. Fig. 5A). Interestingly, the experiment further reveals that MβCD treatment causes the basal, unstimulated activity of both AC8 species to be significantly higher than in control cells (P < 0.0005), which might suggest the dissociation of a raft-based protein that inhibits AC8 activity (Fig. 5A). Even in the MβCD/cholesterol-treated cells, some residue of elevated basal activity remained. Ca2+ measurements in cell populations loaded with Fura-2 established that CCE was unaffected by the MβCD treatment (Fig. 5C).
Fig. 5.
Disruption of lipid rafts ablates the regulation by CCE of the N-glycosylation-defective mutant of AC8. A: in vivo cAMP accumulation on disruption of lipid rafts by cholesterol extraction (methyl-β-cyclodextrin; MβCD) followed by cholesterol replenishment (cholesterol-MβCD complexes). HEK-293 cells transiently transfected with either WT or triple N-glycosylation-defective mutant of AC8 (AC8N814Q/N818Q/N855E) were incubated with 2-[3H]adenine for 45 min, followed by incubation for 45 min with both 2-[3H]adenine and 10 mM MβCD in medium without serum, which was followed, or not, by incubation with cholesterol-MβCD complexes for 45 min (all assays were ±4 mM extracellular Ca2+; see Fig. 2). Data represent means ± SE of at least 3 separate experiments performed in triplicate. Conditions were compared using two-tailed Student's t-test. P < 0.05 was considered significant (***P < 0.0005, **P < 0.005, *P < 0.05; ns, not significant). Statistical significances drawn within bars are relative to their own control (i.e., without MβCD). Inset: in vivo cAMP accumulation on disruption of lipid rafts by sphingomyelinase treatment. Shown are means ± SE of one representative experiment. B: fractionation of raft and non-raft membranes on disruption of lipid rafts by cholesterol extraction. HEK-293 cells stably expressing WT AC8 were incubated for 1 h in medium without serum ±10 mM MβCD. Raft and non-raft membranes were then prepared (see experimental procedures). Aliquots from each fraction were probed for AC8 and caveolin by SDS-PAGE followed by immunoblotting. Signals from fractions 2 and 7 are shown, representative of raft and non-raft membranes, respectively. Fraction 2: 7.5–23% sucrose (top to bottom of the fraction); fraction 7: 33–39.5% sucrose (top to bottom of the fraction). CTR, control. C: measurement of intracellular Ca2+ concentration was performed as indicated in experimental procedures in HEK-293 cells treated with and without 10 mM MβCD for 1 h in medium without serum. Application of 100 nM TG and 4 mM extracellular Ca2+ is indicated by arrows.
As an independent means of assessing the importance of raft integrity to the regulation of both WT and mutant AC8 by CCE, cells were treated with sphingomyelinase (an enzyme with sphingomyelin-specific phospholipase C activity), which, through degrading sphingomyelins, allows cholesterol to be reassimilated from the PM into the ER (34). Sphingomyelinase treatment resulted in a directly analogous increase in basal activity of both AC8 forms and a loss of stimulation by CCE (Fig. 5A, inset).
DISCUSSION
This study has been the first to attempt to address the targeting and regulation of a Ca2+-regulated AC and a possible role of the leucine zipper in this process. Overall, the data suggest an essential role of N-glycosylation in the targeting of AC8 to lipid rafts and additionally that AC8 can respond to CCE even when residing outside of lipid rafts.
Initially, we endeavored to explore whether the leucine zipper motif present in the C1a domain of AC8 served a functional role in promoting the multimeric assembly and/or targeting of AC8. Mutagenesis (Fig. 2B) showed that the integrity of the leucine zipper motif is important for promoting efficient N-linked glycosylation of monomeric AC8, while multimeric species persisted (Fig. 2B). Indeed, it turns out that the leucine zipper motif (spanning residues 429–458) is contained within the C1a catalytic domain of AC8, between the key aspartate residues (D416 and D460) that coordinate Mg2+ atoms in the catalytic site (Fig. 4A). From the crystal structure of the catalytic domain of AC5, which is highly (60%) homologous to that of AC8 (51), it is clear that in the mature structure of associated C1a and C2a domains no leucine zipper coiled coil three-dimensional structure can be adopted. All of the leucines belong to the same α-helix, are orientated toward the deep part of the C1a domain, and are involved in interactions with the side chains of other amino acids, so that they are not exposed to the surface and are not available for interactions with side chains of amino acids of other proteins. Therefore, it seems likely that during the processing of ACs the leucine zipper sequences are a basis for interaction with chaperone molecules, and that by such an interaction the correct trafficking of the protein is controlled. In this regard, it is known that one of the molecular mechanisms whereby chaperones act is through the interaction of coiled coils with hydrophobic regions of unfolded proteins (31, 45). Mutation of the leucine zipper residues results in forms that are unglycosylated and are excluded from lipid rafts (Fig. 2E), and, indeed, they do not even reach the PM (Fig. 4F) and so are grossly mistargeted. These data led us to speculate that N-glycosylation of AC8 is required for, or reflects, the targeting of AC8 into lipid rafts.
