Abstract
Background
Human noroviruses are the major viral pathogens of epidemic acute gastroenteritis. These genetically diverse viruses comprise two major genogroups (GI and GII) and approximately 30 genotypes. Noroviruses recognize human histo-blood group antigens (HBGAs) in a diverse, strain-specific manner. Recently the crystal structures of the HBGA-binding interfaces of the GI Norwalk virus and the GII VA387 have been determined, which allows us to examine the genetic and structural relationships of the HBGA-binding interfaces of noroviruses with variable HBGA-binding patterns. Our hypothesis is that, if HBGAs are the viral receptors necessary for norovirus infection and spread, their binding interfaces should be under a selection pressure in the evolution of noroviruses.
Methods and Findings
Structural comparison of the HBGA-binding interfaces of the two noroviruses has revealed shared features but significant differences in the location, sequence composition, and HBGA-binding modes. On the other hand, the primary sequences of the HBGA-binding interfaces are highly conserved among strains within each genogroup. The roles of critical residues within the binding sites have been verified by site-directed mutagenesis followed by functional analysis of strains with variable HBGA-binding patterns.
Conclusions and Significance
Our data indicate that the human HBGAs are an important factor in norovirus evolution. Each of the two major genogroups represents an evolutionary lineage characterized by distinct genetic traits. Functional convergence of strains with the same HBGA targets subsequently resulted in acquisition of analogous HBGA binding interfaces in the two genogroups that share an overall structural similarity, despite their distinct locations and amino acid compositions. On the other hand, divergent evolution may have contributed to the observed overall differences between and within the two lineages. Thus, both divergent and convergent evolution, as well as the polymorphic human HBGAs, likely contribute to the diversity of noroviruses. The finding of genogroup-specific conservation of HBGA binding interfaces will facilitate the development of rational strategies to control and prevent norovirus-associated gastroenteritis.
Introduction
Noroviruses, a group of single-stranded, positive sense RNA viruses, constitute one of the six genera of the family Caliciviridae [1], [2], [3]. Noroviruses are genetically highly diverse containing 5 genogroups (G) and ∼30 genotypes [4]. GI, GII and GIV infect humans and cause acute gastroenteritis [1], [5], [6], GIII infect cattle causing similar diseases [7], while GV infect mice and cause disease only in immune compromised mice [8]. GI and GII are studied extensively owing to their importance in human disease. Although some genogroup-specific features related to the epidemiology and environmental transmission have been analyzed [9], [10], [11], the biological significance and evolutionary relationships regarding the HBGA interaction of the two major genogroups of human noroviruses requires further elucidation.
Noroviruses contain a major structural protein (VP1) that forms the viral capsid [12]. The VP1 has two principle domains, the shell (S) and the protruding (P) domains, linked by a short hinge. The S domain forms the interior icosahedral shell, while the P domain constitutes the arch-like P dimer protruding from the shell. The P domain can be further divided into two subdomains, P1 and P2, with the P2 subdomain at the outermost surface of the viral capsid [12], [13], [14], [15]. The S and P domains appear to be structurally and functionally independent, as suggested by the facts that the P domain alone forms a dimer and P particle (12 P dimers), and both P dimer and P particle retain receptor-binding function [16], [17], [18], while the S domain alone forms S particle without receptor-binding function [16]. In addition, a large amount of soluble P protein has been found in the stools of Norwalk virus-infected patients [18], [19], [20], although its biological significance remains unknown.
Human noroviruses recognize human histo-blood group antigens (HBGAs), most likely as receptors or co-receptors ([21], [22], [23], [24], [25], [26], [27], [28], [29], reviewed in [30], [31]). HBGAs are complex carbohydrates linked to proteins or lipids on the surface of red blood cells and mucosal epithelia of the respiratory, genitourinary, and digestive tracts, or as free oligosaccharide in biological fluids such as milk and saliva. A number of distinct binding patterns of noroviruses to HBGAs have been described according to the ABO, Lewis and secretor types of the human HBGAs [21], [22], [32]. The prototype Norwalk virus (GI-1) represents one of these patterns and binds to saliva of A and O secretors. Other binding patterns include the A, B, O secretor binder of VA387 (GII-4), A, B binder of MOH (GII-5), and A, O secretor and nonsecretor binder of VA207 (GII-9) and Boxer (GI-8) [22]. The variable binding patterns have been further sorted into two major binding groups, the A/B binding group and Lewis binding group based on shared HBGA targets within binding groups. The A/B binding group recognizes mainly the A/B/H but not the Lewis epitopes, while the Lewis binding group binds the Lewis epitopes but not the A/B epitopes ([22], reviewed in [30], [31]). Strains of both A/B and Lewis binding groups can be found in the two major genogroups of human noroviruses. In addition, virus-HBGA interaction has also been found in two other genera of caliciviruses. For example, the rabbit hemorrhagic disease virus (RHDV) binds to H antigen [33], while the Tulane virus binds to B antigen [34], suggesting that the involvement of HBGAs in calicivirus infection may be a common phenomenon.
High resolution 3D structures of HBGA-binding interfaces of Norwalk virus (GI-1) and VA387 (GII-4) have recently been determined [13], [14], [15]. As shown by these studies, the binding interfaces of both strains are located in the same region of the outermost P2 domain, but the positions and amino acid composition of individual binding sites and the HBGA binding modes are different. The HBGA-binding interface of Norwalk virus is located within a single monomer of the P dimer (Fig. 1, left panel), while the binding interface of VA387 involves both monomers of the P dimer (Fig. 1, right panel), although in both cases dimerization of the P domains appears to be required for their binding function [13], [14], [35]. While both Norwalk virus and VA387 belong to the A/B binding group and share the same A and H antigens, the primary sequences of their binding sites and the binding modes to HBGAs are different. Norwalk virus interacts with the α-GalNAc and α-Fus of the A trisaccharide or α-Fuc and β-Gal of the H tetrasaccharide [13], [15], while VA387 interacts with all three terminal sugars of A (α-GalNAc-β-Gal-α-Fuc) and B (α-Gal-β-Gal-α-Fuc) trisaccharides [14].
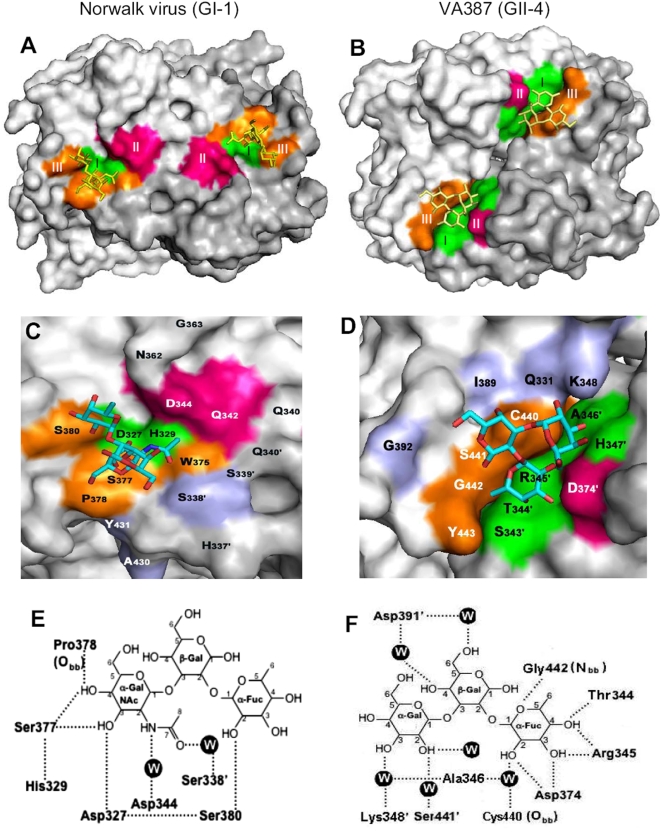
Figure 1. The crystal structures of the HBGA-binding interfaces of Norwalk virus (GI-1) and VA387 (GII-4).
