Abstract
Hippocampal area CA3 is critically involved in the formation of nonoverlapping neuronal subpopulations (“pattern separation”) to store memory representations as distinct events. Efficient pattern separation relies on the strong and sparse excitatory input from the mossy fibers (MFs) to pyramidal cells and feedforward inhibitory interneurons. However, MF synapses on CA3 pyramidal cells undergo long-term potentiation (LTP), which, if unopposed, will degrade pattern separation because MF activation will now recruit additional CA3 pyramidal cells. Here, we demonstrate MF LTP in stratum lacunosum-moleculare (L-M) interneurons induced by the same stimulation protocol that induces MF LTP in pyramidal cells. This LTP was NMDA receptor (NMDAR) independent and occurred at MF Ca2+-impermeable AMPA receptor synapses. LTP was prevented by with voltage clamping the postsynaptic cell soma during high-frequency stimulation (HFS), intracellular injections of the Ca2+ chelator BAPTA (20 mm), or bath applications of the L-type Ca2+ channel blocker nimodipine (10 μm). We propose that MF LTP in L-M interneurons preserves the sparsity of pyramidal cell activation, thus allowing CA3 to maintain its role in pattern separation. In the presence of the mGluR1α antagonist LY367385 [(S)-(+)-a-amino-4-carboxy-2-methylbenzeneacetic acid] (100 μm), the same HFS that induces MF LTP in naive slices triggered NMDAR-independent MF LTD. This LTD, like LTP, required activation of the L-type Ca2+ channel and also was induced after blockade of IP3 receptors with heparin (4 mg/ml) or the selective depletion of receptor-gated Ca2+ stores with ryanodine (10 or 100 μm). We conclude that L-M interneurons are endowed with Ca2+ signaling cascades suitable for controlling the polarity of MF long-term plasticity induced by joint presynaptic and postsynaptic activities.
Keywords: mossy fiber, LTP, LTD, calcium-impermeable AMPARs, CA3 interneurons, feedforward inhibition
Introduction
The circuitry of area CA3 is believed to transform neocortical inputs in a way that overlap between different activity patterns is minimized (“pattern separation”) (McNaughton and Morris, 1987; Treves and Rolls, 1992; O'Reilly and McClelland, 1994; Leutgeb and Leutgeb, 2007). This pattern separation facilitates storage of distinct episodic memories and reduces recall errors. Computational models of hippocampal connectivity (Treves and Rolls, 1992) have shown that pattern separation requires parallel activation of two excitatory afferent inputs to area CA3: the perforant path (PP), the axons of stellate cells in entorhinal cortex (EC) layer II, and the mossy fibers (MFs), the axons of dentate gyrus granule cells. The PP input is diffuse but weak, and conveys the compressed representations of neocortical activity from EC. The MF input is sparse but strong and selects subpopulations of pyramidal cells to establish nonoverlapping representations via strengthening of their recurrent collateral synapses. However, the MF input innervates more interneurons than pyramidal cells (Acsády et al., 1998), and although MF synapses onto CA3 interneurons have only one or two active zones (Acsády et al., 1998), the interneuron activation from the dentate gyrus is highly effective (Henze et al., 2002). When multiple cortical input patterns to CA3 arrive simultaneously, feedforward inhibition driven by MFs will limit the number of pyramidal cells activated by the same input. This synergistic excitatory action onto feedforward inhibitory interneurons could further increase the sparsity of the activity patterns (Mori et al., 2007) and the network storage capacity in CA3 (Treves and Rolls, 1992).
Like most cortical glutamatergic pathways, the MF input exhibits long-term potentiation (LTP) at synapses on pyramidal cells in response to high-frequency stimulation (HFS) (Jaffe and Johnston, 1990; Staubli et al., 1990; Zalutsky and Nicoll, 1990; Urban and Barrionuevo, 1996; Yeckel et al., 1999; Contractor et al., 2002). The resulting increase in pyramidal cell depolarization may promote the induction of Hebbian potentiation at their recurrent collateral synapses during the transfer and decorrelation of the compressed memory representations (Nakazawa et al., 2002). Such plasticity would allow future presentations of similar patterns to drive the same group of CA3 cells without the strong input from MFs. However, an increased gain in synaptic efficacy at MF synapses on pyramidal cells would likely be accompanied by degradation in pattern separation as many more CA3 pyramidal cells are activated. If MF input to pyramidal cells and feedforward interneurons simultaneously undergoes LTP, then the balance between excitation and inhibition is restored and the sparsity in the spatiotemporal segregation of pyramidal cell assemblies is maintained. Indeed, preservation of a narrow temporal window for synaptic integration after CA1 pyramidal cell LTP requires concurrent LTP at excitatory synapses on feedforward interneurons (Lamsa et al., 2005). However, extensive studies on CA3 feedforward inhibitory interneurons in the stratum (s.) lucidum have shown that the preferential form of plasticity at MF synapses is long-term depression (LTD) (Lawrence and McBain, 2003; Pelkey and McBain, 2007) (cf. Nicoll and Schmitz, 2005), which would further reduce pattern separation in the CA3 network. Given that interneuron classes in the hippocampus represent a diverse cell population (Freund and Buzsáki, 1996), the lack of MF LTP in stratum lucidum interneurons may be attributable to target cell-specific differences (Kullmann and Lamsa, 2007; Pelkey and McBain, 2008). In the present study, we performed a detailed analysis of the mechanisms underlying the induction of long-term plasticity at MF synapses on feedforward inhibitory interneurons with soma location in the stratum lacunosum-moleculare (L-M) of area CA3 in rat hippocampal slices.
Materials and Methods
Slice preparation.
Animal use was in accordance with the University Institutional Animal Care and Use Committee. Male Sprague Dawley rats (22 ± 4 d of age; Zivic-Miller Laboratories) were deeply anesthetized (Nembutal; 5 mg/100 g body weight, i.p.) and perfused intracardially with a modified artificial CSF in which sucrose substituted for NaCl (concentrations in mm): 230 sucrose, 1.9 KCl, 1.2 Na2PO4·7H2O, 25.0 NaHCO3, 10.0 glucose, 1.0 CaCl2, 4.0 MgCl2 at 4°C. After 1–2 min of perfusion, animals were decapitated and the brains were removed. Blocks of hippocampus were glued to the stage of a Leica VT1000S and cut in 350-μm-thick sections. Slices were maintained for at least 60 min in an incubation solution of the following composition (in mm): 125 NaCl, 2.0 KCl, 1.2 NaH2PO4, 25 NaHCO3, 10 glucose, 1 CaCl2, and 6 MgCl2, pH 7.3 maintained with bubbled O2 (95%)/CO2 (5%) at room temperature. The slices were then transferred to a submersion recording chamber and superfused at constant flow (2.5 ml/min) with the following solution (in mm): 125 NaCl, 3 KCl, 1.25 Na2HPO4, 25 NaHCO3, 2 CaCl2, 1 MgCl2, 10 glucose, 0.01 (−)-bicuculline methobromide), and 0.05 d-2-amino-5-phosphonopentanoic acid (d-AP5). The temperature of the solution in the recording chamber was set at 33 ± 1°C.
Whole-cell recordings.
Interneurons were identified visually with infrared video microscopy and differential interference contrast optics. Patch pipettes were pulled from borosilicate glass and had resistances of 3–6 MΩ when filled with a solution containing the following (in mm): 120 K-methylsulfate, 10 NaCl, 10 KCl, 10 HEPES, 0.5 EGTA, 4.5 Mg·ATP, 0.3 Na2·GTP, 14 phosphocreatine. Biocytin (0.1%) was routinely added to the pipette solution to allow subsequent morphological identification and reconstruction of the interneurons (see Fig. 1A,B). For 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetra-acetic acid (BAPTA) experiments, the final K-methylsulfate concentration was decreased to 75, and 20 mm tetrapotassium BAPTA (4K+-BAPTA; Sigma-Aldrich) was added. Osmolarity was routinely checked and adjusted to 295–300 mOsm with pH 7.2–7.3.
Figure 1.
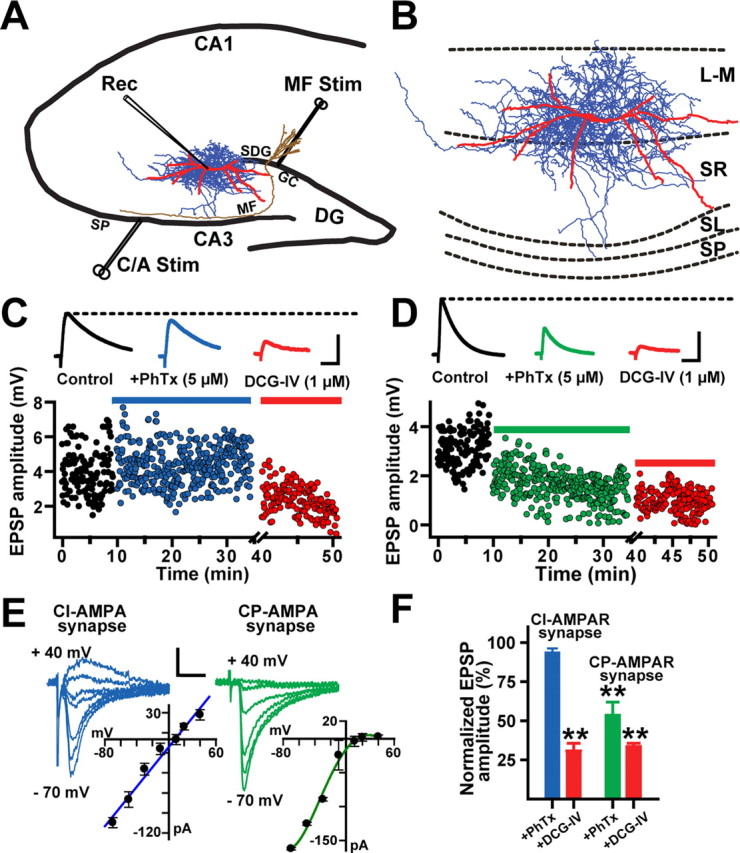
Composition of AMPARs at MF synapses on L-M interneurons. A, Schematic illustration of the hippocampal slice preparation showing the position of the concentric bipolar electrode in the SDG to stimulate MFs (MF Stim). In some experiments, a second bipolar electrode was placed in the s. pyramidale to activate the commissural/associational fibers (C/A Stim). The whole-cell recording pipette (Rec) was placed in the stratum lacunosum-moleculare (L-M) of area CA3. B, Computer reconstruction of a biocytin-filled L-M interneuron. Dendritic (red) and axonal arbors (blue) were reconstructed from serial coronal slices using the Neurolucida software. Abbreviations: SP, Stratum pyramidale; SL, stratum lucidum; SR, stratum radiatum; L-M, stratum lacunosum moleculare. C, Scatter plot from one representative experiment showing weak sensitivity of MF EPSPs (≤10%) to PhTx (5 μm). MF EPSPs were reduced by DCG-IV (1 μm) confirming their MF origin. Each dot represents a single MF EPSP evoked at 0.3 Hz. The inset shows average MF EPSP traces (10 sweeps) from the same experiment. MF EPSPs were recorded in the presence of bicuculline (10 μm) and d-AP5 (50 μm). Calibration: 2 mV, 25 ms. D, In a second group of L-M interneurons, MF EPSPs were highly sensitive to PhTx (≥50%) and were also significantly reduced by DCG-IV (1 μm), confirming their MF origin. The insets show average MF EPSP traces (10 sweeps) from the same experiment. Calibration: 2 mV, 25 ms. E, Representative example of a linear current–voltage relationship (I–V) for a CI-AMPAR MF synapse (r = 0.97; left) and a strongly inward-rectifying I–V for a CP-AMPAR MF synapse (right). Calibration: 100 pA, 10 ms. F, Summary graph showing the number CI-AMPA MF synapses on L-M interneurons (N = 36) and CP-AMPA MF synapses (N = 14) and the average block by PhTx and DCG-IV. **p < 0.001 or higher statistical significance. Error bars indicate SEM.
Electrophysiological measurements of membrane properties.
The membrane potential was measured immediately after cell break-in. After the membrane potential of the cell was stabilized in current clamp, a series of inward and outward current steps (500 ms duration; 30 pA increments) were injected via the whole-cell pipette to assess input resistance (Ri), action potential (AP) amplitude, and afterhyperpolarization (AHP) amplitude. Ri was calculated as the slope of linear fit between voltage and injected current AHP amplitude was measured from AP threshold to the hyperpolarization peak. Experiments were analyzed only if the access resistance was <10 MΩ or it changed <15% of the initial value. Access resistance was monitored throughout the length of experiments.
Stimulation techniques.
MF synaptic responses were evoked by extracellular stimulation using concentric bipolar electrodes (12.5 μm inner pole diameter, 125 μm outer pole diameter; FHC) positioned on the suprapyramidal blade of the dentate gyrus (SDG) (Calixto et al., 2008) (see Fig. 1A). In some experiments, a second stimulating electrode was placed in the s. pyramidale of area CA3 to activate the commissural/associational (C/A) fibers. Stimulation consisted of single monopolar pulses (100–300 μA; 50–100 μs duration) at 0.25 Hz. To reduce the probability of antidromic activation of CA3 pyramidal cells via stimulation of their axon collaterals, we used low current intensities, which resulted in composite EPSP with amplitudes <30% of the threshold amplitude required to evoke action potentials in the recorded interneurons. Paired-pulse facilitation (PPF) (60 ms ISI) was assessed in current-clamp mode. PPF was calculated as the paired-pulse ratio (PPR) of the amplitude of the second EPSP to that of the first in the pair.
Identification of MF synaptic input.
Current- and voltage-clamp recordings were obtained with an Axoclamp-1D amplifier (Molecular Devices) in the presence of (−)-bicuculline methiodide (10 μm) and d-AP5 (50 μm) to block GABAA and NMDA receptor (NMDAR)-mediated responses, respectively [supplemental Table 1 (available at www.jneurosci.org as supplemental material) summarizes the properties MF EPSPs on L-M interneurons]. The group II metabotropic glutamate receptor agonist (2S,2′R,3′R)-2-(2′,3′-dicarboxycyclopropyl)glycine (DCG-IV) (1 μm) was applied at the end of every experiment to confirm the MF origin of the EPSP (see Fig. 1C,D). Although DGC-IV inhibition of MF transmission in pyramidal cells is consistently complete (≥90%) (Kamiya et al., 1996), it is variable in interneurons (Alle et al., 2001; Lawrence and McBain, 2003; Calixto et al., 2008). Therefore, synaptic responses were classified to be of MF origin if their DCG-IV sensitive was >50% inhibition (Lawrence and McBain, 2003).
Criteria for LTP and LTD.
LTP and LTD were induced by HFS consisting of three trains of 100 pulses each at 100 Hz, repeated every 10 s paired with a postsynaptic depolarizing current step (30 ± 0.6 pA) in current-clamp mode. This induction protocol produced strong postsynaptic firing. Changes in the strength of the compound EPSP were assessed by measuring the initial slope of the EPSP waveform (20–80% from the EPSP onset). Minimally evoked EPSPs and EPSCs were quantified measuring peak amplitude of the waveform. We defined LTP as stable potentiation of >25% with respect to baseline for at least 30 min after HFS (Lamsa et al., 2005). LTD was defined as a reduction of synaptic efficacy of <25% for at least 30 min after HFS. Values from each experiment normalized to its pre-HFS baseline magnitude for comparison across experiments. Signals were low-pass filtered at 5 kHz, digitized at 10 kHz, and stored on disk for off-line analysis. Data acquisition and analysis were performed using customized LabView programs (National Instruments)
Minimal stimulation techniques.
These experiments were performed and analyzed as previously reported by others (Stevens and Wang, 1994; Xiang et al., 1994; Perez et al., 2001; Lawrence et al., 2004). To identify putative single MF synaptic connections, the stimulus strength was gradually reduced until no EPSPs were detected and then increased in increments of 5 μA until at an abrupt stimulation threshold at which responses were reliably evoked at a failure rate between 30 and 60% (failures were identified by visual inspection). Cells were held at 69 ± 0.1 mV and access resistance was monitored every 10 min throughout the experiments. MF EPSPs were included according to the following criteria: invariant EPSPs latency and shape, and monoexponential EPSP decay. Under these conditions, we assumed that the EPSP recording was predominantly monosynaptic and attributable to the activation of a single MF fiber. Minimally, EPSPs were evoked at 0.25 Hz. After a 5 min stable baseline period, LTP was induced by injecting a postsynaptic depolarizing current step (30 ± 0.6 pA) that evoked action potentials when paired to the HFS train. EPSPs were recorded for at least 30 min after HFS, and then DCG-IV was applied to confirm their MF origin.
Statistical analysis.
Group measures are expressed as means ± SEM. To determine the statistical significance of the changes in EPSP amplitude, the mean EPSP amplitude baseline was compared with the mean EPSP amplitude at 25–30 min after HFS. Normality of the populations were tested with Kolmogorov–Smirnov test (p < 0.05), followed by one-way ANOVA and Student–Newman–Keuls all-pairwise comparisons (p < 0.05). In all cases, differences were considered significant if p < α = 0.05.
Drugs.
d-AP5, (−)-bicuculline methobromide, DCG-IV, (1)-2-methyl-4-carboxyphenylglycine (LY367385), and 2-methyl-6-(phenylethynyl)-pyridine hydrochloride (MPEP) were purchased from Tocris. Philanthotoxin 433, BAPTA, nimodipine, and low-molecular-weight heparin (H-3400) were acquired from Sigma-Aldrich. Purified Ryanodine was obtained from BIOMOL Research Laboratories.
Morphological reconstruction.
After recordings, slices were fixed in cold 4% paraformaldehyde for 72 h, transferred into an antifreeze solution (1:1 mixture of glycerol and ethylene glycol in 0.1 m phosphate buffer), and stored at −80°C. Slices were then cut into 60 μm sections on a vibratome, reacted with 1% H2O2, and placed in blocking serum with 0.5% Triton X-100 for 2 h at room temperature. Biocytin-labeled neurons were incubated with ABC-peroxidase and developed using the Ni-enhanced DAB (3,3′-diaminobenzidine) chromogen. Interneurons were reconstructed using the Neurolucida tracing system (MicroBrightField) on a Axioplan 2 Zeiss microscope equipped with differential interference contrast, a 100× (numerical aperture, 1.4) planapochromatic lens and additional Optovar magnification of 1.6× (final optical magnification, 1600×; screen magnification, 7200×). For the reconstructions, all sections containing the cell were used.
Results
AMPA receptor composition of the MF synapse on L-M interneurons
Visually guided whole-cell recordings in combination with biocytin cell labeling were obtained from the somata of interneurons positioned in the s. lacunosum-moleculare at ∼278 ± 20 μm from the boundary between s. pyramidale and s. lucidum, 150 ± 20 μm from the SDG (Fig. 1A), and 60–100 μm below the slice surface. Post hoc analysis of labeled interneurons revealed that somata were positioned at −2.45 ± 0.67 mm from bregma, and 2.33 ± 0.16 mm from midline (G. A. Ascoli, K. M. Brown, E. Calixto, J. P. Card, and G. Barrionuevo, unpublished observations). L-M interneurons gave rise to complex axonal arbors, which extended beyond the somatic layer position into the stratum radiatum and stratum lucidum (Fig. 1A,B). Most dendritic branches exhibited a conspicuous lack of spines. Some of the dendrites branches in the s. lacunosum-moleculare course near the SDG where they could receive input from the MF axons traveling to the s. lucidum or from MF collateral plexuses in the hilus of the dentate gyrus (Claiborne et al., 1986; Acsády et al., 1998). In addition, these interneurons also may receive MF input from synapses en passant in the s. lucidum or from filipodial extensions arising from MF boutons on CA3 pyramidal cells in the s. pyramidale (Acsády et al., 1998). However, our previous observations suggest that MF input elicited from the SDG stimulation site is predominantly evoked from axonal projections confined to dendrites near the hilus (Calixto et al., 2008). We previously reported the passive and active membrane properties of L-M interneurons (Calixto et al., 2008).
In hippocampal pyramidal cells, AMPA receptors (AMPARs) are calcium-impermeable (CI) composed of heteromeric GluR1, GluR2, and GluR3 subunits, whereas hippocampal interneurons also express GluR2-lacking calcium-permeable (CP) AMPARs (Dingledine et al., 1999; Tóth et al., 2000; Bischofberger and Jonas, 2002). The GluR2-lacking AMPARs are selectively blocked by the extracellular application of the polyamine toxin philanthotoxin (PhTx). Based on the sensitivity of MF EPSPs to PhTx, L-M interneurons were classified in two subpopulations (Fig. 1F). In 18 of 28 cells, PhTx (5 μm) produced a small (≤10%) but significant reduction in EPSP amplitudes (93 ± 2.6% of baseline; p < 0.05, one-way ANOVA) (Fig. 1C; supplemental Table 1, available at www.jneurosci.org as supplemental material). EPSPs were subsequently depressed by DCG-IV (1 μm; 66.2 ± 4.6% inhibition; p < 0.001, one-way ANOVA) (Fig. 1C) confirming that they were originated from MF synapses (Tóth et al., 2000; Alle et al., 2001; Calixto et al., 2008). These EPSPs were classified as produced by CI-AMPARs (Lei and McBain, 2002). In the 10 remaining cells, MF EPSP amplitudes were substantially reduced (≥50%) by PhTx (53.3 ± 8.4% of baseline) (Fig. 1D,F) and further depressed by DCG-IV (63.2 ± 8% inhibition; p < 0.001, one-way ANOVA). These MF EPSPs were categorized as originating from CP-AMPARs (Lei and McBain, 2002). These two subpopulations of interneurons appear to coexist in close proximity to each other within the stratum lacunosum moleculare. In some experiments, we also assessed the current–voltage relationship (I–V) of AMPAR EPSCs. In agreement with previous reports (Tóth and McBain, 1998; Laezza et al., 1999), the PhTx-insensitive AMPAR EPSCs exhibited near linear I–V relationships, whereas the PhTx-sensitive AMPAR EPSCs showed a strong inward rectification (Fig. 1E).
MF LTP is input specific and induced at predominantly CI-AMPAR synapses
Previous studies demonstrated that MF LTP in pyramidal cells is input specific (Zalutsky and Nicoll, 1990) and induced at synapses containing CI-AMPARs (Tóth et al., 2000) in the absence of NMDAR activation (Harris and Cotman, 1986). Given the considerable proportion of MF synapses that were weakly sensitive to the PhTx block (62%), we postulated that CI-AMPARs on L-M interneurons may be capable of supporting input-specific, NMDAR-independent MF LTP. To test this hypothesis, MF EPSCs and C/A (Fig. 1A) EPSCs were recorded simultaneously (Vh = 69 ± 3 mV) in the presence of PhTx (5 μm) in perfusion bath. In 11 of 18 cells (61% of total), PhTx modestly reduced MF EPSC amplitudes (93.5 ± 3% of baseline; p < 0.05, one-way ANOVA) (Fig. 2A,C) but did not affect C/A EPSC amplitudes (97.2 ± 8% of baseline; p > 0.5, one-way ANOVA) (Fig. 2A,C). At the end of the PhTx application, HFS was delivered to the MF input. The depolarized interneuron fired a train of action potentials during each of the HFS trains. After the HFS, the isolated CI-AMPAR component of the MF EPSC exhibited a robust posttetanic potentiation (PTP) (203.3 ± 6.4% of baseline) followed by a sustained LTP (163.1 ± 17% of baseline at 30 min after HFS; p < 0.001, one-way ANOVA; N = 11). Bath application of DCG-IV (1 μm) confirmed that the EPSCs were generated by MF synapses (67.3 ± 10.8% inhibition) (Fig. 2B,C). In contrast, C/A EPSCs were not affected by the HFS of MFs (95 ± 10.5% at 1 min after HFS, and 95.2 ± 16% at 30 min after HFS; p > 0.5, one-way ANOVA; N = 11) and were insensitive to DCG-IV (3.4 ± 12.4% inhibition) (Fig. 2A–C). Collectively, these data indicate that MF LTP in L-M interneurons is input specific, and not the consequence of contamination from NMDAR-independent LTP at C/A synapses on the recorded cell. Additional data obtained in our laboratory show that similar HFS delivered to the C/A input to L-M interneurons in the presence of d-AP5 induced LTD (57.2 ± 6.8% of baseline; N = 3) (T. Perez-Rosello and G. Barrionuevo, unpublished observations).
Figure 2.
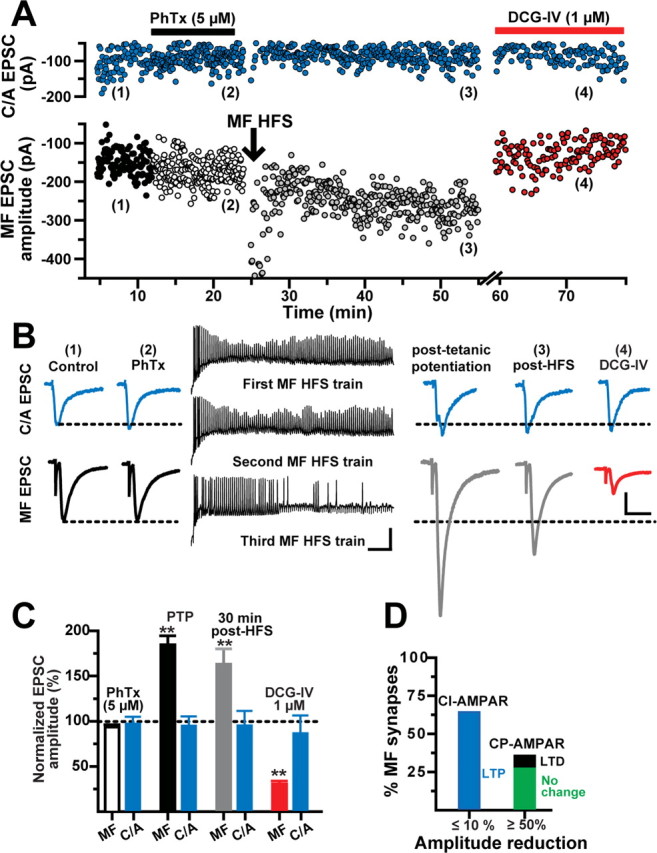
MF LTP is input specific and expressed at predominantly CI-AMPAR synapses. A, Representative experiment showing the time course of MF and C/A EPSC amplitudes before (1), during PhTx (2), after HFS of MFs (3), and during DCG-IV (4). Each circle represents a single EPSC recorded at 0.2 Hz. MF EPSCs were weakly (<10%) sensitive to PhTx (5 μm). LTP was induced at MFs but not C/A synapses. B, Left and right traces, Average MF and C/A EPSCs (10 consecutive sweeps) from the same experiment before and after HFS of MFs, respectively. Calibration: 50 pA, 10 ms. Middle traces, Membrane voltage responses show robust spiking during HFS of MFs. Calibration: 25 mV, 100 ms. C, Summary of the changes in MF and C/A EPSC amplitude (N = 11) during PTP, at 30 min after HFS, and during DCG-IV application. MF synapses were classified as containing CI-AMPARs (MF EPSC block by PhTx, ≤10%). D, Bar graph summarizing the proportion of MF synapses containing predominantly CI-AMPARs (N = 11) and CP-AMPARs (N = 7). All of the CI-AMPAR synapses underwent MF LTP. In contrast, in MF synapses predominantly containing CP-AMPAR the isolated CI-AMPAR-mediated response was unchanged after MF HFS (N = 5) or exhibited MF LTD (N = 2). **p < 0.001 or higher statistical significance.
In 7 of 18 interneurons treated with PhTx, MF EPSCs were substantially reduced (53.3 ± 8.4% of baseline) and classified as predominantly containing CP-AMPARs. After HFS, the isolated CI-AMPAR-mediated responses in 5 of 7 cells (28%) exhibited a smaller PTP (135.5 ± 15.7% relative to PhTx baseline; p < 0.002) and remained unchanged at 30 min after HFS (95.9 ± 6%) despite strong postsynaptic firing during the HFS (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). In the remaining two interneurons (11%), the isolated CI-AMPAR-mediated MF EPSC underwent LTD (73.7 ± 8.2% at 30 min after HFS; p < 0.0001, unpaired t test) (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
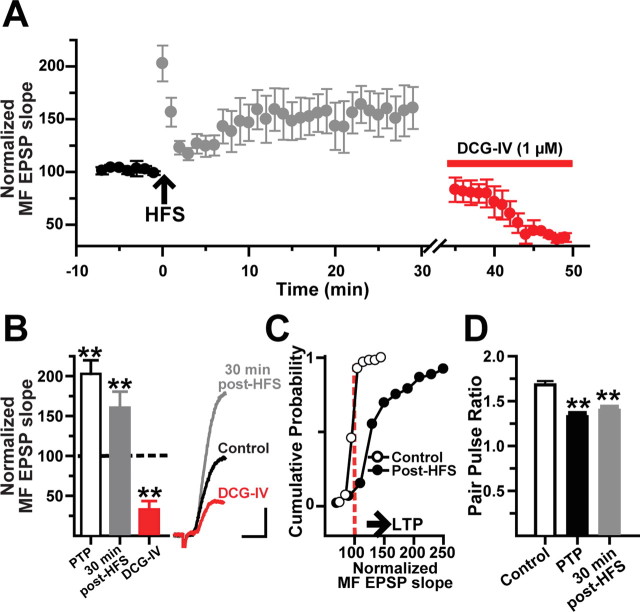
We further characterize the magnitude and time course of MF LTP in L-M interneurons in current-clamp conditions (Vm = 69.6 ± 1 mV) (supplemental Table 1, available at www.jneurosci.org as supplemental material). In the majority of these cells (25 of 32; 78%), the initial slope of the MF EPSP showed robust PTP (202.8 ± 17% of baseline) followed by a sustained LTP (160.6 ± 19% at 30 min after HFS; N = 25 of 32; p < 0.001, one-way ANOVA) (Fig. 3A–C). DCG-IV subsequently reduced the EPSP slopes by 64.7 ± 4% (p < 0.001, one-way ANOVA). We also measured PPR during baseline (1.73 ± 0.05), at 1 min after HFS (1.52 ± 0.03; one-way ANOVA, p < 0.001), and at 30 min after HFS (1.48 ± 0.04; one-way ANOVA, p < 0.05) (Fig. 2D). The reduction in PPF during LTP is suggestive of a presynaptic expression of MF LTP in L-M interneurons, as previously shown for MF LTP in pyramidal cells (Staubli et al., 1990; Zalutsky and Nicoll, 1990; Xiang et al., 1994; Sokolov et al., 2003) and dentate gyrus basket cells (Alle et al., 2001). In the remaining seven cells, MF EPSP slopes remained unchanged after HFS (N = 5; 16% of total) or underwent LTD (N = 2; 6% of total).
Figure 3.
MF LTP is accompanied by a decrease in paired-pulse facilitation. A, Time course of normalized MF EPSP slopes before and after the induction of LTP. Each circle represents average ± SEM from 25 interneurons. The MF origin of the EPSPs was confirmed by DCG-IV application at the end of the experiment. B, Bar graph (N = 25) summarizes the changes in MF EPSP slope during PTP, at 30 min after HFS, and in the presence of DCG-IV. The inset shows the average MF EPSP slope (10 sweeps) from one representative cell showing LTP and DCG-IV sensitivity. Calibration: 2 mV, 5 ms. C, Cumulative probability distribution of normalized EPSP slopes before HFS (open circles) and during LTP measured after the first 5 min after HFS to exclude PTP (closed circles) for the same cells. Each point represents the magnitude of change relative to a normalized baseline computed from the average EPSP slope. The start of the horizontal arrow corresponds to the minimum criteria for LTP (stable synaptic enhancement >25% above baseline for 30 min). D, Decrease in paired-pulse facilitation during PTP and LTP. **p < 0.001 or higher statistical significance. Error bars indicate SEM.
LTP of minimally evoked MF EPSPs
Another possible source of interpretational error is that the potentiation observed in L-M interneurons may have resulted from the recruitment of additional CA3 pyramidal cells because of the parallel induction of MF LTP in CA3 pyramidal cells. Hence, HFS potentiated the MF input and also the synaptic drive from the polysynaptic recurrent collateral input. To reduce the likelihood of polysynaptic contamination, we examined changes in synaptic efficacy evoked by minimal stimulation (Fig. 4A), which putatively activates single axons that terminate onto L-M interneurons (Stevens and Wang, 1994; Xiang et al., 1994; Perez et al., 2001; Lawrence et al., 2004). During baseline period, minimal stimulation elicited MF EPSPs with average amplitudes of 0.66 ± 0.02 mV (failures included). HFS was paired with a postsynaptic depolarizing current pulse (30 ± 0.6 pA), and in all cases the cells fired action potentials in response to the HFS (Fig. 4B, inset). This pairing protocol yielded a sustained increase in EPSP amplitude in six of nine cells (1.10 ± 0.03 mV at 25–30 min after HFS; p < 0.001, one-way ANOVA) (Fig. 4B,C), and no change in the remaining three cells (0.64 ± 0.07 mV). LTP induced with the minimal stimulation protocol was associated with a decrease in both failure rate (30–65% during baseline; 8–30% at 25–30 min after HFS) and coefficient of variation (CV) (0.7 ± 0.06 during baseline; 0.5 ± 0.05 at 25–30 min after HFS; N = 6; p < 0.02, one-way ANOVA) (Fig. 4D–F). After 30 min of sustained LTP, the application of DCG-IV (1 μm) decreased EPSP amplitude to 0.45 ± 0.03 mV (N = 6) and increased the failure rate by 45–60%. Together, these data indicate that LTP was induced directly at MF synapses on L-M interneurons and resulted from an increase in the probability of transmitter release from MF presynaptic terminals.
Figure 4.
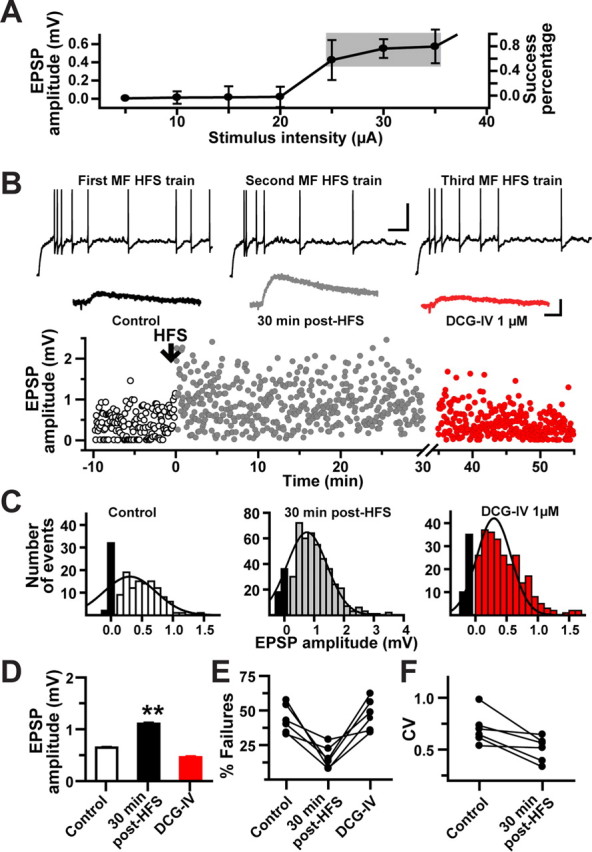
LTP of minimally evoked MF EPSP. A, Putative single-fiber MF EPSPs were identified by plotting EPSP amplitudes as a function of stimulus intensity. The gray area indicates the typical range of stimulation intensity, which was kept constant throughout the experiment. This stimulation intensity produced 30–60% of failure rate of the evoked MF EPSPs. B, Scatter plot of minimal MF EPSP amplitudes from one representative cell showing the occurrence of LTP after the pairing of HFS with postsynaptic depolarization. Each dot in the scatter plot represents a single MF EPSP evoked at 0.3 Hz. Inset, Top traces show postsynaptic spiking during MF HFS. Calibration: 25 mV, 100 ms. Bottom traces are average minimal MF EPSPs (10 consecutive sweeps) exhibiting LTP at 30 min after HFS and the subsequent amplitude reduction by DCG-IV confirming their MF origin. Calibration: 0.5 mV, 10 ms. C, Amplitude histograms of the normalized distribution of minimal MF EPSPs from the same cell. The black bars represent the number of failures. D, Summary bar graph for all cells (N = 6) depicting LTP of minimal MF EPSPs and their inhibition by DCG-IV. E, The increase in the potency of MF synaptic transmission during LTP was associated with a significant decrease in the failure rate. F, Likewise, there was a significant decrease in the CV during MF LTP. **p < 0.001 or higher statistical significance. Error bars indicate SEM.
MF LTP requires postsynaptic depolarization and calcium elevation
To test the involvement of postsynaptic activation in the induction of LTP, we applied HFS to MFs while simultaneously voltage clamping the membrane potential of the cell at −100 mV to prevent postsynaptic spiking (Fig. 5A, inset). Under these conditions, no significant changes after HFS were detected in the EPSP slope compared with baseline responses (98.7 ± 8% at 5 min after HFS; p > 0.05; 102 ± 6% at 15 min after HFS; p > 0.05; N = 10) (Fig. 5A). A subsequent HFS applied in current-clamp mode (Vm = 69 ± 3 mV) elicited a pronounced and sustained increase in the initial EPSP slope (165.7 ± 19% at 30 min after HFS; N = 6) (Fig. 4A–C) (p < 0.001, one-way ANOVA). Application of DCG-IV inhibited the initial slope of the potentiated EPSPs by 64.6 ± 6% (Fig. 5A,C).
Figure 5.
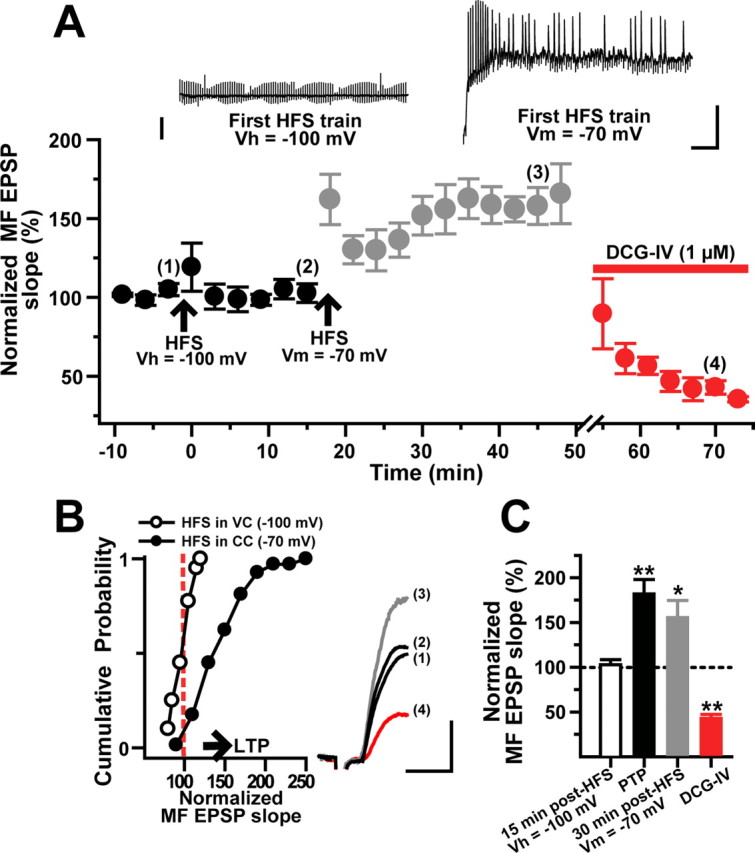
The induction of MF LTP in L-M interneurons requires simultaneous afferent stimulation and postsynaptic depolarization. A, HFS applied while cells (N = 8) were held in voltage clamp at −100 mV failed to induce LTP. Subsequent HFS applied in current-clamp recordings (−70 mV) evoked robust LTP. MF EPSPs were sensitive to DCG-IV. Inset, The left trace is the membrane current response from a representative cell held in voltage-clamp conditions showing the absence of postsynaptic spiking during the HFS. The right trace is the membrane voltage response from the same cell in current-clamp conditions displaying action potential discharge throughout the HFS. Calibration: 25 mV, 100 ms. B, Cumulative probability distribution of normalized MF EPSP slopes for the same cells showing absence of LTP (HFS in voltage-clamp mode) and the occurrence of LTP (HFS in current-clamp mode) in the same cells. The start of the horizontal arrow corresponds to the minimum criteria for LTP (stable synaptic enhancement >25% above baseline for 30 min). The inset shows average MF EPSP slopes (10 consecutive sweeps) from a representative experiment obtained at the time points indicated by the numbers in A. Calibration: 2 mV, 5 ms. C, Population data (N = 10) showing lack of MF LTP when HFS was applied in voltage-clamp conditions. In the same cells, MF LTP was induced by a second HFS delivered during current-clamp recordings. *p < 0.05 and **p < 0.001 or higher statistical significance. Error bars indicate SEM.
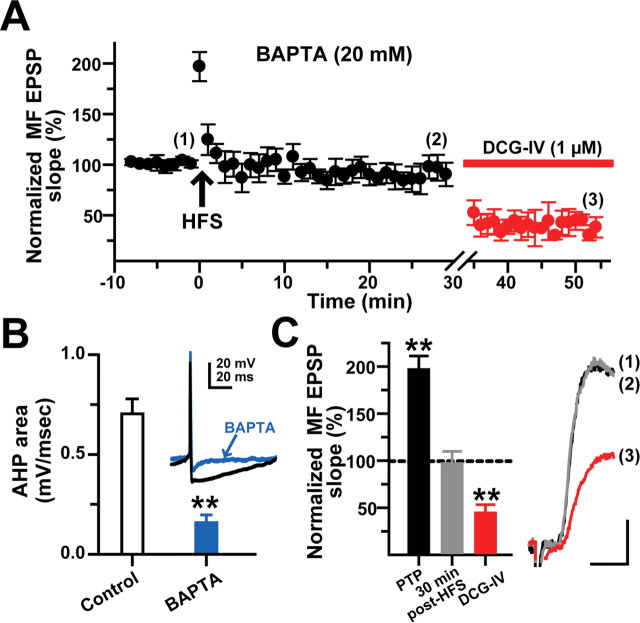
It has been shown that elevation of postsynaptic Ca2+ influx is a necessary condition for the induction of MF LTP in pyramidal cells (Jaffe and Johnston, 1990; Yeckel et al., 1999; Sokolov et al., 2003) (but see Zalutsky and Nicoll, 1990) and basket cells in the dentate gyrus (Alle et al., 2001). To investigate whether MF LTP in L-M interneurons depends on a rise in postsynaptic [Ca2+] elicited by the HFS, the patch pipette was filled with an internal solution containing the fast calcium chelator BAPTA (20 mm). BAPTA loading did not significantly affect the EPSP kinetics during the baseline period (supplemental Table 1, available at www.jneurosci.org as supplemental material) but significantly reduced the area of the Ca2+-dependent afterhyperpolarization potential (AHP) after trains of action potentials (77.4 ± 4.6% of baseline; p < 0.000, unpaired t test; N = 10) (Fig. 6B). BAPTA loading did not affect PTP of the EPSP slope (202.8 ± 16.9% in control cells; N = 31; 216.3 ± 42.3% in BAPTA loaded cells; p > 0.05, one-way ANOVA; N = 11) but prevented LTP induction in 11 of 15 cells (96.9 ± 14% at 5 min, p > 0.05; 90.4 ± 11% at 30 min after HFS, p > 0.05, one-way ANOVA) (Fig. 6A,C). The application of DCG-IV (1 μm) confirmed the MF origin of the EPSPs (62 ± 9% inhibition; p < 0.001, one-way ANOVA) (Fig. 6A,C). In the remaining four cells, BAPTA loading failed to block LTP (159.5 ± 8.6% of baseline at 30 min after HFS; p < 0.001, unpaired t test; 66.2 ± 2.57% inhibition by DCG-IV) (data not shown). It has been reported that, in pyramidal cells, block of MF LTP induced with similar HFS trains requires 30–50 mm BAPTA concentrations (Yeckel et al., 1999). Failure to prevent LTP in some L-M interneurons loaded with 20 mm BAPTA may indicate that some accumulation of Ca2+ ions took place near the Ca2+ sensor that triggers LTP (see Discussion).
Figure 6.
The induction of MF LTP requires elevation of postsynaptic [Ca2+]. A, Time course of normalized MF EPSP slopes before and after HFS from BAPTA-loaded cells (N = 11). At 30 min after HFS, MF EPSP slopes were unchanged. The DCG-IV sensitivity confirmed the MF origin of the EPSPs. Intracellular BAPTA (20 mm) prevented MF LTP without altering the baseline properties of MF EPSPs (see supplemental Table 1, available at www.jneurosci.org as supplemental material). B, AHP reduction in BAPTA-loaded cells. The inset shows a superimposed single trace of the AHP from one control and one BAPTA-loaded cell elicited by a suprathreshold somatic current injection (33 pA; 500 ms duration). Calibration: 20 mV, 20 ms. C, Summarized data indicating that BAPTA did not affect PTP but suppressed LTP. Inset, Average MF EPSP slopes (10 consecutive sweeps) from a representative experiment. Calibration: 2 mV, 5 ms. **p < 0.001 or higher statistical significance. Error bars indicate SEM.
MF LTP requires L-type Ca2+ channel activation
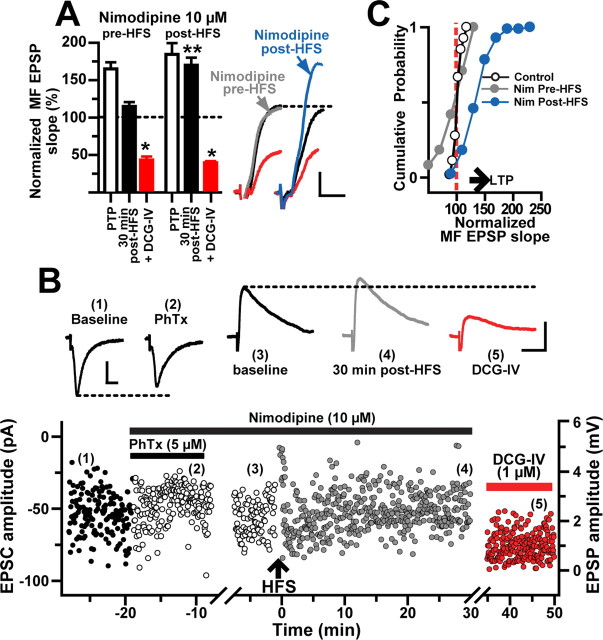
On the basis of the findings described above, we hypothesized that the induction of MF LTP in L-M interneurons requires postsynaptic Ca2+ influx via voltage-gated channels. It has been shown that activation of the L-type Ca2+ channel is necessary for the induction of NMDAR-independent forms of LTP in the hippocampus (Grover and Teyler, 1992; Kullmann et al., 1992; Huang and Malenka, 1993; Chen et al., 1998; Kapur et al., 1998; Lauri et al., 2003) and amygdala (Weisskopf et al., 1999). These data are compatible with the observations that L-type channels are abundantly expressed on the proximal dendrites and soma of hippocampal pyramidal cells and interneurons (Ahlijanian et al., 1990; Westenbroek et al., 1990) and capable of producing considerable increases in cytosolic Ca2+ (Miyakawa et al., 1992; Denk et al., 1996). To test whether L-type channel activation is required for MF LTP induction in L-M interneurons, the L-type channel antagonist nimodipine (10 μm) was added to the perfusion bath before the delivery of HFS. We found that nimodipine did not alter the properties of the evoked MF EPSPs (supplemental Table 1, available at www.jneurosci.org as supplemental material) but prevented LTP of the EPSP slope (98.4 ± 9% at 5 min, p > 0.05; 106.7 ± 10% at 30 min after HFS, p > 0.05; N = 9) (Fig. 7A,C). In three of these cells, we assessed the effect of nimodipine on the isolated CI-AMPAR component by including PhTx (5 μm) in the perfusion bath before HFS. In voltage-clamp conditions (Vh = −70 mV), PhTx moderately reduced MF EPSC amplitudes (91.2 ± 4% of baseline; p < 0.001, unpaired t test) (Fig. 7B). At the end of the PhTx application, recording was switched to current-clamp conditions (−70 mV) and HFS was delivered to the MF input. After the HFS, the isolated CI-AMPAR component of the MF EPSP exhibited PTP (154.8 ± 6.5% of baseline) but did not show LTP (110.8 ± 6% of control at 30 min after HFS; p > 0.5, one-way ANOVA). Bath application of DCG-IV (1 μm) confirmed that the EPSCs were generated by MF synapses (66.1 ± 4.4% inhibition) (Fig. 7B). We also examined the effect of L-type channel blockade on the maintenance of LTP by adding nimodipine 10 min after the delivery of the HFS. The results of these experiments indicated that nimodipine did not affect the potentiated MF EPSP slope (169.6 ± 10% at 30 min after HFS, p < 0.001; N = 5) (Fig. 7A,C). In both cases, DCG-IV applied at the end of the experiments reduced the responses (59 ± 5 and 60.3 ± 2% with nimodipine before and after, respectively; p < 0.001, one-way ANOVA) (Fig. 7A). Together, these findings show that the induction but not the maintenance of LTP at MF synapses on L-M interneurons required the activation of L-type channels.
Figure 7.
The induction of MF LTP requires L-type Ca2+ channel activation. A, Time course of normalized MF EPSP slopes demonstrating that nimodipine (10 μm) added before HFS blocked LTP (N = 9) but did not affect LTP maintenance when it was included in the bath solution 5–10 min after HFS (N = 5). Summary bar graph for the same cells showing the effect of nimodipine added to the bathing solution before or after HFS. The EPSP amplitude was similarly reduced by DCG-IV in both conditions. The inset depicts average MF EPSP slopes (10 consecutive sweeps) from two representative experiments. Calibration: 2 mV, 5 ms. B, Representative experiment showing the time course of MF amplitude before (1), in PhTx and nimodipine (2), during baseline in current clamp (3), at 30 min after HFS (4), and during DCG-IV (5). Each circle represents a single EPSC or EPSP recorded at 0.2 Hz. First break in timescale represents the switch from voltage clamp to current clamp. MF EPSCs were weakly (<10%) sensitive to PhTx (5 μm) and LTP was blocked in the presence of nimodipine. The inset shows average traces (10 sweeps) from the same experiment at time indicated by the numbers. Calibration: 2 mV, 25 ms. C, Cumulative probability distribution of normalized EPSP slopes from all the cells treated with nimodipine before or after HFS. Each circle represents the magnitude of change relative to a normalized baseline computed from the average of the baseline EPSPs. The start of the horizontal arrow corresponds to the minimum criteria for LTP. *p < 0.05 and **p < 0.001 or higher statistical significance. Error bars indicate SEM.
Blockade of mGluR1α, IP3 receptors, or ryanodine receptors results in MF LTD
The group I mGluRs (mGluR1/mGluR5) are expressed in the periphery of the postsynaptic densities in the s. lacunosum moleculare of area CA1 (Lujan et al., 1996). In particular, the mGluR1α isoform is mainly located postsynaptically in GABA-containing interneurons (Baude et al., 1993; Ferraguti et al., 2004) and is required for LTP induction in CA1 alveus/oriens interneurons (Lapointe et al., 2004; Topolnik et al., 2006). To investigate whether mGluR1α activation is also involved in the induction of MF LTP in L-M interneurons, HFS was applied in the presence of the selective antagonist LY367385 (100 μm). Although PTP was similar to baseline (173 ± 7%), the same HFS protocol that induces MF LTP in naive slices induced MF LTD in LY367385-treated slices (73 ± 12% at 30 min after HFS; p < 0.001; N = 11; 66.8 ± 4.5% inhibition by DCG-IV; p < 0.001, one-way ANOVA) (Fig. 8A). We also tested the effect of LY367385 on the isolated CI-AMPAR component by applying PhTx (5 μm) to the perfusion bath before HFS. In voltage-clamp conditions (Vh = −70 mV), PhTx modestly reduced MF EPSC amplitudes (95.4 ± 3% of baseline; p < 0.05, unpaired t test) (Fig. 8B). At the end of the PhTx application, recordings were switched to current-clamp conditions (−70 mV) before the delivery of HFS to MFs. After a robust PTP (165.6 ± 1%), MF EPSPs underwent a pronounced LTD (71 ± 3.6 at 30 min after HFS; p < 0.001; N = 3) and were further inhibited by DCG-IV (69.3 ± 9.1% of control).
Figure 8.
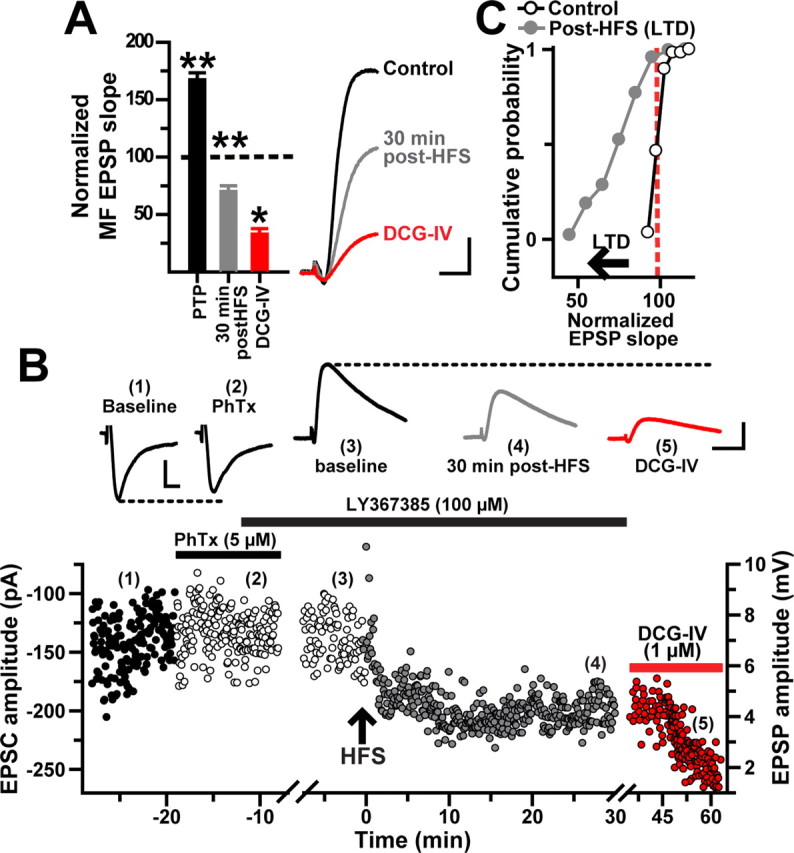
Blockade of mGluR1α reveals MF LTD at CI-AMPARs. A, Time course of normalized MF EPSP slopes (N = 14) showing LTD induced in the presence of LY367385 (100 μm) by the same HFS that induces MF LTP. The bar chart summarizes the effect of LY367385 and DCG-IV in the same cells. Inset, Average MF EPSPs (10 consecutive sweeps) from one representative experiment. B, Representative experiment showing the time course of MF amplitude before (1), in PhTx (2), during baseline in current clamp in presence of LY3657385 (100 μm) (3), at 30 min after HFS (4), and during DCG-IV (5). Each circle represents a single EPSC or EPSP recorded at 0.2 Hz. First break in timescale represents the switch from voltage clamp to current clamp. MF EPSCs were weakly (<5%) sensitive to PhTx (5 μm) and LTD was induced in the presence of LY367385. The inset shows average traces (10 sweeps) from the same experiment at time indicated by the numbers. Calibration: voltage-clamp recordings, 50 pA,10 ms; current-clamp recordings, 2 mV, 25 ms. C, Cumulative probability distribution of normalized EPSP slopes showing a leftward shift of control values after HFS was applied in the presence of LY367385. The start of the horizontal arrow corresponds to the minimum criteria for LTD (depression of the synaptic transmission ≤25% below baseline for 30 min). *p < 0.05 and **p < 0.001 or higher statistical significance. Error bars indicate SEM.
To determine the locus of LTD expression, we examined the percentage of failures and CV using a minimal stimulation protocol. In the presence of LY367385, HFS paired with a postsynaptic depolarization pulse (∼33 pA) resulted in a depression of MF EPSP amplitudes (0.55 ± 0.09 mV in baseline; 0.41 ± 0.06 mV at 25–30 min after HFS; p < 0.05, one-way ANOVA; N = 5) (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material). The EPSP depression was associated with an increase in the failure rate (35–65% during baseline; 60–80% at 25–30 min after HFS; p < 0.001, one-way ANOVA) (supplemental Fig. 3C, available at www.jneurosci.org as supplemental material) and increase in the CV (0.6 ± 0.08 during baseline, 0.89 ± 0.08 at 25–30 min after HFS; p < 0.001, one-way ANOVA; N = 5) (supplemental Fig. 1D, available at www.jneurosci.org as supplemental material). After 30 min of LTD, the application of DCG-IV (1 μm) decreased EPSP amplitude to 0.33 ± 0.02 mV (p < 0.01; N = 4) (supplemental Fig. 3A,B, available at www.jneurosci.org as supplemental material) and increased the failure rate by 55–90% (p < 0.001, one-way ANOVA) (supplemental Fig. 3C,D, available at www.jneurosci.org as supplemental material). These data indicate that MF LTD, like MF LTP, is presynaptically expressed. In contrast to the marked effects on synaptic plasticity mediated by the mGluR1α, application of the mGluR5-selective antagonist MPEP (25 μm) did not affect the polarity of synaptic plasticity induced by MF HFS (172 ± 16% at 30 min after HFS; p < 0.001, one-way ANOVA; N = 10; DCG-IV sensitivity, 64.5 ± 3% of baseline; p < 0.05) (supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
Activation of mGluR1α is known to increase L-type Ca2+ channel conductance and Ca2+ release from intracellular stores in the endoplasmic reticulum (ER) (Chavis et al., 1996; Woodhall et al., 1999; Fagni et al., 2000; Ouardouz et al., 2003; Bardo et al., 2006) via stimulation of the inositol (1,4,5)-trisphosphate receptor (IP3R). To test the involvement of the IP3–IP3R cascade initiated during the induction of MF LTP, we loaded the cells with heparin (4 mg/ml), an antagonist of IP3Rs (Taylor and Broad, 1998). The dialysis with heparin did not affect PTP (155.9 ± 8.3%) but resulted in MF LTD (76.9 ± 5.7% at 30 min after HFS; N = 8; p < 0.001, one-way ANOVA; 76.1 ± 4.7% inhibition by DCG-IV; p < 0.001, one-way ANOVA) (Fig. 9A–C).
Figure 9.
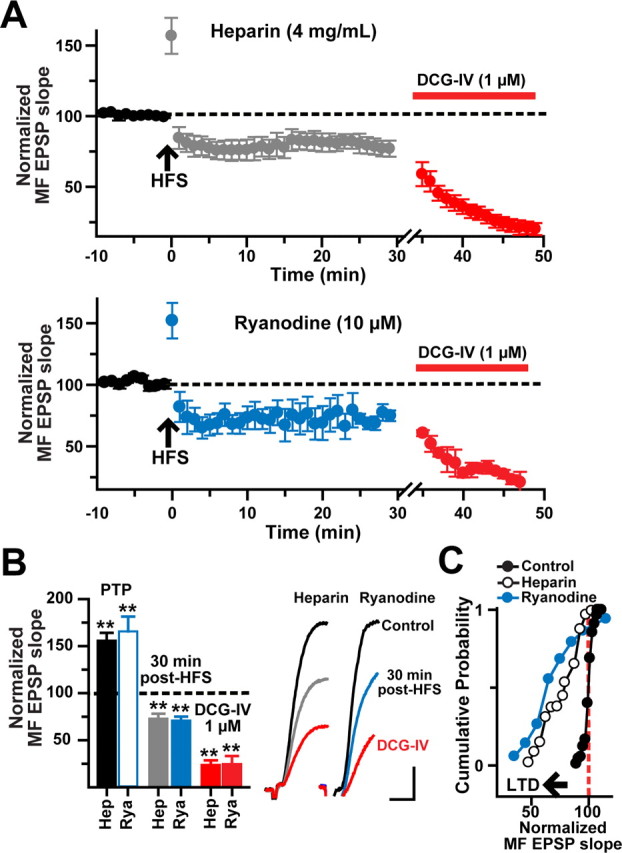
Induction of MF LTP requires Ca2+ release from intracellular stores. A, Time course of normalized MF EPSP slopes before and after HFS from heparin- (top graph; N = 8) and ryanodine- (bottom graph; N = 8) loaded cells showing PTP but not LTP. The DCG-IV sensitivity confirmed the MF origin of EPSPs. B, Bar chart summarizing the post-HFS effect of postsynaptic loading with heparin or ryanodine. The insets are average MF EPSP slopes (10 consecutive sweeps) from a representative cell loaded with heparin or ryanodine. Calibration: 2 mV, 5 ms. C, Cumulative probability distribution of normalized EPSP slopes before and during LTD (measured after the first 5 min after HFS to exclude PTP) for the same cells. Each point represents the magnitude of change relative to a normalized baseline computed from the average EPSP slope in heparin- or ryanodine-loaded cells. The start of the horizontal arrow corresponds to the minimum criteria for LTD (depression of the synaptic transmission ≤25% below baseline for 30 min). **p < 0.001 or higher statistical significance. Error bars indicate SEM.
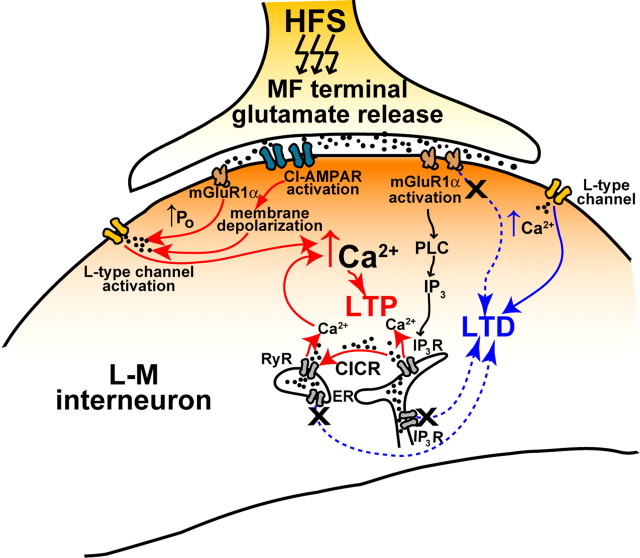
The rise in [Ca2+]i caused by activation of L-type channel and IP3 receptors could result in Ca2+-induced Ca2+ release (CICR) from the ER via activation of the ryanodine receptor (RyR) (Wang et al., 1996; Balschun et al., 1999; Nakamura et al., 2000; Lu and Hawkins, 2002; Raymond and Redman, 2002; Morikawa et al., 2003; Sokolov et al., 2003; Tozzi et al., 2003; Lapointe et al., 2004; Mellentin et al. 2007). In dentate gyrus and CA3 hippocampal cells, RyR levels are higher than IP3R levels (Bardo et al., 2006). Furthermore, a recent study has shown that the distribution of RyR isotype 3 immunoreactivity is strong in the s. lacunosum-moleculare (Hertle and Yeckel, 2007). To examine whether blockade of the RyR also may induce LTD, ryanodine was included in the whole-cell pipette (10 μm; N = 4; or 100 μm; N = 4). Because both concentrations produced the same effect, the data were pooled for statistical analysis. After PTP (176 ± 15%), MF EPSP slopes decayed to a stable LTD (75 ± 4.3% at 30 min after HFS; N = 8; p < 0.001; 75.2 ± 2.4% inhibition by DCG-IV; p < 0.001, one-way ANOVA) (Fig. 9A–C). Together, these results would suggest that the dynamic regulation of [Ca2+]i by the interaction between IP3R and RyR (Nash et al., 2002) determines the balance between MF LTP and LTD (see Fig. 11).
Figure 11.
Schematic representation of the mechanisms for induction of bidirectional plasticity at MF synapses onto spineless dendrites of L-M interneurons. MF LTP has a stronger calcium dependency than MF LTD and requires the initial activation of two sources of calcium: (1) from the extracellular space via L-type Ca2+ channels and (2) from the ER via mGluR1α-dependent phospholipase C (PLC) pathway to promote IP3-mediated Ca2+ mobilization. Enhancement of the L-type channel conductance by mGluR1α activation produces additional increase in [Ca2+]i. Calcium signals from these two sources trigger CICR from RyR-operated Ca2+ stores. The RyR-mediated component of the intracellular Ca2+ signal amplifies Ca2+ liberated through IP3R. In contrast, Ca2+ influx via L-type channels is sufficient to induce LTD in the absence of mGluR1α activation.
MF LTD induced by blockade of mGluR1α requires Ca2+ influx through L-type channel
The lack of long-term changes at MF synapses in the presence of nimodipine (Fig. 7) suggests that LTD, like LTP, also depends on Ca2+ influx through the L-type channel. In the hippocampus, NMDAR-independent forms of LTD have been shown to be dependent on L-type Ca2+ activation (Christie and Abraham, 1994; Christie et al., 1997; Wang et al., 1997). We hypothesized that, in the absence of the mGluR1α signal transduction cascade, the source for [Ca2+]i increase responsible for LTD is through the L-type channels. Indeed, we found that HFS applied in the presence of LY367385 (100 μm) and nimodipine (10 μm) did not change the MF EPSP slope (92 ± 7.3% of control at 30 min after HFS; N = 10; p > 0.216, paired t test) (Fig. 10). After 30 min after HFS, DCG-IV reduced the EPSP slope by 68.5 ± 7.4% of control (p < 0.0001, unpaired t test).
Figure 10.
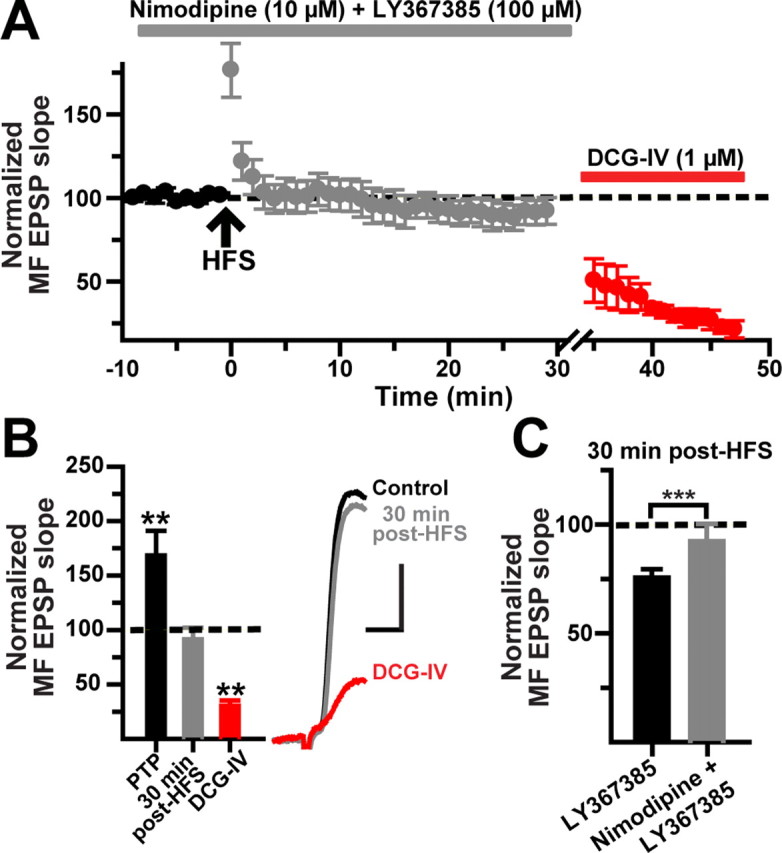
MF LTD requires L-type Ca2+ channel activation. A, Time course of normalized MF EPSP slopes (N = 10) showing that nimodipine added before the HFS prevents the induction of LTD induced in the presence of the mGluR1α antagonist LY367385 (100 μm). B, Summary bar graph for the same cells showing the post-HFS effect of nimodipine plus LY367385 applications and the subsequent reduction by DCG-IV. The inset shows average MF EPSP slopes (10 consecutive sweeps) from one representative experiment. Calibration: 2 mV, 5 ms. C, Population data showing 30 min after HFS changes in EPSP slope in the presence of LY367385 versus LY367385 plus nimodipine. ***p < 0.00002 or higher statistical significance. Error bars indicate SEM.
Discussion
Previous reports have established that LTD mediated by CP-AMPAR or NMDAR is the preferential form of long-term plasticity at MF synapses on stratum lucidum interneurons (Maccaferri et al., 1998; Tóth and McBain, 1998; Tóth et al., 2000; Lei and McBain, 2002). Here, we demonstrate that the majority of L-M interneurons (78%) recorded near the SDG exhibit robust PTP and input-specific NMDAR-independent LTP at predominantly CI-AMPAR MF synapses. These observations support the notion that the induction of pathway-specific LTP does not require dendritic spines (Lamsa et al., 2005). In contrast, in the majority of PhTx-sensitive MF synapses, the isolated CI-AMPAR component showed a reduced PTP and were unaffected by HFS. Only in few of the CP-AMPAR synapses, the CI-AMPAR-mediated EPSP exhibited a lack of PTP followed by LTD. Given that the AMPAR sensitivity to PhTx was not assessed in every experiment, the generality of our conclusion that only synapses predominantly containing CI-AMPARs are able to undergo MF LTP remains to be determined.
The axons of L-M interneurons branch in strata lacunosum-moleculare, radiatum, and pyramidale, and make direct symmetrical synaptic contacts with pyramidal cells (Kunkel et al., 1988) that provide feedforward inhibition to pyramidal cells (Lacaille and Schwartzkroin, 1988; Williams et al., 1994; Vida et al., 1998). Therefore, our results are consistent with the claim that LTP occurs at excitatory inputs to GABAergic interneurons inhibiting principal cells in a feedforward manner (Kullmann and Lamsa, 2007). We also found that MF synapses on L-M interneurons are bidirectionally modifiable. LTP induction required the joint activation of L-type Ca2+ channels and mGluR1α receptors. During blockade of mGluR1α, the same stimulation protocol that induces MF LTP in naive slices induced MF LTD. The requirement for conjunctive presynaptic and postsynaptic activity to induce both LTP and LTD fulfills the condition for Hebbian forms of synaptic plasticity.
To ensure that MF LTP in L-M interneurons is not attributable to the induction of NMDAR-independent LTP at polysynaptic recurrent synapses from intercalated pyramidal cells, we first systematically identified MF EPSPs based on their differential sensitivity to DCG-IV. Second, we explicitly tested the lack of C/A LTP by applying HFS to C/A fibers in the absence of NMDAR activation. Third, LTP was detected with a minimal stimulation protocol during which only one or a few synapses were activated (Stevens and Wang, 1994; Xiang et al., 1994; Perez et al., 2001). Finally, LTP was prevented with somatic voltage clamping during HFS and by intracellular injections of the Ca2+ chelator BAPTA. Together, these data clearly show that MF LTP was induced postsynaptically and expressed directly at MF synapses on L-M interneurons.
MF synapses onto interneurons are made either by small en passant varicosities originating from the axon trunks of MFs traveling in the s. lucidum of CA3 or from dentate gyrus hilar collaterals or filipodial extensions from the large synaptic boutons innervating the pyramidal cells (Claiborne et al., 1986; Acsády et al., 1998; Bischofberger and Jonas, 2002). In contrast, MF synapses on pyramidal cells have large presynaptic boutons with small finger-like extensions (Hamlyn, 1962; Amaral, 1979). Despite significant morphological differences in presynaptic terminals, MF synapses on pyramidal cells and the subpopulation of L-M interneurons containing MF CI-AMPARs share some similarities in the mechanisms of synaptic transmission and use-dependent plasticity. Pyramidal cells, which are predominantly innervated by MFs at CI-AMPAR-containing synapses (Tóth et al., 2000), exhibit robust PTP and LTP, and LTP is induced postsynaptically (Jaffe and Johnston, 1990; Yeckel et al., 1999; Contractor et al., 2002; Sokolov et al. 2003) (but see Zalutsky and Nicoll, 1990). However, the PTP and LTP reported here are comparatively smaller than the PTP and LTP at MF synapses made by the axon collaterals of granule cells on basket cells in the dentate gyrus (Alle et al., 2001). However, MF LTP in these interneurons was only partially attenuated by BAPTA, indicating that the induction mechanisms are more resilient to chelation of postsynaptic Ca2+ than those underlying LTP induction in L-M interneurons.
Both LTP and LTD were prevented by nimopidine applications before HFS. An essential role of L-type channels in long-term synaptic plasticity in CA3 interneurons has not previously been demonstrated. Our results also indicate that mGluR1α serves as a molecular switch that changes the outcome of the synaptic modification triggered by activation of L-type Ca2+ channels (Fig. 11). Activation of this switch by glutamate release results in LTP induction; however, if the switch is inoperative LTD is triggered by the Ca2+ influx through activation of the L-type channel after the postsynaptic depolarization mediated by CI-AMPA receptor activation (Fig. 11). These findings would be consistent with the idea that the magnitude of the activity-dependent rise in postsynaptic Ca2+ is a major determinant of the direction of change in synaptic efficacy (Bear et al., 1987; Artola and Singer, 1993; Hansel et al., 1997; Cormier et al., 2001). Experimental observations suggest that the induction threshold for LTP requires higher levels of postsynaptic [Ca2+]i than for LTD (Cummings et al., 1996; Cho et al., 2001; Nevian and Sakmann, 2006). Therefore, LTP at MF synapses onto L-M interneurons may only be induced after an increase in the L-type channel conductance (Chavis et al., 1996) supplemented by Ca2+ mobilization via IP3R, and RyR-mediated CICR (Nakamura et al., 2000) (Fig. 11). However, LTP may not depend solely on global [Ca2+]i but on a separate Ca2+ sensor (Karmarkar and Buonomano, 2002; Bender et al., 2006; Nevian and Sakmann, 2006), enabled by mGluR1α activation. Another obligatory step in the downstream signaling cascade leading to MF LTP may involve the activation of PKA (protein kinase A) and PKC (E. J. Galván and G. Barrionuevo, unpublished observations). In turn, PKC activation could mediate a phosphorylation-triggered desensitization of mGluR1α (Dale et al., 2002; Sato et al., 2004) perhaps via coactivation of mGluR5 (Poisik et al., 2003), which would prime potentiated MF synapses for a subsequent induction of LTD. Such metaplasticity (Abraham and Bear, 1996) could provide with a homeostatic mechanism to reset synaptic weights and prevent synaptic saturation (Turrigiano and Nelson, 2004) at MF synapses on L-M interneurons.
Previous reports have demonstrated that MF synapses on lucidum interneurons and dentate gyrus basket cells are able to undergo bidirectional plasticity. However, the mechanism that regulates the polarity of synaptic efficacy in these interneuron populations is different from that reported here. In lucidum interneurons in which MF LTD at naive synapses requires presynaptically located mGluR7 activation, HFS elicited MF LTP/dedepression only after L-AP4 applications induced chemical LTD and internalization of mGluR7 localized at MF terminals (Pelkey et al., 2005). The requirement for strong presynaptic receptor activation to elicit LTP indicates that this synaptic plasticity is more suitable for maintaining network stability during sustained activation of granule cells (e.g., during epileptic bursting) (Pelkey et al., 2005). In dentate gyrus basket cells, MF synapses can exhibit either LTP or LTD depending on the postsynaptic membrane potential during HFS (Alle et al., 2001).
Although many mechanisms for NMDAR-independent LTP/LTD have been described in hippocampal interneurons, the expression of this plasticity appears solely determined by changes in transmitter release probability (Kullmann and Lamsa, 2007). Indeed, the changes in PPR, coefficient of variation, and failure rate that were detected during MF LTP/LTD are all indicative of a presynaptic locus of expression for the changes in synaptic efficacy. Presynaptic changes at MF synapses on pyramidal cells and interneurons have been previously reported during both LTP (Staubli et al., 1990; Zalutsky and Nicoll, 1990; Xiang et al., 1994; Alle et al., 2001) and LTD (Domenici et al., 1998; Lei and McBain, 2002). We speculate that the postsynaptic events in response to HFS are expressed presynaptically by a retrograde signaling messenger.
According to computational and behavioral studies on hippocampal function (McNaughton and Morris, 1987; Treves and Rolls, 1992; O'Reilly and McClelland, 1994; Lisman, 1999; Leutgeb et al., 2007), the strong and sparse input from the MF input selects nonoverlapping subpopulations of pyramidal cells to encode new memory representations in a way that reduces interference between stored information. Feedforward inhibitory interneurons also may play a role in regulating the transfer and decorrelation of the compressed memory representation in area CA3. For example, the parallel activation of feedforward inhibitory interneurons by MFs could contribute to pattern separation by restricting the temporal window for synaptic integration and action potential firing in pyramidal cells (Pouille and Scanziani, 2001; Perez-Orive et al., 2002). Therefore, the fine “tuning” of the pyramidal cell network during pattern separation requires a functional balance between monosynaptic excitation and disynaptic inhibition (Mariño et al., 2005). If MF LTP were confined to synapses on pyramidal cells alone, this could limit the sparseness of the memory representation unless it is accompanied by a compensatory LTP on feedforward inhibitory interneurons (Lamsa et al., 2005). The present results demonstrate that the feedforward inhibitory drive to CA3 mediated by the MF input to L-M interneurons has the ability to undergo Hebbian LTP in response to the same stimulation protocol that induces MF LTP in pyramidal cells (cf. Henze et al., 2000; Nicoll and Schmitz, 2005). Similar strengthening of MF connections on both postsynaptic targets by the same presynaptic activity could contribute to preserve the focus of the excitatory drive from the dentate gyrus to the CA3 neuronal network. This would presumably be important for invoking a distributed encoding that minimizes the overlap between incoming cortical patterns that underlie different memory representations.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS24288. We thank John Cavaretta for help with the interneuron reconstruction and technical support.
References
- Abraham WC, Bear MF. Metaplasticity: the plasticity of synaptic plasticity. Trends Neurosci. 1996;19:126–130. doi: 10.1016/s0166-2236(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlijanian MK, Westenbroek RE, Catterall WA. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990;4:819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Alle H, Jonas P, Geiger JR. PTP and LTP at a hippocampal mossy fiber-interneuron synapse. Proc Natl Acad Sci U S A. 2001;98:14708–14713. doi: 10.1073/pnas.251610898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG. Synaptic extensions from the mossy fibers of the fascia dentata. Anat Embryol (Berl) 1979;155:241–251. doi: 10.1007/BF00317638. [DOI] [PubMed] [Google Scholar]
- Artola A, Singer W. Long-term depression of excitatory synaptic transmission and its relationship to long-term potentiation. Trends Neurosci. 1993;16:480–487. doi: 10.1016/0166-2236(93)90081-v. [DOI] [PubMed] [Google Scholar]
- Balschun D, Manahan-Vaughan D, Wagner T, Behnisch T, Reymann KG, Wetzel W. A specific role for group I mGluRs in hippocampal LTP and hippocampus-dependent spatial learning. Learn Mem. 1999;6:138–152. [PMC free article] [PubMed] [Google Scholar]
- Bardo S, Cavazzini MF, Emptage N. The role of the endoplasmic reticulum Ca2+ store in the plasticity of central neurons. Trends Neurosci. 2006;27:78–84. doi: 10.1016/j.tips.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bear MF, Cooper LN, Ebner FF. A physiological basis for a theory of synapse modification. Science. 1987;237:42–48. doi: 10.1126/science.3037696. [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. J Neurosci. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Jonas P. TwoB or not twoB: differential transmission at glutamatergic mossy fiber-interneuron synapses in the hippocampus. Trends Neurosci. 2002;25:600–603. doi: 10.1016/s0166-2236(02)02259-2. [DOI] [PubMed] [Google Scholar]
- Calixto E, Galvan EJ, Card JP, Barrionuevo G. Coincidence detection of convergent perforant path and mossy fibre inputs by CA3 interneurons. J Physiol. 2008;586:2695–2712. doi: 10.1113/jphysiol.2008.152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavis P, Fagni L, Lansman JB, Bockaert J. Functional coupling between ryanodine receptors and L-type calcium channels in neurons. Nature. 1996;382:719–722. doi: 10.1038/382719a0. [DOI] [PubMed] [Google Scholar]
- Chen HX, Hanse E, Pananceau M, Gustafsson B. Distinct expressions for synaptic potentiation induced by calcium through voltage-gated calcium and N-methyl-d-aspartate receptor channels in the hippocampal CA1 region. Neuroscience. 1998;86:415–422. doi: 10.1016/s0306-4522(98)00042-6. [DOI] [PubMed] [Google Scholar]
- Cho K, Aggleton JP, Brown MW, Bashir ZI. An experimental test of the role of postsynaptic calcium levels in determining synaptic strength using perirhinal cortex of rat. J Physiol. 2001;532:459–466. doi: 10.1111/j.1469-7793.2001.0459f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Abraham WC. L-Type voltage-sensitive calcium channel antagonists block heterosynaptic long-term depression in the dentate gyrus of anaesthetized rats. Neurosci Lett. 1994;167:41–45. doi: 10.1016/0304-3940(94)91023-5. [DOI] [PubMed] [Google Scholar]
- Christie BR, Schexnayder LK, Johnston D. Contribution of voltage-gated Ca2+ channels to homosynaptic long-term depression in the CA1 region in vitro. J Neurophysiol. 1997;77:1651–1655. doi: 10.1152/jn.1997.77.3.1651. [DOI] [PubMed] [Google Scholar]
- Claiborne BJ, Amaral DG, Cowan WM. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986;246:435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Contractor A, Rogers C, Maron C, Henkemeyer M, Swanson GT, Heinemann SF. Trans-synaptic Eph receptor-ephrin signaling in hippocampal mossy fiber LTP. Science. 2002;296:1864–1869. doi: 10.1126/science.1069081. [DOI] [PubMed] [Google Scholar]
- Cormier RJ, Greenwood AC, Connor JA. Bidirectional synaptic plasticity correlated with the magnitude of dendritic calcium transients above a threshold. J Neurophysiol. 2001;85:399–406. doi: 10.1152/jn.2001.85.1.399. [DOI] [PubMed] [Google Scholar]
- Cummings JA, Mulkey RM, Nicoll RA, Malenka RC. Ca2+ signaling requirements for long-term depression in the hippocampus. Neuron. 1996;16:825–833. doi: 10.1016/s0896-6273(00)80102-6. [DOI] [PubMed] [Google Scholar]
- Dale LB, Babwah AV, Ferguson SSG. Mechanisms of metabotropic glutamate receptor desensitization: role in the patterning of effector enzyme activation. Neurochem Int. 2002;41:319–326. doi: 10.1016/s0197-0186(02)00073-6. [DOI] [PubMed] [Google Scholar]
- Denk W, Yuste R, Svoboda K, Tank DW. Imaging calcium dynamics in dendritic spines. Curr Opin Neurobiol. 1996;6:372–378. doi: 10.1016/s0959-4388(96)80122-x. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Domenici MR, Berretta N, Cherubini E. Two distinct forms of long-term depression coexist at the mossy fiber-CA3 synapse in the hippocampus during development. Proc Natl Acad Sci U S A. 1998;95:8310–8315. doi: 10.1073/pnas.95.14.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L, Chavis P, Ango F, Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23:80–88. doi: 10.1016/s0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Cobden P, Pollard M, Cope D, Shigemoto R, Watanabe M, Somogyi P. Immunolocalization of metabotropic glutamate receptor 1alpha (mGluR1alpha) in distinct classes of interneuron in the CA1 region of the rat hippocampus. Hippocampus. 2004;14:193–215. doi: 10.1002/hipo.10163. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. N-Methyl-d-aspartate receptor-independent long-term potentiation in area CA1 of rat hippocampus: input-specific induction and preclusion in a non-tetanized pathway. Neuroscience. 1992;49:7–11. doi: 10.1016/0306-4522(92)90072-a. [DOI] [PubMed] [Google Scholar]
- Hamlyn LH. The fine structure of the mossy fibre endings in the hippocampus of the rabbit. J Anat. 1962;96:112–120. [PMC free article] [PubMed] [Google Scholar]
- Hansel C, Artola A, Singer W. Relation between dendritic Ca2+ levels and the polarity of synaptic long-term modifications in rat visual cortex neurons. Eur J Neurosci. 1997;9:2309–2322. doi: 10.1111/j.1460-9568.1997.tb01648.x. [DOI] [PubMed] [Google Scholar]
- Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl-d-aspartate antagonists. Neurosci Lett. 1986;70:132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Urban NN, Barrionuevo G. The multifarious hippocampal mossy fiber pathway: a review. Neuroscience. 2000;98:407–427. doi: 10.1016/s0306-4522(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Henze DA, Wittner L, Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Hertle DN, Yeckel MF. Distribution of inositol-1,4,5-trisphosphate receptor isotypes and ryanodine receptor isotypes during maturation of the rat hippocampus. Neuroscience. 2007;150:625–638. doi: 10.1016/j.neuroscience.2007.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YY, Malenka RC. Examination of TEA-induced synaptic enhancement in area CA1 of the hippocampus: the role of voltage-dependent Ca2+ channels in the induction of LTP. J Neurosci. 1993;13:568–576. doi: 10.1523/JNEUROSCI.13-02-00568.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe D, Johnston D. Induction of long-term potentiation at hippocampal mossy-fiber synapses follows a Hebbian rule. J Neurophysiol. 1990;64:948–960. doi: 10.1152/jn.1990.64.3.948. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shinozaki H, Yamamoto C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapses. J Physiol. 1996;493:447–455. doi: 10.1113/jphysiol.1996.sp021395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapur A, Yeckel MF, Gray R, Johnston D. L-Type calcium channels are required for one form of hippocampal mossy fiber LTP. J Neurophysiol. 1998;79:2181–2190. doi: 10.1152/jn.1998.79.4.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmarkar UR, Buonomano DV. A model of spike-timing dependent plasticity: one or two coincidence detectors? J Neurophysiol. 2002;88:507–513. doi: 10.1152/jn.2002.88.1.507. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Lamsa KP. Long-term synaptic plasticity in hippocampal interneurons. Nat Rev Neurosci. 2007;8:687–699. doi: 10.1038/nrn2207. [DOI] [PubMed] [Google Scholar]
- Kullmann DM, Perkel DJ, Manabe T, Nicoll RA. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992;9:1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Kunkel DD, Lacaille JC, Schwartzkroin PA. Ultrastructure of stratum lacunosum-moleculare interneurons of hippocampal CA1 region. Synapse. 1988;2:382–394. doi: 10.1002/syn.890020405. [DOI] [PubMed] [Google Scholar]
- Lacaille JC, Schwartzkroin PA. Intracellular responses of rat hippocampal granule cells in vitro to discrete applications of norepinephrine. Neurosci Lett. 1988;89:176–181. doi: 10.1016/0304-3940(88)90377-1. [DOI] [PubMed] [Google Scholar]
- Laezza F, Doherty JJ, Dingledine R. Long-term depression in hippocampal interneurons: joint requirement for pre- and postsynaptic events. Science. 1999;285:1411–1414. doi: 10.1126/science.285.5432.1411. [DOI] [PubMed] [Google Scholar]
- Lamsa K, Heeroma JH, Kullmann DM. Hebbian LTP in feed-forward inhibitory interneurons and the temporal fidelity of input discrimination. Nat Neurosci. 2005;8:916–924. doi: 10.1038/nn1486. [DOI] [PubMed] [Google Scholar]
- Lapointe V, Morin F, Ratte S, Croce A, Conquet F, Lacaille JC. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J Physiol. 2004;555:125–135. doi: 10.1113/jphysiol.2003.053603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, Bleakman D, Ornstein PL, Lodge D, Isaac JT, Collingridge GL. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, McBain CJ. Interneuron diversity series: containing the detonation–feedforward inhibition in the CA3 hippocampus. Trends Neurosci. 2003;26:631–640. doi: 10.1016/j.tins.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Grinspan ZM, McBain CJ. Quantal transmission at mossy fibre targets in the CA3 region of the rat hippocampus. J Physiol. 2004;554:175–193. doi: 10.1113/jphysiol.2003.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei S, McBain CJ. Distinct NMDA receptors provide differential modes of transmission at mossy fiber-interneuron synapses. Neuron. 2002;33:921–933. doi: 10.1016/s0896-6273(02)00608-6. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MB, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315:961–966. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learn Mem. 2007;14:745–757. doi: 10.1101/lm.703907. [DOI] [PubMed] [Google Scholar]
- Lisman JE. Relating hippocampal circuitry to function: recall of memory sequences by reciprocal dentate-CA3 interactions. Neuron. 1999;22:233–242. doi: 10.1016/s0896-6273(00)81085-5. [DOI] [PubMed] [Google Scholar]
- Lu YF, Hawkins RD. Ryanodine receptors contribute to cGMP-induced late-phase LTP and CREB phosphorylation in the hippocampus. J Neurophysiol. 2002;88:1270–1278. doi: 10.1152/jn.2002.88.3.1270. [DOI] [PubMed] [Google Scholar]
- Lujan R, Nusser Z, Roberts JD, Shigemoto R, Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. Eur J Neurosci. 1996;8:1488–1500. doi: 10.1111/j.1460-9568.1996.tb01611.x. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Tóth K, McBain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- Mariño J, Schummers J, Lyon DC, Schwabe L, Beck O, Wiesing P, Obermayer K, Sur M. Invariant computations in local cortical networks with balanced excitation and inhibition. Nat Neurosci. 2005;8:194–201. doi: 10.1038/nn1391. [DOI] [PubMed] [Google Scholar]
- McNaughton N, Morris RG. Chlordiazepoxide, an anxiolytic benzodiazepine, impairs place navigation in rats. Behav Brain Res. 1987;24:39–46. doi: 10.1016/0166-4328(87)90034-9. [DOI] [PubMed] [Google Scholar]
- Mellentin C, Jahnsen H, Abraham WC. Priming of long-term potentiation mediated by ryanodine receptor activation in rat hippocampal slices. Neuropharmacology. 2007;52:118–125. doi: 10.1016/j.neuropharm.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Miyakawa H, Ross WN, Jaffe D, Callaway JC, Lasser-Ross N, Lisman JE, Johnston D. Synaptically activated increases in Ca2+ concentration in hippocampal CA1 pyramidal cells are primarily due to voltage-gated Ca2+ channels. Neuron. 1992;9:1163–1173. doi: 10.1016/0896-6273(92)90074-n. [DOI] [PubMed] [Google Scholar]
- Mori M, Gähwiler BH, Gerber U. Recruitment of an inhibitory hippocampal network after bursting in a single granule cell. Proc Natl Acad Sci U S A. 2007;104:7640–7645. doi: 10.1073/pnas.0702164104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Nakamura K, Lasser-Ross N, Barbara JG, Sandler VM, Ross WN. Inositol 1,4,5-trisphosphate (IP3)-mediated Ca2+ release evoked by metabotropic agonists and backpropagating action potentials in hippocampal CA1 pyramidal neurons. J Neurosci. 2000;20:8365–8376. doi: 10.1523/JNEUROSCI.20-22-08365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazawa K, Quirk MC, Chitwood RA, Watanabe M, Yeckel MF, Sun LD, Kato A, Carr CA, Johnston D, Wilson MA, Tonegawa S. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash MS, Schell MJ, Atkinson PJ, Johnston NR, Nahorski SR, Challiss RAJ. Determinants of metabotropic glutamate receptor-5-mediated Ca2+ and inositol 1,4,5-trisphosphate oscillation frequency. J Biol Chem. 2002;277:35947–35960. doi: 10.1074/jbc.M205622200. [DOI] [PubMed] [Google Scholar]
- Nevian T, Sakmann B. Spine Ca2+ signaling in spike-timing-dependent plasticity. J Neurosci. 2006;26:11001–11013. doi: 10.1523/JNEUROSCI.1749-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, McClelland JL. Hippocampal conjunctive encoding, storage, and recall: avoiding a trade-off. Hippocampus. 1994;4:661–682. doi: 10.1002/hipo.450040605. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Nikolaeva MA, Coderre E, Zamponi GW, McRory JE, Trapp BD, Yin X, Wang W, Woulfe J, Stys PK. Depolarization-induced Ca2+ release in ischemic spinal cord white matter involves L-type Ca2+ channel activation of ryanodine receptors. Neuron. 2003;40:53–63. doi: 10.1016/j.neuron.2003.08.016. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Differential regulation at functionally divergent release sites along a common axon. Curr Opin Neurobiol. 2007;17:366–373. doi: 10.1016/j.conb.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Pelkey KA, McBain CJ. Target-cell-dependent plasticity within the mossy fibre-CA3 circuit reveals compartmentalized regulation of presynaptic function at divergent release sites. J Physiol. 2008;586:1495–1502. doi: 10.1113/jphysiol.2007.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelkey KA, Lavezzari G, Racca C, Roche KW, McBain CJ. mGluR7 is a metaplastic switch controlling bidirectional plasticity of feedforward inhibition. Neuron. 2005;46:89–102. doi: 10.1016/j.neuron.2005.02.011. [DOI] [PubMed] [Google Scholar]
- Perez Y, Morin F, Lacaille JC. A Hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc Natl Acad Sci U S A. 2001;98:9401–9406. doi: 10.1073/pnas.161493498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Raymond CR, Redman SJ. Different calcium sources are narrowly tuned to the induction of different forms of LTP. J Neurophysiol. 2002;88:249–255. doi: 10.1152/jn.2002.88.1.249. [DOI] [PubMed] [Google Scholar]
- Sato M, Tabata T, Hashimoto K, Nakamura K, Nakao K, Katsuki M, Kitano J, Moriyoshi K, Kano M, Nakanishi S. Altered agonist sensitivity and desensitization of neuronal mGluR1 responses in knock-in mice by a single amino acid substitution at the PKC phosphorylation site. Eur J Neurosci. 2004;20:947–955. doi: 10.1111/j.1460-9568.2004.03552.x. [DOI] [PubMed] [Google Scholar]
- Sokolov MV, Rossokhin AV, M Kasyanov A, Gasparini S, Berretta N, Cherubini E, Voronin LL. Associative mossy fibre LTP induced by pairing presynaptic stimulation with postsynaptic hyperpolarization of CA3 neurons in rat hippocampal slice. Eur J Neurosci. 2003;17:1425–1437. doi: 10.1046/j.1460-9568.2003.02563.x. [DOI] [PubMed] [Google Scholar]
- Staubli U, Larson J, Lynch G. Mossy fiber potentiation and long-term potentiation involve different expression mechanisms. Synapse. 1990;5:333–335. doi: 10.1002/syn.890050410. [DOI] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature. 1994;371:704–707. doi: 10.1038/371704a0. [DOI] [PubMed] [Google Scholar]
- Taylor CW, Broad LM. Pharmacological analysis of intracellular Ca2+ signalling: problems and pitfalls. Trends Pharmacol Sci. 1998;19:370–375. doi: 10.1016/s0165-6147(98)01243-7. [DOI] [PubMed] [Google Scholar]
- Topolnik L, Azzi M, Morin F, Kougioumoutzakis A, Lacaille JC. mGluR1/5 subtype-specific calcium signalling and induction of long-term potentiation in rat hippocampal oriens/alveus interneurones. J Physiol. 2006;575:115–131. doi: 10.1113/jphysiol.2006.112896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tóth K, McBain CJ. Afferent-specific innervation of two distinct AMPA receptor subtypes on single hippocampal interneurons. Nat Neurosci. 1998;1:572–578. doi: 10.1038/2807. [DOI] [PubMed] [Google Scholar]
- Tóth K, Suares G, Lawrence JJ, Philips-Tansey E, McBain CJ. Differential mechanisms of transmission at three types of mossy fiber synapse. J Neurosci. 2000;20:8279–8289. doi: 10.1523/JNEUROSCI.20-22-08279.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational constraints suggest the need for two distinct input systems to the hippocampal CA3 network. Hippocampus. 1992;2:189–199. doi: 10.1002/hipo.450020209. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Nelson SB. Homeostatic plasticity in the developing nervous system. Nat Rev Neurosci. 2004;5:97–107. doi: 10.1038/nrn1327. [DOI] [PubMed] [Google Scholar]
- Urban NN, Barrionuevo G. Induction of Hebbian and non-Hebbian mossy fiber long-term potentiation by distinct patterns of high-frequency stimulation. J Neurosci. 1996;16:4293–4299. doi: 10.1523/JNEUROSCI.16-13-04293.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vida I, Halasy K, Szinyei C, Somogyi P, Buhl EH. Unitary IPSPs evoked by interneurons at the stratum radiatum-stratum lacunosum-moleculare border in the CA1 area of the rat hippocampus in vitro. J Physiol. 1998;506:755–773. doi: 10.1111/j.1469-7793.1998.755bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wu J, Rowan MJ, Anwyl R. Ryanodine produces a low frequency stimulation-induced NMDA receptor-independent long-term potentiation in the rat dentate gyrus in vitro. J Physiol. 1996;495:755–767. doi: 10.1113/jphysiol.1996.sp021631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Rowan MJ, Anwyl R. Induction of LTD in the dentate gyrus in vitro is NMDA receptor independent, but dependent on Ca2+ influx via low-voltage-activated Ca2+ channels and release of Ca2+ from intracellular stores. J Neurophysiol. 1997;77:812–825. doi: 10.1152/jn.1997.77.2.812. [DOI] [PubMed] [Google Scholar]
- Weisskopf MG, Bauer EP, LeDoux JE. L-Type voltage-gated calcium channels mediate NMDA-independent associative long-term potentiation at thalamic input synapses to the amygdala. J Neurosci. 1999;19:10512–10519. doi: 10.1523/JNEUROSCI.19-23-10512.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990;347:281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Williams S, Samulack DD, Beaulieu C, LaCaille JC. Membrane properties and synaptic responses of interneurons located near the stratum lacunosum-moleculare/radiatum border of area CA1 in whole-cell recordings from rat hippocampal slices. J Neurophysiol. 1994;71:2217–2235. doi: 10.1152/jn.1994.71.6.2217. [DOI] [PubMed] [Google Scholar]
- Woodhall G, Gee CE, Robitaille R, Lacaille JC. Membrane potential and intracellular Ca2+ oscillations activated by mGluRs in hippocampal stratum oriens/alveus interneurons. J Neurophysiol. 1999;81:371–382. doi: 10.1152/jn.1999.81.1.371. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Greenwood AC, Kairiss EW, Brown TH. Quantal mechanism of long-term potentiation in hippocampal mossy-fiber synapses. J Neurophysiol. 1994;71:2552–2556. doi: 10.1152/jn.1994.71.6.2552. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Kapur A, Johnston D. Multiple forms of LTP in hippocampal CA3 neurons use a common postsynaptic mechanism. Nat Neurosci. 1999;2:625–633. doi: 10.1038/10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalutsky RA, Nicoll RA. Comparison of two forms of long-term potentiation in single hippocampal neurons. Science. 1990;248:1619–1624. doi: 10.1126/science.2114039. [DOI] [PubMed] [Google Scholar]