Abstract
Background
The basolateral complex of the amygdala (BLA) is uniquely affected by steroid hormones. While glucocorticoids, the adrenal hormones released during stress, increase the excitability of BLA neurons, estrogen decreases it.
Methods
In this study, we used a vector designed to express a chimeric gene that contains the GC-binding domain of the GC receptor (GR) and the DNA binding domain of the E receptor (ER) (“ER/GR”) in infected neurons; as a result, it transduces GC signals into estrogenic ones. We microinfused ER/GR bilaterally into the BLA of rats to determine whether it would impair fear conditioning, a valuable BLA-dependent paradigm for studying the neural basis of emotional memory.
Results
Expression of ER/GR in the BLA caused robust expression of the transgene and a significant disruption of both auditory and contextual long-term fear memory consolidation, while fear learning and post-shock freezing remained intact.
Conclusions
These data show that dual gene therapy with ER/GR may be a useful tool for understanding the role of steroid hormones in the storage of traumatic memories.
Keywords: Fear conditioning, glucocorticoids, estrogen, amygdala, gene therapy, memory
Introduction
Emotionally aversive events activate the hypothalamic-pituitary-adrenal axis, resulting in release of adrenal stress hormones glucocorticoids (GCs). GCs bind to high-affinity mineralocorticoid receptors (MR) and low-affinity glucocorticoid receptors (GR) in target tissues. These and other steroid receptors bind to responsive elements in DNA and alter gene transcription. GRs contribute to the deleterious effects of major stressors, including the disruption of synaptic plasticity, neuron survival, and hippocampal-dependent memory (1).
The basolateral complex of the amygdala (BLA, including the lateral, basal and accessory basal nuclei), crucial to emotional learning and memory (2), is affected by GCs. The BLA contains numerous GRs (3, 4), whose activation is pivotal to aversive memory formation (5, 6). Moreover, high GC concentrations and stress increase the activity of BLA neurons by enhancing excitability (7), increasing dendritic arborization, and heightening anxiety (8, 9).
In contrast to GCs, another steroid hormone, estrogen, acting via the estrogen receptor (ER), has opposite effects, reducing excitatory postsynaptic potential amplitude and excitatory neurotransmission in the BLA (10). While the precise influence of ERs remains unclear, they can influence affective processing and aversive memory formation (11).
These data suggest that blunting GR actions and mimicking ER actions in tandem may synergistically reduce negative memories. Thus, we employed a herpes simplex virus-1 (HSV) amplicon vector which expresses a chimeric gene containing the GC-binding domain of the GR and the DNA binding domain of the ER (“ER/GR”). We show that bilateral infusions of ER/GR into the BLA impair auditory and contextual fear memories.
Methods and Materials
Subjects
Male Long Evans rats (Charles Rivers, Wilmington, MA; 240-320g), were housed with a 12-hr light/dark cycle, food and water ad libitum. Procedures were approved by Stanford University's Administrative Panel on Laboratory Animal Care.
Vector construction and intraamygdala infusion
The construction of an HSV vector expressing ER/GR plus a green fluorescent protein (GFP) reporter has been described (12). Rats were anesthetized with a ketamine/xylazine/acepromazine mixture for stereotaxic, bilateral intraaamygdala delivery of virus with a10μl Hamilton syringe connected to a 28g injection needle and pump (2μl/hemisphere administered over 10min with a titer of ∼1×107) dorsal to the BLA (coordinates: anteroposterior, 2.4mm from bregma; mediolateral, 5.2mm from midline; dorsoventral, 6.5mm from dura). Behavioral experiments started 1d later.
Histology
Rats were overdosed with isoflurane (Abbott Laboratories, North Chicago, IL) and perfused transcardially with 4% paraformaldehyde (PFA). Brains were cryoprotected in 30% sucrose in 4% PFA, blocked, cryostat sectioned at 30μm, and Nissl- or 4′,6-diamidino-2-phenylindole- stained. Data were excluded from animals with incorrect infusion sites. For demonstration of reporter gene signal, brains were sectioned at 30μm on a sliding freezing microtome and viewed at a 490λ excitation to locate GFP-positive neurons. The target region was examined at high and low magnifications to determine the density and span of cells infected with vector.