A combination of mutagenesis studies and experiments using specific inhibitors of N-glycosylation revealed that N-glycosylation is not required for the regulation of AC8 by Ca2+ in vitro. This was somewhat unexpected because N-glycosylation has previously been shown to influence the regulatory properties of AC6 (56). However, N-glycosylation does affect the targeting of AC8. Although confocal microscopy revealed that N-glycosylation was not required to target AC8 to the PM (Fig. 4B), fractionation studies showed that, unlike AC8, the AC8 N-glycosylation-deficient mutant was excluded from lipid rafts (Fig. 4C). The cytoplasmic domains of AC8 were previously demonstrated to be important for raft targeting (8). Those data led us to propose that protein-protein interactions involving the cytoplasmic domains of AC8 govern targeting into lipid raft domains. However, the AC8 N-glycosylation-deficient mutant possesses intact cytoplasmic domains, and yet it is excluded from lipid rafts. It is therefore possible that N-glycosylation is important for the mediation of any protein-protein interaction (possibly through the cytoplasmic domains) that targets AC8 into rafts.
Despite being apparently excluded from lipid rafts, the AC8 N-glycosylation-deficient mutant is fully responsive to CCE (Fig. 4D). This was a surprise, since it was previously believed that the residence of AC8 in lipid rafts was necessary for its regulation by CCE (48). This conclusion had been based on experiments in which the regulation of AC8 by CCE was lost following the disruption of rafts using MβCD (48). Indeed, again in the present study, MβCD treatment ablated the wild-type AC8 response to CCE (Fig. 5A). Furthermore, we now show that MβCD treatment moves AC8 out of rafts (Fig. 5B). However, the regulation of the non-raft-residing N-glycosylation mutant of AC8 was also ablated following MβCD treatment. These data raise a number of possibilities. 1) The AC8 N-glycosylation mutant depends on the integrity of lipid rafts to respond to CCE, despite not being localized directly—or measurably by these methods—within them. Protein-protein interactions have previously been implicated in the targeting of AC8 into lipid raft domains (8). It is conceivable that the N-glycosylation-deficient mutant of AC8 still associates loosely with lipid raft associated proteins through such an interaction, an interaction that is destroyed following MβCD treatment. This model could also help explain the increase in the basal activity that is observed following MβCD treatment with the AC8 N-glycosylation-deficient mutants and wild-type AC8, for instance, reflecting a direct or indirect association between AC8 and a putative lipid raft associated protein, which exerts a tonic inhibitory influence on basal activity. 2) Lipid rafts are not static structures (26, 41), and it is conceivable that AC8 moves between raft and non-raft membranes. Our fractionation studies reveal a biochemical— necessarily artificial—snapshot of the membrane distribution of AC8, which cannot address the dynamics of AC8-raft association. It is therefore possible that, unlike the wild-type AC8, the AC8 N-glycosylation-deficient mutant associates with lipid rafts but is more mobile/dynamic in these associations, which makes it harder to detect its presence in biochemically isolated raft domains. Techniques such as fluorescence recovery after photobleaching (FRAP) could compare the relative mobility of wild-type AC8 and the AC8 N-glycosylation-deficient mutant. 3) A final possibility is that MβCD exerts a nonspecific effect, which is independent of lipid raft integrity, and that raft localization is not at all implicated in the regulation of AC8 by CCE. This possibility seems to be excluded by the reversibility of the MβCD effect by treating depleted cells with an MβCD/cholesterol mixture (Fig. 5A). Mimicking of the MβCD ablation of CCE responsiveness by the sphingomyelinase treatment (Fig. 5A, inset) speaks very strongly to the critical role of the lipid environment in maintaining responsiveness to CCE. Sphingomyelinase, by producing ceramide and phosphorylcholine, allows cholesterol to be removed from the PM less harshly (34) and strongly underlines the importance of these cholesterol/sphingomyelin platforms for the integrity of the CCE-AC8 interaction.