The surface models of the P dimers (top views) with indications of the HBGA-binding interfaces (colored regions) are shown in (A) and (B) with one monomer being shown in darker gray than another. Enlargements of the HBGA-binding interfaces are shown in (C) and (D) correspondingly with labels of individual amino acids, in which the prime symbol indicates a residue of another protomer. The three major components of the binding interfaces are colored in green (site I), red (site II), and orange (site III), respectively, while the trisaccharides binding to the interface are in yellow in (A) and (B) or in variable colors (C-cyans, O-red, and N-blue) in (C) and (D). The amino acids around the interface that affect the binding specificity are in light blue. (E) and (F) are schematic diagrams of hydrogen bonding network (dash lines) between the amino acids of the P dimers of Norwalk virus (E), or VA387 (F) and the A- or B- type trisaccharides. The water-bridged hydrogen bonds are indicated by W. (A) to (D) were prepared by software PyMOL version 1.0 (Delano Scientific), while (E) and (F) by software ChemDraw Pro version 11.0 (Adept Scientific). (E) is adapted from [13] with permission. The original data were published in [13], [14], [15].
In this study, we characterized the genetic relatedness of human noroviruses in the context of their host carbohydrate-binding specificity by sequence alignment and structural analysis, followed by site-directed mutagenesis and functional assay for strains representing both GI and GII and both A/B and Lewis binding groups. We showed that the HBGA-binding interfaces are highly conserved among strains within, but not between, the two genogroups, and that the conserved binding interfaces are able to interact with variable HBGAs of the ABH and Lewis families. Our results suggested that human HBGA may be a selection factor in norovirus evolution. The polymorphic human HBGAs and the highly adaptative nature of noroviruses may underlie the observed diversity of noroviruses. The high conservation of HBGA-binding interfaces within genogroups may also help in the development of new strategies to control and prevent norovirus infection.
Materials and Methods
Construction of mutant P particles
Bacterial expression constructs for wild type P particles were made by cloning of the P domain-encoding sequences into the plasmid pGEX-4T-1 (Amersham Bioscience, Piscataway, NJ). To enhance the efficiency of P particle formation a cysteine-containing short peptide was linked to either the N- (Norwalk virus, CNGRC) or C- (Boxer, MOH, and VA207, CDCRGDCFC) termini of the P domains, as reported previously [16], [17], [18], [35], [36]. Mutant P particles with single amino acid substitutions were designed and constructed by site-directed mutagenesis using the corresponding wild type constructs as templates. Site-directed mutagenesis was performed using the QuickChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and the corresponding primer pairs with mutations (Table 1) as described previously [16], [17], [18], [35], [36]. The wild type and mutant P particles were expressed and purified as described previously [16], [17], [18], [35], [36]. Briefly, after sequence confirmation through DNA sequencing, the mutant constructs were expressed in E. coli strain BL21 with an induction of IPTG (0.25 mM) at room temperature (∼25°C) overnight. The P protein-GST fusion was purified by the Glutathione Sepharose 4 flow (GE Healthcare Life Sciences, Piscataway, NJ) according to the manufacturer's protocol. P proteins were released from GST tag by thrombin (GE Healthcare Life Sciences, Piscataway, NJ) digestion. The formation of P particle and P dimer was determined by gel filtration using a size-exclusion column Superdex 200 (GE Healthcare Life Sciences, Piscataway, NJ) powered by an AKTA-FPLC system (model 920, GE Healthcare Life Sciences, Piscataway, NJ) followed by SDS-PAGE electrophoresis, in which the P particles form a peak at ∼830 kDa and the P dimer at ∼69 kDa, respectively previously [16], [17], [18], [35], [36]. The efficiency of P particle formation for Norwalk virus, Boxer, MOH, and VA207 were ∼70%, ∼80%, ∼80%, and ∼90%, respectively. None of the designed single residue mutations in this study affected P particle formation.
Table 1. Primers used for cloning of P domain and site-directed mutagenesis to generate single mutation in the HBGA-binding interface and around regions.
| Name | Primer sequence (5′ to 3′) | Sense | Mutation |
| Primers for Norwalk virus (GI-1) | |||
| P588 | gcgtggatcctgcaacggccgttgctttttagtccctcctacggtg | + | Wild type |
| P494 | ggacgcggccgcttatcggcgcagaccaagcct | − | Wild type |
| P1227 | cctcggtggttgtgcttggcatatcaatatg | + | D327A |
| P1228 | catattgatatgccaagcacaaccaccgagg | − | D327A |
| P1122 | ggtggttgtgattgggctatcaatatgacacag | + | H329A |
| P1123 | ctgtgtcatattgatagcccaatcacaaccacc | − | H329A |
| P1124 | atgacacagtttggcgcttctagccagacccag | + | H337A |
| P1125 | ctgggtctggctagaagcgccaaactgtgtcatattg | − | H337A |
| P1126 | acacagtttggccatgctagccagacccagtatg | + | S338A |
| P1127 | atactgggtctggctagcatggccaaactgtgtc | − | S338A |
| P1271 | acacagtttggccataatagccagacccagtatg | + | S338N |
| P1272 | cacactgggtctggctattatggccaaactgtgt | − | S338N |
| P1128 | cagtttggccattctgcccagacccagtatgatg | + | S339A |
| P1129 | atcatactgggtctgggcagaatggccaaactgtg | − | S339A |
| P1231 | gcatatcaatatgacagcgacccagtatgatg | + | Q340A |