Fear conditioning procedure
One day after surgery, rats were habituated to the training and tone testing chambers (MedAssociates, Georgia, VT) for 10-15min. The Plexiglas training chamber had a metal grid floor, was dimly lit, scented with 70% ethanol, and ventilated with a small fan. The tone testing chamber contained a soft plastic floor, was brightly lit, and scented with 1% acetic acid. The next day, rats acclimated for 2-3min to the conditioning chamber immediately before behavioral training. For fear conditioning, rats were presented with 3 pairings of a 20-sec tone conditioned stimulus (CS) (5kHz, 75dB) that coterminated with a foot shock unconditioned stimulus (US) (0.5sec, 0.6mA, 60-100sec intertrial interval (ITI)). Rats remained in their conditioning boxes for 3min after the last conditioning trial.
Testing of conditioned fear responses
1d after conditioning, long-term fear memories to the conditioning apparatus (context) and to the tone were tested separately. Testing for contextual conditioning occurred in the same environment as fear conditioning. Rats were placed in the chamber and freezing responses recorded for 5min after animals were given 5min to recognize the context. 1hr later, responses conditioned to the tone CS were measured in the novel context (described above). Rats acclimated for 5min, after which freezing responses were recorded while subjects were presented with 10 test tones (20sec, 5kHz, 75dB; ITI, 90-120sec).
Data analysis
Learning data are reported as average percentage freezing during each tone of the training paradigm. Post-shock freezing levels were measured during the 20-sec period preceding each tone and the final measure occurred after a typical ITI. Long-term tone memory was measured during each test tone, and the 20-sec period preceding all tone presentations was analyzed for baseline movement. Long-term contextual memory was measured after animals were given time to recognize context. ER/GR (n=11) and control (n=9) data were compared with two-tailed, paired Student t-tests.
Results
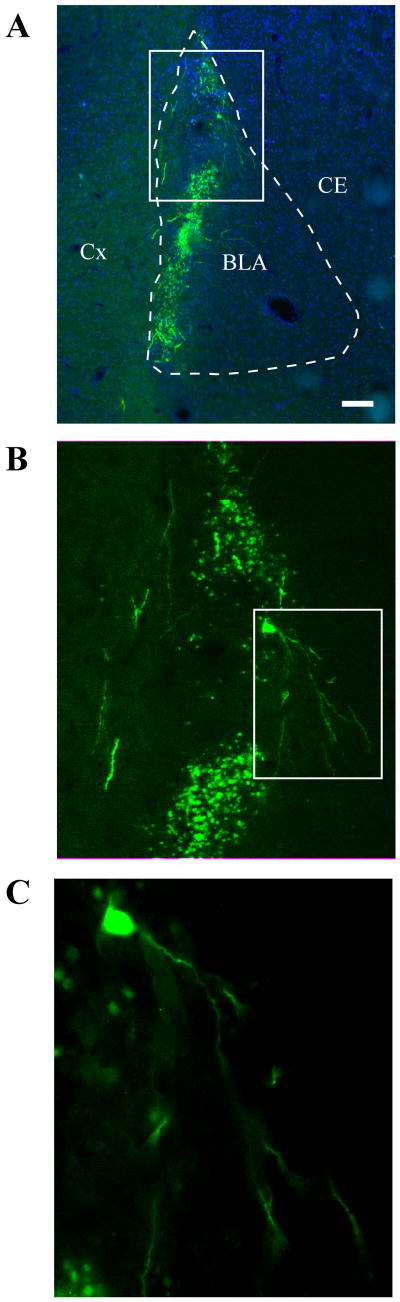
Intraamygdala infusion of ER/GR causes robust expression of the transgene in the BLA (Figure 1)
Figure 1.