The present study has illuminated the trafficking and function of a Ca2+-sensitive adenylyl cyclase. The leucine zipper turns out to be a critical targeting motif, although the protein or chaperones with which it may associate to ensure its appropriate processing remain to be determined. Clearly, if this motif is mutated, AC8 is not processed—or N-glycosylated—appropriately. Further down the chain, N-glycosylation seems essential for stable raft localization; however, demonstrably stable, or biochemically robust, raft association is not essential for the regulation of AC8 by CCE. Consequently, we are refining our understanding of raft association of AC8 so that whereas the integrity of lipid rafts continues to be essential, transient or ephemeral associations may be adequate to allow the regulation of AC8 by CCE.
GRANTS
This work was supported by the Wellcome Trust. D. M. F. Cooper is a Royal Society Wolfson Research fellow.
Acknowledgments
The authors are grateful to our colleagues Drs. A. C. Martin and D. Willoughby for critical discussion of the manuscript and invaluable advice, and to Dr. Luca Pellegrini for helpful discussions.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Baragli A, Grieco ML, Trieu P, Villeneuve LR, Hebert TE. Heterodimers of adenylyl cyclases 2 and 5 show enhanced functional responses in the presence of Galpha s. Cell Signal 20: 480–492, 2008. [DOI] [PubMed] [Google Scholar]
- 2.Cali JJ, Parekh RS, Krupinski J. Splice variants of type VIII adenylyl cyclase. Differences in glycosylation and regulation by Ca2+/calmodulin. J Biol Chem 271: 1089–1095, 1996. [DOI] [PubMed] [Google Scholar]
- 3.Cali JJ, Zwaagstra JC, Mons N, Cooper DMF, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem 269: 12190–12195, 1994. [PubMed] [Google Scholar]
- 4.Chen-Goodspeed M, Lukan AN, Dessauer CW. Modeling of Galpha(s) and Galpha(i) regulation of human type V and VI adenylyl cyclase. J Biol Chem 280: 1808–1816, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol 7: 2745–2752, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooper DMF, Karpen JW, Fagan KA, Mons NE. Ca2+-sensitive adenylyl cyclases. Adv Second Messenger Phosphoprotein Res 32: 23–51, 1998. [PubMed] [Google Scholar]
- 7.Cooper DMF, Crossthwaite AJ. Higher-order organization and regulation of adenylyl cyclases. Trends Pharmacol Sci 27: 426–431, 2006. [DOI] [PubMed] [Google Scholar]
- 8.Crossthwaite AJ, Seebacher T, Masada N, Ciruela A, Dufraux K, Schultz JE, Cooper DMF. The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J Biol Chem 280: 6380–6391, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Dehaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW Jr. Complex actions of 2-aminoethyldiphenyl borate on store-operated calcium entry. J Biol Chem 283: 19265–19273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ding Q, Gros R, Chorazyczewski J, Ferguson SS, Feldman RD. Isoform-specific regulation of adenylyl cyclase function by disruption of membrane trafficking. Mol Pharmacol 67: 564–571, 2005. [DOI] [PubMed] [Google Scholar]
- 11.Elbein AD Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J 5: 3055–3063, 1991. [DOI] [PubMed] [Google Scholar]
- 12.Evans T, Smith MM, Tanner LI, Harden TK. Muscarinic cholinergic receptors of two cell lines that regulate cyclic AMP metabolism by different molecular mechanisms. Mol Pharmacol 26: 395–404, 1984. [PubMed] [Google Scholar]
- 13.Fagan KA, Mahey R, Cooper DMF. Functional co-localization of transfected Ca2+-stimulable adenylyl cyclases with capacitative Ca2+ entry sites. J Biol Chem 271: 12438–12444, 1996. [DOI] [PubMed] [Google Scholar]
