Abstract
Family and twin studies have indicated that genetic factors play a role in the development of eating disorders, such as anorexia and bulimia nervosa, but novel views and tools may enhance the identification of neurobiological mechanisms underlying these conditions. Here we propose an integrative genetic approach to reveal novel biological substrates of eating disorder traits analogous in mouse and human. For example, comparable to behavioral hyperactivity that is observed in 40-80% of anorexia nervosa patients, inbred strains of mice with different genetic backgrounds are differentially susceptible to develop behavioral hyperactivity when food restricted. In addition, a list of characteristics that are relevant to eating disorders and approaches to their measurement in humans together with potential analogous rodent models has been generated. Interspecies genetics of neurobehavioral characteristics of eating disorders has the potential to open new roads to identify and functionally test genetic pathways that influence neurocircuits relevant for these heterogeneous psychiatric disorders.
1. Introduction
Although the past decade has witnessed a rapid infusion of family, twin, and molecular genetic studies into the literature on eating disorders (for reviews, see 1,2), it is critical to maintain a balanced appraisal of the current status of the research and to be clear about what we know, what we have yet to determine, and how best to move the field forward.
Family and twin studies, now replicated across primarily European populations converge to support the familial aggregation of eating disorders and related traits 2. Yet, heritability estimates are not uniform, indicating clearly that environment also plays a role. Moreover, considerable heterogeneity in the extent to which additive genetic and environmental factors influence liability to various component symptoms within discrete eating disorders diagnostic categories suggests that our current diagnostic system might capture syndromes that include both symptoms that are more biologically mediated as well as symptoms that are more environmentally mediated and reflect the cultural context in which the symptoms arise 3-5.
The initial family and twin studies of anorexia nervosa were instrumental in determining the estimated heritability (>50%) of this disorder 6-9 and in providing scientific support and rationale for both human linkage and association studies for anorexia nervosa. The published linkage study to date, points towards a locus on chromosome 1 10,11 with evidence of association to both serotonergic and opioidergic genes stationed under that peak 12,13. A somewhat bewildering array of both positive and negative association studies have explored various candidate genes across a range of systems 2,14-17. Without reiterating the plethora of results here, in general, the typical pattern has been one or two positive studies accompanied by several non-replications. Overall, although the score card is balanced, small sample sizes, inadequate statistical power, and inconsistent phenotyping have also contributed to the overall inconsistency in the literature 2,4.
If eating disorders follow a similar path as other complex traits (e.g., for obesity 18,19), we anticipate that consistently positive findings will emerge for some candidate genes, but that the greatest discoveries will emerge not from single-gene association studies but from genomewide approaches.
Regardless, forethought is important to plan the next stages of research. One potentially fruitful approach is the application of complementary translational studies to functionally test human candidate genes for eating disorders and to identify novel genes for neurobehavioral traits relevant to eating disorders in a controlled environmental and genetic background. In order to design these studies, in the first instance, we require appropriate animal models for complex traits associated with eating disorders. This need provides challenging opportunities for the behavioral neuroscientist. As we will not develop animal models that capture the complete biological and psychological “package” of anorexia and bulimia nervosa, we rely on a host of second generation family and twin studies which drill down to the critical components of eating disorders in attempts to reduce phenotypic heterogeneity and theoretically enhance our efforts at gene-finding 4,20-25.
While heritability contributes to the development of eating disorders, it is likely that environmental factors (e.g., socio-cultural and/or stressful life events) also play an important role. Gene—environment interaction is a potential explanatory model in which individuals are differentially vulnerable to, for example, strict dieting, because of differences in their genotypes. This differential vulnerability could then be the first step in the development of an eating disorder. Although gene-environmental studies for eating disorders are difficult to perform, animal studies may offer a unique opportunity to understand the mechanisms underlying these interactions, since eating disorder characteristics can be studied using standardized genetic background and laboratory conditions.
2. The ways forward
2.1 Genomewide association studies
We have entered the era of genomewide association studies (GWAS). New, large studies are appearing in high-profile journals, findings are impressive and unexpected. In many cases, the candidate regions implicated by GWAS were not genes that had been previously prioritized as implicated in the disease under study. By the end of 2007, tens of billions of genotypes became available for five major psychiatric disorders — autism, attention-deficit hyperactivity disorder, bipolar disorder, major depressive disorder, and schizophrenia. The potential for both within and cross-disorder analyses is staggering.
While the findings from the currently performed GWAS are striking, they still explain only a small proportion of the diseases studied. For example, recent studies of memory function 26 and obesity 27each identified a single significantly associated SNP but with relatively small effect. These results demonstrate that GWAS alone will not suffice to unravel the genetic basis of complex disorders and that alternative complementary approaches should also be pursued.
2.2 Genetic cascades versus single candidate genes
Future GWAS for anorexia nervosa will reveal novel candidate genes for anorexia nervosa, but it is also possible that the identified candidate genes will not be replicated consistently in subsequent case-control genetic association studies of anorexia nervosa patients. While these contradictory findings may reflect phenotypic heterogeneity within the human eating disorder population, an alternative explanation is also plausible. For example, it is conceivable that the candidate genes identified are expressed in defined cell groups of certain tissues and are part of an integrated network of regulatory mechanisms. Therefore, mutations in upstream and/or downstream signaling molecules of a particular candidate gene will affect phenotypes similar to those expected in individuals with mutations in the candidate gene.
The orexin/hypocretin gene, for instance, is expressed in a well-defined cell population of the hypothalamus and has been implicated in the regulation of feeding behavior and arousal state 28,29. Terminals of these hypocretin/orexin producing neurons are spread throughout the brain 30 and activate orexin/hypocretin receptors. A forward genetic study (from phenotype to genotype) in dogs showed that a hypocretin receptor mutation and canine narcolepsy are linked 31. However, a subsequent study in human narcoleptic patients did not reveal an association between the hypocretin receptor gene and narcolepsy. Interestingly, post-mortem analysis revealed a lack of hypocretin/orexin production in hypothalamic neurons of narcoleptic patients 32. These findings indicate that searching for an association between a candidate gene (e.g., hypocretin receptor) and a certain phenotype (e.g., narcolepsy) may not be successful, since the disruption of the expression of other genes (e.g., hypocretin/orexin) in the genetic pathway of the candidate gene may be responsible for the phenotype. Therefore, unraveling the genetics of human eating disorder populations has to take into account genetic pathways rather than relying solely on individual candidate genes.
Genetic pathway analysis software is becoming available to identify and to further explore the involvement of a particular pathway in the development of a complex disorder. For example, Franke and co-workers 33 developed a functional human gene network that integrates information on genes and the functional relationships between genes. This is based on various data sets, such as the Biomolecular Interaction Network Database, the Human Protein Reference Database, the Gene Ontology database, predicted protein-protein interactions, human yeast two-hybrid interactions, and microarray co-expressions. The combination of these various layers of information about a certain candidate gene will reveal novel and/or known genetic pathways that interact with a particular candidate gene. These tools, therefore, will provide new opportunities to identify gene-gene relationships underlying complex disorders.
In light of this, various linkage and genetic association studies in anorexia nervosa point to the serotonin system as being involved in the disease 12,13,34-37. While these findings may be biased by the intense search for associations between serotonin system components and anorexia nervosa, linkage studies have shown that region-wide association with the 5-HT1D gene can be replicated in independent anorexia nervosa patient populations 12,13 pointing to a possible involvement of the serotonin signaling pathway in anorexia nervosa.