| P1232 | catcatactgggtcgctgtcatattgatatgc | − | Q340A |
| P1130 | cattctagccagaccgcgtatgatgtagacaccacc | + | Q342A |
| P1131 | ggtgtctacatcatacgcggtctggctagaatggc | − | Q342A |
| P1255 | cagacccagtatgvtgtagacaccacc | + | D344A |
| P1256 | ggtggtgtctacagcatactgggtctg | − | D344A |
| P1132 | ggttcaattcaggcagctggcattggcagtgg | + | N362A |
| P1133 | accactgccaatgccagctgcctgaattgaacc | − | N362A |
| P1134 | ggttcaattcaggcaaatgccattggcagtggtaattatg | + | G363A |
| P1135 | attaccactgccaatggcatttgcctgaattgaacc | − | G363A |
| P1136 | gttggtgttcttagcgcgatttcccccccatcac | + | W375A |
| P1137 | tgatgggggggaaatcgcgctaagaacaccaac | − | W375A |
| P1240 | gttcttagctggattgcccccccatcacac | + | S377A |
| P1239 | gtgtgatgggggggcaatccagctaagaac | − | S377A |
| P1241 | cttagctggatttccgccccatcacacccg | + | P378A |
| P1242 | cgggtgtgatggggcggaaatccagctaag | − | P378A |
| P1243 | ggatttcccccccagcacacccgtctggc | + | S380A |
| P1244 | gccagacgggtgtgctgggggggaaatcc | − | S380A |
| P1138 | atgccaggtcctggtacttataatttgccctgtc | + | A430S |
| P1139 | gacagggcaaattataagtaccaggacctggc | − | A430S |
| P1140 | ccaggtcctggtgctgctaatttgccctgtctattacc | + | Y431A |
| P1141 | tagacagggcaaattagcagcaccaggacctgg | − | Y431A |
| Primers for Boxer (GI-8) | |||
| P744 | cgcggatcccaaagaaccaagccatttagtg | + | Wild type |
| P746 | ataagaatgcggccgcttagcaaaagctaactgccacggcaatcgca | ||
| tgatctcctgagaccaagcct | − | Wild type | |
| P1011 | ggaaattgtgatttggctatgacctttgttaag | + | H334A |
| P1012 | cttaacaaaggtcatagccaaatcacaatttcc | − | H334A |
| P1042 | catatgacctttgttaaggctaatcccactgagttgtcc | + | I340A |
| P1043 | caactcagtgggattagccttaacaaaggtcatatgc | − | I340A |
| P1013 | gacctttgttaagattgctcccactgagttgtcc | + | N341A |
| P1014 | ggacaactcagtgggagcaatcttaacaaaggtc | − | N341A |
| P1044 | cctttgttaagattaatgctactgagttgtccactg | + | P342A |
| P1045 | cagtggacaaatcagtagcattaatcttaacaaagg | − | P342A |
| P1046 | ccactgagttgtccgctggagatccttctggtaag | + | T347A |
| P1047 | accagaaggatctccagcggacaactcagtggg | − | T347A |
| P1015 | cactgagttgtccactgcagatccttctggtaag | + | G348A |
| P1016 | cttaccagaaggatctgcagtggacaactcagtg | − | G348A |
| P1048 | gagttgtccactggagctccttctggtaaggtg | + | D349A |
| P1049 | caccttaccagaaggagctccagtggacaactc | − | D349A |
| P1017 | gttgtccactggagatgcttctggtaaggtggtc | + | P350A |
| P1018 | gaccaccttaccagaagcatctccagtggacaac | − | P350A |
| P1050 | ctggaggataataatgctttagatcagtttgtgg | + | E377A |
| P1051 | cacaaactgatctaaagcattattatcctccag | − | E377A |
| P1052 | gaggataataatgaggctgatcagtttgtgggc | + | L378A |
| P1053 | ccacaaactgatcagcctcattattatcctcc | − | L378A |
| P1019 | gaggataataatgagttagctcagtttgtgggcaag | + | D379A |
| P1020 | cttgcccacaaactgagctaactcattattatcctc | − | D379A |
| P1054 | gataataatgagttagatgcttttgtgggcaaggaag | + | Q380A |
| P1055 | ttccttgcccacaaaagcatctaactcattattatcctc | − | Q380A |
| P1056 | aatgagttagatcaggctgtgggcaaggaagtgg | + | F381A |
| P1057 | cacttccttgcccacagcctgatctaactcattattatcc | − | F381A |
| P1021 | gtgctggagatgacggcggtttccaatagaacgg | + | W392A |
| P1022 | cgttctattggaaaccgccgtcagctccagcag | − | W392A |
| P1058 | catttccaacggtcagtgctccaaaagttccatgtacc | + | N444A |
| P1059 | gtacatggaacttttggagcactgaccgttggaaatgtgg | − | N444A |
| MOH (GII-5) | |||
| P1309 | cgcggatcctcaaagactaagccatttacac | + | Wild type |
| P1310 | ataagaatgcggccgctaagcaaaagcaatctogccacggcaatcgca | ||
| ctgaaaccttctgcgcccattc | − | Wild type | |
| P1344 | gcaacccagcaaacgcggctcatgatgctg | + | R347A |
| P1345 | cagcatcatgagccgcgtttgctgggttgc | − | R347A |
| P1346 | cttggaacaccaatgctgttgaaaaccaacc | + | D376A |
| P1347 | ggttggttttcaacagcattggtgttccaag | − | D376A |
| P1348 | cccattaaaaggtgcatttggaaaccctg | + | G441A |
| P1349 | cagggtttccaaatgcaccttttaatggg | − | G441A |
| VA207 (GII-9) | |||
| P709 | acgcgtcgactctcaaagactaaggcattcac | + | Wild type |
| P702 | gcgtgcggccgcttagcaaaagcaatcgccatggcaatcgcattgg | ||
| atccttctccccccacttcc | − | Wild type | |
| P1338 | caggtgacgccacggcggcccatgaggcaag | + | R346A |
| P1339 | cttgcctcatgggccgccgtggcgtcacctg | − | R346A |
| P1340 | ctcaacctcaagcgcttttgaaacaaacc | + | D374A |
| P1341 | ggtttgtttcaaaagcgcttgaggttgag | − | D374A |
| P1342 | ccaggagctagtgcccacacaaatggg | + | G440A |
| P1343 | cccatttgtgtgggcactagctcctgg | − | G440A |
Saliva-based HBGA binding assay
These were performed as described elsewhere [21], [22], [35], [36]. The affinity-column purified P particles were first diluted to 1 mg/ml as a starting solution. They were then diluted further in a 3-fold-series to indicated concentration directly on the testing Elisa plates that had been coated with different saliva. The different P particles were incubated with coated saliva samples for 60 min at 37°C. Five well-characterized saliva samples representing typical blood types of “O”, “A”, “B”, “AB” secretor and “O” nonsecretor [21], [22] were used for the binding assays.
Crystal structure visualization and analysis
The crystal structures of the P dimers of Norwalk virus and VA387 complexed with type A-, B-trisaccharides, and/or H-pentasaccharide were analyzed using the PyMOL software (DeLano Scientific LLC, Palo Alto, CA) and the Polyview-3D server (http://polyview.cchmc.org). The PDB files of the Norwalk virus P protein in complex with A-trisaccharide (3D26 and 2ZL7) or H-pentasaccharide (2ZL6) [13], [15] and VA387 P protein in complex with A-trisaccharide (2OBS) or with B-trisaccharide (2OBT) [14] were downloaded from the Protein Data Bank at Rutgers University, New Brunswick, NJ (http://www.rcsb.org).