- 14.Fagan KA, Mons N, Cooper DMF. Dependence of the Ca2+-inhibitable adenylyl cyclase of C6–2B glioma cells on capacitative Ca2+ entry. J Biol Chem 273: 9297–9305, 1998. [DOI] [PubMed] [Google Scholar]
- 15.Fagan KA, Smith KE, Cooper DMF. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J Biol Chem 275: 26530–26537, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Fujiki Y, Hubbard AL, Fowler S, Lazarow PB. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol 93: 97–102, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- 18.Gu C, Cali JJ, Cooper DMF. Dimerization of mammalian adenylate cyclases. Eur J Biochem 269: 413–421, 2002. [DOI] [PubMed] [Google Scholar]
- 19.Gu C, Sorkin A, Cooper DMF. Persistent interactions between the two transmembrane clusters dictate the targeting and functional assembly of adenylyl cyclase. Curr Biol 11: 185–190, 2001. [DOI] [PubMed] [Google Scholar]
- 20.Hancock JF Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol 7: 456–462, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanoune J, Pouille Y, Tzavara E, Shen T, Lipskaya L, Miyamoto N, Suzuki Y, Defer N. Adenylyl cyclases: structure, regulation and function in an enzyme superfamily. Mol Cell Endocrinol 128: 179–194, 1997. [DOI] [PubMed] [Google Scholar]
- 22.Head BP, Patel HH, Roth DM, Murray F, Swaney JS, Niesman IR, Farquhar MG, Insel PA. Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J Biol Chem 281: 26391–26399, 2006. [DOI] [PubMed] [Google Scholar]
- 23.Hulme JT, Westenbroek RE, Scheuer T, Catterall WA. Phosphorylation of serine 1928 in the distal C-terminal domain of cardiac CaV1.2 channels during beta1-adrenergic regulation. Proc Natl Acad Sci USA 103: 16574–16579, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kenworthy AK Peering inside lipid rafts and caveolae. Trends Biochem Sci 27: 435–438, 2002. [DOI] [PubMed] [Google Scholar]
- 25.Klein U, Gimpl G, Fahrenholz F. Alteration of the myometrial plasma membrane cholesterol content with beta-cyclodextrin modulates the binding affinity of the oxytocin receptor. Biochemistry 34: 13784–13793, 1995. [DOI] [PubMed] [Google Scholar]
- 26.Kusumi A, Suzuki K. Toward understanding the dynamics of membrane-raft-based molecular interactions. Biochim Biophys Acta 1746: 234–251, 2005. [DOI] [PubMed] [Google Scholar]
- 27.Landschulz WH, Johnson PF, McKnight SL. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science 240: 1759–1764, 1988. [DOI] [PubMed] [Google Scholar]
- 28.Lenne PF, Wawrezinieck L, Conchonaud F, Wurtz O, Boned A, Guo XJ, Rigneault H, He HT, Marguet D. Dynamic molecular confinement in the plasma membrane by microdomains and the cytoskeleton meshwork. EMBO J 25: 3245–3256, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lockwich TP, Liu X, Singh BB, Jadlowiec J, Weiland S, Ambudkar IS. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J Biol Chem 275: 11934–11942, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Martin AC, Cooper DMF. Capacitative and 1-oleyl-2-acetyl-sn-glycerol-activated Ca2+ entry distinguished using adenylyl cyclase type 8. Mol Pharmacol 70: 769–777, 2006. [DOI] [PubMed] [Google Scholar]
- 31.Martin J, Gruber M, Lupas AN. Coiled coils meet the chaperone world. Trends Biochem Sci 29: 455–458, 2004. [DOI] [PubMed] [Google Scholar]
- 32.Mons N, Decorte L, Jaffard R, Cooper DMF. Ca2+-sensitive adenylyl cyclases, key integrators of cellular signalling. Life Sci 62: 1647–1652, 1998. [DOI] [PubMed] [Google Scholar]
- 33.Munro S Lipid rafts: elusive or illusive? Cell 115: 377–388, 2003. [DOI] [PubMed] [Google Scholar]
- 34.Neufeld EB, Cooney AM, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem 271: 21604–21613, 1996. [DOI] [PubMed] [Google Scholar]
- 35.Nichols BJ GM1-containing lipid rafts are depleted within clathrin-coated pits. Curr Biol 13: 686–690, 2003. [DOI] [PubMed] [Google Scholar]
