Abstract
Recent biosocial theories postulate that both biological risk and the social context influence the development of mental health problems (Boyce & Ellis, 2005). Guided by this framework, we examined whether basal cortisol and its diurnal rhythm were associated with mental health symptoms in early adolescence. Because cross-sectional and longitudinal investigations sometimes reveal different cortisol-mental health associations, we examined the association both concurrently and longitudinally when children transition to middle school, a time which entails a major change in social context from single to multiple teachers, classrooms, and sets of classmates. Salivary cortisol was measured three times a day (waking, afternoon, and bedtime) across three days when adolescents were 5th graders. Mental health was measured when adolescents were in 5th and 7th grades, just before and after the transition to middle school. To deal with the substantial comorbidity of internalizing and externalizing symptoms at this developmental stage, mental health measures distinguished overall symptom severity from the preponderance of internalizing vs. externalizing symptoms (i.e., directionality). A three-level Hierarchical Linear Model was used to extract basal cortisol and its diurnal rhythm separate from the day-to-day and within-the-day fluctuations in cortisol in response to daily experiences. Results were specific to symptom severity, suggesting that cortisol is a nonspecific risk factor for mental health symptoms in young adolescents. At 5th grade, low basal cortisol was associated with concurrent symptom severity. However, longitudinally, it was adolescents with high cortisol at 5th grade who were at risk for increasing mental health symptoms by 7th grade. Flat diurnal rhythms in 5th grade were related to levels of symptom severity at both 5th and 7th grades. Considering the change in social context, as defined by the transition to middle school, helped resolve seemingly inconsistent evidence that both hypo- and hyper-arousal were associated with mental health symptoms in early adolescence.
Keywords: Cortisol, mental health, adolescence, longitudinal, hierarchical linear modeling, stress
Introduction
A growing body of literature links Hypothalamic-Pituitary-Adrenal (HPA) axis activity with mental health problems across the lifespan, including both internalizing and externalizing problems (Gunnar & Vazquez, 2006; van Goozen, Fairchild, Snoek, & Harold, 2007). Cortisol is important for a wide variety of adaptive functions (de Kloet, 2003), is implicated directly in affect-related brain areas and neural processes (Gunnar & Quevedo, 2007), and thus may provide a window into one of the broad mechanisms whereby children’s biosocial development puts them at risk for mental health problems. Although there is considerable research on the linkages between cortisol and mental health symptoms and disorders in adults (Burke, Davis, Otte, & Mohr, 2005; Raine, 2002), relatively little attention has been paid to the period of early adolescence even though it is a time of developmental alterations in the stress system and heightened risk for mental health problems (Spear, 2000). Further, for several conceptual and methodological reasons, many inconsistencies exist in the findings. In the present study, we address these issues to investigate whether and how cortisol is associated with internalizing and externalizing symptoms in early adolescence.
Studies in children and adolescents have shown associations of both HPA hypo-arousal (i.e., low cortisol) and hyper-arousal (i.e., high cortisol) with both internalizing (i.e., anxiety and depression) and externalizing (i.e., oppositional/conduct and inattention/impulsivity) problems (Angold, 2003; Gunnar & Vazquez, 2006; van Goozen et al., 2007). Some studies considered only one internalizing or externalizing disorder or symptom type (e.g., depression or conduct problems), but because of the very high comorbidity within internalizing and externalizing problems in childhood and early adolescence (Angold, Costello, & Erkanli, 1999), we will generally review this literature within these two broader domains of problems.
Cross-sectional studies have generally shown that low cortisol is associated with internalizing symptoms in children (De Bellis, Dahl, Perel, & Birmaher, 1996; Granger et al., 1998); however, one study of normally developing adolescents found high cortisol was associated with internalizing problems (Colomina, Canals, Carbajo, & Domingo, 1997). Similarly the majority of cross-sectional studies have shown an association between low cortisol and externalizing problems (Kariyawasam, Zaw, & Handley, 2002; Pajer, Gardner, Rubin, Perel, & Neal, 2001; Shirtcliff, Granger, Booth, & Johnson, 2005; van Goozen, Matthys, Cohen-Kettenis, Gispen-de Wied et al., 1998; Vanyukov et al., 1993).
Longitudinal studies have shown somewhat different results. High cortisol in normally developing preschoolers predicted internalizing symptoms as they transitioned into primary school (Smider et al., 2002). Further, children who later developed clinical depression had higher morning cortisol (Goodyer, Herbert, Moor, & Altham, 1991; Goodyer, Herbert, Tamplin, & Altham, 2000; Goodyer, Park, & Herbert, 2001) and evening cortisol (Goodyer et al., 1991; Goodyer et al., 1996; Herbert et al., 1996; Rao et al., 1996). Conversely, several longitudinal investigations have revealed that children and adolescents with low cortisol levels have higher externalizing symptoms in clinical samples (McBurnett, Lahey, Rathouz, & Loeber, 2000) and children at risk (Moss, Vanyukov, & Martin, 1995).
In addition to the direction of the findings being controversial, some studies have failed to find cortisol links with either internalizing (Dahl et al., 1989) or externalizing problems (Kruesi, Schmidt, Donnelly, Hibbs, & Hamburger, 1989; Targum, Clarkson, Magac-Harris, Marshall, & Skwerer, 1990). There may be several reasons for the inconsistencies in the findings of the association between child and adolescent cortisol and mental health problems. First, some of the inconsistencies may result from a general lack of consideration of the high comorbidity of internalizing and externalizing problems in younger populations (Angold et al., 1999). Early adolescence represents a developmental window in which trajectories are still being established, so adolescents may frequently express comorbid symptoms because they have not yet canalized along a particular pathway (Boyce & Ellis, 2005; Boyce et al., 2002). Although not always replicated (McBurnett et al., 2000), studies have found that cortisol levels are higher in comorbid youth than when externalizing behavior is more pure (McBurnett et al., 1991; van Goozen, Matthys, Cohen-Kettenis, Thijssen, & van Engeland, 1998). Similarly, Essex and colleagues (2002) showed preschoolers with high afternoon cortisol are most likely to develop comorbid internalizing and externalizing symptoms at the end of the transition to primary school. Studies which have examined the diurnal rhythm have found that comorbid youth have the most blunted diurnal rhythms (Cicchetti & Rogosch, 2001) and that the afternoon cortisol decline was evident in control, but not internalizing or comorbid adolescents (Klimes-Dougan, Hastings, Granger, Usher, & Zahn-Waxler, 2001). These findings suggest flattened diurnal rhythms may be associated with a gradation of problem behavior, with comorbid youth often displaying the most mental health problems.
Second, some of the inconsistencies in previous findings may derive from the difficulties of measuring cortisol, which is highly responsive to the social context (Eisenberger, Taylor, Gable, Hilmert, & Lieberman, 2007; Kirschbaum, Pirke, & Hellhammer, 1993; Stroud, Tanofsky-Kraff, Wilfley, & Salovey, 2000). Consequently, cortisol fluctuates greatly within and across days, and this variability may have implications for the expression of mental health symptoms (Peeters, Nicolson, & Berkhof, 2004). Statistical analyses that separate within person (i.e., situation-specific, state) from between person variability (i.e., trait, basal) are useful because they partial out spurious social contextual influences from stable, basal cortisol (Shirtcliff et al., 2005). In addition to day-to-day fluctuations, cortisol changes within each day according to its diurnal rhythm with levels highest in the morning and declining across the day. Dysregulated diurnal rhythms can be thought of as an index of biological risk. A wide variety of behavioral changes are observed when rhythms are dysregulated or flattened, including studies which have revealed that flattened diurnal rhythms are related to mental health symptoms (Peeters et al., 2004; Stetler, Dickerson, & Miller, 2004; Stone et al., 2001; van Eck, Berkhof, Nicolson, & Sulon, 1996) and may normalize with successful pharmacological treatment (Linkowski, 2003; Mendlewicz, Linkowski, Van Cauter, & Kerkhofs, 1994).