Results
Characterization of the HBGA-binding interface of Norwalk virus
The prototype Norwalk virus (GI-1) and strain VA387 (GII-4), each representing genogroups (G) I and II of human noroviruses, exhibit distinct HBGA binding patterns but share the ability to bind to the A and H antigens [22]. Crystallographic studies indicated that these two strains use distinct binding interfaces and modes of interaction with HBGA receptors (Fig. 1, [13], [14], [15]). Our recent site-directed mutagenesis analysis of VA387 has identified a number of additional amino acids around the carbohydrate binding interface that are also involved in HBGA binding [35]. To further elucidate the differences and similarities between the two HBGA-binding interfaces and modes, we extended such mutagenesis analysis to the Norwalk virus.
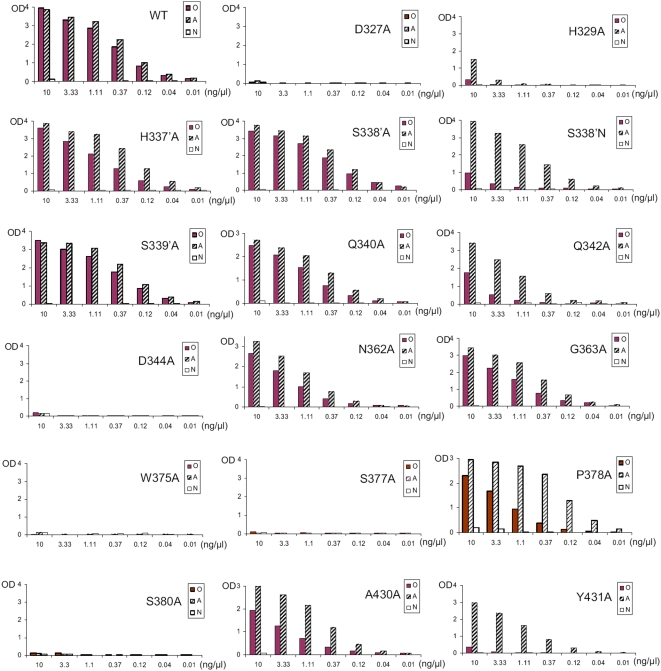
Among 17 mutant P particles with single amino acid changes into alanines/serines in and around the HBGA-binding interface of the Norwalk virus (Fig. 2 and Table 2), six mutants (D327A, H329A, D344A, W375A, S377A, and S380A) lost their binding to HBGAs completely or nearly completely (H329A) (Fig. 1 and Fig. 2, [13]), indicating that these amino acids are critical for the structural integrity of the binding interface. Residue Q342 that was shown to interact with the type H but not the type A oligosaccharide [13], [15] affected binding mainly to the H when it was replaced by an alanine (Fig. 2). Similar effects were also seen in mutants P378A, A430S, and Y431A, respectively, although P378 was predicted to interact with both types H and A oligosaccharides, while A430 and Y431 do not appear to interact with either of the two oligosaccharides [13], [15]. Furthermore, a replacement of S338 by an alanine (S338A) did not affect binding to either A or H antigens (Fig. 2 and [13]), but a change to an asparagine (S338N) wiped out binding to H without affecting binding to A antigen (Fig. 2), although S338 from a heterologous P monomer interacted with the α-N-acetylgalacosamine (α-GalNAc) of the A antigen via a water-bridged hydrogen bond [13]. The involvement of residues S338, P378, A430, and Y431 in HBGA-binding specificity may be supported by their common location in a region on one side of the binding interface (Fig. 1C), although the mechanism remains to be elucidated. In contrast, although residues H337, S339, Q340, N362, and G363 are also located around the binding interface, their mutations into alanines did not have significant impact on binding to HBGAs (Fig. 2 and Table 2).
Figure 2. Binding of various mutant P particles with single amino acid changes in or around the HBGA-binding interface of Norwalk virus to the saliva samples.
X-axes show protein concentrations of the P particles and Y axes indicate the optical densities at 450 nm (OD450) that were the average values of triplicate experiments. “O”, “A” and “N” represent the saliva samples of type O secretor (containing H antigen), type A secretor, and type O nonsecretor, respectively. Data of mutations D327A, H329A, S338'A, and S377A are adapted with permission from [13]. Mutants with prime symbol (') indicate the mutated residues of another P monomer.
Table 2. Summary of the mutagenesis study of the amino acid residues in and around the predicted HBGA-binding interface of Norwalk virus.
| Mutants1 | Binding to type O saliva2 | Binding to type A saliva2 | Binding to saliva of nonsecretor2 | Predict to interact with HBGAs3 |
| WT | ++++ | ++++ | − | |
| D327A | − | − | − | H and A |
| H329A | − | + | − | H and A |
| H337A | +++ | ++++ | − | no |
| S338A | ++++ | ++++ | − | A |
| S338N | + | ++++ | − | A |
| S339A | ++++ | ++++ | − | no |
| Q340A | +++ | +++ | − | no |
| Q342A | + | +++ | − | H |
| D344A | − | − | − | H and A |
| N362A | ++ | +++ | − | no |
| G363A | +++ | ++++ | − | no |
| W375A | − | − | − | H and A |
| S377A | − | − | − | H and A |
| P378A | ++ | +++ | − | H and A |
| S380A | − | − | − | A |
| A430S | ++ | +++ | − | no |
| Y431A | − | +++ | − | no |
The P particle formation of the wild type (WT) and all mutants were confirmed by gel filtration. The italicized mutants in indicate the mutated residues of another P monomer.
The number of “+” indicated the relative binding affinity of the wild type and mutant P particles at 3.33 ng/µl to HBGAs: “++++” indicates an OD450 >3.0; “+++” between 2.0 and 3.0; “++” between 1.0 and 2.0; “+” between 0.3 and 1.0; while “−” indicates an OD450 <0.3, suggesting a complete loss of binding. Type O and A saliva were from secretors containing H and A antigen, respectively.
Predictions were made by two independent crystallographic studies (ref13 and 15).
Distinct HBGA-binding interfaces and modes between Norwalk virus and VA387
The crystal structures of the binding interfaces [13], [14], [15] and the site-directed mutagenesis ([35]and data in the previous section) suggest that the HBGA-binding interfaces of both Norwalk virus and VA387 can be divided into three major analogous regions, representing the bottom (site I) and the walls (site II and III) of the interface (Fig. 1). These sites are composed of either a single or a cluster of sterically closed amino acids, including D327 and H329 (site I), Q342 and D344 (site II), and W375, S377, and S380 (site III) for Norwalk virus and S343 to H347 (site I), D374 (site II), and S441 and G442 (site III) for VA387, but none of these sites are shared by the two strains. It should be noted that all three sites of Norwalk virus are formed by residues of a single P2 subdomain without direct interactions with the P1 subdomain or the dimer-related P2 subdomain, while site III (S441 and G442) of VA387 is formed by the top of an exposed loop of P1 subdomain, and it involves the other chain of the VA387 P dimer. In both strains these three sites interact with at least two sugars of the A, B or H antigens. However, in Norwalk virus the major contacts are on the α-N-acetyl galactosamine (α-GalNAc) of the A trisaccharide or the β-galactose (β-Gal) of H pentasaccharide [13], [15], while in VA387 the major contacts are on the α-fucose (α-Fuc) of the A and B trisaccharides [14].