- 36.Ohtani Y, Irie T, Uekama K, Fukunaga K, Pitha J. Differential effects of alpha-, beta- and gamma-cyclodextrins on human erythrocytes. Eur J Biochem 186: 17–22, 1989. [DOI] [PubMed] [Google Scholar]
- 37.Oliveria SF, Dell'Acqua ML, Sather WA. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55: 261–275, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pani B, Ong HL, Liu X, Rauser K, Ambudkar IS, Singh BB. Lipid rafts determine clustering of STIM1 in ER-plasma membrane junctions and regulation of SOCE. J Biol Chem 283: 17333–17340, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Putney JW Pharmacology of capacitative calcium entry. Mol Interv 1: 84–94, 2001. [PubMed] [Google Scholar]
- 40.Putney JW Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here). Cell Calcium 42: 103–110, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajendran L, Simons K. Lipid rafts and membrane dynamics. J Cell Sci 118: 1099–1102, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Rothberg KG, Heuser JE, Donzell WC, Ying YS, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell 68: 673–682, 1992. [DOI] [PubMed] [Google Scholar]
- 43.Schneider EG, Nguyen HT, Lennarz WJ. The effect of tunicamycin, an inhibitor of protein glycosylation, on embryonic development in the sea urchin. J Biol Chem 253: 2348–2355, 1978. [PubMed] [Google Scholar]
- 44.Shuttleworth TJ, Thompson JL. Discriminating between capacitative and arachidonate-activated Ca2+ entry pathways in HEK293 cells. J Biol Chem 274: 31174–31178, 1999. [DOI] [PubMed] [Google Scholar]
- 45.Siegert R, Leroux MR, Scheufler C, Hartl FU, Moarefi I. Structure of the molecular chaperone prefoldin: unique interaction of multiple coiled coil tentacles with unfolded proteins. Cell 103: 621–632, 2000. [DOI] [PubMed] [Google Scholar]
- 46.Simons K, Ikonen E. Functional rafts in cell membranes. Nature 387: 569–572, 1997. [DOI] [PubMed] [Google Scholar]
- 47.Simpson RE, Ciruela A, Cooper DMF. The role of calmodulin recruitment in Ca2+-stimulation of adenylyl cyclase type 8. J Biol Chem 281: 17379–17389, 2006. [DOI] [PubMed] [Google Scholar]
- 48.Smith KE, Gu C, Fagan KA, Hu B, Cooper DMF. Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca2+ entry. J Biol Chem 277: 6025–6031, 2002. [DOI] [PubMed] [Google Scholar]
- 49.Sunahara RK, Taussig R. Isoforms of mammalian adenylyl cyclase: multiplicities of signaling. Mol Interv 2: 168–184, 2002. [DOI] [PubMed] [Google Scholar]
- 50.Takatsuki A, Arima K, Tamura G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J Antibiot (Tokyo) 24: 215–223, 1971. [DOI] [PubMed] [Google Scholar]
- 51.Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα.GTPγS. Science 278: 1907–1916, 1997. [DOI] [PubMed] [Google Scholar]
- 52.Toya Y, Schwencke C, Couet J, Lisanti MP, Ishikawa Y. Inhibition of adenylyl cyclase by caveolin peptides. Endocrinology 139: 2025–2031, 1998. [DOI] [PubMed] [Google Scholar]
- 53.Van Deurs B, Roepstorff K, Hommelgaard AM, Sandvig K. Caveolae: anchored, multifunctional platforms in the lipid ocean. Trends Cell Biol 13: 92–100, 2003. [DOI] [PubMed] [Google Scholar]
- 54.Vinson C, Myakishev M, Acharya A, Mir AA, Moll JR, Bonovich M. Classification of human B-ZIP proteins based on dimerization properties. Mol Cell Biol 22: 6321–6335, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Willoughby D, Cooper DMF. Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol Rev 87: 965–1010, 2007. [DOI] [PubMed] [Google Scholar]
- 56.Wu GC, Lai HL, Lin YW, Chu YT, Chern Y. N-glycosylation and residues Asn805 and Asn890 are involved in the functional properties of type VI adenylyl cyclase. J Biol Chem 276: 35450–35457, 2001. [DOI] [PubMed] [Google Scholar]