Intraamygdala infusion of ER/GR produces robust expression of the transgene in the BLA. (A) Low-power micrograph (2×) shows expression of the GFP-tagged ER/GR vector (green) and 4′,6-diamidino-2-phenylindole DNA binding counterstain (blue) in the BLA. Nearby structures, including the cortex (Cx) and central nucleus of the amygdala (CE), are labeled to aid orientation. White box indicates area shown in (B). Scale bar = 250 μm. (B) Medium-power magnification (20×) of ER/GR in the BLA. White box indicates neuron displayed in (C). (C) High-power magnification of an ER/GR-infected neuron.
All subjects had robust expression in both the lateral and basal nuclei (with weak to significant expression in the accessory basal nucleus, and weak to no expression in the central nucleus), precluding single-nucleus comparisons in subject behavior. There was 15-40% of BLA infection in all animals, with no correlation between behavioral measures and extent of expression (data not shown); possibly because peak transgene expression levels occurred during behavioral experiments, rather than at time of sacrifice.
Dual-hormone gene therapy with ER/GR does not impair fear learning or post-shock freezing (Figure 2)
Figure 2.
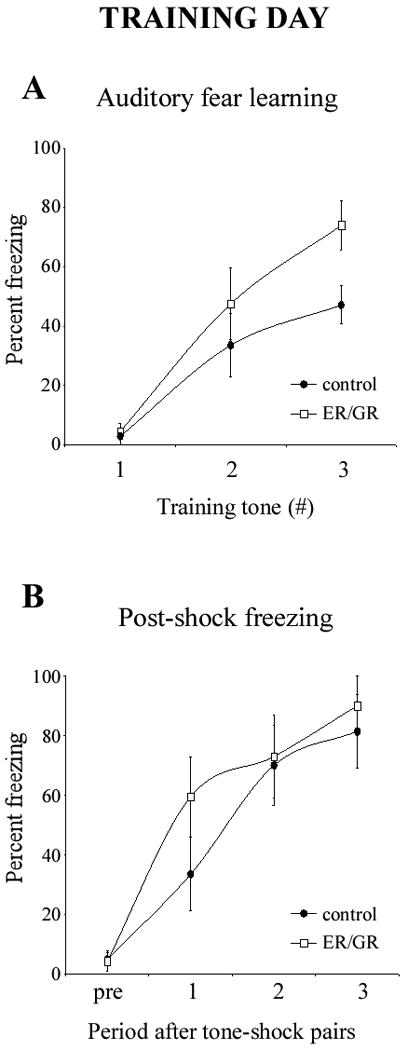
ER/GR does not impair fear learning or post-shock freezing levels. (A) Bilateral intraamygdala microinjections of ER/GR (n=11; white squares) did not impair the animals' ability to learn the CS-US association as compared to controls (n=9; black circles). The first data point illustrates the lack of freezing during the presentation of the first tone that immediately preceded its pairing to the US. Error bars represent SEM. (B) Bilateral intraamygdala microinjections of ER/GR (n=11; white squares) does not alter post-shock freezing levels as compared to controls (n=9; black circles). The first data point (“pre”) represents freezing levels before any CS-US pairings. Error bars represent SEM.
To distinguish the effects of ER/GR on fear learning versus consolidation, we analyzed the freezing levels during tone presentation over the conditioning session. Neither group froze to the tone before it was paired with the US, but both increasingly froze to subsequent tones after the CS-US association was learned. Therefore, learning rates were not impaired by ER/GR (p=0.20).
To assess effects of ER/GR on locomotion and shock sensitivity, rats were observed throughout the training procedure. ER/GR rats ran, jumped, and vocalized normally in response to the shock. Furthermore, ER/GR did not alter post-shock freezing levels (p=0.22), implying no effect on sensory processing, or immediate (short-term) fear memory.
Dual-hormone gene therapy with ER/GR disrupts both auditory and contextual fear conditioning (Figure 3)
Figure 3.
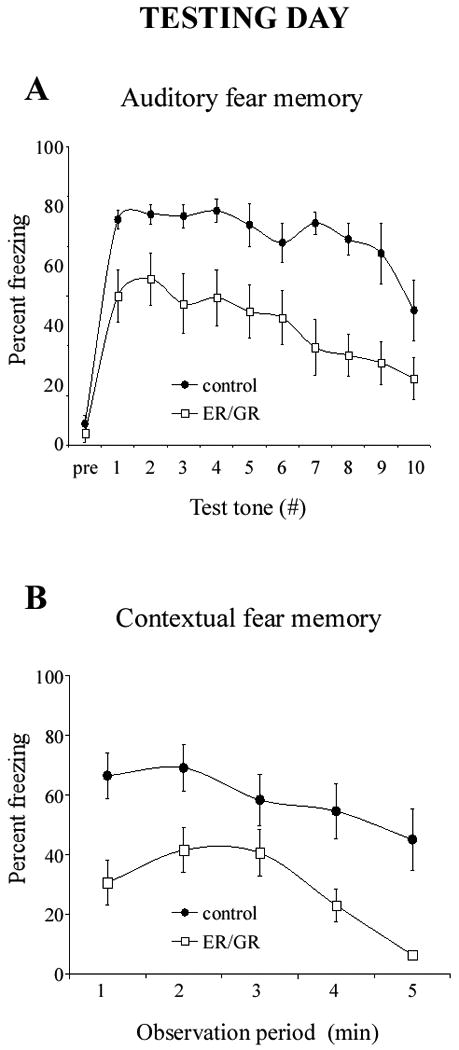
Disruption of fear memory through dual-hormone gene therapy. Bilateral intraamygdala microinjections of ER/GR (n=11; white squares) causes a severe disruption of both auditory (A) and contextual (B) fear memory at 24 hours after conditioning as compared to controls (n=9; black circles). Animals were given a 5-minute acclimation period before testing. The first data point in B (“pre”) illustrates the freezing levels before the test tone presentations. Error bars represent SEM.
We next tested whether ER/GR impairs BLA-dependent fear conditioning. In controls, the memory of the association of tone and context to shock remained 24hr later, with high levels of freezing. ER/GR decreased both long-term auditory and contextual fear memory (p < 0.000001).
Discussion
While hormones have long been known to influence emotional processing, no study has examined how combined manipulation of estrogen and GC signaling in the BLA effects fear conditioning. In the present study, we used a unique vector, ER/GR, which transforms GCs actions at the receptor level into estrogenic signals at the nuclear level, thereby converting disruptive GC effects into salutary estrogenic ones. ER/GR expression decreases neurotoxicity following a neurological insult (12), and rescues stress-induced impairments of hippocampal-dependent memory (13), reflecting the adverse and protective effects of GCs and estrogen, respectively. In the present study, intraamygdala expression of ER/GR disrupted auditory and contextual fear conditioning.
ER/GR animals acquired the tone-shock association and exhibited post-shock freezing levels normally during training, but showed blunted fear memories 24hr later, suggesting that ER/GR altered genomic, rather than rapid synaptic events during experience-dependent short-term learning (14). These data agree with reports that GR blockade in the BLA does not affect pre- and post-shock freezing, but does impair fear memories 24hr later (5). Thus, ER/GR did not influence emotional learning or the ability to express fear responses during conditioning, but impaired consolidation processes, as evidenced by impaired auditory and contextual fear memory the following day.
ER/GR may simultaneously halt the excitatory effects of GCs and promote the inhibitory influences of estrogen on BLA neuronal firing. As another example of a GC/estrogen contrast, GCs decrease activity and cause dendritic atrophy in the hippocampus, while estrogen does the opposite (8, 9, 11). Gene therapy with ER or GR alone may produce intermediate impairments given the dramatic effect of boosting the signals of the former while blunting those of the latter.
It was not possible to analyze the effect of ER/GR on individual BLA nuclei, but given that the lateral nucleus is the main nucleus for sensory input and integration (2, 15), and the basal nucleus receives projections from the hippocampus (2), we believe that ER/GR expression in both the lateral and basal nuclei contributed to the significant impairments found in auditory and contextual fear memories. It is unknown whether GCs or estrogen affect the firing rates of neurons in these nuclei differently.
ER/GR in the BLA produced a striking emotional memory deficit despite modest expression of the transgene. Commensurate with this, vector-driven transgene expression in ∼10-25% of lateral nucleus neurons is sufficient to impair fear memory (16, 17), while a similar magnitude of expression in the hippocampus can also alter its function, (18, 19).
The neural circuit underlying fear conditioning is pertinent to emotional disorders. Therefore, these data may aid understanding endocrine effects on the storage of traumatic memories and for identifying targets for the treatment of emotional disorders. Future studies must explore further the molecular role of these hormone receptors in fear memory consolidation.
Acknowledgments
This research was supported NIH grant 5R01 AG020633. We thank M.Y. Cheng for ER/GR DNA and A.L. Lee for technical assistance.
Footnotes
Drs. Rodrigues and Sapolsky reported no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- 3.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Johnson LR, Farb C, Morrison JH, McEwen BS, LeDoux JE. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 5.Donley MP, Schulkin J, Rosen JB. Glucocorticoid receptor antagonism in the basolateral amygdala and ventral hippocampus interferes with long-term memory of contextual fear. Behav Brain Res. 2005;164:197–205. doi: 10.1016/j.bbr.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 6.Roozendaal B, McGaugh JL. Glucocorticoid receptor agonist and antagonist administration into the basolateral but not central amygdala modulates memory storage. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 7.Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra R, Sapolsky RM. Acute corticosterone treatment is sufficient to induce anxiety and amygdaloid dendritic hypertrophy. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:5573–5578. doi: 10.1073/pnas.0705615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Womble MD, Andrew JA, Crook JJ. 17beta-Estradiol reduces excitatory postsynaptic potential (EPSP) amplitude in rat basolateral amygdala neurons. Neurosci Lett. 2002;331:83–86. doi: 10.1016/s0304-3940(02)00871-6. [DOI] [PubMed] [Google Scholar]
- 11.Daniel JM. Effects of oestrogen on cognition: what have we learned from basic research? J Neuroendocrinol. 2006;18:787–795. doi: 10.1111/j.1365-2826.2006.01471.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaufer D, Ogle WO, Pincus ZS, Clark KL, Nicholas AC, Dinkel KM, et al. Restructuring the neuronal stress response with anti-glucocorticoid gene delivery. Nat Neurosci. 2004;7:947–953. doi: 10.1038/nn1296. [DOI] [PubMed] [Google Scholar]
- 13.Nicholas A, Munhoz CD, Ferguson D, Campbell L, Sapolsky R. Enhancing cognition after stress with gene therapy. J Neurosci. 2006;26:11637–11643. doi: 10.1523/JNEUROSCI.3122-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McEwen B. Estrogen actions throughout the brain. Recent Prog Horm Res. 2002;57:357–384. doi: 10.1210/rp.57.1.357. [DOI] [PubMed] [Google Scholar]
- 15.Rodrigues SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. doi: 10.1016/j.neuron.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 16.Han JH, Kushner SA, Yiu AP, Cole CJ, Matynia A, Brown RA, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. doi: 10.1126/science.1139438. [DOI] [PubMed] [Google Scholar]
- 17.Rumpel S, LeDoux J, Zador A, Malinow R. Postsynaptic receptor trafficking underlying a form of associative learning. Science. 2005;308:83–88. doi: 10.1126/science.1103944. [DOI] [PubMed] [Google Scholar]
- 18.Dumas TC, Sapolsky RM. Gene therapy against neurological insults: sparing neurons versus sparing function. Trends in neurosciences. 2001;24:695–700. doi: 10.1016/s0166-2236(00)01956-1. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson D, Sapolsky R. Mineralocorticoid receptor overexpression differentially modulates specific phases of spatial and nonspatial memory. J Neurosci. 2007;27:8046–8052. doi: 10.1523/JNEUROSCI.1187-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]