The HBGA-binding interfaces are conserved within but not between genogroups
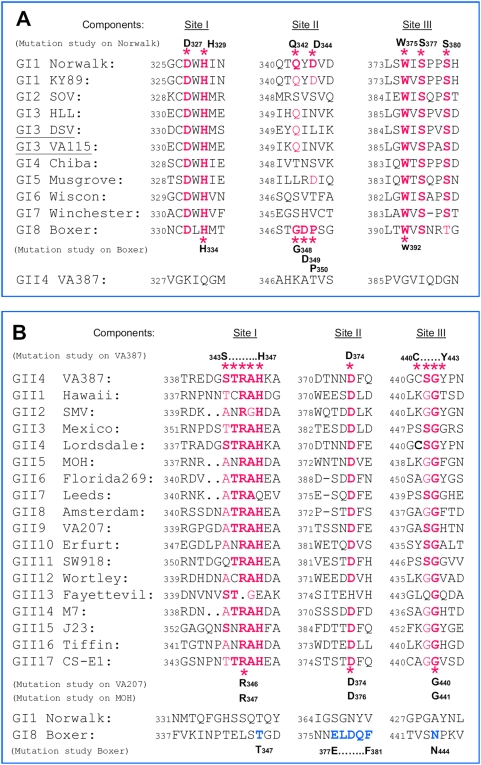
Sequence alignments of the P domains of noroviruses representing 8 GI and 17 GII genotypes (Fig. 3) show that the three sites of the HBGA-binding interfaces are highly conserved within each genogroup. Sites I and III are more conserved than site II for the GI viruses, while all three sites are highly conserved among GII viruses except for strains of GII-13, including site III that is in the P1 subdomain of the capsid (Fig. 1 and 3). The overall sequence identities of the P2 subdomains are only 31–56% for strains within each of the two genogroups, further indicating the selective pressures of the HBGAs on the receptor binding interfaces.
Figure 3. Sequence alignments of the HBGA-binding interfaces of various GI and GII noroviruses.
Sequence of the three major components (red letters) of the HBGA-binding interfaces of 10 genogroup I (GI) (A) and 17 genogroup II (GII) (B) noroviruses, representing each of the 8 GI and 17 GII genetic types, respectively, are aligned based on the two known binding interfaces of Norwalk virus (GI) and VA387 (GII). Star symbols label the residues that have been experimentally shown to be required for binding to HBGAs. The two strains that have no detectable binding to examined HBGAs are underlined. The accession numbers of the sequence are: M87661 (Norwalk virus), L23828 (KY 89), L07418 (SOV), AF414403 (HLL), U04469 (DSV), AY038598 (VA115), AB042808 (Chiba), AJ277614 (Musgrove), AY502008 (Wiscon), AJ277609 (Winchester), AF538679 (Boxer), AY038600 (VA387), U07611 (Hawaii), AY134748 (SMV), U22498 (Mexico), X86557 (Lordsdale), AF397156 (MOH), AF414407 (Florida269), AJ277608 (Leeds), AF195848 (Amsterdam), AAK84676 (VA207), AF427118 (Erfurt), AB074893 (SW918), AJ277618 (Wortley), AY113106 (Fayettevil), AY130761 (M7), AY130762 (J23), AY502010 (Tiffin), AY502009 (CS-E1).
All three conserved sites are required for Boxer (GII-8) binding to HBGAs
The high conservation of the HBGA-binding interfaces raises an important question on the role of human HBGAs in norovirus evolution. For strains with similar binding patterns within the same genogroups, such as Norwalk virus (GI-1) and C59 (GI-2) in the A/B binding group that both bind to types A and H antigens of secretors but not to the Lewis antigens of the non-secretors [22], such conservation is understandable. However, the conservation of HBGA binding interfaces among strains with distinct binding patterns, such as Boxer (GI-8) of the Lewis binding group [22], seems to challenge the hypothesis that HBGAs confer selective pressure on norovirus evolution.
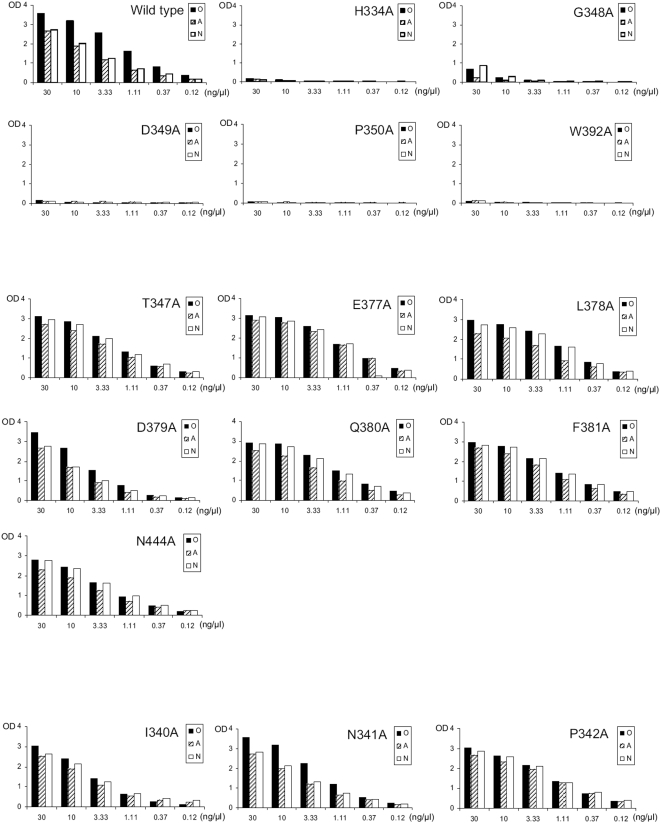
To address this apparent inconsistency and further elucidate the relationship between structure and HBGA binding patterns, we performed mutagenesis analysis on the role of the three conserved sites in HBGA binding of the Boxer virus. Three sets of mutant P particles were constructed. The first set contained 5 mutants with single residue mutations in each of the three GI conserved sites (Fig. 3A), including H334A (site I), G348A, D349A, P350A (site II), and W392A (site III); the second set were 7 mutants with mutations in regions corresponding to each of the three GII conserved sites (Fig. 3B), including T347A (site I), E377A, L378A, D379A, Q380A, F381A (site II), and N444A (site III); while the third set contained 3 mutants with mutations (I340A, N341A and P342A) away from the predicted binding interface as control (Fig. 4 and Table 3). The saliva-based binding results showed that all 5 mutants of the first set but none of the 7 mutants in the second set lost their binding to HBGAs completely or nearly completely (Fig. 4 and Table 3). As expected, the three mutants in the third set did not affect the binding. Therefore, all three conserved sites deduced from the GI Norwalk virus for the A/B binding group are also involved in HBGA-binding of the GI Boxer virus of the Lewis binding group. These data provided functional evidence for the conservation of the HBGA binding interface of GI noroviruses.
Figure 4. Saliva-based binding results of various mutant P particles of Boxer (GI-8) with single amino acid changes at the three GI conserved sites (upper panel), at the regions corresponding to the three GII-conserved sites (middle panel), and at regions away from the predicted binding interface (lower panel).
The X-axes show the protein concentrations of the P particles and the Y axes indicate the optical densities at 450 nm (OD450) that were the average value of triplicate experiments. “O” and “A” represent the saliva samples of type O (containing H antigen) and A secretor, respectively, while “N” one of nonsecretor.
Table 3. Summary of the mutagenesis study of the three sites of the HBGA-binding interface of Boxer virus (GI-8) predicted by sequence alignment with Norwalk virus (GI) and VA387 (GII).
| Mutants | Binding to type O saliva1 | Binding to type A saliva1 | Binding to saliva of nonsecretor1 | Components of the binding interface |
| WT | ++++ | +++ | +++ | |
| H334A | − | − | − | GI-site I |
| G348A | − | − | + | GI-site II |
| D349A | − | − | − | GI-site II |
| P350A | − | − | − | GI-site II |
| W392A | − | − | − | GI-site III |
| T347A | ++++ | +++ | +++ | GII-site I |
| E377A | ++++ | +++ | +++ | GII-site II |
| L378A | +++ | +++ | +++ | GII-site II |
| D379A | +++ | ++ | ++ | GII-site II |
| Q380A | +++ | +++ | +++ | GII-site II |
| F381A | +++ | +++ | +++ | GII-site II |
| N444A | +++ | ++ | +++ | GII-site III |
| I340A | +++ | +++ | +++ | control |
| N341A | ++++ | +++ | +++ | control |
| P342A | +++ | +++ | +++ | control |
The number of “+” indicated the relative binding affinity of the wild type (WT) and mutant P particles at 10 ng/µl to HBGAs: “++++” indicates an OD450 >3.0; “+++” between 2.0 and 3.0; “++” between 1.0 and 2.0; “+” between 0.3 and 1.0; while “−” indicates an OD450 <0.3, suggesting a complete loss of binding. Type O and A saliva were from secretors containing H and A antigen, respectively.
Similar genetic relatedness also exists among GII noroviruses
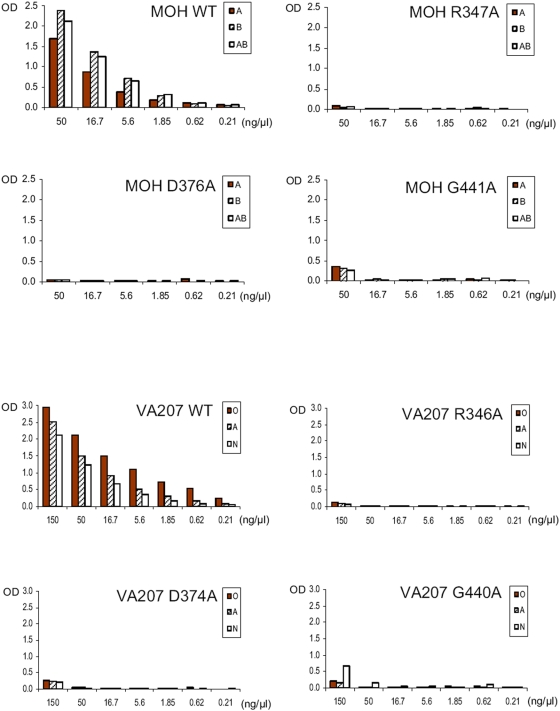
Similar variations of HBGA binding have also been found among GII strains. For example, strains MOH (GII-5), Buds (GII-2), Parris Island (GII-13), MxV (GII-3), members of the A/B binding group [22], target the common A, B and/or H antigens as VA387 (GII-4) does. Using a similar approach we constructed three mutant P particles (R347A, D376A and G441A) of MOH, each with a single residue change in the three GII conserved sites deduced from VA387 (Fig. 3B) and the binding to HBGAs of all three mutants were completely (R347A and D376A) or nearly completely (G441A) lost (Fig. 5 and Table 4). These results demonstrated that MOH shares the three conserved binding sites with VA387 which is consistent with their recognition of the common A and B antigens.
Figure 5. Saliva-based binding results of various mutant P particles of MOH (GII-5, upper panel) and VA207 (GII-9, lower panel) with single amino acid changes at the three GII conserved sites.
The X-axes show the protein concentrations of the P particles and the Y axes indicate the optical densities at 450 nm (OD450) that were the average value of triplicate experiments. “O”, “A”, “B” and “AB” represent the saliva samples of type O (containing H antigen), A, B, and AB secretor, respectively, while “N” one of nonsecretor.
Table 4. Summary of the mutagenesis study of the three sites of the HBGA-binding interface of MOH (GII-5) and VA207 (GII-9) predicted by sequence alignment with that of Norwalk virus (GI) and VA387 (GII).
| Mutants of MOH | Binding to type A saliva1 | Binding to type B saliva1 | Binding to type AB saliva1 | Components of the binding interface |
| WT | + | ++ | ++ | |
| R347A | − | − | − | GII-site I |
| D376A | − | − | − | GII-site II |
| D349A | − | − | − | GII-site III |
The number of “+” indicated the relative binding affinity of the wild type (WT) and mutant P particles at 16.7 ng/µl to HBGAs: “+++” indicates an OD450 >2.0; “++” between 1.0 and 2.0; “+” between 0.3 and 1.0; while “−” indicates an OD450 <0.3, suggesting a complete loss of binding. Saliva samples of type A, B, AB, and O were from secretors containing A, B, A/B and H antigen, respectively.
We then examined the role of the three GII conserved sites in HBGA-binding of VA207 (GII-9), a strain of the Lewis binding group that recognizes the Lex and Ley but not the A and B antigens. Construction of three mutants (R346A, D374A and G440A) with a single residue mutation at each of the three binding sites (Fig. 3B) resulted in complete (R346A) or nearly complete (D374A and G440A) loss of binding to HBGAs (Fig. 5 and Table 4). These data showed that VA207 shares the common HBGA-binding interface with those A/B binding strains within GII noroviruses and this has been recently confirmed by the crystal structure of VA207 P dimer in complex with Lewis antigen (Y. Chen, X. Jiang and X. Li to be published data, also see discussion).
Discussion
In this study, we used sequence alignment, structural analysis and site-directed mutagenesis to examine the evolutionary relatedness of human noroviruses in terms of their interaction with the HBGA receptors. We showed that strains with distinct HBGA binding patterns within genogroups share common receptor binding interfaces in their interactions with variable HBGAs, likely tuned up by subtle structural differences within the binding interfaces. At the same time, strains in different genogroups that use different binding interfaces, as defined by their locations and sequence motifs, can recognize the same HBGA-targets, pointing to the overall functional and structural similarity of these distinct binding sites. These results provide evidence that the human HBGAs exert an important selection pressure in norovirus evolution. The two major genogroups (G I and GII) of human noroviruses that cause acute gastroenteritis represent two major evolutionary lineages, while strains in the A/B and Lewis binding groups within the two genogroups, such as those represented by the Norwalk virus and Boxer in GI and those by VA387 and VA207 in GII, may further divide into evolutionary sub-lineages as a result of divergent evolution within each branch (Fig. 6).
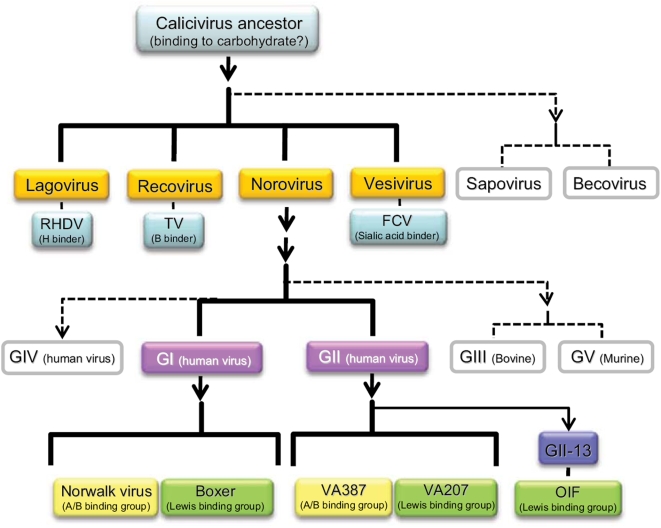
Figure 6. A schematic relationship of the known carbohydrate-binding phenotypes of caliciviruses.
Six calicivirus genera may be evolved from a common calicivirus ancestor and at least one strain from four genera (orange) has been shown to bind to carbohydrates. Similarly, five norovirus genogroups (G) may be evolved from a common norovirus ancestor and two of the three human norovirus genogroups have been demonstrated to recognize HBGAs (purple). GI and GII noroviruses share conserved genogroup-specific HBGA-binding interfaces and both genogroups contain strains binding to either A/B/H antigens (A/B binding groups, yellow) or Lewis antigens (Lewis binding group, green). GII-13 (Blue) is a unique genotype that does not share conserved binding sites with other GII genotypes and thus may represent a sublineage parallel to other GII genotypes, in which a strain (OIF) has been shown to bind to Lewis antigens (green). Arrows indicate the direction of evolution. Solid line shows the evolutionary lineages with defined binding to HBGAs, while the dashed line shows the lineages with unknown interaction with carbohydrates. RHDV, rabbit hemorrhagic disease virus; TV, Tulane virus; FCV, feline calicivirus; OIF, norovirus strain that was isolated from troops deployed to the Operation of Iraqi Freedom.
The HBGA-binding interfaces of the two major genogroups of human noroviruses share some similarity in the overall structure and location; both are located in the outermost P2 regions of the capsids [13], [14], [15] and both are composed of three major structural components, corresponding to the bottom and the walls of the binding pocket (Fig. 1). However, the two binding interfaces differ in their primary sequences, detailed locations, and modes of interaction with the HBGA-receptors [13], [14], [15]. The binding interface of the GI strains (Norwalk virus) is constituted by three groups of amino acids from the P2 subdomain and positioned mainly in one P monomer, although it is near the interface of two P monomers of the Norwalk virus P dimer. On the other hand, the binding interface of the GII viruses (VA387) is composed of residues from both P1 and P2 subdomains and is located right at the interface of two monomers in the VA387 P dimer [13], [14], [15], [35]. The conservation of the binding interfaces within GII has been confirmed by the crystal structures of the VA207 P dimers in complex with the Lewis x and Lewis y tetrasaccharides, respectively, in which the binding interface of VA207, a Lewis binding strain, is constituted by the conserved amino acids and interacts with the α-1,3/4 fucose of the Lewis y antigen in a similar way like that of VA387 (Y. Chen, X. Jiang and X. Li, to be published data).
The two types of binding interfaces differ also in their binding modes to HBGAs. Based on crystal structures of the P dimers complexed with oligosaccharides, Norwalk virus has a smaller or narrower binding interface, while VA387 has a larger or broader one (Fig. 1, [13], [14], [15]). As a result only two sugars of the A trisaccharide and the H pentasaccharide are involved in interaction with Norwalk virus [13], [15], as opposed to all three sugars of the A and B trisaccharides in case of VA387 [14], [35]. In addition, more amino acid residues of VA387 appear to be involved in binding to HBGAs, compared to Norwalk virus. Specifically, crystal structures revealed 11 residues of VA387 P domain interacting with the B trisaccharides, as opposed to only 7 in case of Norwalk virus P domain binding to the A or H oligosaccharides [13], [14], [15]. Furthermore, mutagenesis studies mapped another 8 amino acids around the binding interface of VA387 affecting the binding function [35], while only 2 such residues of Norwalk virus were found (this report). Nevertheless, the aforementioned binding modes are based solely on the P dimers interacting with oligosaccharides under the condition of co-crystallization. The native interactions between norovirus and HBGAs in vivo remain to be elucidated.
Another observation emerging from this study is the possibility of interplay between convergent and divergent evolution of noroviruses. The two major genogroups (GI and GII) of human noroviruses are characterized by distinct genetic traits with significant differences in the primary sequence within their P domains. These two distinct lineages may have evolved in the course of divergent evolution from a common ancestor. On the other hand, the acquisition of the common function of binding to HBGAs by distinct binding interfaces and modes is consistent with functional convergence as a result of adaptation to and selection by the same niche of human HBGAs. The two strains described in this study, VA387 and Norwalk virus, provide strong support for this hypothesis. Convergent evolution of protein function and/or structure in conjunction with acquired ligand binding specificity has been observed previously [37], [38], [39]. One such example includes sugar binding families of LacI/GalR repressors and their PBP analogues, in which evolutionarily divergent lineages acquired independently similar ligand binding patterns through convergent evolution [40].
The fact that almost all known HBGAs have their noroviral counterparts suggests that noroviruses are highly adaptive human pathogens. In addition, it has been noted that some strains with conserved binding interfaces appear not to recognize HBGAs, such as the Desert Shield virus (DSV, GI-3) [22] and Hunter virus (GII-4) [41], while other strains lacking the conserved binding interfaces retain the HBGA-binding ability, such as OIF of the GII-13 noroviruses [22], [42]. These variations further highlight the adaptive nature of noroviruses that may recognize other carbohydrates or even non-carbohydrates as receptors. As long as noroviruses remain a human pathogen, the diversity of HBGA-binding patterns seen today will probably extend into the future.
Limited studies have shown that the GI and GII noroviruses are biologically different. For example, the GI noroviruses are more involved in environmental contamination and cause outbreaks year around without apparent seasonal peaks, while GII strains are easier to spread via person-to-person contact [9], [10], [11] and commonly cause outbreaks with clear fall/winter peaks. While future studies are required to identify factors and genetic markers responsible for these differences, this work can help to elucidate the evolutionary relatedness of the GI and GII noroviruses and improve the classification of caliciviruses (Figure 6). Each of the four major genera and the two newly discovered “Becovirus” [3] and “Recovirus” [2] genera should represent an evolutionary lineage in this virus family. While each of them has adapted well into individual host species, the binding to carbohydrates has apparently been maintained or acquired in at least some strains of most genera of caliciviruses, other than human noroviruses. For example, the rabbit hemorrhagic disease virus (RHDV) of the Lagovirus and the Tulane virus (TV) of the Recovirus recognize HBGAs [33], [34], while feline calicivirus (FCV) bind to sialic acid [43]. Since the common ancestor of these genetically distinct species might not possess the HBGA binding trait, one might speculate that these common characteristics were acquired independently as a result of adapting to similar biological niches, suggesting a possible convergent evolution of caliciviruses.
Our mutagenesis study further demonstrated that, in addition to the conserved binding sites, a number of nearby amino acids also play an important role in the binding specificity to HBGAs, possibly by contributing to the conformational flexibility of the carbohydrate binding interfaces, and these residues are less conserved. For example, residues Q331, K348, I389, and G392 of VA387 are likely involved in the binding to the A but not the B antigens [35], while S338, A430 and Y431 of Norwalk virus affect the binding strongly to H but weakly to A antigen (this study). Similar role of D393 of another GII-4 strain was also observed [41]. The recent studies on the globally dominant GII-4 noroviruses suggests that the host herd immunity may play a role in the epochal evolution of GII-4 viruses [41], [44]. Future studies focusing on these non-conserved residues for their potential roles in the antigenicity and immunogenicity of the viruses may be necessary.
In this study 39 mutant P particles of four strains (Norwalk, Boxer, MOH, and VA207) have been generated to address the conservation issue of the HBGA binding interfaces of noroviruses. This task would be very difficult to complete by using the VLPs as the model, because VLP production are very time-consuming compared to P particles. In our previous studies we have demonstrated that the P particle is a good model for studying norovirus-HBGAs interaction by the observations that P particle uses the same HBGA binding interface and shares very similar HBGA binding profile as that of its VLP counterpart [17], [18], [35], [36]. In addition, we used the saliva binding assay for its simplicity, convenience and sensitivity. All saliva samples used in this report have been well characterized for their phenotypes and binding patterns to noroviruses in our previous studies [17], [18], [21], [22], [35], [36], [45]. We do not expect significant differences with respect to synthetic oligosaccharide-based assays in evaluation of the importance of HBGA binding sites.
The findings of the conservation of HBGA-binding interfaces within genogroups can greatly facilitate the design and development of therapeutics against noroviruses. For example, a single compound that inhibits the function of the conserved HBGA-binding interface may be capable of blocking infection of all strains with the same type of HBGA binding interface. Thus, only two compounds might be sufficient to block most noroviruses in the two genogroups studied here, each group sharing a similar binding interface that could be blocked by one common inhibitor.
Acknowledgments
We thank Weiming Zhong for technical support in performing the protein expression and Dr. Xuejun C. Zhang for helpful comments to this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The research described in this article was supported by the National Institute of Health, the National Institute of Allergy and Infectious diseases (R01 AI37093 and R01 AI55649) and National Institute of Child Health (PO1 HD13021), and the Department of Defense (PR033018) to X.J. This work was also supported by a grant from the Translational Research Initiative of Cincinnati Children's Hospital Medical Center (SPR102032) to M.T. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Green K, Chanock R, Kapikian A. Human Calicivirus. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields Virology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 841–874. [Google Scholar]
- 2.Farkas T, Sestak K, Wei C, Jiang X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J Virol. 2008;82:5408–5416. doi: 10.1128/JVI.00070-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver SL, Asobayire E, Dastjerdi AM, Bridger JC. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology. 2006;350:240–250. doi: 10.1016/j.virol.2006.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng DP, Ando T, Fankhauser RL, Beard RS, Glass RI, et al. Norovirus classification and proposed strain nomenclature. Virology. 2006;346:312–323. doi: 10.1016/j.virol.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Estes MK, Prasad BV, Atmar RL. Noroviruses everywhere: has something changed? Curr Opin Infect Dis. 2006;19:467–474. doi: 10.1097/01.qco.0000244053.69253.3d. [DOI] [PubMed] [Google Scholar]
- 6.Tan M, Jiang X. Norovirus gastroenteritis, increased understanding and future antiviral options. Curr Opin Investig Drugs. 2008;9:146–151. [PubMed] [Google Scholar]
- 7.Scipioni A, Mauroy A, Vinje J, Thiry E. Animal noroviruses. Vet J. 2008;178:32–45. doi: 10.1016/j.tvjl.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 8.Wobus CE, Thackray LB, Virgin HWt. Murine norovirus: a model system to study norovirus biology and pathogenesis. J Virol. 2006;80:5104–5112. doi: 10.1128/JVI.02346-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Silva AK, Le Saux JC, Parnaudeau S, Pommepuy M, Elimelech M, et al. Evaluation of removal of noroviruses during wastewater treatment, using real-time reverse transcription-PCR: different behaviors of genogroups I and II. Appl Environ Microbiol. 2007;73:7891–7897. doi: 10.1128/AEM.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moe C, Honorat E, Leon J, Eisenberg J. Epidemiologic patterns of published norovirus outbreak reports, 1981–2006. Program and Abstracts of Third International Calicivirus Conference. 2007:39. [Google Scholar]
- 11.Hedlund K, Anderson Y, Hjertqvist M, Lysen M. Norovirus genogroup I dominates in Swedish Waterborne Outbreaks. Program and Abstracts of Third International Calicivirus Conference. 2007:38. [Google Scholar]
- 12.Prasad BV, Hardy ME, Dokland T, Bella J, Rossmann MG, et al. X-ray crystallographic structure of the Norwalk virus capsid. Science. 1999;286:287–290. doi: 10.1126/science.286.5438.287. [DOI] [PubMed] [Google Scholar]
- 13.Bu W, Mamedova A, Tan M, Xia M, Jiang X, et al. Structural basis for the receptor binding specificity of Norwalk virus. J Virol. 2008;82:5340–5347. doi: 10.1128/JVI.00135-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao S, Lou Z, Tan M, Chen Y, Liu Y, et al. Structural basis for the recognition of blood group trisaccharides by norovirus. J Virol. 2007;81:5949–5957. doi: 10.1128/JVI.00219-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choi JM, Hutson AM, Estes MK, Prasad BV. Atomic resolution structural characterization of recognition of histo-blood group antigens by Norwalk virus. Proc Natl Acad Sci U S A. 2008;105:9175–9180. doi: 10.1073/pnas.0803275105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan M, Hegde RS, Jiang X. The P domain of norovirus capsid protein forms dimer and binds to histo-blood group antigen receptors. J Virol. 2004;78:6233–6242. doi: 10.1128/JVI.78.12.6233-6242.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tan M, Jiang X. The p domain of norovirus capsid protein forms a subviral particle that binds to histo-blood group antigen receptors. J Virol. 2005;79:14017–14030. doi: 10.1128/JVI.79.22.14017-14030.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tan M, Meller J, Jiang X. C-terminal arginine cluster is essential for receptor binding of norovirus capsid protein. J Virol. 2006;80:7322–7331. doi: 10.1128/JVI.00233-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greenberg HB, Valdesuso JR, Kalica AR, Wyatt RG, McAuliffe VJ, et al. Proteins of Norwalk virus. J Virol. 1981;37:994–999. doi: 10.1128/jvi.37.3.994-999.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy ME, White LJ, Ball JM, Estes MK. Specific proteolytic cleavage of recombinant Norwalk virus capsid protein. J Virol. 1995;69:1693–1698. doi: 10.1128/jvi.69.3.1693-1698.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang P, Farkas T, Marionneau S, