Abstract
Multiple evidences indicate that inflammation is an event occurring prior to infection in patients with cystic fibrosis. The self-perpetuating inflammatory cycle may play a pathogenic part in this disease. The role of the NF-κB pathway in enhanced production of inflammatory mediators is well documented. The pathophysiologic mechanisms through which the intrinsic inflammatory response develops remain unclear. The unfolded mutated protein cystic fibrosis transmembrane conductance regulator (CFTRΔF508), accounting for this pathology, is retained in the endoplasmic reticulum (ER), induces a stress, and modifies calcium homeostasis. Furthermore, CFTR is implicated in the transport of glutathione, the major antioxidant element in cells. CFTR mutations can alter redox homeostasis and induce an oxidative stress. The disturbance of the redox balance may evoke NF-κB activation and, in addition, promote apoptosis. In this review, we examine the hypotheses of the integrated pathogenic processes leading to the intrinsic inflammatory response in cystic fibrosis.
Introduction
Cystic Fibrosis (CF) is the most common inherited autosomal recessive and lethal disease in caucasian population [1]. It is due to mutations in the product of the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) [2]. This ATP-binding cassette (ABC) transporter is a membrane glycoprotein that functions not only as a cyclic AMP-regulated chloride channel in epithelial cells but also, as a reduced glutathione transporter [3,4]. Moreover, CFTR can regulate other channels such as the outwardly rectifying Cl- channels (ORCC), epithelial Na+ channels (ENaC), renal outer medullary K+ channels (ROMK) or other inwardly rectifying K+ channels [5]. It is expressed in various organs such as lung, pancreas, liver, gastrointestinal tract, and sweat glands [1].
More than 1,500 mutations of CFTR have been identified [6]. Mutations affect localization of CFTR at the apical plasma membrane and could interfere with its function and regulation. The most common mutation, deletion of phenylalanine at position 508 (CFTRΔF508), results in a misfolded protein that is retained in the endoplasmic reticulum (ER) [7]. The lack of functional CFTR at the plasma membrane correlates with impaired ionic balance and modification of cellular homeostasis. This pathological aspect is well documented and is characterized by thick secretions [8-11]. Thus, viscous and hyper-concentrated pancreatic secretions obstruct pancreatic ducts in CF patients accounting for the destruction of pancreas epithelium, which could promote inflammation [12,13]. Concerning the respiratory system, airway obstruction resulting from the thick mucus and reduced clearance of inhaled particles, including bacteria, promotes persistent infection and chronic inflammation [14] that are the major causes of death [15]. However, even in the absence of bacterial or viral pathogens, exacerbated inflammation has been reported in the respiratory tract of CF infants [16-18]. Hence, the origin of inflammation in CF has been a matter of debate and, recent data suggest that the retention of the misfolded protein could play a key role in the development and maintenance of the inflammatory response.
In the present review, we examine hypotheses of the coordinated and integrated pathophysiologic processes associated with CFTR defect retention in the ER that could lead to intrinsic inflammatory and apoptotic responses.
Defects in CFTR and inflammatory responses
Although several studies have shown that CF and normal cells did not differ in both cytokine profile secretion and NF-κB activation [19,20], a large amount of data show that CF cells appear to produce excessive quantity of pro-inflammatory cytokines such as interleukin (IL)-8, IL-6 or RANTES [21-23]. Concretely, airway tract, and more particularly lungs, of CF patients present a high density of acute inflammatory cells, chiefly infiltrated neutrophils and macrophages, even in the absence of bacteria [24,25]. Moreover, airway infiltrated neutrophils are associated with high levels of pro-inflammatory mediators and cytokines like IL-8. In addition to a specific cytokine secretion profile, it has been observed that neutrophils from patients with CF show phenotype alteration(s) associated with functional and signaling changes, such as increased lipid raft assembly and increased levels of the cytoskeleton-associated phospho-Syk kinase [26]. Besides, blood and airway neutrophils in CF produce high basal levels of IL-8 that increase after lipopolysaccharide (LPS) treatment, suggesting an exaggerated basal pro-inflammatory cytokine secretion that could further augment under inflammatory conditions [27,28] (Figure 1). Co-cultures of polymorphonuclear (PMN) cells from CF and non-CF airways with CFTR-mutated and CFTR-corrected epithelial cell lines, respectively, have shown that PMN from CF airways and CFTR-mutated epithelial cell lines are responsible for pro-inflammatory cytokine production [29]. Furthermore, CFTR correction in epithelial cells did not allow return to levels of cytokine expression towards levels of normal cells, revealing an activated inflammatory intrinsic pathway in CF epithelial cells. In addition, a disturbance of the balance between inflammatory versus anti-inflammatory mediator production has also been described in CF cells [30,31]. Thus, the release of IL-10, the anti-inflammatory cytokine known to reduce IκB degradation (an inhibitor of NF-κB) [32], is reduced [33], whereas pro-inflammatory cytokines are over-expressed in association with an increase of both NF-κB and AP-1 activities that are under the control of IκB kinase and ERK signaling pathway (Figure 1). Moreover, pro-inflammatory cytokines, like IL-1β and FGF, promote activation of NF-κB and AP-1, participating in the perpetuation of the vicious cycle of inflammation [34]. Indeed, implication of NF-κB in inflammatory status in CF is well documented; AP-1 also appears to play an important role in inflammation [34,35]. However, the beneficial effect of miglustat [36], without affecting NF-κB and AP-1 status, is in favor of the implication of other transcription factors, notably ATF-6 implicated in the unfolded protein response (UPR) (see below) and Nrf-2 implicated in defense against oxidative stress [37].
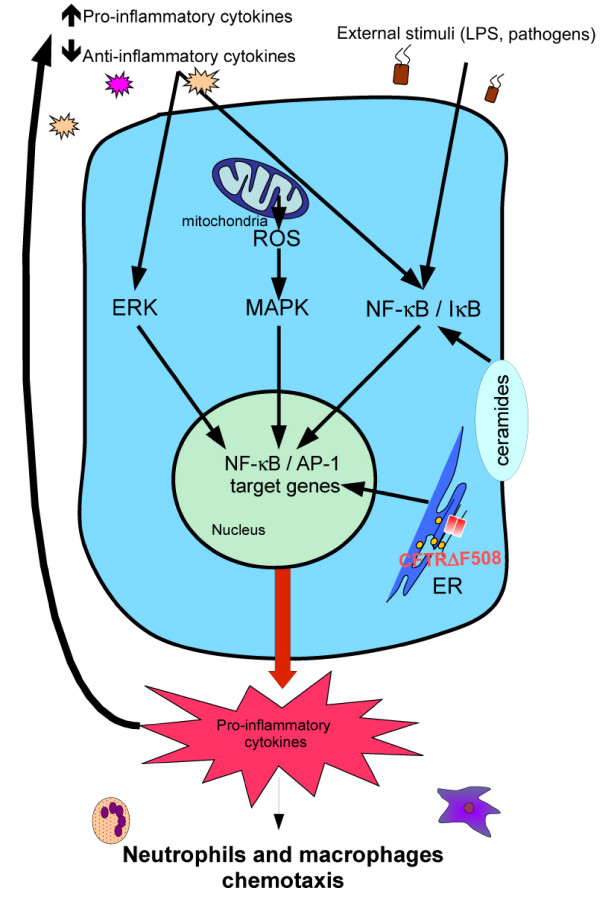
Figure 1.
Representation of the noxious inflammatory cycle in CF cell: Pro-inflammatory cytokines and external stimuli, such as pathogens, activate ERK pathway and promote dissociation of NF-κB/IκB complexe leading to NF-κB activation. Retention of CFTRΔF508 in endoplasmic reticulum (ER), excessive production of reactive oxygen species (ROS) by mitochondria and alteration of ceramide levels leads to NF-κB activation, resulting in inflammation.
In agreement with results obtained in cells cultured directly from uninfected CF tissues, Weber et al., [38] have found that defects of CFTR, in function of localization, contribute to endogenous activation of NF-κB, and consequently to the exaggerated production of the pro-inflammatory cytokine IL-8, even in the absence of bacteria. It should be noted that the link between CFTR defects and NF-κB activation must be strengthened during bacterial infection as described for Pseudomonas aeruginosa [39-41]. In addition, after LPS treatment a pro-inflammatory environment induces excessive NF-κB activation in CF macrophages, as above described for neutrophils [27,28], that is associated with a high production of pro-inflammatory cytokines [42,43]. This excessive production of cytokines results from CFTR dysfunction because the effect of LPS was also observed in healthy heterozygotes [44], suggesting that a single allelic CFTR mutation is sufficient for increase of the inflammatory response.
CFTR and apoptosis
Contradictory data on the sensitivity of CF cells to apoptosis have been reported. Whereas intestinal epithelial CF cells show a higher fragmentation of DNA [45], suggesting an elevated susceptibility to programmed cell death, respiratory epithelial CF cells undergo delayed Pseudomonas aeruginosa-induced apoptosis [46]. Also, neutrophils from CF patients present a prolonged survival when compared to normal counterparts [47]. In agreement with these observations, an over-expression of the antiapoptotic protein Bcl-2 has been evidenced in CF patients [48]. By contrast, we have recently demonstrated [22] that CF epithelial cells from pancreas or trachea are more sensitive to apoptosis compared to normal cells. In addition, conditioned medium from apoptotic CF cells promotes apoptosis in normal cells, indicating that the release of mediators of inflammation during apoptosis is in turn able to evoke apoptosis [22]. Furthermore, macrophages from CF patients produce more TNF-α than their counterparts [49], and the increased secretion of Fas and FasLigand by CF epithelial cells [50], consolidate these observations. Increased susceptibility to apoptosis in epithelial cells and failed apoptosis in neutrophils would contribute to the self-perpetuating inflammatory cycle in CF. Independently of the susceptibility to apoptosis of CF cells, it has been shown that clearance of apoptotic cells is defective and that accumulation of such cells could contribute to ongoing inflammation in CF patients [51].
CFTR and endoplasmic reticulum
ER has several specialized functions: (i) it is implicated in maturation, folding and transport of newly synthesized proteins; (ii) it is the site of biosynthesis for many lipids; and (iii) it acts as a calcium reservoir [52]. The ER contains intraluminal machinery composed of a large number of chaperone proteins implicated in the "quality control system" [53]. Proteins translocated into the ER lumen interact with chaperone proteins to acquire their conformation and then they are transported to the cell membrane or are secreted. Acquisition of conformation and formation of disulfide bonds needs resident chaperone proteins, high levels of calcium and an oxidative environment specific to the ER [54]. Accumulation of misfolded proteins, alterations of calcium homeostasis, inflammation or hypoxia, causing perturbations in environment, lead to disrupted ER function, also referred as to "ER stress" [55]. The most common mutation of CFTR produces an incorrectly folded protein, CFTRΔF508, which is present within the ER, and accumulates in the ER-Golgi intermediate compartment [56] causing an ER stress. In addition, in ER CFTRΔF508 interacts with calcium-dependent chaperones, modifying calcium homeostasis [57] and generating further stress [58].
Under ER stress conditions, three different pathways are activated in order to reduce the synthesis of new proteins and to increase degradation of uncorrected proteins (Figure 2): (i) the UPR that leads to a reduction of protein synthesis and transcription of chaperone target genes, (ii) the ER-associated degradation (ERAD) in order to eliminate misfolded proteins by proteasome, and (iii) in case these responses are unsuccessful, the ER-overload response (EOR) leading to apoptosis [59]. ER stress generated by misfolded CFTRΔF508 protein activates the UPR and the over-expression of several ER resident chaperones such as Grp78 [60], resulting in decreased CFTR expression [61,62]. In addition, in CFTRΔF508 cells, Kerbiriou et al. [60] have described an increase of ATF6 form, an initiator of UPR that is accompanied with its translocation into the nuclei. Using cells expressing different mutations of CFTR, Weber et al. [38] have demonstrated that accumulation of CFTR in the ER contributes to the endogenous activation of NF-κB. Also, Verhaege et al. [34] have shown that activation of NF-κB and AP-1 transcription factors are dependent on the ER sequestration of the misfolded CFTRΔF508 protein.
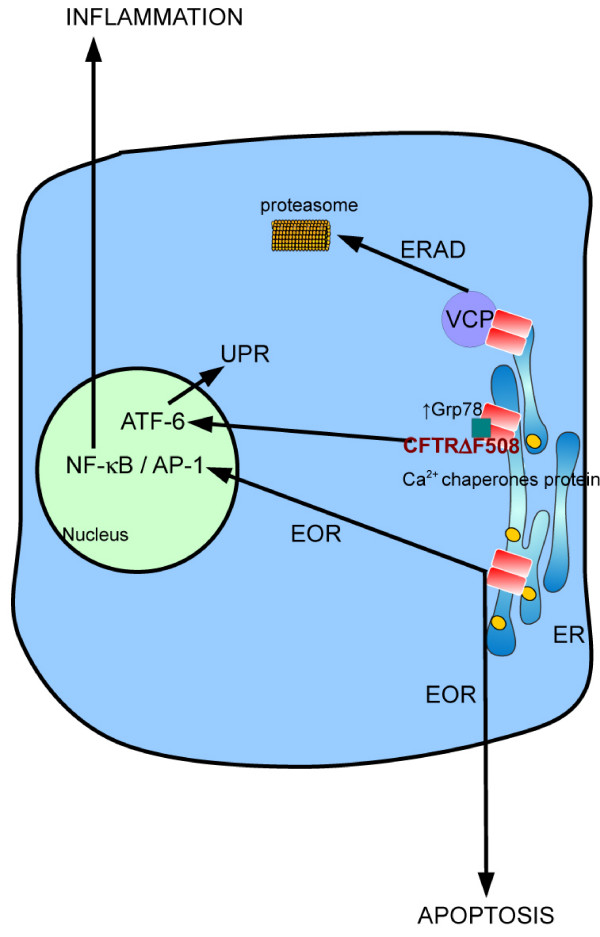
Figure 2.
Involvement of misfolded CFTRΔF508 in endoplasmic reticulum (ER) stress in CF cell: Retention of CFTRΔF508 is associated with an increase of Ca2+ concentration in the lumen of ER and with the interaction with chaperone proteins. This induces activation of ERAD in order to degrade misfolded CFTRΔF508 by proteasome. In addition, increase of Grp78 induces activation of the UPR via the ATF-6. Whether ERAD and UPR are not sufficient to restore normal cellular parameters, EOR is activated resulting in apoptosis induction.
ERAD, a component of a coordinated cellular response to ER stress, can be activated and induces protein removal from the ER and degradation in the cytosol, via the ubiquitin-proteasome system. Thus, after being selected by quality control system, the ubiquitin proteasome system interacts with unfolded ER proteins and cofactors that permit their translocation from ER to cytosol, where they are polyubiquitynated by ubiquitin ligase system, and then degraded by cytosolic proteasome system. Among the misfolded proteins processed by ERAD and the ubiquitin proteasome system, CFTRΔF508 is detected in the cytosol and within the ER membrane by the ERAD components, and finally, it can be degraded by proteasome [63,64]. In particular, in CF bronchial epithelial cells from CFTRΔF508 homozygous patients, the p97/valosin-containing protein (VCP), which is an integral component of ERAD, is over-expressed compared with non-CF bronchial epithelial cells. Moreover, inhibition of VCP induces the rescue of CFTRΔF508 protein to the plasma membrane that is associated with the diminution of NF-κB activation and the reduction of IL-8 secretion. The fact that the ERAD system, or at least some of the components, is up-regulated in CF could explain, in part, the exaggerated NF-κB activation in cells presenting CFTR defects [65].
When UPR and ERAD signaling pathways are not sufficient to restore normal cell parameters, cells could activate EOR in order to induce apoptosis [59]. It has been described that, independently of UPR activation, the accumulation of unfolded proteins in the ER membranes provides an NF-κB-activating stimulus [66]. Several messengers have been proposed as signals from the ER for NF-κB activation, such as the efflux of calcium or the reactive oxygen species (ROS) [67]. It is possible that the constitutive NF-κB activation observed in CF cells could be the consequence of EOR in response to CFTR accumulation in the ER membranes as described by Knorre and colleagues [68]. Exaggerated NF-κB activation, apoptosis and pro-inflammatory mediator productions have also been described in CF pancreatic and tracheal cells [22] suggesting that EOR may be implicated in theses events.
Altogether, prevention of the retention of CFTR in the ER and its rescue at the plasma membrane may be a key element in CF pathogenesis, and CFTRΔF508 correction based on the activation of different ER-linked systems can represent new approaches of this disease [69-71].
CFTR and calcium homeostasis
In agreement with the observations that ER plays a major role in the CF pathology, it has been shown that calcium homeostasis is altered in cells presenting CFTR mutations. Antigny et al. [57] have shown that the release of calcium from ER stores by agonists, such as histamine or ATP, is increased in CF cells. More important, the rescue of CFTRΔF508 at the plasma membrane by incubating cells at low temperature (27°C) restored calcium mobilization at similar levels than those measured in normal cells.
In CF human airway epithelia, other studies have described a higher calcium mobilization in response of G protein-coupled receptors to nucleotides or bradykinin, suggesting that the apical ER/calcium store compartment is expanded [72]. However, these authors propose that the increase in ER volume could be attributable to airway infection and inflammation rather than the intrinsic CFTR defects since in the absence of infection or after long term culturing CF cells, ER expansion is abolished. In addition, same authors have established a relationship between inflammation and ER expansion [73]. Indeed, the increase in IL-8 secretion induced after bradykinin treatment was mediated by an increased calcium mobilization consecutive to ER expansion. The link between inflammation and calcium mobilization has been confirmed by the work from Tabary et al. [74]. Using a model of individual living airway epithelial cell monitoring, they have shown that inflammatory mediators such as IL-1β are able to induce an increased calcium release, which is accompanied by activation of NF-κB in CF cells. Depletion of calcium stores from ER or inhibition of NF-κB activation leads to a decrease in calcium responses suggesting that inflammatory cytokines regulate calcium handling in CF cells, and in turn, calcium mobilization controls NF-κB activation contributing to a noxious cycle. In this context, the most common pathogens in CF, Pseudomonas aeruginosa and Staphylococcus aureus, promote an increase of intracellular calcium concentration in CF cells that lead to NF-κB activation and proinflammatory cytokine production via ERK1/2 and p38 pathways [75].
Other evidences suggest that the excess of calcium sequestration in ER may contribute to the abnormal trafficking of CFTRΔF508. Indeed, it has been demonstrated that the decrease and the maintenance of low calcium levels in ER, by using SERCA inhibitors, prevent the interaction between CFTRΔF508 and chaperones and thus this restores CFTRΔF508 at the plasma membrane [76,77].
In addition to ER expansion, mitochondria from CF lymphocytes display calcium accumulation [78], that could be explained by the higher activities of several enzymes involved in the cell energy metabolism such as NADH oxidase, NADH- and succinate-cytochrome c reductases [79].
CFTR and oxidative stress
When the balance between antioxidants and oxidants is no longer able to prevent the alteration of physiological functions, oxidative stress takes place. In airways in particular, ROS, a general term for a number of various and highly reactive oxygen derived ions or molecules, including both radicals and non-radicals, are the main oxidant species. ROS display beneficial and deleterious effects, depending of their concentration; an increase occurs when cells present an imbalance between the production and the neutralization of free radicals by antioxidant defense systems. Thus, ROS excess has been described to promote inflammatory gene transcription [80]. In addition, the interaction of ROS with nitric oxide (NO), resulting in reactive nitrogen species formation, can further enhance their potential deleterious effects [81,82]. Among the effects due to excessive production of ROS, the oxidation of macromolecules causes irreversible damage in molecular targets like proteins, DNA and lipids [83].
CFTR and GSH
Glutathione, an ubiquitous tripeptide, is one of the most important antioxidant molecules. Glutathione exists in reduced monomeric (GSH) and oxidized dimeric forms (GSSG). GSH is found in extracellular fluids, in lung and in cells at high concentrations of its reduced form. Extracellular GSH neutralizes free radicals produced by neutrophils during inflammation [84]. Taking into consideration that CFTR permits the transport of GSH between cells and apical extracellular media [85], it is reasonable to imagine that intracellular GSH content may be altered in CF. In this respect, contradictory data on GSH content in lung fluid of CF patients have been reported (for review, see [86]). Indeed, in epithelial lining fluids, like plasma, the concentration of GSH, but not GSSG, is reduced compared with normal subjects [25]. The level of GSH in plasma of CF patients is also reduced suggesting that GSH deficiency is not limited to the site of inflammation but is rather systemic [25]. Gao and collaborators [84] have shown that, in cell lines expressing CFTR mutations or transfected with normal CFTR, GSH efflux is lower in the former indicating an abnormal transport of GSH associated with a defective CFTR. Interestingly, it has been reported that nebulized buffered GSH or the combination of oral GSH and inhaled buffered GSSG attenuates CF disease [87,88], suggesting that new approaches against altered cellular redox status may represent potential treatments of CF. Not only cellular content of GSH is altered in CF, low mitochondria GSH levels have also been described in both lung from CFTR-deficient mice and human lung epithelial cells lines expressing CFTR mutations [89]. Because GSH is known to inhibit IκBα degradation [90,91], low levels of GSH in CF cells may promote NF-κB activation and participate in the maintenance of inflammation. By contrast, Jungas et al. [92] have measured an increase of intracellular GSH levels in epithelial cells (HeLa) transfected with CFTRΔF508 compared to wild-type cells, and this is associated with a defective apoptosis in CF cells probably due to a slower GSH depletion. In agreement with this work, Day et al. [93] have shown an increase of GSSG in epithelial lining fluid from CFTR-deficient mice after Pseudomonas aeruginosa infection resulting in a reduction of the GSH/GSSG ratio indicating an increased oxidative stress.
CFTR and reactive oxygen species
Elevated markers of oxidative stress, like lipid hydroperoxidation and protein oxidation, have been measured in plasma from CF patients and are associated with diminished concentration of plasma antioxidants [94,95]. Thus, elevated lipid peroxidation could be associated with pulmonary dysfunction leading to damage of structural membranes. An elevation of urine concentration of a marker of ROS-induced DNA damage, 8-hydroxydeoxyguanosine, has also been described in CF patients, suggesting that in CF an increased susceptibility to oxidative-induced DNA damage may explain the further incidence of malignancy [96] (Figure 3).
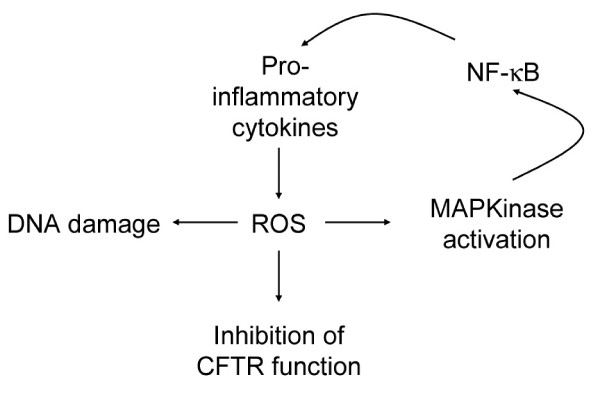
Figure 3.
Reactive oxygen species (ROS) affect DNA integrity, CFTR function and can participate in the noxious cycle of inflammation in CF cells.
In epithelial lining fluid in lung of CFTR-deficient mice, Velsor et al. [89] have shown an increase of markers of oxidative stress related to lipid and DNA oxidation. Furthermore, in a CF epithelial cell line, they have reported high intracellular levels of hydrogen peroxide, reflecting oxidative stress. Moreover, mitochondrial levels of ROS (superoxide anion and hydrogen peroxide) are enhanced in CFTR-deficient cell line [97]. Altogether, these results indicate that elevated levels of mitochondrial and cellular ROS are associated with a CFTR-deficient state. The effects of ROS may be double in CF, on the one hand, it is known that cellular stress induced by ROS inhibits CFTR maturation, levels and function [58] (Figure 3). On the other hand, an increase of ROS leads to MAPK signaling pathway activation [83]. Because this cascade is known to regulate pro-inflammatory gene expression in CF cells [34], it is reasonable to hypothesize that ROS are implicated in the initiation or/and the maintenance of the inflammatory state in CFTR deficiency. In addition, an excessive production of pro-inflammatory cytokines could increase ROS production [98] perpetuating the vicious circle of inflammation in CF (Figure 3).
Defective neutralization of ROS can also elicit oxidative stress. Three superoxide dismutases (SOD) have been described in mammals, Cu/Zn-SOD or SOD1, Mn-SOD or SOD2, and extracellular-SOD or SOD3 (for review see [99]). These enzymes are implicated in decreasing superoxide anion levels that damage cells at excessive concentration [100]. Alterations in the expression or/and activity of SODs have been described in several pathologies such as amyotrophic lateral sclerosis for SOD1 [101], cardiomyopathies for SOD2 [102] and lung diseases for SOD3 [103]. Indeed, SOD3 is highly expressed in lungs and is associated with decreased recruitment of neutrophils, suggesting an important role in regulating pulmonary inflammation [99]. Although no direct evidence has shown the involvement of SOD3 in CF, the fact that it is highly expressed in airways raises the possibility that it may play a role in CF. In addition, pro-inflammatory cytokines increase SOD3 expression, in culture and in animal models of lung injury [104,105].
In agreement with these studies, we have observed a diminution of the expression of three isoforms of SOD, in pancreatic and tracheal CF cells compared to their respective controls. In addition, direct measurements of superoxide anion production by electronic paramagnetic resonance correlate with the diminution of SOD expression suggesting an adaptive response to oxidative stress, in CF cells (unpublished results).
Taken together, deficiency in antioxidant systems seems to be associated with all CFTR mutations. Thus, in CF patients, inadequate antioxidant defenses are associated with the elevated oxidative stress, which contributes to the decline of pulmonary function.
CFTR and NO
NO plays a dual role under inflammatory conditions. On the one hand, NO acts as a bronchodilator molecule, is able to modulate immune responses, possesses antimicrobial activities and acts as an important signaling molecule. One the other hand, reactions of NO with ROS can originate intermediate reactive species that have deleterious properties. Three different isoforms of NO synthase are expressed in normal lung tissue but only one, iNOS, leads to production of NO that has antimicrobial properties by reducing Pseudomonas aeruginosa adherence. Whereas expression of iNOS is upregulated in inflammatory diseases [106], expression of this enzyme is decreased in CF [107,108], which may explain, at least partially, the chronic airway infection. Although sites and techniques for NO measurement are subjects at debate, patients with CF have lower NO levels, certainly in parallel with low expression of iNOS, but present high levels of peroxynitrite, nitrite and nitrates [109-114]. A possible explanation has been proposed by Jones et al. [115], suggesting the formation of nitrates and other NO metabolites by reaction with superoxide anion, and then NO levels are reduced. In addition, there is increasing evidence that decreased NO formation contributes to airway obstruction in CF [111,116-118]. Interestingly, low NO levels are correlated to both pancreatic insufficiency and chronic Pseudomnas aeruginosa infection, suggesting that airway NO could be associated with genotype and could be considered a risk factor for infection [111]. S-Nitrosothiols, NO adducts that can mediate several effects of NO, are also decreased in CF airways [119]. Thus, treatment with S-nitrosothiols is able to increase expression, maturation and function of both wild-type and mutated CFTR [120]. Moreover, low levels of S-nitrosoglutathione, a physiological NO donor that acts as an innate-immune mediator, can enhance the deleterious effects of Pseudomonas aeruginosa by affecting its metabolism [118]. Furthermore, treatment of CF homozygote ΔF508 mice with LPS has no effect on NO production, suggesting that decreased expression of iNOS could contribute to chronic infection of the airways [119].
CFTR and ceramides
Ceramides are sphingolipids, located in vesicles and cell intermembranes. They are generated by hydrolysis of sphingomyelin by acid sphingomyelinase (ASM) or by de novo synthesis by ceramide synthase. They are degraded by ceramidase to form fatty acid and sphingosin. Ceramides play crucial rules in signaling pathways. Recent data suggest that ceramides may be involved in the pathogenesis of CF. For instance, infection through Pseudomonas aeruginosa favors interaction with rafts and activates ASM, the latter translocates from intracellular vesicles to the extracellular leaflet of the cell plasma membrane and generates ceramides. Then, ceramides induce rafts fusion to form membrane platforms with receptors and molecule clusters, like CD95 and CFTR, initiating signalization [121], leading to cell death [48], regulation of NF-κB pathway [122], and expression of pro-inflammatory cytokines (Figure 1). Therefore, ceramides may represent an important target for understanding and treating CF. Like for apoptosis, levels of ceramides in CF cells are a matter of debate. Vilela et al. [48] have shown a decreased ceramide production in CFTRΔF508 lung epithelial cells after treatment with TNF-α. For these authors, low levels of ceramide production is due to high GSH levels that inhibit ASM. This results in an exaggerated inflammation due to absence of NF-κB inhibition by ceramides. Recently, Teichgräber and colleagues [122] observed a ceramide accumulation, age-dependent, in ciliated respiratory, nasal epithelial cells, submucosal glands, and macrophages from CFTR-deficient mice. In addition, accumulation of ceramides, probably through an imbalance between generation and degradation, is associated with constitutive pulmonary inflammation and epithelial cell death. Most interestingly, pharmacological inhibition of ASM, in CFTR-deficient mice concomitantly normalizes ceramide production and decreases pathological parameters [122]. Altogether, these results and those of Vilela et al. [48] demonstrate a modification of ceramide homeostasis in CF associated with an exaggerated inflammatory response to various stress inducers.
Conclusion and Perspectives
Intrinsic inflammation in CF, in the absence of pathogens, has multiple origins that make it difficult to resolve. In addition, contradictory data on apoptosis in CF highlight the need of further studies in order to explore the status of programmed cell death in this pathology. As detailed above, whether ER stress remains sustained, as this occurs with CFTRΔF508 retention, cells initiate programmed death, and this could explain the activation of NF-κB-associated pathways and inflammation observed in CF. Although gene therapy may symbolize a real solution for the disease, the enthusiasm has been rapidly tempered in view of the difficulties associated with the use of viral and non-viral vectors, as well as the complexity of the pathways controlling CFTR function. In this line, carrier proteins delivering pharmacological chaperones may be considered good candidates to new therapies leading to correction of mutated CFTR [123]. However, very recently, several pathways, up to date not linked to CFTR deficiency, have been proposed to modulate inflammatory response in CF and may represent new therapeutic approaches.
Among these pathways, the peroxisome proliferator-activated receptor-γ (PPARγ) may be considered because of its ability to participate in the transcription of various genes involved in the regulation of the inflammatory response [124]. In this respect, it has been recently described that up-regulation of tissue transglutaminase may account for the reduction of PPARγ expression in CFTR-defective cell lines, and in this way, tissue transglutaminase inhibition could regulate inflammation in CF cells [125]. In addition, these authors have shown that increase of intracellular calcium and excess of oxidative stress modulate tissue transglutaminase activity, reinforcing the notion that both calcium and ROS are implicated in the inflammatory response in CF. In the same way, Perez et al. [126] have shown that PPARγ agonists reduce cytokine secretion in vitro and airway inflammation in response to Pseudomonas aeroginosa in CF mice.
Natural products, mainly from vegetables, may also represent potential therapy in CF. In particular, flavonoids and other bioactive compounds, with a large spectrum of biological effects such as anti-oxidant potential, could affect different stages of CF pathogenesis. For instance, several plant extracts have been found able to decrease secretion of inflammatory cytokines from CF cells [127]. Others correct defective electrolyte transport in CF airways acting in parallel on second messengers and channel activities [128]. Curcumin has also been described as a corrector of the CFTR defect by eliciting increased CFTR traffic towards the plasma membrane [129].
Nevertheless, until gene therapy is completely successful, inflammation, in addition to elimination of infection mucus, remains the main target in the treatment of CF. To improve the effects of the different therapies, a better knowledge of the intracellular and molecular mechanisms involved in the regulation of CFTR traffic and function seems essential for correction of mutated CFTR at different stages of impaired functions.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
MR participated in the design of the study and wrote all chapters; JMF contributed to the final revision of the manuscript; MCM designed and wrote the paper, participated in the revision and editing of the manuscript. All authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
This work was supported in part by Vaincre la Mucoviscidose. M.R. is a recipient of a doctoral fellowship from Fondation pour la Recherche Médicale.
Contributor Information
Mathilde Rottner, Email: mathilde.rottner@hemato-ulp.u-strasbg.fr.
Jean-Marie Freyssinet, Email: jean-marie.freyssinet@hemato-ulp.u-strasbg.fr.
M Carmen Martínez, Email: carmen.martinez@univ-angers.fr.
References
- Aschcroft F. Ion Channel and Disease. Academic Press. San Diego; 1999. Cystic fibrosis transmembrane conductance regulator; pp. 211–232. [Google Scholar]
- Akabas MH. Cystic fibrosis transmembrane conductance regulator. Structure and function of an epithelial chloride channel. J Biol Chem. 2000;275:3729–3732. doi: 10.1074/jbc.275.6.3729. [DOI] [PubMed] [Google Scholar]
- Hudson VM. Rethinking cystic fibrosis pathology: the critical role of abnormal reduced glutathione (GSH) transport caused by CFTR mutation. Free Radic Biol Med. 2001;30:1440–1461. doi: 10.1016/s0891-5849(01)00530-5. [DOI] [PubMed] [Google Scholar]
- Kogan I, Ramjeesingh M, Li C, Kidd JF, Wang Y, Leslie EM, Cole SP, Bear CE. CFTR directly mediates nucleotide-regulated glutathione flux. EMBO J. 2003;22:1981–1989. doi: 10.1093/emboj/cdg194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Benos DJ, Egan ME, Stutts MJ, Guggino WB. CFTR is a conductance regulator as well as a chloride channel. Physiol Rev. 1999;79:S145–166. doi: 10.1152/physrev.1999.79.1.S145. [DOI] [PubMed] [Google Scholar]
- Cystic Fibrosis Mutation Database http://www.genet.sickkids.on.ca/cftr/app
- Yoo JS, Moyer BD, Bannykh S, Yoo HM, Riordan JR, Balch WE. Non-conventional trafficking of the cystic fibrosis transmembrane conductance regulator through the early secretory pathway. J Biol Chem. 2002;277:11401–11409. doi: 10.1074/jbc.M110263200. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Liedtke CM. Electrolyte transport in the epithelium of pulmonary segments of normal and cystic fibrosis lung. FASEB J. 1992;6:3076–3084. doi: 10.1096/fasebj.6.12.1521739. [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Chang XB, Kartner N, Rotstein OD, Riordan JR, Grinstein S. The cystic fibrosis transmembrane regulator is present and functional in endosomes. Role as a determinant of endosomal pH. J Biol Chem. 1992;267:14568–14572. [PubMed] [Google Scholar]
- Rowntree RK, Harris A. The phenotypic consequences of CFTR mutations. Ann Hum Genet. 2003;67:471–485. doi: 10.1046/j.1469-1809.2003.00028.x. [DOI] [PubMed] [Google Scholar]
- Lim M, Zeitlin PL. Therapeutic strategies to correct malfunction of CFTR. Paediatr Respir Rev. 2001;2:159–164. doi: 10.1053/prrv.2000.0124. [DOI] [PubMed] [Google Scholar]
- Taylor CJ, Aswani N. The pancreas in cystic fibrosis. Paediatr Respir Rev. 2002;3:77–81. doi: 10.1053/prrv.2002.0183. [DOI] [PubMed] [Google Scholar]
- Boucher RC. An overview of the pathogenesis of cystic fibrosis lung disease. Adv Drug Deliv Rev. 2002;54:1359–1371. doi: 10.1016/s0169-409x(02)00144-8. [DOI] [PubMed] [Google Scholar]
- Heijerman H. Infection and inflammation in cystic fibrosis: a short review. J Cyst Fibros. 2005;4:3–5. doi: 10.1016/j.jcf.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Dakin CJ, Numa AH, Wang H, Morton JR, Vertzyas CC, Henry RL. Inflammation, infection, and pulmonary function in infants and young children with cystic fibrosis. Am J Respir Crit Care Med. 2002;165:904–910. doi: 10.1164/ajrccm.165.7.2010139. [DOI] [PubMed] [Google Scholar]
- Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med. 1995;151:1075–1082. doi: 10.1164/ajrccm/151.4.1075. [DOI] [PubMed] [Google Scholar]
- Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160:186–191. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- Becker MN, Sauer MS, Muhlebach MS, Hirsh AJ, Wu Q, Verghese MW, Randell SH. Cytokine secretion by cystic fibrosis airway epithelial cells. Am J Respir Crit Care Med. 2004;169:645–653. doi: 10.1164/rccm.200207-765OC. [DOI] [PubMed] [Google Scholar]
- Hybiske K, Fu Z, Schwarzer C, Tseng J, Do J, Huang N, Machen TE. Effects of cystic fibrosis transmembrane conductance regulator and DeltaF508CFTR on inflammatory response, ER stress, and Ca2+ of airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2007;293:L1250–1260. doi: 10.1152/ajplung.00231.2007. [DOI] [PubMed] [Google Scholar]
- Escotte S, Tabary O, Dusser D, Majer-Teboul C, Puchelle E, Jacquot J. Fluticasone reduces IL-6 and IL-8 production of cystic fibrosis bronchial epithelial cells via IKK-beta kinase pathway. Eur Respir J. 2003;21:574–581. doi: 10.1183/09031936.03.00031803. [DOI] [PubMed] [Google Scholar]
- Rottner M, Kunzelmann C, Mergey M, Freyssinet JM, Martinez MC. Exaggerated apoptosis and NF-kappaB activation in pancreatic and tracheal cystic fibrosis cells. Faseb J. 2007;21:2939–2948. doi: 10.1096/fj.06-7614com. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan A, Stecenko AA, King G, Blackwell TR, Brigham KL, Christman JW, Blackwell TS. Exaggerated activation of nuclear factor-kappaB and altered IkappaB-beta processing in cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol. 2000;23:396–403. doi: 10.1165/ajrcmb.23.3.3949. [DOI] [PubMed] [Google Scholar]
- Bergoin C, Gosset P, Lamblin C, Bolard F, Turck D, Tonnel AB, Wallaert B. Cell and cytokine profile in nasal secretions in cystic fibrosis. J Cyst Fibros. 2002;1:110–115. doi: 10.1016/s1569-1993(02)00072-3. [DOI] [PubMed] [Google Scholar]
- Roum JH, Buhl R, McElvaney NG, Borok Z, Crystal RG. Systemic deficiency of glutathione in cystic fibrosis. J Appl Physiol. 1993;75:2419–2424. doi: 10.1152/jappl.1993.75.6.2419. [DOI] [PubMed] [Google Scholar]
- Tirouvanziam R, Gernez Y, Conrad CK, Moss RB, Schrijver I, Dunn CE, Davies ZA, Herzenberg LA. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc Natl Acad Sci USA. 2008;105:4335–4339. doi: 10.1073/pnas.0712386105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conese M, Copreni E, Di Gioia S, De Rinaldis P, Fumarulo R. Neutrophil recruitment and airway epithelial cell involvement in chronic cystic fibrosis lung disease. J Cyst Fibros. 2003;2:129–135. doi: 10.1016/S1569-1993(03)00063-8. [DOI] [PubMed] [Google Scholar]
- Corvol H, Fitting C, Chadelat K, Jacquot J, Tabary O, Boule M, Cavaillon JM, Clement A. Distinct cytokine production by lung and blood neutrophils from children with cystic fibrosis. Am J Physiol Lung Cell Mol Physiol. 2003;284:L997–1003. doi: 10.1152/ajplung.00156.2002. [DOI] [PubMed] [Google Scholar]
- Tabary O, Corvol H, Boncoeur E, Chadelat K, Fitting C, Cavaillon JM, Clement A, Jacquot J. Adherence of airway neutrophils and inflammatory response are increased in CF airway epithelial cell-neutrophil interactions. Am J Physiol Lung Cell Mol Physiol. 2006;290:L588–596. doi: 10.1152/ajplung.00013.2005. [DOI] [PubMed] [Google Scholar]
- Courtney JM, Ennis M, Elborn JS. Cytokines and inflammatory mediators in cystic fibrosis. J Cyst Fibros. 2004;3:223–231. doi: 10.1016/j.jcf.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Virella-Lowell I, Herlihy JD, Liu B, Lopez C, Cruz P, Muller C, Baker HV, Flotte TR. Effects of CFTR, interleukin-10, and Pseudomonas aeruginosa on gene expression profiles in a CF bronchial epithelial cell Line. Mol Ther. 2004;10:562–573. doi: 10.1016/j.ymthe.2004.06.215. [DOI] [PubMed] [Google Scholar]
- Schottelius AJ, Mayo MW, Sartor RB, Baldwin AS., Jr Interleukin-10 signaling blocks inhibitor of kappaB kinase activity and nuclear factor kappaB DNA binding. J Biol Chem. 1999;274:31868–31874. doi: 10.1074/jbc.274.45.31868. [DOI] [PubMed] [Google Scholar]
- Saadane A, Soltys J, Berger M. Role of IL-10 deficiency in excessive nuclear factor-kappaB activation and lung inflammation in cystic fibrosis transmembrane conductance regulator knockout mice. J Allergy Clin Immunol. 2005;115:405–411. doi: 10.1016/j.jaci.2004.10.044. [DOI] [PubMed] [Google Scholar]
- Verhaeghe C, Remouchamps C, Hennuy B, Vanderplasschen A, Chariot A, Tabruyn SP, Oury C, Bours V. Role of IKK and ERK pathways in intrinsic inflammation of cystic fibrosis airways. Biochem Pharmacol. 2007;73:1982–1994. doi: 10.1016/j.bcp.2007.03.019. [DOI] [PubMed] [Google Scholar]
- Tchilibon S, Zhang J, Yang Q, Eidelman O, Kim H, Caohuy H, Jacobson KA, Pollard BS, Pollard HB. Amphiphilic pyridinium salts block TNF alpha/NF kappa B signaling and constitutive hypersecretion of interleukin-8 (IL-8) from cystic fibrosis lung epithelial cells. Biochem Pharmacol. 2005;70:381–393. doi: 10.1016/j.bcp.2005.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechecchi MC, Nicolis E, Norez C, Bezzerri V, Borgatti M, Mancini I, Rizzotti P, Ribeiro CM, Gambari R, Becq F, Cabrini G. Anti-inflammatory effect of miglustat in bronchial epithelial cells. J Cyst Fibros. 2008;7:555–565. doi: 10.1016/j.jcf.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Chen J, Kinter M, Shank S, Cotton C, Kelley TJ, Ziady AG. Dysfunction of Nrf-2 in CF epithelia leads to excess intracellular H2O2 and inflammatory cytokine production. PLoS ONE. 2008;3:e3367. doi: 10.1371/journal.pone.0003367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, Soong G, Bryan R, Saba S, Prince A. Activation of NF-kappaB in airway epithelial cells is dependent on CFTR trafficking and Cl- channel function. Am J Physiol Lung Cell Mol Physiol. 2001;281:L71–78. doi: 10.1152/ajplung.2001.281.1.L71. [DOI] [PubMed] [Google Scholar]
- Dechecchi MC, Nicolis E, Bezzerri V, Vella A, Colombatti M, Assael BM, Mettey Y, Borgatti M, Mancini I, Gambari R, Becq F, Cabrini G. MPB-07 reduces the inflammatory response to Pseudomonas aeruginosa in cystic fibrosis bronchial cells. Am J Respir Cell Mol Biol. 2007;36:615–624. doi: 10.1165/rcmb.2006-0200OC. [DOI] [PubMed] [Google Scholar]
- Machen TE. Innate immune response in CF airway epithelia: hyperinflammatory? Am J Physiol Cell Physiol. 2006;291:C218–230. doi: 10.1152/ajpcell.00605.2005. [DOI] [PubMed] [Google Scholar]
- Saadane A, Soltys J, Berger M. Acute Pseudomonas challenge in cystic fibrosis mice causes prolonged nuclear factor-kappa B activation, cytokine secretion, and persistent lung inflammation. J Allergy Clin Immunol. 2006;117:1163–1169. doi: 10.1016/j.jaci.2006.01.052. [DOI] [PubMed] [Google Scholar]
- Andersson C, Zaman MM, Jones AB, Freedman SD. Alterations in immune response and PPAR/LXR regulation in cystic fibrosis macrophages. J Cyst Fibros. 2008;7:68–78. doi: 10.1016/j.jcf.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Bruscia EM, Zhang PX, Ferreira E, Caputo C, Emerson JW, Tuck D, Krause DS, Egan ME. Macrophages directly contribute to the exaggerated inflammatory response in CFTR-/- mice. Am J Respir Cell Mol Biol. 2009;40:295–304. doi: 10.1165/rcmb.2008-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman MM, Gelrud A, Junaidi O, Regan MM, Warny M, Shea JC, Kelly C, O'Sullivan BP, Freedman SD. Interleukin 8 secretion from monocytes of subjects heterozygous for the deltaF508 cystic fibrosis transmembrane conductance regulator gene mutation is altered. Clin Diagn Lab Immunol. 2004;11:819–824. doi: 10.1128/CDLI.11.5.819-824.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri L, Raia V, De Marco G, Coletta S, de Ritis G, Londei M, Auricchio S. DNA fragmentation is a feature of cystic fibrosis epithelial cells: a disease with inappropriate apoptosis? FEBS Lett. 1997;408:225–231. doi: 10.1016/s0014-5793(97)00347-5. [DOI] [PubMed] [Google Scholar]
- Cannon CL, Kowalski MP, Stopak KS, Pier GB. Pseudomonas aeruginosa-induced apoptosis is defective in respiratory epithelial cells expressing mutant cystic fibrosis transmembrane conductance regulator. Am J Respir Cell Mol Biol. 2003;29:188–197. doi: 10.1165/rcmb.4898. [DOI] [PubMed] [Google Scholar]
- McKeon DJ, Condliffe AM, Cowburn AS, Cadwallader KC, Farahi N, Bilton D, Chilvers ER. Prolonged survival of neutrophils from patients with Delta F508 CFTR mutations. Thorax. 2008;63:660–661. doi: 10.1136/thx.2008.096834. [DOI] [PubMed] [Google Scholar]
- Vilela RM, Lands LC, Meehan B, Kubow S. Inhibition of IL-8 release from CFTR-deficient lung epithelial cells following pre-treatment with fenretinide. Int Immunopharmacol. 2006;6:1651–1664. doi: 10.1016/j.intimp.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Pfeffer KD, Huecksteadt TP, Hoidal JR. Expression and regulation of tumor necrosis factor in macrophages from cystic fibrosis patients. Am J Respir Cell Mol Biol. 1993;9:511–519. doi: 10.1165/ajrcmb/9.5.511. [DOI] [PubMed] [Google Scholar]
- Durieu I, Amsellem C, Paulin C, Chambe MT, Bienvenu J, Bellon G, Pacheco Y. Fas and Fas ligand expression in cystic fibrosis airway epithelium. Thorax. 1999;54:1093–1098. doi: 10.1136/thx.54.12.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandivier RW, Fadok VA, Hoffmann PR, Bratton DL, Penvari C, Brown KK, Brain JD, Accurso FJ, Henson PM. Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J Clin Invest. 2002;109:661–670. doi: 10.1172/JCI13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer-Hansen M, Jaattela M. Connecting endoplasmic reticulum stress to autophagy by unfolded protein response and calcium. Cell Death Differ. 2007;14:1576–1582. doi: 10.1038/sj.cdd.4402200. [DOI] [PubMed] [Google Scholar]
- Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–327. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ. 2004;11:372–380. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- Gilbert A, Jadot M, Leontieva E, Wattiaux-De Coninck S, Wattiaux R. Delta F508 CFTR localizes in the endoplasmic reticulum-Golgi intermediate compartment in cystic fibrosis cells. Exp Cell Res. 1998;242:144–152. doi: 10.1006/excr.1998.4101. [DOI] [PubMed] [Google Scholar]
- Antigny F, Norez C, Becq F, Vandebrouck C. Calcium homeostasis is abnormal in cystic fibrosis airway epithelial cells but is normalized after rescue of F508del-CFTR. Cell Calcium. 2008;43:175–183. doi: 10.1016/j.ceca.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Rab A, Bartoszewski R, Jurkuvenaite A, Wakefield J, Collawn JF, Bebok Z. Endoplasmic reticulum stress and the unfolded protein response regulate genomic cystic fibrosis transmembrane conductance regulator expression. Am J Physiol Cell Physiol. 2007;292:C756–766. doi: 10.1152/ajpcell.00391.2006. [DOI] [PubMed] [Google Scholar]
- Lai E, Teodoro T, Volchuk A. Endoplasmic reticulum stress: signaling the unfolded protein response. Physiology (Bethesda) 2007;22:193–201. doi: 10.1152/physiol.00050.2006. [DOI] [PubMed] [Google Scholar]
- Kerbiriou M, Le Drevo MA, Ferec C, Trouve P. Coupling cystic fibrosis to endoplasmic reticulum stress: Differential role of Grp78 and ATF6. Biochim Biophys Acta. 2007;1772:1236–1249. doi: 10.1016/j.bbadis.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Bartoszewski R, Rab A, Jurkuvenaite A, Mazur M, Wakefield J, Collawn JF, Bebok Z. Activation of the Unfolded Protein Response by {Delta}F508 CFTR. Am J Respir Cell Mol Biol. 2008;39:448–457. doi: 10.1165/rcmb.2008-0065OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoszewski R, Rab A, Twitty G, Stevenson L, Fortenberry J, Piotrowski A, Dumanski JP, Bebok Z. The mechanism of cystic fibrosis transmembrane conductance regulator transcriptional repression during the unfolded protein response. J Biol Chem. 2008;283:12154–12165. doi: 10.1074/jbc.M707610200. [DOI] [PubMed] [Google Scholar]
- Meusser B, Hirsch C, Jarosch E, Sommer T. ERAD: the long road to destruction. Nat Cell Biol. 2005;7:766–772. doi: 10.1038/ncb0805-766. [DOI] [PubMed] [Google Scholar]
- Turnbull EL, Rosser MF, Cyr DM. The role of the UPS in cystic fibrosis. BMC Biochem. 2007;8:S11. doi: 10.1186/1471-2091-8-S1-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij N, Fang S, Zeitlin PL. Selective inhibition of endoplasmic reticulum-associated degradation rescues DeltaF508-cystic fibrosis transmembrane regulator and suppresses interleukin-8 levels: therapeutic implications. J Biol Chem. 2006;281:17369–17378. doi: 10.1074/jbc.M600509200. [DOI] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. A novel signal transduction pathway from the endoplasmic reticulum to the nucleus is mediated by transcription factor NF-kappa B. EMBO J. 1995;14:2580–2588. doi: 10.1002/j.1460-2075.1995.tb07256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahl HL, Baeuerle PA. The ER-overload response: activation of NF-kappa B. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- Knorre A, Wagner M, Schaefer HE, Colledge WH, Pahl HL. DeltaF508-CFTR causes constitutive NF-kappaB activation through an ER-overload response in cystic fibrosis lungs. Biol Chem. 2002;383:271–282. doi: 10.1515/BC.2002.029. [DOI] [PubMed] [Google Scholar]
- Loo TW, Bartlett MC, Clarke DM. Correctors promote folding of the CFTR in the endoplasmic reticulum. Biochem J. 2008;413:29–36. doi: 10.1042/BJ20071690. [DOI] [PubMed] [Google Scholar]
- Singh OV, Pollard HB, Zeitlin PL. Chemical rescue of deltaF508-CFTR mimics genetic repair in cystic fibrosis bronchial epithelial cells. Mol Cell Proteomics. 2008;7:1099–1110. doi: 10.1074/mcp.M700303-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga K, Goldstein RF, Jurkuvenaite A, Chen L, Matalon S, Sorscher EJ, Bebok Z, Collawn JF. Enhanced cell-surface stability of rescued DeltaF508 cystic fibrosis transmembrane conductance regulator (CFTR) by pharmacological chaperones. Biochem J. 2008;410:555–564. doi: 10.1042/BJ20071420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro CM, Paradiso AM, Carew MA, Shears SB, Boucher RC. Cystic fibrosis airway epithelial Ca2+ i signaling: the mechanism for the larger agonist-mediated Ca2+ i signals in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280:10202–10209. doi: 10.1074/jbc.M410617200. [DOI] [PubMed] [Google Scholar]
- Ribeiro CM, Paradiso AM, Schwab U, Perez-Vilar J, Jones L, O'Neal W, Boucher RC. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280(18):17798–17806. doi: 10.1074/jbc.M410618200. [DOI] [PubMed] [Google Scholar]
- Tabary O, Boncoeur E, de Martin R, Pepperkok R, Clement A, Schultz C, Jacquot J. Calcium-dependent regulation of NF-(kappa)B activation in cystic fibrosis airway epithelial cells. Cell Signal. 2006;18:652–660. doi: 10.1016/j.cellsig.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ratner AJ, Bryan R, Weber A, Nguyen S, Barnes D, Pitt A, Gelber S, Cheung A, Prince A. Cystic fibrosis pathogens activate Ca2+-dependent mitogen-activated protein kinase signaling pathways in airway epithelial cells. J Biol Chem. 2001;276:19267–19275. doi: 10.1074/jbc.M007703200. [DOI] [PubMed] [Google Scholar]
- Egan ME, Glockner-Pagel J, Ambrose C, Cahill PA, Pappoe L, Balamuth N, Cho E, Canny S, Wagner CA, Geibel J, et al. Calcium-pump inhibitors induce functional surface expression of Delta F508-CFTR protein in cystic fibrosis epithelial cells. Nat Med. 2002;8:485–492. doi: 10.1038/nm0502-485. [DOI] [PubMed] [Google Scholar]
- Norez C, Pasetto M, Dechecchi MC, Barison E, Anselmi C, Tamanini A, Quiri F, Cattel L, Rizzotti P, Dosio F, Cabrini G, Colombatti M. Chemical conjugation of DeltaF508-CFTR corrector deoxyspergualin to transporter human serum albumin enhances its ability to rescue Cl- channel functions. Am J Physiol Lung Cell Mol Physiol. 2008;295:L336–347. doi: 10.1152/ajplung.00059.2008. [DOI] [PubMed] [Google Scholar]
- Waller RL, Brattin WJ, Dearborn DG. Cytosolic free calcium concentration and intracellular calcium distribution in lymphocytes from cystic fibrosis patients. Life Sci. 1984;35:775–781. doi: 10.1016/0024-3205(84)90347-3. [DOI] [PubMed] [Google Scholar]
- von Ruecker AA, Bertele R, Harms HK. Calcium metabolism and cystic fibrosis: mitochondrial abnormalities suggest a modification of the mitochondrial membrane. Pediatr Res. 1984;18:594–599. doi: 10.1203/00006450-198407000-00005. [DOI] [PubMed] [Google Scholar]
- Bartling TR, Drumm ML. Oxidative stress causes IL8 promoter hyperacetylation in cystic fibrosis airway cell models. Am J Respir Cell Mol Biol. 2009;40:58–65. doi: 10.1165/rcmb.2007-0464OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkerts G, Kloek J, Muijsers RB, Nijkamp FP. Reactive nitrogen and oxygen species in airway inflammation. Eur J Pharmacol. 2001;429:251–262. doi: 10.1016/s0014-2999(01)01324-3. [DOI] [PubMed] [Google Scholar]
- Martinez MC, Andriantsitohaina R. Reactive nitrogen species: Molecular mechanisms and potential significance in health and disease. Antioxid Redox Signal. 2009;11:669–702. doi: 10.1089/ars.2007.1993. [DOI] [PubMed] [Google Scholar]
- Genestra M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007;19:1807–1819. doi: 10.1016/j.cellsig.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Gao L, Kim KJ, Yankaskas JR, Forman HJ. Abnormal glutathione transport in cystic fibrosis airway epithelia. Am J Physiol. 1999;277:L113–118. doi: 10.1152/ajplung.1999.277.1.L113. [DOI] [PubMed] [Google Scholar]
- Linsdell P, Evagelidis A, Hanrahan JW. Molecular determinants of anion selectivity in the cystic fibrosis transmembrane conductance regulator chloride channel pore. Biophys J. 2000;78:2973–2982. doi: 10.1016/S0006-3495(00)76836-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson VM. New insights into the pathogenesis of cystic fibrosis: pivotal role of glutathione system dysfunction and implications for therapy. Treat Respir Med. 2004;3:353–363. doi: 10.2165/00151829-200403060-00003. [DOI] [PubMed] [Google Scholar]
- Bishop C, Hudson VM, Hilton SC, Wilde C. A pilot study of the effect of inhaled buffered reduced glutathione on the clinical status of patients with cystic fibrosis. Chest. 2005;127:308–317. doi: 10.1378/chest.127.1.308. [DOI] [PubMed] [Google Scholar]
- Visca A, Bishop CT, Hilton SC, Hudson VM. Improvement in clinical markers in CF patients using a reduced glutathione regimen: An uncontrolled, observational study. J Cyst Fibros. 2008;7:433–436. doi: 10.1016/j.jcf.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Velsor LW, van Heeckeren A, Day BJ. Antioxidant imbalance in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Physiol Lung Cell Mol Physiol. 2001;281:L31–38. doi: 10.1152/ajplung.2001.281.1.L31. [DOI] [PubMed] [Google Scholar]
- Haddad JJ. Redox regulation of pro-inflammatory cytokines and IkappaB-alpha/NF-kappaB nuclear translocation and activation. Biochem Biophys Res Commun. 2002;296:847–856. doi: 10.1016/s0006-291x(02)00947-6. [DOI] [PubMed] [Google Scholar]
- Rahman I, MacNee W. Role of transcription factors in inflammatory lung diseases. Thorax. 1998;53:601–612. doi: 10.1136/thx.53.7.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungas T, Motta I, Duffieux F, Fanen P, Stoven V, Ojcius DM. Glutathione levels and BAX activation during apoptosis due to oxidative stress in cells expressing wild-type and mutant cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2002;277:27912–27918. doi: 10.1074/jbc.M110288200. [DOI] [PubMed] [Google Scholar]
- Day BJ, van Heeckeren AM, Min E, Velsor LW. Role for cystic fibrosis transmembrane conductance regulator protein in a glutathione response to bronchopulmonary pseudomonas infection. Infect Immun. 2004;72:2045–2051. doi: 10.1128/IAI.72.4.2045-2051.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RK, Wyatt H, Price JF, Kelly FJ. Pulmonary dysfunction in cystic fibrosis is associated with oxidative stress. Eur Respir J. 1996;9:334–339. doi: 10.1183/09031936.96.09020334. [DOI] [PubMed] [Google Scholar]
- Collins CE, Quaggiotto P, Wood L, O'Loughlin EV, Henry RL, Garg ML. Elevated plasma levels of F2 alpha isoprostane in cystic fibrosis. Lipids. 1999;34:551–556. doi: 10.1007/s11745-999-0397-1. [DOI] [PubMed] [Google Scholar]
- Brown RK, McBurney A, Lunec J, Kelly FJ. Oxidative damage to DNA in patients with cystic fibrosis. Free Radic Biol Med. 1995;18:801–806. doi: 10.1016/0891-5849(94)00172-g. [DOI] [PubMed] [Google Scholar]
- Velsor LW, Kariya C, Kachadourian R, Day BJ. Mitochondrial oxidative stress in the lungs of cystic fibrosis transmembrane conductance regulator protein mutant mice. Am J Respir Cell Mol Biol. 2006;35:579–586. doi: 10.1165/rcmb.2005-0473OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozben T. Oxidative stress and apoptosis: impact on cancer therapy. J Pharm Sci. 2007;96:2181–2196. doi: 10.1002/jps.20874. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Crapo JD. Oxidative stress in airways: is there a role for extracellular superoxide dismutase? Am J Respir Crit Care Med. 2002;166:S38–43. doi: 10.1164/rccm.2206014. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Nicks M, Tran K, Tanner G, Chang LY, Young SK, Worthen GS. Extracellular superoxide dismutase attenuates lipopolysaccharide-induced neutrophilic inflammation. Am J Respir Cell Mol Biol. 2004;31:432–439. doi: 10.1165/rcmb.2004-0057OC. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Robinson BH. The role of manganese superoxide dismutase in health and disease. J Inherit Metab Dis. 1998;21:598–603. doi: 10.1023/a:1005427323835. [DOI] [PubMed] [Google Scholar]
- Smith LJ, Shamsuddin M, Sporn PH, Denenberg M, Anderson J. Reduced superoxide dismutase in lung cells of patients with asthma. Free Radic Biol Med. 1997;22:1301–1307. doi: 10.1016/s0891-5849(96)00550-3. [DOI] [PubMed] [Google Scholar]
- Bowler RP, Arcaroli J, Abraham E, Patel M, Chang LY, Crapo JD. Evidence for extracellular superoxide dismutase as a mediator of hemorrhage-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2003;284:L680–687. doi: 10.1152/ajplung.00191.2002. [DOI] [PubMed] [Google Scholar]
- Marklund SL. Regulation by cytokines of extracellular superoxide dismutase and other superoxide dismutase isoenzymes in fibroblasts. J Biol Chem. 1992;267:6696–6701. [PubMed] [Google Scholar]
- Gotoh T, Mori M. Nitric oxide and endoplasmic reticulum stress. Arterioscler Thromb Vasc Biol. 2006;26:1439–1446. doi: 10.1161/01.ATV.0000223900.67024.15. [DOI] [PubMed] [Google Scholar]
- Darling KE, Evans TJ. Effects of nitric oxide on Pseudomonas aeruginosa infection of epithelial cells from a human respiratory cell line derived from a patient with cystic fibrosis. Infect Immun. 2003;71:2341–2349. doi: 10.1128/IAI.71.5.2341-2349.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotsch J, Demirakca S, Terbrack HG, Huls G, Rascher W, Kuhl PG. Airway nitric oxide in asthmatic children and patients with cystic fibrosis. Eur Respir J. 1996;9:2537–2540. doi: 10.1183/09031936.96.09122537. [DOI] [PubMed] [Google Scholar]
- Dotsch J, Puls J, Klimek T, Rascher W. Reduction of neuronal and inducible nitric oxide synthase gene expression in patients with cystic fibrosis. Eur Arch Otorhinolaryngol. 2002;259:222–226. doi: 10.1007/s00405-001-0436-8. [DOI] [PubMed] [Google Scholar]
- Grasemann H, Ratjen F. Cystic fibrosis lung disease: the role of nitric oxide. Pediatr Pulmonol. 1999;28:442–448. doi: 10.1002/(sici)1099-0496(199912)28:6<442::aid-ppul10>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Keen C, Olin AC, Edentoft A, Gronowitz E, Strandvik B. Airway nitric oxide in patients with cystic fibrosis is associated with pancreatic function, Pseudomonas infection, and polyunsaturated fatty acids. Chest. 2007;131:1857–1864. doi: 10.1378/chest.06-2635. [DOI] [PubMed] [Google Scholar]
- Mhanna MJ, Ferkol T, Martin RJ, Dreshaj IA, van Heeckeren AM, Kelley TJ, Haxhiu MA. Nitric oxide deficiency contributes to impairment of airway relaxation in cystic fibrosis mice. Am J Respir Cell Mol Biol. 2001;24:621–626. doi: 10.1165/ajrcmb.24.5.4313. [DOI] [PubMed] [Google Scholar]
- Texereau J, Fajac I, Hubert D, Coste J, Dusser DJ, Bienvenu T, Dall'Ava-Santucci J, Dinh-Xuan AT. Reduced exhaled NO is related to impaired nasal potential difference in patients with cystic fibrosis. Vascul Pharmacol. 2005;43:385–389. doi: 10.1016/j.vph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Vliet A van der, Cross CE. Phagocyte oxidants and nitric oxide in cystic fibrosis: new therapeutic targets? Curr Opin Pulm Med. 2000;6:533–539. doi: 10.1097/00063198-200011000-00013. [DOI] [PubMed] [Google Scholar]
- Jones KL, Bryan TW, Jinkins PA, Simpson KL, Grisham MB, Owens MW, Milligan SA, Markewitz BA, Robbins RA. Superoxide released from neutrophils causes a reduction in nitric oxide gas. Am J Physiol. 1998;275:L1120–1126. doi: 10.1152/ajplung.1998.275.6.L1120. [DOI] [PubMed] [Google Scholar]
- Elphick HE, Demoncheaux EA, Ritson S, Higenbottam TW, Everard ML. Exhaled nitric oxide is reduced in infants with cystic fibrosis. Thorax. 2001;56:151–152. doi: 10.1136/thorax.56.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasemann H, Michler E, Wallot M, Ratjen F. Decreased concentration of exhaled nitric oxide (NO) in patients with cystic fibrosis. Pediatr Pulmonol. 1997;24:173–177. doi: 10.1002/(sici)1099-0496(199709)24:3<173::aid-ppul2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Wood SR, Firoved AM, Ornatowski W, Mai T, Deretic V, Timmins GS. Nitrosative stress inhibits production of the virulence factor alginate in mucoid Pseudomonas aeruginosa. Free Radic Res. 2007;41:208–215. doi: 10.1080/10715760601052610. [DOI] [PubMed] [Google Scholar]
- Grasemann H, Ratjen F. [Pulmonary metabolism of nitric oxide (NO) in patients with cystic fibrosis] Pneumologie. 2002;56:376–381. doi: 10.1055/s-2002-32164. [DOI] [PubMed] [Google Scholar]
- Chen L, Patel RP, Teng X, Bosworth CA, Lancaster JR, Jr, Matalon S. Mechanisms of cystic fibrosis transmembrane conductance regulator activation by S-nitrosoglutathione. J Biol Chem. 2006;281:9190–9199. doi: 10.1074/jbc.M513231200. [DOI] [PubMed] [Google Scholar]
- Grassme H, Jendrossek V, Riehle A, von Kurthy G, Berger J, Schwarz H, Weller M, Kolesnick R, Gulbins E. Host defense against Pseudomonas aeruginosa requires ceramide-rich membrane rafts. Nat Med. 2003;9:322–330. doi: 10.1038/nm823. [DOI] [PubMed] [Google Scholar]
- Teichgraber V, Ulrich M, Endlich N, Riethmuller J, Wilker B, De Oliveira-Munding CC, van Heeckeren AM, Barr ML, von Kurthy G, Schmid KW, Weller M, Tümmler B, Lang J, Grassme H, Döring G, Gulbins E. Ceramide accumulation mediates inflammation, cell death and infection susceptibility in cystic fibrosis. Nat Med. 2008;14:382–391. doi: 10.1038/nm1748. [DOI] [PubMed] [Google Scholar]
- Norez C, Antigny F, Becq F, Vandebrouck C. Maintaining low Ca2+ level in the endoplasmic reticulum restores abnormal endogenous F508del-CFTR trafficking in airway epithelial cells. Traffic. 2006;7:562–573. doi: 10.1111/j.1600-0854.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- Semple RK, Chatterjee VK, O'Rahilly S. PPAR gamma and human metabolic disease. J Clin Invest. 2006;116:581–589. doi: 10.1172/JCI28003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiuri L, Luciani A, Giardino I, Raia V, Villella VR, D'Apolito M, Pettoello-Mantovani M, Guido S, Ciacci C, Cimmino M, Cexus ON, Londei M, Quarantino S. Tissue transglutaminase activation modulates inflammation in cystic fibrosis via PPARgamma down-regulation. J Immunol. 2008;180:7697–7705. doi: 10.4049/jimmunol.180.11.7697. [DOI] [PubMed] [Google Scholar]
- Perez A, van Heeckeren AM, Nichols D, Gupta S, Eastman JF, Davis PB. Peroxisome proliferator-activated receptor-gamma in cystic fibrosis lung epithelium. Am J Physiol Lung Cell Mol Physiol. 2008;295:L303–313. doi: 10.1152/ajplung.90276.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolis E, Lampronti I, Dechecchi MC, Borgatti M, Tamanini A, Bianchi N, Bezzerri V, Mancini I, Grazia Giri M, Rizzotti P, Gambari R, Cabrini G. Pyrogallol, an active compound from the medicinal plant Emblica officinalis, regulates expression of pro-inflammatory genes in bronchial epithelial cells. Int Immunopharmacol. 2008;8:1672–1680. doi: 10.1016/j.intimp.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Sousa M, Ousingsawat J, Seitz R, Puntheeranurak S, Regalado A, Schmidt A, Grego T, Jansakul C, Amaral MD, Schreiber R, Kunzelmann K. An extract from the medicinal plant Phyllanthus acidus and its isolated compounds induce airway chloride secretion: A potential treatment for cystic fibrosis. Mol Pharmacol. 2007;71:366–376. doi: 10.1124/mol.106.025262. [DOI] [PubMed] [Google Scholar]
- Egan ME, Pearson M, Weiner SA, Rajendran V, Rubin D, Glockner-Pagel J, Canny S, Du K, Lukacs GL, Caplan MJ. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 2004;304:600–602. doi: 10.1126/science.1093941. [DOI] [PubMed] [Google Scholar]