2.3. Phenotype refinement
The Diagnostic and Statistic Manual 4th edition (DSM-IV, American Psychiatric Association, 1994) classifies patients as having anorexia nervosa if they refuse to maintain body weight at or above a minimally normal weight for age and height (e.g., weight loss leading to maintenance of body weight less than 85% of that expected or failure to make expected weight gain during period of growth, leading to body weight less than 85% of that expected), demonstrate an intense fear of becoming fat, have disturbed perceptions of body shape and size or deny their illness, and have amenorrhea. Two subgroups are recognized, the restricting type, in which weight loss is the result of dietary restriction, and the binge/purge type, in which episodes of binging or purging and dietary restrictions co-exist. One approach for the future is to focus our attention on the identification and refinement of intermediate phenotypes and endophenotypes for eating disorders. Endophenotypes represent intermediate phenotypes related to a particular disorder that mark the pathway between the genotype and the behavior of interest 38. Examining endophenotypes could theoretically simplify genetic analyses because the number of genes involved in a disorder may be greater than the number of genes influencing a single endophenotype. In the case of eating disorders, it is possible that some of the core symptoms (such as maintenance of low body weight, binge eating, vomiting) could be clearly genetically mediated symptoms, whereas others, such as placing undue influence of shape and weight on self-evaluation could represent more environmentally mediated symptoms. Two lines of evidence support this contention. First, for anorexia nervosa, the drive for thinness and influence of shape and weight are not observed in all cultures although all other aspects of the disorder appear identical 39-41. Second, a novel twin method applied to the individual diagnostic criteria for bulimia nervosa indicates substantial variability across symptoms in terms of the magnitude of genetic contribution. In this maximal likelihood model, the heritability estimates for purging behaviours ranged from .43-.53, for binge eating behaviours, .34-.35; however, the genetic contribution to undue influence of shape and weight on self-evaluation was only .24 5. These results highlight the inherent heterogeneity introduced by our current diagnostic schema. Although there are considerable challenges in correctly identifying endo- and subphenotypes for eating disorders, genetic research looking at these traits may lead to the identification of genetic markers that will ultimately assist in improving diagnostic nosology.
3. “of mice and men”
3.1 Interspecies genetics of eating disorder endophenotypes in mice
While validity of translational research from mouse to human is widely accepted for common physiological processes (e.g., blood pressure regulation), interspecies comparison for psychiatric disorders offers a challenging opportunity for biomedical research 42. Recently, a proof of concept of the confluence between mouse and human was presented by Chen et al 43. A common genetic variant of the brain-derived neurotrophic factor (BDNF) gene in humans is associated with alterations in brain anatomy, memory and has been associated with psychiatric disorders, such as eating disorders, depression and schizophrenia 44-50. BDNF plays a role in neuronal survival, differentiation, and synaptic plasticity. Chen and co-workers showed that when this variant (Val66Met) is genetically introduced in mice, it mimics the phenotypic hallmarks in humans with the variant allele, including anxiety-related behaviors. This key finding demonstrates the potential of comparative neuro-behavioral genetic studies between mouse and human.
With the current availability of a large variety of inbred mouse strains and their known genome sequences 51,52, mouse genetics offer a challenging way to study complex neurobehavioral traits. For example, in contrast to patient populations, mouse strains can be used to control for phenotypic and genetic heterogeneity as well as for complex gene-environment interactions. With the recent generation of genetic reference populations, such as Recombinant Inbred Strains (RIS) 53 and Chromosome Substitution Strains (CSS) 54, rapid QTL analysis can be performed for neurobiological traits in mice. Complementary approaches for mice, such as haplotype mapping 55-57, genome-wide gene expression 58-62, and quantitative complementation studies 63 are readily available and provide a strong technological platform for gene identification.
Having said this, the true challenge for this approach is the development of appropriate animal models for anorexia nervosa. Perhaps the most critical caveat of animal models to bear in mind is whether the observed phenotype is truly being influenced by the same underlying neurobiological processes in human and rodent. Whereas the end-phenotype may be analogous, many biological paths can lead to the same endpoint. Moreover, each analog must be approached with extreme skepticism and alternative explanations must be considered. For example, before concluding that a mouse model represents an “asocial” mouse, it must be ruled out that the mouse does not have impaired eyesight or smell (both contributing to reduced approach behavior). Alternative explanations must be carefully ruled out before a rodent model can be accepted as an appropriate analog to human behavior.
The activity-based anorexia (ABA) or semi-starvation induced hyperactivity model is, among others 64, a known animal model to study pathophysiological processes in anorexia nervosa that has been in existence since the 1960′s 65. ABA is induced in rodents with voluntary access to running wheels and that are exposed to daily scheduled restricted food availability. Reminiscent of anorexia nervosa, certain rat and mouse inbred strains exposed to this daily scheduled feeding paradigm exhibit a paradoxical behavioral hyperactivity with reduced food availability and a subsequent body weight loss 66,67. Excessive behavioral hyperactivity may be a core trait of anorexia nervosa 68-72. In addition, we have shown that Olanzapine, an anti-psychotic drug, can suppress behavioral hyperactivity in both anorexia nervosa patients and rodents exposed to the ABA-model 73. Furthermore, plasma leptin levels are correlated with physical activity levels in anorexia nervosa patients during the acute phase of the illness 74,75 and chronic leptin infusion suppresses behavioral activity in rats exposed to the ABA model 76,77. Moreover, reminiscent to the high incidence of anorexia nervosa in young females (in the age of 15-19 years) 78, young adolescent rodents are more susceptible to ABA than older rodents 79. Taken together, these findings provide some face- and predictive validity of the model for pathophysiological processes observed in anorexia nervosa.
From an ethological perspective, rodents display a variety of critical behavioral, physiological and molecular responses 80 in order to survive during times of reduced food availability. In ABA, mice have daily limited access to food during the first two hours of the dark phase (the habitual eating phase of rodents) for five consecutive days. During these days, mouse strains develop diverse behavioral responses, such as differences in preparing for the upcoming meal (food anticipatory behavior) 81 and/or behavioral hyperactivity 66. Differences in behavioral responses to this scheduled feeding paradigm by mouse strains with different genetic backgrounds allow a systematic search for genetic loci involved in these responses (Figure 1).
Figure 1.
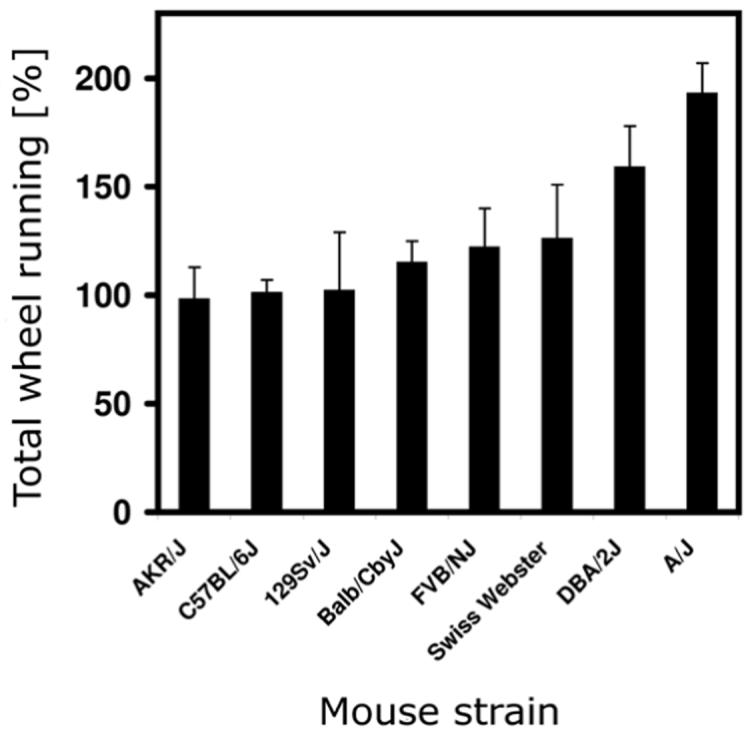
Strain differences in behavioral activity levels when exposed to scheduled daily feeding. Eight inbred mouse strains with different genetic backgrounds were exposed to five days of daily scheduled limited food access (for protocol, see 66) and revealed strain dependent differences in total wheel running levels during these days (relative to their baseline wheel running levels). For example, some strains (AKR/J, C57BL/6J, and 129Sv/J) did not increase their wheel running activity during scheduled feeding, while in contrast, the A/J strain almost doubled its behavioral activity levels.
The question remains, how relevant are these behavioral traits or endophenotypes within the model with respect to the development of an eating disorder? For this purpose, the combination of comparative genomics with susceptibility traits across species may provide an important bridge for translational research of neuropsychiatric traits 82.
Interestingly, a recent study using mu-opioid receptor knock-out mice showed that this receptor mediates food anticipatory activity 83, suggesting that opioid signaling may be relevant for a certain trait within the ABA-model. The involvement of opioid signaling is consistent with the region-wide association of the delta opioid receptor in anorexia nervosa 12,13 and, when considering the role of the opioid system in reward processes, would fit with the concept that self-starvation induces a reward from stress 72. In view of these findings, interspecies trait genetics rather than syndrome genetics 84,85 may offer opportunities to unravel further the biological substrates underlying neurobehavioral traits of anorexia nervosa. New insights in translatable susceptibility traits for anorexia nervosa are desirable.
3.2. Modeling eating disorder characteristics in rodents
In addition to studies that focus on behavioral traits within animal models for eating disorders, such as the development of behavioral hyperactivity during food restriction episodes in the ABA model, one could also consider modelling susceptibility traits of eating disorders. For example, as discussed below, anxiety disorders are highly co-morbid with eating disorders 86-88. Anxiety disorders, such as social phobia, may represent risk factors for eating disorders and could share common mechanisms that are relevant to the development of anorexia and bulimia nervosa. Animal models for these susceptibility traits may reveal new insights into the mechanisms underlying eating disorder development. Table 1 presents a list of characteristics that are relevant to eating disorders and approaches to their measurement in humans together with potential analogous rodent models and their measurement approaches.
Table 1.
Examples of how eating disorder characteristics may be modeled/tested in rodents and measured in humans.
| Domain | Characteristic | Human | 9Rodent |
|---|---|---|---|
| Anxiety and anxiety disorders |
• Generalized anxiety • Social phobis |
• Clinical interview • Laboratory measures of anxiety and arousa • State Trait Anxiety Inventory • Social Phobia and Anxiety Inventory |
• Light-dark box • Open field test • Elevated plus maze •Novelty-suppressed feeding |
| • Social threat perception |
• Internet-base programs to assess social threat perception |
• Social approach behavior | |
| Depression | • Dysphoria • Anhedonia |
• Clinical interview • Beck Depression Inventory |
• Cocaine withdrawal • Sucrose preference test • Anticipatory activity |
| Weigh | • Weight dysregulation low |
• Low BMI | • Strains that do not gain weight even with increased consumption |
| • Weight dysregulation high |
• High BMI | • Strains that gain weight in the absence of increased caloric intake |
|
| Motor activity | • Behavioral activity |
• Actiwatch • Observation Questionnaires |
• Activity-based anorexia model • Home cage activity monitoring • Open field testing |
| Cognition | • Set-shifting | • Trail Making Test (TMT) • Wisconsin Card Sort Test (WCST) • Brixton task • Haptic Illusion CatBat task • Set-shifting subset of the Cambridge Neuropsychological Test Automated Battery (CANTAB). |
• Multidimensional visual stimuli task |
| Obsessionality and Compulsivity |
• OCD traits • Symmetry and exactness • Flaw detection |
• Yale-Brown Obsessive- Compulsive Scale • EatAte Life • Internet delivered tasks |
• Quinpirole-induced compulsive checking • Barbering • Drug seeking behavior |
| Hormonal | • Amenorrhea in response to food deprivation/low BMI |
• Plasma hormone levels |
• Plasma hormone levels • Vaginal cytology |
| Eating behavior |
• Binge eating | • Self- report/laboratory observation • In response to short term food deprivation (disinhibition) |
• Restriction/refeeding and stress- induced eating • Intermittent access to palatable foods • Deprivation-induced binge eating • Novelty-suppressed feeding |
| Impulsivity | • Impulsive behavior |
• Barratt Impulsivity Scale (BIS) • Go/No-go task |
• Go/No-go task |
| Brain activity | • D2/D3 receptor activity in striatum |
• SPECT, fMRI, PET | • SPECT, fMRI, PET, • Gene expression analysis |
| Physiology | • Body temperature |
• Hypothermia | • Hypothermia |
| Perfectionism | • Concern over mistakes |
• Multidimensional Perfectionism Scale (MPS) |
? |
| Drive for thinness |
• Dieting • Fear of weight gain |
• Eating disorder inventory (EDI) |
? |
| Body image distortion |
• Body dissatisfaction |
• Various self-report or IT-delivered measures |
? |
While animal models certainly cannot mimic all eating disorder traits, such as perfectionism, body dissatisfaction, or drive for thinness, behavioral scientists have been working for decades on animal models for other behavioral characteristics relevant to anorexia and bulimia nervosa (such as, for example, depressive symptoms (see 89-93 for review), for compulsive behavior (see 94,95 for review), for impulsivity (see 96-98 for review), for set-shifting 99,100, and for body weight regulation (see 101-105 for review)). Insights into mechanisms underlying these separate components may further contribute to our understanding of the development of heterogeneity within eating disorder populations. Nevertheless, novel developments to further refine assessments of these behavioral components in rodents will be required to further optimize these animal studies.
3.2.1. Anxiety and anxiety disorders
As indicated above, there is substantial co-morbidity of eating disorders and anxiety disorders (for review see 106). Studies have consistently shown that a significant number of patients with anorexia nervosa or bulimia nervosa experience one or more anxiety disorders 107. Lifetime prevalence of at least one anxiety disorder in individuals with eating disorders varies from 25% 108 to 75% 109 in bulimia nervosa and from 23% 110 to 75% 111 in anorexia nervosa. Several studies have shown that anxiety disorders are pre-morbid to the development of an eating disorder 109,111-114, indicating that studies unveiling mechanisms underlying anxiety disorders may provide further insight into susceptibility factors for eating disorders.
In rodents, considerable information exists on the determination of anxiety levels. Standard laboratory tests, such as the open field, elevated plus maze, and light-dark box test, are generally used to measure novelty-induced anxiety levels in rodents. In general, rodent species have an innate preference for sheltered places that have lower light intensities than the outside world and that provide a sense of safety via body contact with the shelter area surface (thigmotaxis). The open field test was one of the first behavioral tests developed for emotionality and that was based on the assessment of these behavioral expressions 115. These relatively brief tests provide insights in novelty-responsiveness of the animal, but are confounded by strain differences in locomotor activity and do not provide baseline measures of anxiety levels. For these reasons, the field will benefit from novel measures that assess baseline anxiety levels and control for strain differences in locomotor activity 116,117. Furthermore, in addition to measures of anxiety levels in relation to novel environments with a non-social context, animal models for social phobia have also been introduced and may be relevant to eating disorder development. For instance, behavioral tests have been developed in which rodents can be tested for their preference for social approach or avoidance 118,119. These further refinements in rodent behavioral testing paradigms will contribute to face, predictive and construct validity of animal models for eating disorder traits.
3.2.2. Neuroimaging
In addition to the development of behavioral testing paradigms to assess eating disorder characteristics in both mouse and human, neuroimaging approaches have recently been initiated across species to picture brain activities in relation to eating disorder development. While imaging studies in anorexia nervosa are in their infancy, they can provide novel insights into the neurocircuits that are involved in eating disorders. For example, recent imaging studies in anorexia nervosa have shown that disturbances of limbic and cognitive circuits have been found in malnourished, underweight anorexia nervosa 120 including involvement of the subgenual cingulate, basal ganglia, parietal lobes as well as other regions. However, for the most part, these studies assess “baseline” levels of brain function and do not provide information on how these regions process information. Furthermore, these studies in ill subjects are confounded by the potential effects of malnutrition. To circumvent potential state dependent effects of malnutrition on brain activity, studies have also been done on individuals with anorexia nervosa after long-term recovery. Such studies also find that individuals recovered from anorexia nervosa have altered function of limbic and cognitive regions 121-124, indicating a possible involvement of these brain regions in anorexia nervosa that is independent of nutritional status.
Recent studies using functional magnetic resonance imaging (fMRI) offer the advantages of higher resolution coupled with tasks designed to activate circuits of interest. For example, a recent study 124 showed that individuals who have recovered from restricting-type anorexia nervosa had altered patterns of response in the ventral and dorsal striatum to positive and negative feedback. That is, an anterior ventral striatum response that distinguished between winning and losing was seen in the comparison women but not in the anorexia nervosa group. These findings suggest that individuals with anorexia nervosa may have difficulty discriminating between positive and negative feedback, relative to healthy comparison subjects. Similarly, in a study using a startle reflex paradigm 125, a generalized failure to activate the appetitive motivational system was observed in individuals with anorexia nervosa. Recently, Barbarich-Marsteller 126 found changes in the striatum, hippocampus, and thalamus in rodents exposed to the ABA-model. Similarly, Van Kuyck 127 found altered activity in the ventral striatum, insula, thalamus, and ventral pontine nuclei, as well as a positive correlation between body weight loss and metabolism in the anterior cingulate and related regions in ABA rodents. When considered together, these human and rodent studies suggest the possibility of involvement of common pathways, but differences in imaging techniques and the effects of nutritional status make direct comparisons problematic.
Integration of genetic, behavioral, and neuroimaging findings may, in the end, provide more complete insight into the mechanisms underlying complex disorders. For example, increased anterior ventral striatum dopamine D2/D3 receptor binding in recovered anorexia nervosa patients could be contributing to the above mentioned alteration in anterior ventral striatum function 25. Disturbed dopamine 2 receptor binding observed by these brain imaging techniques would be consistent with recently observed genetic linkage with the dopamine D2 receptor and anorexia nervosa 128,129. Furthermore, recent animal studies have shown that mice with high susceptibility to develop ABA have increased striatal dopamine D2 receptor mRNA levels when compared to mice that do not develop behavioral hyperactivity in this animal model 130. In view of the relation between dopamine D2 receptor regulation and eating disorders, it is interesting to note that mice with a genetic deletion of this receptor have deficits in reward processes 131-135 that may also translate to certain aspects observed in eating disorders 72.
3.2.3. Some additional considerations
Several eating disorder characteristics have been proposed here as potential susceptibility factors for the development of eating disorders. Some of these clinical characteristics will afford translational research (e.g., behavioral hyperactivity, increased anxiety levels), whereas others may prove to be more difficult to mimic in rodents (e.g., perfectionism, body dissatisfaction). Furthermore, theoretical considerations should also be taken into account. For instance, some of the core characteristics identified may be highly dependent on each other and/or may reflect the same pathophysiological process. For example, do neurocircuits that underlie disturbed set-shifting in anorexia nervosa (partially) overlap with those of compulsive behavior and/or of perfectionism observed in these patients, or are these truly separate components of the disease? In addition, recent studies have indicated that behavioral hyperactivity seen in anorexia nervosa are related to the levels of anxiety and of food restriction 136, indicating that some behavioral characteristics are highly dependent on others. This requires practical considerations about study design to assess these potentially related eating disorder characteristics properly. Future research is necessary to study the relation between these eating disorder characteristics, as well as the genetic pathways underlying the pathophysiological neuro-processes that drive these behavioral characteristics.
3.3. Functional testing of human candidate genes
Human genetic studies will provide novel candidate genes for eating disorders; however, these genes need to be functionally tested. Furthermore, to unravel the contribution of a candidate gene in a neurobiological mechanism underlying eating disorders, translational research will be needed. Gene knock-out technology in mice has provided a tremendous contribution to gene function research in all biomedical disciplines. This has evolved in novel and further refined applications, such as conditional knock-out technology, vector-directed gene expression and short-hairpin interference methodologies to study gene function relationships over time (e.g., genetic deletion during development or adulthood 137 and in a tissue-specific manner). For example, recent studies have shown that the contribution of melanocortin-4 receptor antagonism (with agouti) on overeating behavior endophenotypes highly depends on the brain region in which agouti was over expressed 138. Thus, sufficient gene manipulation technology is available to functionally test human candidate genes for anorexia nervosa. This will, in combination with relevant animal models, further contribute to understanding gene function(s) in neurobiological mechanisms underlying the pathophysiology of self-starvation.
Concluding remarks
Eating disorders, such as anorexia and bulimia nervosa, are psychiatric disorders that are likely determined by a complex interaction between genetic variations, developmental processes, and certain life events. An animal model has limitation to the extent that it cannot mimic the heterogeneous spectrum of an eating disorder. However, interspecies genetics may offer opportunities to identify and functionally test genetic networks that are necessary for proper functioning of neurocircuits underlying relevant eating disorder characteristics, such as set-shifting, increased anxiety levels, and behavioral hyperactivity. Increasing our knowledge about these neurobiological mechanisms will be relevant to develop new therapies applicable for subgroups (e.g., patients with extreme behavioral hyperactivity) within the heterogeneous eating disorder populations. Novel mouse genetic and phenotyping tools offer a way to study these neurobehavioral traits under controlled environmental and genetic background conditions.
Acknowledgments
Research was supported by VIDI-grant (91786327) from The Netherlands Organization for Scientific Research (NWO) to Dr. M.J.H. Kas. Dr. Foulds-Mathes was supported by National Institute of Health grant T32MH076694.
Reference List
- 1.Slof-Op ’t Landt MC, et al. Eating disorders: from twin studies to candidate genes and beyond. Twin.Res.Hum.Genet. 2005;8:467–482. doi: 10.1375/183242705774310114. [DOI] [PubMed] [Google Scholar]
- 2.Bulik CM, Slof-Op’t Landt MC, van Furth EF, Sullivan PF. The genetics of anorexia nervosa. Annu.Rev.Nutr. 2007;27:263–275. doi: 10.1146/annurev.nutr.27.061406.093713. [DOI] [PubMed] [Google Scholar]
- 3.Fairburn CG, Harrison PJ. Eating disorders. Lancet. 2003;361:407–416. doi: 10.1016/S0140-6736(03)12378-1. [DOI] [PubMed] [Google Scholar]
- 4.Bulik CM, et al. Genetic epidemiology, endophenotypes, and eating disorder classification. Int.J.Eat.Disord. 2007;40(Suppl):S52–S60. doi: 10.1002/eat.20398. [DOI] [PubMed] [Google Scholar]
- 5.Mazzeo SE, et al. Assessing the heritability of anorexia nervosa symptoms using a marginal maximal likelihood approach. Psychological Medicine. , in press, doi:10.1017/S0033291708003310.
- 6.Lilenfeld LR, et al. A controlled family study of anorexia nervosa and bulimia nervosa: psychiatric disorders in first-degree relatives and effects of proband comorbidity. Arch.Gen.Psychiatry. 1998;55:603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 7.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychol.Med. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 8.Bulik CM, et al. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch.Gen.Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 9.Wade TD, Bulik CM, Sullivan PF, Neale MC, Kendler KS. The relation between risk factors for binge eating and bulimia nervosa: a population-based female twin study. Health Psychol. 2000;19:115–123. doi: 10.1037//0278-6133.19.2.115. [DOI] [PubMed] [Google Scholar]
- 10.Grice DE, et al. Evidence for a susceptibility gene for anorexia nervosa on chromosome 1. Am.J.Hum.Genet. 2002;70:787–792. doi: 10.1086/339250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devlin B, et al. Linkage analysis of anorexia nervosa incorporating behavioral covariates. Hum.Mol.Genet. 2002;11:689–696. doi: 10.1093/hmg/11.6.689. [DOI] [PubMed] [Google Scholar]
- 12.Bergen AW, et al. Candidate genes for anorexia nervosa in the 1p33-36 linkage region: serotonin 1D and delta opioid receptor loci exhibit significant association to anorexia nervosa. Mol.Psychiatry. 2003;8:397–406. doi: 10.1038/sj.mp.4001318. [DOI] [PubMed] [Google Scholar]
- 13.Brown KM, et al. Further evidence of association of OPRD1 & HTR1D polymorphisms with susceptibility to anorexia nervosa. Biol.Psychiatry. 2007;61:367–373. doi: 10.1016/j.biopsych.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Klump KL, Gobrogge KL. A review and primer of molecular genetic studies of anorexia nervosa. Int.J.Eat.Disord. 2005;37(Suppl):S43–S48. doi: 10.1002/eat.20116. [DOI] [PubMed] [Google Scholar]
- 15.Kas MJ, van Elburg AA, van Engeland H, Adan RA. Refinement of behavioural traits in animals for the genetic dissection of eating disorders. Eur.J.Pharmacol. 2003;480:13–20. doi: 10.1016/j.ejphar.2003.08.088. [DOI] [PubMed] [Google Scholar]
- 16.Hinney A, Remschmidt H, Hebebrand J. Candidate gene polymorphisms in eating disorders. Eur.J.Pharmacol. 2000;410:147–159. doi: 10.1016/s0014-2999(00)00812-8. [DOI] [PubMed] [Google Scholar]
- 17.Mazzeo SE, et al. Associations among postpartum depression, eating disorders, and perfectionism in a population-based sample of adult women. Int.J.Eat.Disord. 2006;39:202–211. doi: 10.1002/eat.20243. [DOI] [PubMed] [Google Scholar]
- 18.Dina C, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat.Genet. 2007;39:724–726. doi: 10.1038/ng2048. [DOI] [PubMed] [Google Scholar]
- 19.Frayling TM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holliday J, Tchanturia K, Landau S, Collier D, Treasure J. Is impaired set-shifting an endophenotype of anorexia nervosa? Am.J.Psychiatry. 2005;162:2269–2275. doi: 10.1176/appi.ajp.162.12.2269. [DOI] [PubMed] [Google Scholar]
- 21.Treasure J. Where do eating disorders lie on the diagnostic spectrum and what does it mean? Nord.J.Psychiatry. 2006;60:27–31. doi: 10.1080/08039480500517984. [DOI] [PubMed] [Google Scholar]
- 22.Shroff H, et al. Features associated with excessive exercise in women with eating disorders. Int.J.Eat.Disord. 2006;39:454–461. doi: 10.1002/eat.20247. [DOI] [PubMed] [Google Scholar]
- 23.Zucker NL, et al. Anorexia nervosa and autism spectrum disorders: guided investigation of social cognitive endophenotypes. Psychol.Bull. 2007;133:976–1006. doi: 10.1037/0033-2909.133.6.976. [DOI] [PubMed] [Google Scholar]
- 24.Halmi KA, et al. Perfectionism in anorexia nervosa: variation by clinical subtype, obsessionality, and pathological eating behavior. Am.J.Psychiatry. 2000;157:1799–1805. doi: 10.1176/appi.ajp.157.11.1799. [DOI] [PubMed] [Google Scholar]
- 25.Frank GK, et al. Increased dopamine D2/D3 receptor binding after recovery from anorexia nervosa measured by positron emission tomography and [11c]raclopride. Biol.Psychiatry. 2005;58:908–912. doi: 10.1016/j.biopsych.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Papassotiropoulos A, et al. Common Kibra alleles are associated with human memory performance. Science. 2006;314:475–478. doi: 10.1126/science.1129837. [DOI] [PubMed] [Google Scholar]
- 27.Herbert A, et al. A common genetic variant is associated with adult and childhood obesity. Science. 2006;312:279–283. doi: 10.1126/science.1124779. [DOI] [PubMed] [Google Scholar]
- 28.de Lecea L, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc.Natl.Acad.Sci.U.S.A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakurai T, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 30.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J.Neurosci. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin L, et al. The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell. 1999;98:365–376. doi: 10.1016/s0092-8674(00)81965-0. [DOI] [PubMed] [Google Scholar]
- 32.Peyron C, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat.Med. 2000;6:991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 33.Franke L, et al. Reconstruction of a functional human gene network, with an application for prioritizing positional candidate genes. Am.J.Hum.Genet. 2006;78:1011–1025. doi: 10.1086/504300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rybakowski F, et al. The 5-HT2A -1438 A/G and 5-HTTLPR polymorphisms and personality dimensions in adolescent anorexia nervosa: association study. Neuropsychobiology. 2006;53:33–39. doi: 10.1159/000090701. [DOI] [PubMed] [Google Scholar]
- 35.Matsushita S, et al. Serotonin transporter regulatory region polymorphism is associated with anorexia nervosa. Am.J.Med.Genet.B Neuropsychiatr.Genet. 2004;128B:114–117. doi: 10.1002/ajmg.b.30022. [DOI] [PubMed] [Google Scholar]
- 36.Hu X, et al. Association of the 5-HT2c gene with susceptibility and minimum body mass index in anorexia nervosa. Neuroreport. 2003;14:781–783. doi: 10.1097/00001756-200305060-00001. [DOI] [PubMed] [Google Scholar]
- 37.Di Bella DD, Catalano M, Cavallini MC, Riboldi C, Bellodi L. Serotonin transporter linked polymorphic region in anorexia nervosa and bulimia nervosa. Mol.Psychiatry. 2000;5:233–234. doi: 10.1038/sj.mp.4000689. [DOI] [PubMed] [Google Scholar]
- 38.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am.J.Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 39.Lee S, Lee AM, Ngai E, Lee DT, Wing YK. Rationales for Food Refusal in Chinese Patients with Anorexia Nervosa. Int.J.Eat.Disord. 2001;29:224–229. doi: 10.1002/1098-108x(200103)29:2<224::aid-eat1012>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Lee S, Ho TP, Hsu LK. Fat phobic and non-fat phobic anorexia nervosa: a comparative study of 70 Chinese patients in Hong Kong. Psychol.Med. 1993;23:999–1017. doi: 10.1017/s0033291700026465. [DOI] [PubMed] [Google Scholar]
- 41.Lee S. How abnormal is the desire for slimness? A survey of eating attitudes and behaviour among Chinese undergraduates in Hong Kong. Psychol.Med. 1993;23:437–451. doi: 10.1017/s0033291700028531. [DOI] [PubMed] [Google Scholar]
- 42.Dennis C. Psychiatric disease: all in the mind of a mouse. Nature. 2005;438:151–152. doi: 10.1038/438151a. [DOI] [PubMed] [Google Scholar]
- 43.Chen ZY, et al. Genetic variant BDNF (Val66Met) polymorphism alters anxiety-related behavior. Science. 2006;314:140–143. doi: 10.1126/science.1129663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lang UE, et al. Association of a functional BDNF polymorphism and anxiety-related personality traits. Psychopharmacology (Berl) 2005;180:95–99. doi: 10.1007/s00213-004-2137-7. [DOI] [PubMed] [Google Scholar]
- 45.Lohoff FW, et al. Confirmation of association between the Val66Met polymorphism in the brain-derived neurotrophic factor (BDNF) gene and bipolar I disorder. Am.J.Med.Genet.B Neuropsychiatr.Genet. 2005;139:51–53. doi: 10.1002/ajmg.b.30215. [DOI] [PubMed] [Google Scholar]
- 46.Neves-Pereira M, et al. BDNF gene is a risk factor for schizophrenia in a Scottish population. Mol.Psychiatry. 2005;10:208–212. doi: 10.1038/sj.mp.4001575. [DOI] [PubMed] [Google Scholar]
- 47.Ribases M, et al. Association of BDNF with restricting anorexia nervosa and minimum body mass index: a family-based association study of eight European populations. Eur.J.Hum.Genet. 2005;13:428–434. doi: 10.1038/sj.ejhg.5201351. [DOI] [PubMed] [Google Scholar]
- 48.Ribases M, et al. Association of BDNF with anorexia, bulimia and age of onset of weight loss in six European populations. Hum.Mol.Genet. 2004;13:1205–1212. doi: 10.1093/hmg/ddh137. [DOI] [PubMed] [Google Scholar]
- 49.Schumacher J, et al. Evidence for a relationship between genetic variants at the brain-derived neurotrophic factor (BDNF) locus and major depression. Biol.Psychiatry. 2005;58:307–314. doi: 10.1016/j.biopsych.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 50.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 51.Frazer KA, et al. A sequence-based variation map of 8.27 million SNPs in inbred mouse strains. Nature. 2007;448:1050–1053. doi: 10.1038/nature06067. [DOI] [PubMed] [Google Scholar]
- 52.Yang H, Bell TA, Churchill GA, Pardo-Manuel d., V. On the subspecific origin of the laboratory mouse. Nat.Genet. 2007;39:1100–1107. doi: 10.1038/ng2087. [DOI] [PubMed] [Google Scholar]
- 53.Plomin R, McClearn GE, Gora-Maslak G, Neiderhiser JM. Use of recombinant inbred strains to detect quantitative trait loci associated with behavior. Behav.Genet. 1991;21:99–116. doi: 10.1007/BF01066330. [DOI] [PubMed] [Google Scholar]
- 54.Singer JB, et al. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 55.Wade CM, Daly MJ. Genetic variation in laboratory mice. Nat.Genet. 2005;37:1175–1180. doi: 10.1038/ng1666. [DOI] [PubMed] [Google Scholar]
- 56.Wade CM, et al. The mosaic structure of variation in the laboratory mouse genome. Nature. 2002;420:574–578. doi: 10.1038/nature01252. [DOI] [PubMed] [Google Scholar]
- 57.Grupe A, et al. In silico mapping of complex disease-related traits in mice. Science. 2001;292:1915–1918. doi: 10.1126/science.1058889. [DOI] [PubMed] [Google Scholar]
- 58.Jansen RC, Nap JP. Genetical genomics: the added value from segregation. Trends Genet. 2001;17:388–391. doi: 10.1016/s0168-9525(01)02310-1. [DOI] [PubMed] [Google Scholar]
- 59.Fernandes C, et al. Hippocampal gene expression profiling across eight mouse inbred strains: towards understanding the molecular basis for behaviour. Eur.J.Neurosci. 2004;19:2576–2582. doi: 10.1111/j.0953-816X.2004.03358.x. [DOI] [PubMed] [Google Scholar]
- 60.Hovatta I, et al. DNA variation and brain region-specific expression profiles exhibit different relationships between inbred mouse strains: implications for eQTL mapping studies. Genome Biol. 2007;8:R25. doi: 10.1186/gb-2007-8-2-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sandberg R, et al. Regional and strain-specific gene expression mapping in the adult mouse brain. Proc.Natl.Acad.Sci.U.S.A. 2000;97:11038–11043. doi: 10.1073/pnas.97.20.11038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Letwin NE, et al. Combined application of behavior genetics and microarray analysis to identify regional expression themes and gene-behavior associations. J.Neurosci. 2006;26:5277–5287. doi: 10.1523/JNEUROSCI.4602-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yalcin B, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat.Genet. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]
- 64.Siegfried Z, Berry EM, Hao S, Avraham Y. Animal models in the investigation of anorexia. Physiol Behav. 2003;79:39–45. doi: 10.1016/s0031-9384(03)00103-3. [DOI] [PubMed] [Google Scholar]
- 65.Routtenberg A, Kuznesof AW. Self-starvation of rats living in activity wheels on a restricted feeding schedule. J Comp Physiol Psychol. 1967;64:414–421. doi: 10.1037/h0025205. [DOI] [PubMed] [Google Scholar]
- 66.Gelegen C, et al. Difference in susceptibility to activity-based anorexia in two inbred strains of mice. Eur.Neuropsychopharmacol. 2007;17:199–205. doi: 10.1016/j.euroneuro.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 67.Kas MJ, van Dijk G, Scheurink AJ, Adan RA. Agouti-related protein prevents self-starvation. Mol.Psychiatry. 2003;8:235–240. doi: 10.1038/sj.mp.4001206. [DOI] [PubMed] [Google Scholar]
- 68.Brewerton TD, Stellefson EJ, Hibbs N, Hodges EL, Cochrane CE. Comparison of eating disorder patients with and without compulsive exercising. Int.J.Eat.Disord. 1995;17:413–416. doi: 10.1002/1098-108x(199505)17:4<413::aid-eat2260170414>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 69.Davis C, Katzman DK, Kirsh C. Compulsive physical activity in adolescents with anorexia nervosa: a psychobehavioral spiral of pathology. J.Nerv.Ment.Dis. 1999;187:336–342. doi: 10.1097/00005053-199906000-00002. [DOI] [PubMed] [Google Scholar]
- 70.Davis C, et al. The prevalence of high-level exercise in the eating disorders: etiological implications. Compr.Psychiatry. 1997;38:321–326. doi: 10.1016/s0010-440x(97)90927-5. [DOI] [PubMed] [Google Scholar]
- 71.Hebebrand J, et al. Hyperactivity in patients with anorexia nervosa and in semistarved rats: evidence for a pivotal role of hypoleptinemia. Physiol Behav. 2003;79:25–37. doi: 10.1016/s0031-9384(03)00102-1. [DOI] [PubMed] [Google Scholar]
- 72.Bergh C, Sodersten P. Anorexia nervosa, self-starvation and the reward of stress. Nat.Med. 1996;2:21–22. doi: 10.1038/nm0196-21. [DOI] [PubMed] [Google Scholar]
- 73.Hillebrand JJ, van Elburg AA, Kas MJ, van Engeland H, Adan RA. Olanzapine reduces physical activity in rats exposed to activity-based anorexia: possible implications for treatment of anorexia nervosa? Biol.Psychiatry. 2005;58:651–657. doi: 10.1016/j.biopsych.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 74.van Elburg AA, Kas MJ, Hillebrand JJ, Eijkemans RJ, van Engeland H. The impact of hyperactivity and leptin on recovery from anorexia nervosa. J.Neural Transm. 2007;114:1233–1237. doi: 10.1007/s00702-007-0740-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Holtkamp K, et al. Elevated physical activity and low leptin levels co-occur in patients with anorexia nervosa. J.Clin.Endocrinol.Metab. 2003;88:5169–5174. doi: 10.1210/jc.2003-030569. [DOI] [PubMed] [Google Scholar]
- 76.Exner C, et al. Leptin suppresses semi-starvation induced hyperactivity in rats: implications for anorexia nervosa. Mol.Psychiatry. 2000;5:476–481. doi: 10.1038/sj.mp.4000771. [DOI] [PubMed] [Google Scholar]
- 77.Hillebrand JJ, Koeners MP, de Rijke CE, Kas MJ, Adan RA. Leptin treatment in activity-based anorexia. Biol.Psychiatry. 2005;58:165–171. doi: 10.1016/j.biopsych.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 78.Hoek HW. Incidence, prevalence and mortality of anorexia nervosa and other eating disorders. Curr.Opin.Psychiatry. 2006;19:389–394. doi: 10.1097/01.yco.0000228759.95237.78. [DOI] [PubMed] [Google Scholar]
- 79.Barbarich-Marsteller N, Pike K, Underwood M, Foltin R, Walsh B. Abstract Annual Meeting of the Eating Disorders Research Society. P8 2007. Vulnerability ot activity-based anorexia in adolescent female rats. [Google Scholar]
- 80.Gelegen C, Collier DA, Campbell IC, Oppelaar H, Kas MJ. Behavioral, physiological, and molecular differences in response to dietary restriction in three inbred mouse strains. Am.J.Physiol Endocrinol.Metab. 2006;291:E574–E581. doi: 10.1152/ajpendo.00068.2006. [DOI] [PubMed] [Google Scholar]
- 81.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci.Biobehav.Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- 82.Kas MJ, Gelegen C, Schalkwyk LC, Collier DA. Interspecies comparisons of functional genetic variations and their implications in neuropsychiatry. Am.J.Med.Genet.B Neuropsychiatr.Genet. in press.
- 83.Kas MJ, et al. Mu-opioid receptor knockout mice show diminished food-anticipatory activity. Eur.J.Neurosci. 2004;20:1624–1632. doi: 10.1111/j.1460-9568.2004.03581.x. [DOI] [PubMed] [Google Scholar]
- 84.Gould TD, Gottesman II. Psychiatric endophenotypes and the development of valid animal models. Genes Brain Behav. 2006;5:113–119. doi: 10.1111/j.1601-183X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 85.Kas MJ, Fernandes C, Schalkwyk LC, Collier DA. Genetics of behavioural domains across the neuropsychiatric spectrum; of mice and men. Mol.Psychiatry. 2007;12:324–330. doi: 10.1038/sj.mp.4001979. [DOI] [PubMed] [Google Scholar]
- 86.Godart NT, Flament MF, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: a review. Int.J.Eat.Disord. 2002;32:253–270. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 87.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: co-morbidity and chronology of appearance. Eur.Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 88.Fornari V, et al. Perception of family functioning and depressive symptomatology in individuals with anorexia nervosa or bulimia nervosa. Compr.Psychiatry. 1999;40:434–441. doi: 10.1016/s0010-440x(99)90087-1. [DOI] [PubMed] [Google Scholar]
- 89.Cryan JF, Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat.Rev.Drug Discov. 2005;4:775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- 90.Cryan JF, Mombereau C. In search of a depressed mouse: utility of models for studying depression-related behavior in genetically modified mice. Mol.Psychiatry. 2004;9:326–357. doi: 10.1038/sj.mp.4001457. [DOI] [PubMed] [Google Scholar]
- 91.Dranovsky A, Hen R. Hippocampal neurogenesis: regulation by stress and antidepressants. Biol.Psychiatry. 2006;59:1136–1143. doi: 10.1016/j.biopsych.2006.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nestler EJ, et al. Preclinical models: status of basic research in depression. Biol.Psychiatry. 2002;52:503–528. doi: 10.1016/s0006-3223(02)01405-1. [DOI] [PubMed] [Google Scholar]
- 93.Redei EE, et al. Novel animal models of affective disorders. Semin.Clin.Neuropsychiatry. 2001;6:43–67. doi: 10.1053/scnp.2001.20882. [DOI] [PubMed] [Google Scholar]
- 94.Joel D. Current animal models of obsessive compulsive disorder: a critical review. Prog.Neuropsychopharmacol.Biol.Psychiatry. 2006;30:374–388. doi: 10.1016/j.pnpbp.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 95.Korff S, Harvey BH. Animal models of obsessive-compulsive disorder: rationale to understanding psychobiology and pharmacology. Psychiatr.Clin.North Am. 2006;29:371–390. doi: 10.1016/j.psc.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 96.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology (Berl) 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 97.Evenden J. Impulsivity: a discussion of clinical and experimental findings. J.Psychopharmacol. 1999;13:180–192. doi: 10.1177/026988119901300211. [DOI] [PubMed] [Google Scholar]
- 98.Lesch KP, Merschdorf U. Impulsivity, aggression, and serotonin: a molecular psychobiological perspective. Behav.Sci.Law. 2000;18:581–604. doi: 10.1002/1099-0798(200010)18:5<581::aid-bsl411>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 99.Brooks SP, Betteridge H, Trueman RC, Jones L, Dunnett SB. Selective extra-dimensional set shifting deficit in a knock-in mouse model of Huntington’s disease. Brain Res.Bull. 2006;69:452–457. doi: 10.1016/j.brainresbull.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 100.Brigman JL, Bussey TJ, Saksida LM, Rothblat LA. Discrimination of multidimensional visual stimuli by mice: intra- and extradimensional shifts. Behav.Neurosci. 2005;119:839–842. doi: 10.1037/0735-7044.119.3.839. [DOI] [PubMed] [Google Scholar]
- 101.Barsh GS, Farooqi IS, O’rahilly S. Genetics of body-weight regulation. Nature. 2000;404:644–651. doi: 10.1038/35007519. [DOI] [PubMed] [Google Scholar]
- 102.Hillebrand JJ, Kas MJ, Adan RA. To eat or not to eat; regulation by the melanocortin system. Physiol Behav. 2006;89:97–102. doi: 10.1016/j.physbeh.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 103.Mercer JG, Tups A. Neuropeptides and anticipatory changes in behaviour and physiology: seasonal body weight regulation in the Siberian hamster. Eur.J.Pharmacol. 2003;480:43–50. doi: 10.1016/j.ejphar.2003.08.091. [DOI] [PubMed] [Google Scholar]
- 104.Rohner-Jeanrenaud E, Jeanrenaud B. Central nervous system and body weight regulation. Ann.Endocrinol.(Paris) 1997;58:137–142. [PubMed] [Google Scholar]
- 105.Buettner R, Scholmerich J, Bollheimer LC. High-fat diets: modeling the metabolic disorders of human obesity in rodents. Obesity.(Silver.Spring) 2007;15:798–808. doi: 10.1038/oby.2007.608. [DOI] [PubMed] [Google Scholar]
- 106.Swinbourne JM, Touyz SW. The co-morbidity of eating disorders and anxiety disorders: a review. Eur.Eat.Disord.Rev. 2007;15:253–274. doi: 10.1002/erv.784. [DOI] [PubMed] [Google Scholar]
- 107.Kaye WH, Bulik CM, Thornton L, Barbarich N, Masters K. Comorbidity of anxiety disorders with anorexia and bulimia nervosa. Am.J.Psychiatry. 2004;161:2215–2221. doi: 10.1176/appi.ajp.161.12.2215. [DOI] [PubMed] [Google Scholar]
- 108.Keck P, et al. A controlled study ofphenomenology and family history in outpatients withbulimia nervosa. Comprehensive Psychiatry. 1990;31:275–283. doi: 10.1016/0010-440x(90)90034-p. [DOI] [PubMed] [Google Scholar]
- 109.Schwalberg MD, Barlow DH, Alger SA, Howard LJ. Comparison of bulimics, obese binge eaters, social phobics, and individuals with panic disorder on comorbidity across DSM-III-R anxiety disorders. J.Abnorm.Psychol. 1992;101:675–681. doi: 10.1037//0021-843x.101.4.675. [DOI] [PubMed] [Google Scholar]
- 110.Laessle RG, et al. A comparison of nutritional management with stress management in the treatment of bulimia nervosa. Br.J.Psychiatry. 1991;159:250–261. doi: 10.1192/bjp.159.2.250. [DOI] [PubMed] [Google Scholar]
- 111.Deep AL, Nagy LM, Weltzin TE, Rao R, Kaye WH. Premorbid onset of psychopathology in long-term recovered anorexia nervosa. Int.J.Eat.Disord. 1995;17:291–297. [PubMed] [Google Scholar]
- 112.Brewerton TD, et al. Comorbidity of axis I psychiatric disorders in bulimia nervosa. J.Clin.Psychiatry. 1995;56:77–80. [PubMed] [Google Scholar]
- 113.Bulik CM. Handbook of eating disorders. Wiley; Chichester, UK: 2003. Anxiety, depression and eating disorders; pp. 193–198. [Google Scholar]
- 114.Godart NT, et al. Anxiety disorders in subjects seeking treatment for eating disorders: a DSM-IV controlled study. Psychiatry Res. 2003;117:245–258. doi: 10.1016/s0165-1781(03)00038-6. [DOI] [PubMed] [Google Scholar]
- 115.Hall CS. Emotional behavior in the rat. III the relationship between emotionality and ambulatory activity. J.Comp.Psychol. 1936;22:345–352. [Google Scholar]
- 116.Kas MJ, Van Ree JM. Dissecting complex behaviours in the post-genomic era. Trends Neurosci. 2004;27:366–369. doi: 10.1016/j.tins.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 117.Kas MJ, de Mooij-van Malsen JG, Olivier B, Spruijt BM, Van Ree JM. Differential genetic regulation of motor activity and anxiety-related behaviors in mice using an automated home cage task. Behav.Neurosci. in press, doi:10.1037/0735-7044.122.3.000.
- 118.Moy SS, et al. Mouse behavioral tasks relevant to autism: phenotypes of 10 inbred strains. Behav.Brain Res. 2007;176:4–20. doi: 10.1016/j.bbr.2006.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nadler JJ, et al. Automated apparatus for quantitation of social approach behaviors in mice. Genes Brain Behav. 2004;3:303–314. doi: 10.1111/j.1601-183X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 120.Frank GK, Bailer UF, Henry S, Wagner A, Kaye WH. Neuroimaging studies in eating disorders. CNS.Spectr. 2004;9:539–548. doi: 10.1017/s1092852900009639. [DOI] [PubMed] [Google Scholar]
- 121.Gordon I, Lask B, Bryant-Waugh R, Christie D, Timimi S. Childhood-onset anorexia nervosa: towards identifying a biological substrate. Int.J.Eat.Disord. 1997;22:159–165. doi: 10.1002/(sici)1098-108x(199709)22:2<159::aid-eat7>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 122.Rastam M, et al. Regional cerebral blood flow in weight-restored anorexia nervosa: a preliminary study. Dev.Med.Child Neurol. 2001;43:239–242. doi: 10.1017/s0012162201000457. [DOI] [PubMed] [Google Scholar]
- 123.Uher R, et al. Recovery and chronicity in anorexia nervosa: brain activity associated with differential outcomes. Biol.Psychiatry. 2003;54:934–942. doi: 10.1016/s0006-3223(03)00172-0. [DOI] [PubMed] [Google Scholar]
- 124.Wagner A, et al. Altered reward processing in women recovered from anorexia nervosa. Am.J.Psychiatry. 2007;164:1842–1849. doi: 10.1176/appi.ajp.2007.07040575. [DOI] [PubMed] [Google Scholar]
- 125.Friederich HC, et al. Differential motivational responses to food and pleasurable cues in anorexia and bulimia nervosa: a startle reflex paradigm. Psychol.Med. 2006;36:1327–1335. doi: 10.1017/S0033291706008129. [DOI] [PubMed] [Google Scholar]
- 126.Barbarich-Marsteller NC, Marsteller DA, Alexoff DL, Fowler JS, Dewey SL. MicroPET imaging in an animal model of anorexia nervosa. Synapse. 2005;57:85–90. doi: 10.1002/syn.20160. [DOI] [PubMed] [Google Scholar]
- 127.van Kuyck K, et al. Motor- and food-related metabolic cerebral changes in the activity-based rat model for anorexia nervosa: a voxel-based microPET study. Neuroimage. 2007;35:214–221. doi: 10.1016/j.neuroimage.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 128.Nisoli E, et al. D2 dopamine receptor DRD2) gene Taq1A polymorphism and the eating-related psychological traits in eating disorders (anorexia nervosa and bulimia) and obesity. Eat.Weight.Disord. 2007;12:91–96. doi: 10.1007/BF03327583. [DOI] [PubMed] [Google Scholar]
- 129.Bergen AW, et al. Association of multiple DRD2 polymorphisms with anorexia nervosa. Neuropsychopharmacology. 2005;30:1703–1710. doi: 10.1038/sj.npp.1300719. [DOI] [PubMed] [Google Scholar]
- 130.Gelegen C, et al. Dopaminergic and BDNF signalling in inbred mice exposed to a restricted feeding schedule. Genes Brain and Behaviour. , in press, doi:10.1111/j.1601-183X.2008.00394.x.
- 131.Cunningham CL, et al. Ethanol-conditioned place preference is reduced in dopamine D2 receptor-deficient mice. Pharmacol.Biochem.Behav. 2000;67:693–699. doi: 10.1016/s0091-3057(00)00414-7. [DOI] [PubMed] [Google Scholar]
- 132.Drew MR, et al. Transient overexpression of striatal D2 receptors impairs operant motivation and interval timing. J.Neurosci. 2007;27:7731–7739. doi: 10.1523/JNEUROSCI.1736-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Elmer GI, et al. Failure of intravenous morphine to serve as an effective instrumental reinforcer in dopamine D2 receptor knock-out mice. J.Neurosci. 2002;22:RC224. doi: 10.1523/JNEUROSCI.22-10-j0004.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tran AH, et al. Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc.Natl.Acad.Sci.U.S.A. 2002;99:8986–8991. doi: 10.1073/pnas.132284599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Maldonado R, et al. Absence of opiate rewarding effects in mice lacking dopamine D2 receptors. Nature. 1997;388:586–589. doi: 10.1038/41567. [DOI] [PubMed] [Google Scholar]
- 136.Holtkamp K, Hebebrand J, Herpertz-Dahlmann B. The contribution of anxiety and food restriction on physical activity levels in acute anorexia nervosa. Int.J.Eat.Disord. 2004;36:163–171. doi: 10.1002/eat.20035. [DOI] [PubMed] [Google Scholar]
- 137.Gross C, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 138.Kas MJ, et al. Induction of brain-region-specific forms of obesity by agouti. J.Neurosci. 2004;24:10176–10181. doi: 10.1523/JNEUROSCI.3442-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