Third, some of the inconsistencies in previous findings, especially cross-sectional vs. longitudinal studies, may be due to a general lack of consideration of the changing social contexts of young adolescents (Boyce et al., 1998). One such normative change is the transition to middle school. School transitions have been examined before as a salient social context (Boyce et al., 1995; Davis, Donzella, Krueger, & Gunnar, 1999; de Haan, Gunnar, Tout, Hart, & Stansbury, 1998; Turner-Cobb, Rixon, & Jessop, 2008), although this study is the first to our knowledge to focus on the transition to middle school. Such times of transition are ideal for distinguishing the concurrent association of cortisol and mental health from the longitudinal association of cortisol with changes in mental health across periods of transition. The transition to middle school, which is marked by a change from single to multiple teachers, classrooms, and sets of classmates, challenges the adolescent to accept new tasks, broaden their social networks, redefine social roles, and navigate an unpredictable and often ambiguous and novel social context (Rudolph, Lambert, Clark, & Kurlakowsky, 2001). Novel environments or events which are socially threatening are particularly salient triggers of cortisol reactivity (Dickerson & Kemeny, 2004). While some adolescents may view this transition as a challenge to mobilize resources and an opportunity for social and psychological growth, some may not adjust well, particularly adolescents who have difficulty with social and emotional regulation (Berndt & Mikos, 1995; Hirsch & Rapkin, 1987; Rudolph et al., 2001; Vanlede, Little, & Card, 2006). Appreciating this normative social challenge may help advance theories about when hypo- or hyper-arousal of the HPA axis is associated with mental health symptoms.
A pattern of hypoarousal (low cortisol, flat diurnal rhythms) has been termed a low biological sensitivity to context (BSC, Boyce & Ellis, 2005; Ellis & Boyce, in press). A low BSC can be expected to disadvantage the developing child, potentially rendering them less able to cognitively process environmental opportunities and threats, with poorer attention, and less efficient priming for memory storage for emotionally and socially relevant events (Ellis & Boyce, in press; Flinn, 2006). Hypoarousal is expected to be associated with mental health symptoms (Gunnar & Vazquez, 2001) because social dysregulation is a central component of psychopathology (Keltner & Kring, 1998); indeed, low cortisol has been directly linked with emotion and social regulation (Peeters et al., 2004; Stetler et al., 2004; Stetler & Miller, 2005).
Hypoarousal is often a component of theories that focus on externalizing problems. One of the most replicated indices of externalizing problems is low fear and physiological arousal (Raine, 2002), leading to the fearlessness theory which posits that individuals with tonic hypoarousal can engage in antisocial behavior because they are not encumbered by extant physiological arousal (Raine, 1993). On the other hand, stimulation seeking theory argues that low arousal is an aversive physiological state and hypoaroused individuals are motivated to seek out stimulation in order to raise arousal to normal or optimal levels (Lovallo, 1997; Snoek, Van Goozen, Matthys, Buitelaar, & van Engeland, 2004; van Goozen et al., 2007). Van Honk and colleagues (2003) combine these ideas by suggesting there is a balance between responding to fear-producing stimuli and seeking out rewarding stimuli. Cortisol helps set this balance so that when cortisol levels are low, the balance shifts towards low sensitivity to punishment and high reward dependency.
While low BSC individuals might display a lack of biological responsivity to subtle environmental challenges and relative insensitivity to social cues, low BSC individuals might be buffered against a broad array of stressful events (Ellis, Essex, & Boyce, 2005). Taken together, these low BSC individuals are expected to generally have more mental health symptoms (due to poorly functioning HPA axis) but to be less likely to show increases in mental health symptoms in response to environmental perturbations (Ellis & Boyce, in press), in this case the transition to middle school.
On the other hand, individuals with hyperarousal (e.g., high cortisol) have higher BSC, which means greater susceptibility to both positive and negative environmental experiences and events. It is noteworthy that theories of hyperarousal uniformly posit it as a risk factor in stressful social contexts, most frequently in relation to internalizing problems (Burke et al., 2005). Hyperarousal may be evident in individuals with internalizing symptoms because they have a heightened physiological response to stress, experience chronic stress that taxes the developing stress systems, or have a lowered threshold of activity in areas of the brain responsive to stressful stimuli. Gotlib and colleagues (2008) and others (Bremner, 2003; Tarullo & Gunnar, 2006) suggest that several of these psychobiological mechanisms may be operating simultaneously in individuals at greatest risk. In particular, the occurrence of stressful life events can set the stage for a cascade of neurobiological changes and concomitant HPA axis alterations, and when these vulnerabilities and events occur together, the individual experiences the greatest expression of underlying risk. High BSC individuals may thus be specifically vulnerable during periods of high stress (e.g., school transitions) while they may be advantaged in stable, supportive environments.
The Present Study
The major goal of the present study was to investigate cortisol and mental health symptoms in early adolescence. We studied the association of cortisol with mental health symptoms both concurrently at grade 5 (age 11) and longitudinally at grade 7 (age 13). This interval includes a normative transition to a new social context: middle school. For all children in this study, the transition includes moving from a single classroom to a setting involving frequent changes in classrooms, teachers and peers. Additionally, for most (80%) of the adolescents in the present study, the interval between 5th and 7th grade includes the transition to a new school. Based on the BSC theory, we held different hypotheses for concurrent and longitudinal associations. We predicted that low cortisol would be associated with concurrent mental health symptoms, but that adolescents with more severe and/or rising levels of mental health symptoms across the middle school transition would show evidence of hyper-arousal. We also predicted that a flattened diurnal rhythm would be associated with mental health symptoms, beyond the contribution of basal cortisol. Although there are distinct theories for understanding the associations of cortisol with internalizing versus externalizing problems, the BSC emphasizes social context over symptom type. We specifically considered comorbid internalizing and externalizing symptoms by distinguishing total symptom level (i.e., symptom severity) from whether youth displayed a preponderance of externalizing vs. internalizing symptoms (i.e., symptom directionality). The hypotheses above regarded symptom severity. Associations between cortisol and symptom directionality were exploratory, since no prior studies have employed this methodology in relation to cortisol.
Methods
Participants
The adolescents in this study are participants in a longitudinal study, the Wisconsin Study of Families and Work (WSFW). Originally, pregnant women and their partners were recruited from prenatal clinics in two Midwestern cities. To be eligible, female participants were required to be over the age of 18, in the second trimester of pregnancy, living with the baby’s biological father, and either employed or a full-time homemaker. Of those eligible to participate, 570 (75%) agreed; 560 subsequently had live births and were eligible to continue in the study. All study procedures were approved according to the University of Wisconsin institutional guidelines; informed consent was obtained from all participants.
The present analyses included the 294 families who lived within geographical proximity to the project offices to participate in a home visit, agreed to participate in the saliva collection, and who had complete mother, teacher, and child reports of children’s 5th grade mental health symptoms. At the time of the 5th grade assessment, 70% of the original sample (N=399) remained in the study. Of these, 42 lived outside geographical proximity to the project offices, 40 did not participate in the saliva collection, and 23 did not complete the teacher interview, and thus were not included in the present analyses. At the time of recruitment, 44% of the 294 mothers and 53% of the fathers had a high school or technical degree or less, the remainder had at least a college degree. Mother’s and father’s average age was 29.6 (SD = 4.2) and 31.7 (SD = 5.2), respectively; 40% were first-time mothers. Most couples (95%) were married and Caucasian (90%); median annual family income was $48,000 (range = <$10,000 to >$180,000). There were no significant differences between the 294 participants and the remaining families in the original sample in terms of parental age or education, marital or ethnic status, or annual family income.
Measures
Salivary Cortisol at Grade 5
Cortisol was assessed in saliva because it can be noninvasively collected and reflects the plasma concentration of the non-protein bound active fraction (Kirschbaum & Hellhammer, 1990). Adolescents were asked to collect saliva for three consecutive days across three target collection times: (1) at waking (before brushing teeth or eating breakfast); (2) between 3:00 PM and 7:00 PM (prior to dinner); and (3) at bedtime. Participants completed Saliva Diary Charts each of the three days, including actual saliva collection times, any major stresses experienced that day (yes/no), and any medications used. Because of the influence of the Cortisol Awakening Response (CAR) on the diurnal rhythm (Dockray, Bhattacharyya, Molloy, & Steptoe, 2008; Pruessner et al., 1997), particular attention was paid to emphasize to participants the importance of collecting the first sample immediately upon waking. All participants provided the waking sample within 17 minutes of waking, and 66% provided the waking sample within 10 minutes of awakening.
Cortisol was assessed in duplicate with a salivary enzymeimmunoassay kit (Salimetrics, State College, PA). The detection limit of the assay (ED80) was 0.007 μg/dL. Mean intra-assay and inter-assay coefficients of variation (CVs) were 3.8% and 7.4%, respectively. Assay results were acceptable only if the CV for the duplicate measurements of a sample were<20% for samples with values >.02 μg/dL and <30% for samples with values <.02 μg/dL. To normalize the distributions, raw cortisol values were log-transformed. Medication use was coded (1) non-narcotic anti-inflammatories (2) antibiotics/non-steroidal cold, allergy medications, (3) non-oral steroids, (4) psychotropics, and (5) other. Analyses revealed no significant impact of medication use on cortisol. Nevertheless, due to previous findings on medication effects on cortisol (Hibel, Granger, Kivlighan, & Blair, 2006; Schreiber et al., 2006), we explored medication usage as a control measure in all analyses.
Puberty
Measuring puberty is necessary to give greater credence to the view that the HPA axis changes are due primarily to a social, and not a biological, transition. Co-occurring pubertal development and change in the activity of the stress system may exacerbate adolescents’ underlying vulnerabilities for mental health problems (Hayward & Sanborn, 2002). We chose to define physical maturation by level of pubertal development rather than age because there is little variability in the age of 5th graders whereas the present sample includes the full range of breast/genital (stage 1 to 4.5) and pubic hair development (stage 1 to 5). Mothers and youth completed a self-administered puberty measure based on descriptions and a visual inspection of line drawings of the five Tanner stages; correspondence with a physical exam Tanner score is high (rs >.60) (Morris & Udry, 1980). Self- and mother- ratings of boys’ genital and pubic hair development, respectively, were highly reliable, Cronbach’s α=0.82, and were averaged. Self-and mother- ratings of girls’ breast and pubic hair development were also highly reliable, Cronbach’s α=0.89, and were averaged.
Mental Health Symptoms
Multiple informants provided information about each adolescent. Mother-, teacher- and self-reports of adolescent mental health symptoms in 5th and 7th grades were based on the mental health scales of the MacArthur Health and Behavior Questionnaire (HBQ) (Boyce et al., 2002; Essex, Boyce et al., 2002). The HBQ, which was derived primarily from the Ontario Child Health Study (OCHS-R) (Boyle et al., 1987; Boyle et al., 1993), has good psychometric properties, discriminates well between clinic-referred children and community controls, and has moderate to high agreement with the Diagnostic Interview Schedule for Children (Essex, Boyce et al., 2002; Lemery-Chalfant et al., in press; Luby et al., 2002). Originally designed and validated for children through age 8 (Essex, Boyce et al., 2002), the HBQ has been used longitudinally throughout the WSFW with only minor word modifications and additions of age-appropriate items for older children and adolescents. Studies of other samples of children (Lemery-Chalfant et al., in press; Luby et al., 2002), as well as a subset of 80 WSFW adolescents (unpublished data), have demonstrated that an HBQ cut-point of approximately 1 standard deviation above the mean discriminates well between children with and without psychiatric diagnoses.
Internalizing symptoms included subscales for depression (n items >13; αs >.84), generalized anxiety (n items >8; αs >.78), and separation anxiety (n items >6; αs >.76). Externalizing symptoms included subscales for oppositional defiance (n items>9; αs >.78), conduct problems (n items >12; αs >.70), inattention (n items >4; αs >.76), impulsivity (n items >8; αs >.79), overt aggression (n items >4; αs >.58), and relational aggression (n items >6; αs >.76). Based on Kraemer and colleagues (2003), principal component analysis (PCA) integrated multi-informant data for each symptom measure. Through the careful selection of reporters who view the child from different perspectives (e.g., child self view vs. adult view) and in different contexts (e.g., home vs. school), this approach removes the core characteristic from sources of error from reporters’ differing perspectives and contexts. This approach has been demonstrated to be more reliable and valid than scores based on a single reporter or a combinational approach that does not control extraneous variance (Kraemer et al., 2003). Separate PCA was used for 5th and 7th grade symptoms, respectively.
As expected, there was substantial overlap within individuals in internalizing and externalizing symptoms, r=0.45, p <.001 for 5th grade and r=0.47, p <.001 for 7th grade. One practical challenge to the high rate of comorbid symptoms in adolescents is that measures of internalizing and externalizing problems are often highly correlated, raising statistical concerns with multicollinearity (Angold et al., 1999; Essex, Boyce et al., 2002). Studies which have considered comorbid symptoms have typically used diagnostic categories (e.g., McBurnett et al., 1991), which causes some loss of statistical power related to dichotomization (MacCallum, Zhang, Preacher, & Rucker, 2002). Our previously validated methodology examines the full range of symptoms in our sample by separating symptom severity from a preponderance of internalizing or externalizing types of symptoms (Essex, Klein, Cho, & Kraemer, 2003; Essex et al., 2006). Specifically, the two multi-informant scores were used to construct measures of symptom Severity (average of the two standardized scores, which reflects what the two scores have in common) and Directionality (half difference of the standardized scores, which reflects what differentiates the two scores; positive score indicates a preponderance of externalizing vs. internalizing symptoms). Severity and Directionality are independent (i.e., uncorrelated) and thus, in contrast to Internalizing and Externalizing scores, can be included together in analyses without problems of multicollinearity.
Severity and Directionality were derived separately for 5th and 7th grade symptoms. Change scores were calculated to examine individual differences in mental health symptoms over time (Llabre, Spitzer, Saab, Ironson, & Schneiderman, 1991).
Analytical Strategy
Repeated measures of salivary cortisol help to reduce the influence of random environmental factors, yet aggregation works slowly in naturalistic settings (Li, Chiou, & Shen, 2007). Statistical analyses that separate within person (i.e., situation-specific, state) from between person variability (i.e., trait, basal) are useful because they extract Basal Cortisol. In a previous study, we found that basal cortisol comprises approximately 25% of the total variance in cortisol, and when basal cortisol was extracted, low cortisol was associated with externalizing symptoms (Shirtcliff et al., 2005). Several others have shown similar results using the same or similar methodologies (Adam, 2005, 2006; Hruschka, Kohrt, & Worthman, 2005; Kirschbaum et al., 1990), although others suggest as much as 40-60% of cortisol variability is Basal (Kertes & van Dulmen, 2005; Ranjit, Young, Raghunathan, & Kaplan, 2005; Wismer Fries, Shirtcliff, & Pollak, in press). These tools can be extended to optimally and simultaneously assess the diurnal rhythm using growth curve analyses (Collins & Sayer, 2001). Cortisol levels and rhythms are highly intercorrelated; growth curves control for the law of initial values at the intraindividual difference level (Berntson, Uchino, & Cacioppo, 1994). Because growth curve models provide independent estimates of levels and rhythms, it is possible to conclude that the diurnal rhythm contributes to cortisol variability beyond basal cortisol levels.
A three level hierarchical linear model separated within-the-day (N=2585, or 9 samples/person), day-to-day (N=877 or 3 days/person) and between individual (N=294) sources of cortisol variability. Level 1 included Time Since Waking (in hours) as a random predictor of cortisol in order to capture the diurnal rhythm at the within-day level and subsequently examine it as an outcome of interest at the between individual level; the intercept reflects basal cortisol levels at waking. Level 2 allowed the level and slope to vary across days. To assess whether cortisol levels or its daily diurnal rhythm were different on days which were novel, stressful, or in which participants used medications, measures of day of saliva collection (i.e., Day 1, 2, 3), daily stressors, and medication/inhaler use from the Saliva Diary Chart were explored as Level 2 predictors.
Between individual difference predictors of interest were introduced in Level 3. We first examined control variables of medication use and - to broadly assess whether maturation affected cortisol - sex and puberty. Mental health symptom severity and directionality were then examined in a single model, before and after controlling for sex, puberty, and medications to determine if basal cortisol or the diurnal rhythm were related to mental health symptoms. We repeated these steps with the two-year time-lagged mental health symptoms. Finally, we examined the change in mental health symptom severity and directionality to test if cortisol was a risk factor for the increase of mental health symptoms in early adolescence before and after accounting for symptoms in grade 5.
Results
Estimating Basal Cortisol and the Diurnal Rhythm
The three-level HLM showed substantial day-to-day variability in cortisol levels, 1ϰ2(578)=823.9, p<.0001, as well as significant variability in cortisol levels across individuals, ϰ2(293)=552.1, p<.0001. After controlling for the diurnal rhythm, basal cortisol comprised 20.8% of the total variability in cortisol levels, day-to-day fluctuations accounted for 1.9% of the total variance, and within-the-day fluctuations from the predicted diurnal decline comprised 77.3% of the total variability in cortisol levels.
Time Since Waking (in hours) was a significant predictor of intra-individual cortisol levels, B=-0.16, t=58.0, p<.0001. Figure 1 illustrates that cortisol levels demonstrated the characteristic decline across daytime hours. There was substantial intra-individual variability in cortisol’s decline across the day, ϰ2(578)=1045.4, p<.0001, illustrating that the diurnal rhythm varied across days. Individual variance in the diurnal rhythm was significant, ϰ2(293)=559.5, p<.0001, suggesting that there was enough variability in the diurnal rhythm across individuals for the diurnal slope to be an outcome of interest.
Figure 1.
Intra-individual variability in cortisol levels measured three times a day across three days in 297 early adolescents. A three-level hierarchical model separated variability into within the day (77.3%), day-to day (1.9%), and between individual (20.8%) sources of variability in salivary cortisol after accounting for the steep diurnal decline.
We next examined variables to help explain the substantial intra-individual and individual variability in cortisol levels. At the intra-individual level, adolescents had higher cortisol, B=0.02, p<.05, and a trend for steeper slopes, B=.004, p=.07, on the first day of collection compared to subsequent days of saliva collection (p>0.4), suggesting that novelty may have systematically influenced cortisol’s daily rhythm. Self-reported stress, inhaler or medication use did not predict cortisol levels. At the individual level, there was evidence for a sex difference such that boys had lower basal cortisol, B=-0.09, t=2.0, p<.05, and flatter diurnal rhythms than girls, B=.02, t= 4.0, p<.0001. Pubertal status did not predict cortisol levels, but more physically mature children had flatter diurnal rhythms, B=0.01, t=2.4, p<.02.
Cortisol and Mental Health Symptoms in Early Adolescence
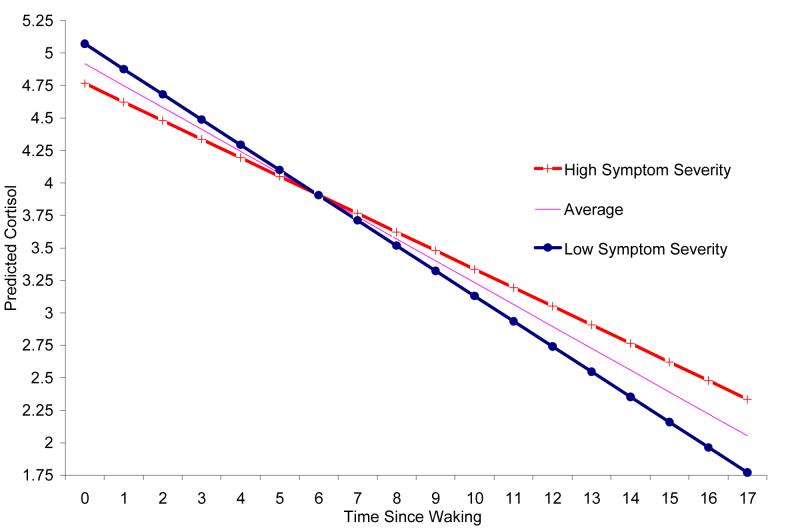
Figure 2 shows that individuals with more severe mental health symptoms at 5th grade had low concurrent basal cortisol, B=-.058, t=2.2, p<.03, and flat diurnal slopes, B=.009, t=3.0, p<.003. Effects were still significant after accounting for sex, puberty, and medication use, ps<.05. Symptom directionality was not associated with basal cortisol or the diurnal decline, ps>.3. This suggests that individuals with a preponderance of externalizing or internalizing problems do not have unique concurrent cortisol patterns, but rather that low basal cortisol and flat diurnal rhythms may be non-specific correlates of mental health symptoms.
Figure 2.
Predicted cortisol levels across the day for early adolescents with high and low mental health symptoms (three standard deviations above and below the mean, respectively) illustrate how youth with more severe mental health symptoms had lower morning basal cortisol and flatter diurnal slopes.
Next, we examined whether basal and diurnal cortisol at 5th grade were risk factors for levels of mental health symptoms measured two years later in 7th grade, after the transition to middle school1. We were also interested in whether basal and diurnal cortisol predicted if adolescents showed increasing mental health symptoms across the transition to middle school. Basal cortisol in 5th grade did not predict levels of symptom severity in 7th graders, p=.3. Yet, figure 3 shows that 5th graders with higher basal cortisol had rising levels of mental health symptoms across the middle school transition, B=.07, t=2.1, p<.03; and this association remained significant after controlling for symptom severity levels at 5th grade, p<.05. This longitudinal finding with change in symptoms may explain why low basal cortisol did not predict levels of mental health symptoms at 7th grade. Adolescents with flatter diurnal slopes as 5th graders also had more mental health symptoms as 7th graders, B=.009, t=2.7, p<.007. All associations remained significant after controlling sex, puberty and medications. There were no associations with symptom directionality across the two-year period.
Figure 3.
Predicted cortisol levels across the day for adolescents with increasing and decreasing mental health symptom severity from 5th to 7th grade, respectively, illustrate how youth with high cortisol levels had increasing mental health symptoms across the transition to middle school.
Discussion
The primary purpose of this study was to examine the associations of adolescent basal cortisol and the diurnal rhythm in 5th grade with mental health symptoms both concurrently and longitudinally. The results of the cross-sectional analyses showed that low basal cortisol and flat diurnal rhythms were associated with the severity of mental health symptoms in 5th grade. In contrast, longitudinal analyses showed that high basal cortisol in 5th grade was associated with increasing mental health symptoms from 5th to 7th grades, which included the transition to middle school. School transitions, concomitant social and life stress, and pubertal development may all render early adolescents particularly vulnerable to risk factors for emerging mental health symptoms. Apparently divergent cross-sectional and longitudinal findings may be integrated by recognizing the importance of the social context and an adolescent’s biological sensitivity to context (Boyce & Ellis, 2005; Ellis & Boyce, in press; Ellis et al., 2005).
The BSC framework suggests that hypoarousal should generally be associated with mental health problems since cortisol functions to enhance mental activities in localized domains, focus attention, prime memory for emotionally or socially relevant events, improve cognitive processes for dealing with environmental opportunities and threats, and regulate social and emotional responses (Ellis & Boyce, in press; Flinn, 2006). Accordingly, some theoretical models link tonic hypoarousal with mental health problems, mainly externalizing symptoms (Raine, 1993). Our cross-sectional findings support this view in that low cortisol and flat diurnal slopes were associated with greater symptom severity. Nevertheless, being less susceptible to social contextual cues may provide a buffer during times of transition.
Children with a high BSC operate well in stable social contexts, but may be at risk when they experience changes in their social contexts, such as the transition to middle school. Consistent with this view, several theoretical models implicate altered stress responsivity (e.g., heightened physiological stress response, lowered threshold for responding to stressful stimuli, etc), rather than tonic arousal, to explain why cortisol hyperarousal may be associated with internalizing problems (Gunnar & Vazquez, 2006). Concurrent 5th grade associations did not capture the high BSC adolescents because their school contexts were largely stable throughout elementary school, or changes were random across high and low BSC adolescents. However, longitudinal findings illustrated that adolescents with high basal cortisol at 5th grade were at risk for rising mental health symptoms across the change in social context associated with the transition to middle school. These findings dovetail with earlier findings of Essex and colleagues (2002) who showed that children with high cortisol expressed more mental health symptoms across the transition to elementary school. Another study likewise found high cortisol and stress reactivity was associated with behavior problems in boys after the transition to kindergarten (Hatzinger et al., 2007). High basal cortisol may be a risk for mental health symptoms in a subset of children with a high BSC during periods of transition when they are exposed to unstable social contexts.
The BSC provides some potentially informative leads for how cortisol is mechanistically linked with mental health problems. An individual’s BSC is thought to be relatively heritable. Cortisol is likewise influenced by both genetic and environmental factors (Bartels, de Geus, Kirschbaum, Sluyter, & Boomsma, 2003; Bartels, Van den Berg, Sluyter, Boomsma, & de Geus, 2003). Cortisol responsivity to stress exposure is one pathway through which genetic heritability is translated into genetic expression (De Kloet, 2004). For example, individuals with high risk for internalizing symptoms (as a function of family history and homozygosity for the short serotonin transporter allele) were most likely to show cortisol elevations and more prolonged cortisol responsivity to a laboratory stressor, suggesting that one’s genetic heritage informs their biological stress reactivity and may increase their susceptibility to psychopathology after stress exposure (Gotlib et al., 2008). Across early development, a child’s BSC becomes expressed as they experience a range of life stressors and differential quality of their early environment (Essex, Klein et al., 2002; Gunnar & Quevedo, 2007). Over time, basal activity of the HPA axis can get canalized in a state of consistent hypo- or hyper-arousal depending on the child’s threshold for activating the HPA axis. The mechanism for this threshold is not yet clear, but likely involves a combination of psychological coping strategies and concomidant activation in ventromedial prefrontal and amygdalar circuitry (Susman, 2006; Urry et al., 2006). In a subset of individuals born with a high BSC, stress exposure and alterations in HPA activity and related neural circuitry provide one pathway for mental health problems.
Because of the substantial comorbidity between internalizing and externalizing symptoms, we examined symptom Severity and Directionality rather than more traditional methods. All of our findings were with symptom severity and not whether adolescents had a preponderance of internalizing or externalizing symptoms, suggesting that the HPA axis may operate as a nonspecific correlate or risk factor for mental health symptoms in young adolescents. Whether youth express a preponderance of internalizing or externalizing symptoms may emerge later when problem behavior canalizes in adulthood or when symptoms become psychopathology (Essex, Boyce et al., 2002). It is difficult to determine how theoretical models which posit opposing predictions for internalizing versus externalizing behavior problems can account for comorbid symptomotology and cortisol as a generalized risk factor for both behavior problem categories. Our explanatory models need to be modified, or a new theory should be adopted which better explains why youth generally express comorbid symptoms (Angold et al., 1999).
We found evidence that flat slopes were associated with more mental health symptoms at both 5th and 7th grade assessments. Obtaining information about both morning and afternoon levels provides information about the confluence of both intrinsic biological (i.e., circadian) processes as well as extrinsic contextual processes such as social and emotional events (i.e., zeitgebers) (Carskadon, Acebo, & Jenni, 2004). Morning cortisol is influenced by unique factors which are largely genetic and under strong pituitary control (Bartels, de Geus et al., 2003). Afternoon levels are not highly genetic (Schreiber et al., 2006), but are more easily influenced by the proximate social context. Our findings are in keeping with the view that flat diurnal rhythms are associated with psychopathology because they indicate desynchrony between an individual’s internal biological processes and extrinsic social contextual cues (Carskadon et al., 2004; Stetler et al., 2004). Significant findings with the diurnal rhythm may also help explain why our earlier investigation, which focused only on early morning basal cortisol, did not uncover associations with internalizing behavior. It is possible that alterations in the diurnal rhythm, more so than absolute cortisol levels, manifest risk for mental health symptoms. Nevertheless, had we focused exclusively on rhythms, we would have missed observing that high basal cortisol is a risk factor for rising mental health symptoms.
Longitudinally, a flat diurnal rhythm was a risk factor for symptom severity two years later, suggesting this psychobiological index of desynchrony may be a persistent indicator of mental health symptoms. Nevertheless, another study revealed that the diurnal rhythm normalized as participants experienced unique improvements in social contexts, and the change in the diurnal rhythm mirrored a reduction in mental health symptoms (Fisher, Gunnar, Chamberlain, & Reid, 2000; Fisher, Stoolmiller, Gunnar, & Burraston, 2007). Efforts at improving the fit of the individual with their social context may change the shape of the diurnal rhythm, although this may be particularly challenging in adolescents who experience unique challenges in their social contexts as well as biological changes (Carskadon, Labyak, Acebo, & Seifer, 1999).
Limitations
Because we only studied adolescents, findings are limited to this developmental stage. It will be interesting to observe whether early biological events in adolescents persist or magnify as the participants in Wisconsin Study of Family and Work enter early adulthood. It is possible that the HPA axis is still malleable during adolescence and it is not until adulthood that psychopathological cortisol profiles are fully expressed. We do not necessarily expect that this pattern would be observed if we had studied adults because the endocrine system behaves differently in this age youth. These considerations support the idea that researchers should be careful with developmental issues, especially with early adolescents.
Although a subset of adolescents are expected to have clinical level mental health problems, this is a community sample and so caution should be maintained regarding the generalizability of the findings to clinical samples. We do not know whether this pattern would generalize to adolescents with clinical level of behavior problems because their mental health problems are already fully expressed, but their endocrine system is still actively developing.
Cortisol’s diurnal rhythm is complicated by a sharp rise approximately 30 min after awakening which could potentially make noncompliant participants appear to have a steeper diurnal rhythm. While all of our participants provided the waking sample within 17 minutes of awakening (prior to the peak in the awakening response), one limitation of the present study is that we did not measure the awakening response, nor did we standardize the time of awakening (Dockray et al., 2008). We do not believe this explains away the cortisol-mental health associations, however, because one would predict that those with higher symptoms would be less compliant (and thus would have steeper diurnal slopes). Future studies should measure both waking levels and the awakening response.
Finally, we should note that this sample represents largely middle class, white, and - at the outset - intact families in a Midwestern state. Yet, even here there is a range of socioeconomic status. The findings need to be replicated in other samples in order to conclude that the findings are robust.
Summary and Conclusion
In summary, basal cortisol and flat diurnal slopes were associated with adolescent mental health symptoms. At 5th grade, low cortisol and flat diurnal rhythms were concurrently associated with the severity of mental health symptoms, indicating hypoarousal as a correlate of mental health symptoms. However, it was high cortisol levels and flat diurnal rhythms that predicted increases in symptom severity from 5th to 7th grade, indicating hyperarousal as a risk for rising mental health problems as children experience a normative social challenge. These findings suggest that HPA axis alterations may be a nonspecific risk factor for mental health symptoms, highlighting the need for theoretical models to incorporate ideas regarding comorbid mental health symptoms. Recognizing adolescents’ social contexts, especially during periods of transition, may help resolve when low or high cortisol is related to mental health. Results support the utility of Boyce and Ellis’s (2005) model of a biological sensitivity to context, in which adolescents with a high BSC appear at risk when social contexts are unstable.
Acknowledgments
Funding was provided to MJE by NIMH Grants P50-MH52354 and P50-MH69315, and the HealthEmotions Research Institute, University of Wisconsin-Madison. EAS was supported by a NIMH training grant, T32-MH18931, and a Roadmap Interdisciplinary Award, T32-MH75880. EAS was at the University of Wisconsin when this paper was written. We thank Dr. Ned Kalin for invaluable consultation and provision of the cortisol assays, Drs. Bruce Ellis and W. Tom Boyce for helpful comments, and the adolescents and families for their continued participation in the study over many years.
Footnotes
One interpretational challenge is that mental health symptoms in 7th grade were statistical predictors of 5th grade cortisol. This was necessary since we wanted to rigorously examine cortisol variability. Nevertheless, we extracted Empirical Bayes estimates of Basal Cortisol and the Diurnal Rhythm for each individual and then used linear regression to confirm that cortisol predicts symptom severity in 7th graders. Parallel with the HLM models, basal cortisol predicted the rise in symptom severity, p=.03, and flat diurnal slopes predicted symptom severity, p<.008.
Copyright © 2008 John Wiley & Sons, Inc.
References
- Adam EK. Momentary emotion and cortisol levels in the everyday lives of working parents. In: Schneider B, Waite L, editors. Being together, working apart: Dual-career families and the work-life balance. University Press; Cambridge: 2005. pp. 105–133. [Google Scholar]
- Adam EK. Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology. 2006;31(5):664–679. doi: 10.1016/j.psyneuen.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Angold A. Adolescent depression, cortisol and DHEA. Psychol Med. 2003;33(4):573–581. doi: 10.1017/s003329170300775x. [DOI] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Erkanli A. Comorbidity. J Child Psychol Psychiatry. 1999;40(1):57–87. [PubMed] [Google Scholar]
- Bartels M, de Geus EJ, Kirschbaum C, Sluyter F, Boomsma DI. Heritability of daytime cortisol levels in children. Behav Genet. 2003;33(4):421–433. doi: 10.1023/a:1025321609994. [DOI] [PubMed] [Google Scholar]
- Bartels M, Van den Berg M, Sluyter F, Boomsma DI, de Geus EJ. Heritability of cortisol levels: review and simultaneous analysis of twin studies. Psychoneuroendocrinology. 2003;28(2):121–137. doi: 10.1016/s0306-4530(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Berndt TJ, Mikos D. Adolescent’s perceptions of the stressful and desirable aspects of the transition to junior high school. Journal of Research on Adolescence. 1995;5:123–142. [Google Scholar]
- Berntson GG, Uchino BN, Cacioppo JT. Origins of baseline variance and the Law of Initial Values. Psychophysiology. 1994;31(2):204–210. doi: 10.1111/j.1469-8986.1994.tb01042.x. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Adams S, Tschann JM, Cohen F, Wara D, Gunnar MR. Adrenocortical and behavioral predictors of immune responses to starting school. Pediatr Res. 1995;38(6):1009–1017. doi: 10.1203/00006450-199512000-00030. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Woodward HR, Measelle JR, Ablow JC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: exploring the “head waters” of early life morbidities. J Am Acad Child Adolesc Psychiatry. 2002;41(5):580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Frank E, Jensen PS, Kessler RC, Nelson CA, Steinberg L. Social context in developmental psychopathology: recommendations for future research from the MacArthur Network on Psychopathology and Development. The MacArthur Foundation Research Network on Psychopathology and Development. Dev Psychopathol. 1998;10(2):143–164. doi: 10.1017/s0954579498001552. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Hofmann HG, Catlin GP, Byles JA, Cadman DT, et al. Ontario Child Health Study. I. Methodology. Arch Gen Psychiatry. 1987;44(9):826–831. doi: 10.1001/archpsyc.1987.01800210078012. [DOI] [PubMed] [Google Scholar]
- Boyle MH, Offord DR, Racine Y, Fleming JE, Szatmari P, Sanford M. Evaluation of the revised Ontario Child Health Study scales. J Child Psychol Psychiatry. 1993;34(2):189–213. doi: 10.1111/j.1469-7610.1993.tb00979.x. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Long-term effects of childhood abuse on brain and neurobiology. Child Adolesc Psychiatr Clin N Am. 2003;12(2):271–292. doi: 10.1016/s1056-4993(02)00098-6. [DOI] [PubMed] [Google Scholar]
- Burke HM, Davis MC, Otte C, Mohr DC. Depression and cortisol responses to psychological stress: a meta-analysis. Psychoneuroendocrinology. 2005;30(9):846–856. doi: 10.1016/j.psyneuen.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Acebo C, Jenni OG. Regulation of adolescent sleep: implications for behavior. Ann N Y Acad Sci. 2004;1021:276–291. doi: 10.1196/annals.1308.032. [DOI] [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260(2):129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Development and Psychopathology. 2001;13:783–804. [PubMed] [Google Scholar]
- Collins LM, Sayer AG. 1st American Psychological Association; Washington, DC: 2001. New methods for the analysis of change. [Google Scholar]
- Colomina MT, Canals J, Carbajo G, Domingo JL. Salivary cortisol in a young population: Relationship with psychopathological disorders. Research Communications in Biological Psychology and Psychiatry. 1997;22(12):1–10. [Google Scholar]
- Dahl R, Puig-Antich J, Ryan N, Nelson B, Novacenko H, Twomey J, et al. Cortisol secretion in adolescents with major depressive disorder. Acta Psychiatr Scand. 1989;80(1):18–26. doi: 10.1111/j.1600-0447.1989.tb01295.x. [DOI] [PubMed] [Google Scholar]
- Davis EP, Donzella B, Krueger WK, Gunnar M. The start of a new school year: individual differences in salivary cortisol response in relation to child temperament. Dev Psychobiol. 1999;35(3):188–196. doi: 10.1002/(sici)1098-2302(199911)35:3<188::aid-dev3>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Dahl R, Perel J, Birmaher B. Nocturnal ACTH, cortisol, growth hormone, and prolactin secretion in prepubertal depression. Journal of the American Academy of Child and Adolescent Psychiatry. 1996;35(9):1130–1138. doi: 10.1097/00004583-199609000-00010. [DOI] [PubMed] [Google Scholar]
- de Haan M, Gunnar M, Tout K, Hart J, Stansbury K. Familiar and novel contexts yield different associations between cortisol and behavior among 2-year-old children. Dev Psychobiol. 1998;33(1):93–101. doi: 10.1002/(sici)1098-2302(199807)33:1<93::aid-dev8>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37(2):51–68. [PubMed] [Google Scholar]
- De Kloet ER. Hormones and the stressed brain. Ann N Y Acad Sci. 2004;1018:1–15. doi: 10.1196/annals.1296.001. [DOI] [PubMed] [Google Scholar]
- Dickerson SS, Kemeny ME. Acute stressors and cortisol responses: a theoretical integration and synthesis of laboratory research. Psychol Bull. 2004;130(3):355–391. doi: 10.1037/0033-2909.130.3.355. [DOI] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, Steptoe A. The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology. 2008;33(1):77–82. doi: 10.1016/j.psyneuen.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Taylor SE, Gable SL, Hilmert CJ, Lieberman MD. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT. (in press) Biological Sensitivity to Context Current Directions in Psychological Science
- Ellis BJ, Essex MJ, Boyce WT. Biological sensitivity to context: II. Empirical explorations of an evolutionary-developmental theory. Dev Psychopathol. 2005;17(2):303–328. doi: 10.1017/s0954579405050157. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: developing the Macarthur health and Behavior Questionnaire. J Am Acad Child Adolesc Psychiatry. 2002;41(5):588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol Psychiatry. 2002;52(8):776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kraemer HC. Exposure to maternal depression and marital conflict: gender differences in children’s later mental health symptoms. J Am Acad Child Adolesc Psychiatry. 2003;42(6):728–737. doi: 10.1097/01.CHI.0000046849.56865.1D. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Kraemer HC, Armstrong JM, Boyce WT, Goldsmith HH, Klein MH, et al. Exploring risk factors for the emergence of children’s mental health problems. Arch Gen Psychiatry. 2006;63(11):1246–1256. doi: 10.1001/archpsyc.63.11.1246. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Gunnar MR, Chamberlain P, Reid JB. Preventive intervention for maltreated preschool children: impact on children’s behavior, neuroendocrine activity, and foster parent functioning. J Am Acad Child Adolesc Psychiatry. 2000;39(11):1356–1364. doi: 10.1097/00004583-200011000-00009. [DOI] [PubMed] [Google Scholar]
- Fisher PA, Stoolmiller M, Gunnar MR, Burraston BO. Effects of a therapeutic intervention for foster preschoolers on diurnal cortisol activity. Psychoneuroendocrinology. 2007 doi: 10.1016/j.psyneuen.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinn MV. Evolution and ontogeny of stress response to social challenges in the human child. Developmental Review. 2006;26:138–174. [Google Scholar]
- Goodyer I, Herbert J, Moor S, Altham P. Cortisol hypersecretion in depressed school-aged children and adolescents. Psychiatry Res. 1991;37(3):237–244. doi: 10.1016/0165-1781(91)90060-3. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion during major depression in 8- to 16-year-olds, I. Altered diurnal rhythms in salivary cortisol and dehydroepiandrosterone (DHEA) at presentation. Psychol Med. 1996;26(2):245–256. doi: 10.1017/s0033291700034644. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Herbert J, Tamplin A, Altham PM. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high-risk adolescents. British journal of psychiatry. 2000;177:499–504. doi: 10.1192/bjp.177.6.499. [DOI] [PubMed] [Google Scholar]
- Goodyer IM, Park RJ, Herbert J. Psychosocial and endocrine features of chronic first-episode major depression in 8-16 year olds. Biol Psychiatry. 2001;50(5):351–357. doi: 10.1016/s0006-3223(01)01120-9. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J, Minor KL, Hallmayer J. HPA axis reactivity: a mechanism underlying the associations among 5-HTTLPR, stress, and depression. Biol Psychiatry. 2008;63(9):847–851. doi: 10.1016/j.biopsych.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Serbin LA, Schwartzman AE, Lehoux PM, Cooperman JM, Ikeda S. Children’s salivary cortisol, internalizing behavior problems, and family environment: Results from the Concordia Longitudinal Risk Project. International Journal of Behavioral Development. 1998;22:707–728. [Google Scholar]
- Gunnar M, Quevedo K. The neurobiology of stress and development. Annu Rev Psychol. 2007;58:145–173. doi: 10.1146/annurev.psych.58.110405.085605. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez D. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental psychopathology. 2nd Vol. 2. John Wiley & Sons; Hoboken, NJ: 2006. pp. 533–577. [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev Psychopathol. 2001;13(3):515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Hatzinger M, Brand S, Perren S, von Wyl A, von Klitzing K, Holsboer-Trachsler E. Hypothalamic-pituitary-adrenocortical (HPA) activity in kindergarten children: importance of gender and associations with behavioral/emotional difficulties. J Psychiatr Res. 2007;41(10):861–870. doi: 10.1016/j.jpsychires.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Hayward C, Sanborn K. Puberty and the emergence of gender differences in psychopathology. J Adolesc Health. 2002;30(4 Suppl):49–58. doi: 10.1016/s1054-139x(02)00336-1. [DOI] [PubMed] [Google Scholar]
- Herbert J, Goodyer IM, Altham PM, Pearson J, Secher SM, Shiers HM. Adrenal secretion and major depression in 8- to 16-year-olds, II. Influence of comorbidity at presentation. Psychol Med. 1996;26(2):257–263. doi: 10.1017/s0033291700034656. [DOI] [PubMed] [Google Scholar]
- Hibel LC, Granger DA, Kivlighan KT, Blair C. Individual differences in salivary cortisol: associations with common over-the-counter and prescription medication status in infants and their mothers. Horm Behav. 2006;50(2):293–300. doi: 10.1016/j.yhbeh.2006.03.014. [DOI] [PubMed] [Google Scholar]
- Hirsch BJ, Rapkin BD. The transition to junior high school: A longitudinal study of self-esteem, psychological symptomatology, school life and social support. Child Development. 1987;58(5):1235–1243. doi: 10.1111/j.1467-8624.1987.tb01454.x. [DOI] [PubMed] [Google Scholar]
- Hruschka DJ, Kohrt BA, Worthman CM. Estimating between- and within-individual variation in cortisol levels using multilevel models. Psychoneuroendocrinology. 2005;30(7):698–714. doi: 10.1016/j.psyneuen.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Kariyawasam SH, Zaw F, Handley SL. Reduced salivary cortisol in children with comorbid attention deficit hyperactivity disorder and oppositional defiant disorder. Neuroendocrinology Letters. 2002;23:45–48. [PubMed] [Google Scholar]
- Keltner D, Kring AM. Emotion, social function, and psychopathology. Review of General Psychology. 1998;2(3):320–342. [Google Scholar]
- Kertes DA, van Dulmen MH.Latent state trait modeling of children’s cortisol at two points of the diurnal cycle 2005 Paper presented at the Society for Research on Child Development, Atlanta, GA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Hellhammer D. Methodological aspects of salivary cortisol measurement. In: Kirschbaum C, Read GF, Hellhammer D, editors. Assessment of hormones and drugs in saliva in biobehavioral research. Hogrefe & Huber Publishers; Gottingen, Germany: 1990. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ’Trier Social Stress Test’--a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28(12):76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Steyer R, Eid M, Patalla U, Schwenkmezger P, Hellhammer DH. Cortisol and behavior: 2. Application of a latent state-trait model to salivary cortisol. Psychoneuroendocrinology. 1990;15(4):297–307. doi: 10.1016/0306-4530(90)90080-s. [DOI] [PubMed] [Google Scholar]
- Klimes-Dougan B, Hastings PD, Granger DA, Usher BA, Zahn-Waxler C. Adrenocortical activity in at-risk and normally developing adolescents: individual differences in salivary cortisol basal levels, diurnal variation, and responses to social challenges. Dev Psychopathol. 2001;13(3):695–719. doi: 10.1017/s0954579401003157. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, Kupfer DJ. A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. Am J Psychiatry. 2003;160(9):1566–1577. doi: 10.1176/appi.ajp.160.9.1566. [DOI] [PubMed] [Google Scholar]
- Kruesi MJP, Schmidt ME, Donnelly H, Hibbs ED, Hamburger SP. Urinary free cortisol output and disruptive behavior in children. Journal of the American Academy of Child and Adolescent Psychiatry. 1989;28:441–443. doi: 10.1097/00004583-198905000-00024. [DOI] [PubMed] [Google Scholar]
- Lemery-Chalfant K, Schreiber JE, Schmidt NL, Van Hulle C, Essex MJ, Goldsmith HH. Assessing internalizing, externalizing, and attention problems in young children: Validation of the MacArthur HBQ. Journal of the American Academy of Child and Adolescent Psychiatry. doi: 10.1097/chi.0b013e3180f616c6. [DOI] [PubMed] [Google Scholar]
- Li I, Chiou HH, Shen PS. Correlations between cortisol level and internalizing disposition of young children are increased by selecting optimal sampling times and aggregating data. Dev Psychobiol. 2007;49(6):633–639. doi: 10.1002/dev.20239. [DOI] [PubMed] [Google Scholar]
- Linkowski P. Neuroendocrine profiles in mood disorders. Int J Neuropsychopharmacol. 2003;6(2):191–197. doi: 10.1017/S1461145703003407. [DOI] [PubMed] [Google Scholar]
- Llabre MM, Spitzer SB, Saab PG, Ironson GH, Schneiderman N. The reliability and specificity of delta versus residualized change as measures of cardiovascular reactivity to behavioral challenges. Psychophysiology. 1991;28(6):701–711. doi: 10.1111/j.1469-8986.1991.tb01017.x. [DOI] [PubMed] [Google Scholar]
- Lovallo W. Stress and health: Biological and psychological interactions. Vol. 1. Sage publications; Thousand Oaks: 1997. [Google Scholar]
- Luby JL, Heffelfinger A, Measelle JR, Ablow JC, Essex MJ, Dierker L, et al. Differential performance of the macarthur HBQ and DISC-IV in identifying DSM-IV internalizing psychopathology in young children. J Am Acad Child Adolesc Psychiatry. 2002;41(4):458–466. doi: 10.1097/00004583-200204000-00019. [DOI] [PubMed] [Google Scholar]
- MacCallum RC, Zhang S, Preacher KJ, Rucker DD. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7(1):19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- McBurnett K, Lahey BB, Frick PJ, Risch C, Loeber R, Hart EL, et al. Anxiety, inhibition, and conduct disorder in children: II. Relation to salivary cortisol. J Am Acad Child Adolesc Psychiatry. 1991;30(2):192–196. doi: 10.1097/00004583-199103000-00005. [DOI] [PubMed] [Google Scholar]
- McBurnett KM, Lahey BB, Rathouz PJ, Loeber R. Low salivary cortisol and persistent aggression in boys referred for disruptive behavior. Archives of General Psychiatry. 2000;57:38–43. doi: 10.1001/archpsyc.57.1.38. [DOI] [PubMed] [Google Scholar]
- Mendlewicz J, Linkowski P, Van Cauter E, Kerkhofs M. Chronobiological aspects of depression. Psychiatria Fennica. 1994;25:9–19. [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Adolesc Health. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Moss HB, Vanyukov MM, Martin CS. Salivary cortisol responses and the risk for substance abuse in prepubertal boys. Biological Psychiatry. 1995;38:547–555. doi: 10.1016/0006-3223(94)00382-D. [DOI] [PubMed] [Google Scholar]
- Pajer K, Gardner W, Rubin RT, Perel J, Neal S. Decreased cortisol levels in adolescent girls with conduct disorder. Archives of General Psychiatry. 2001;58(3):297–302. doi: 10.1001/archpsyc.58.3.297. [DOI] [PubMed] [Google Scholar]
- Peeters F, Nicolson NA, Berkhof J. Levels and variability of daily life cortisol secretion in major depression. Psychiatry Res. 2004;126(1):1–13. doi: 10.1016/j.psychres.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, et al. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61(26):2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Raine A. The psychopathology of crime: Criminal behavior as a clinical disorder. Academic Press; San Diego: 1993. [Google Scholar]
- Raine A. Biosocial studies of antisocial and violent behavior in children and adults: A review. Journal of Abnormal Child Psychology. 2002;30(4):311–326. doi: 10.1023/a:1015754122318. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30(7):615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Rao DC, Dahl R, Ryan N, Birmaher B, Williamson D, Giles DE. The relationship between longitudinal clinical course and sleep and cortisol changes in adolescent depression. Biological Psychiatry. 1996;40(6):474–484. doi: 10.1016/0006-3223(95)00481-5. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Lambert SF, Clark AG, Kurlakowsky KD. Negotiating the transition to middle school: the role of self-regulatory processes. Child Dev. 2001;72(3):929–946. doi: 10.1111/1467-8624.00325. [DOI] [PubMed] [Google Scholar]
- Schreiber JE, Shirtcliff E, Van Hulle C, Lemery-Chalfant K, Klein MH, Kalin NH, et al. Environmental influences on family similarity in afternoon cortisol levels: twin and parent-offspring designs. Psychoneuroendocrinology. 2006;31(9):1131–1137. doi: 10.1016/j.psyneuen.2006.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Granger DA, Booth A, Johnson D. Low salivary cortisol levels and externalizing behavior problems in youth. Dev Psychopathol. 2005;17(1):167–184. doi: 10.1017/s0954579405050091. [DOI] [PubMed] [Google Scholar]
- Smider NA, Essex MJ, Kalin NH, Buss KA, Klein MH, Davidson RJ, et al. Salivary cortisol as a predictor of socioemotional adjustment during kindergarten: A prospective study. Child Development. 2002;73(1):75–92. doi: 10.1111/1467-8624.00393. [DOI] [PubMed] [Google Scholar]
- Snoek H, Van Goozen SH, Matthys W, Buitelaar JK, van Engeland H. Stress responsivity in children with externalizing behavior disorders. Dev Psychopathol. 2004;16(2):389–406. doi: 10.1017/s0954579404044578. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24(4):417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Stetler C, Dickerson SS, Miller GE. Uncoupling of social zeitgebers and diurnal cortisol secretion in clinical depression. Psychoneuroendocrinology. 2004;29(10):1250–1259. doi: 10.1016/j.psyneuen.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contacts. J Abnorm Psychol. 2005;114(4):697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Stone AA, Schwartz JE, Smyth J, Kirschbaum C, Cohen S, Hellhammer D, et al. Individual differences in the diurnal cycle of salivary free cortisol: a replication of flattened cycles for some individuals. Psychoneuroendocrinology. 2001;26(3):295–306. doi: 10.1016/s0306-4530(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Tanofsky-Kraff M, Wilfley DE, Salovey P. The Yale Interpersonal Stressor (YIPS): affective, physiological, and behavioral responses to a novel interpersonal rejection paradigm. Ann Behav Med. 2000;22(3):204–213. doi: 10.1007/BF02895115. [DOI] [PubMed] [Google Scholar]
- Susman EJ. Psychobiology of persistent antisocial behavior: stress, early vulnerabilities and the attenuation hypothesis. Neurosci Biobehav Rev. 2006;30(3):376–389. doi: 10.1016/j.neubiorev.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Targum S, Clarkson LL, Magac-Harris K, Marshall LE, Skwerer RG. Measurement of cortisol and lymphocyte subpopulations in depressed and conduct-disordered adolescents. Journal of Affective Disorders. 1990;18:91–96. doi: 10.1016/0165-0327(90)90064-f. [DOI] [PubMed] [Google Scholar]
- Tarullo AR, Gunnar MR. Child maltreatment and the developing HPA axis. Horm Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Turner-Cobb JM, Rixon L, Jessop DS. A prospective study of diurnal cortisol responses to the social experience of school transition in four-year-old children: anticipation, exposure, and adaptation. Dev Psychobiol. 2008;50(4):377–389. doi: 10.1002/dev.20298. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. J Neurosci. 2006;26(16):4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eck M, Berkhof H, Nicolson N, Sulon J. The effects of perceived stress, traits, mood states, and stressful daily events on salivary cortisol. Psychosom Med. 1996;58(5):447–458. doi: 10.1097/00006842-199609000-00007. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Fairchild G, Snoek H, Harold GT. The evidence for a neurobiological model of childhood antisocial behavior. Psychol Bull. 2007;133(1):149–182. doi: 10.1037/0033-2909.133.1.149. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Gispen-de Wied C, Wiegant VM, van Engeland H. Salivary cortisol and cardiovascular activity during stress in oppositional-defiant disorder boys and normal controls. Biol Psychiatry. 1998;43(7):531–539. doi: 10.1016/S0006-3223(97)00253-9. [DOI] [PubMed] [Google Scholar]
- van Goozen SH, Matthys W, Cohen-Kettenis PT, Thijssen JH, van Engeland H. Adrenal androgens and aggression in conduct disorder prepubertal boys and normal controls. Biol Psychiatry. 1998;43(2):156–158. doi: 10.1016/S0006-3223(98)00360-6. [DOI] [PubMed] [Google Scholar]
- van Honk J, Schutter DJ, Hermans EJ, Putman P. Low cortisol levels and the balance between punishment sensitivity and reward dependency. Neuroreport. 2003;14(15):1993–1996. doi: 10.1097/00001756-200310270-00023. [DOI] [PubMed] [Google Scholar]
- Vanlede M, Little TD, Card NA. Action-control beliefs and behaviors as predictors of change in adjustment across the transition to middle school. Anxiety, Stress & Coping. 2006;19(2):111–127. [Google Scholar]
- Vanyukov MM, Moss HB, Plail JA, Blackson T, Mezzich AC, Tarter RE. Antisocial symptoms in preadolescent boys and in their parents: Associations with cortisol. Psychiatry Research. 1993;46:9–17. doi: 10.1016/0165-1781(93)90003-y. [DOI] [PubMed] [Google Scholar]
- Wismer Fries AB, Shirtcliff EA, Pollak SD. (in press). Neuroendocrine dysregulation following early social deprivation in children Developmental Psychobiology [DOI] [PMC free article] [PubMed] [Google Scholar]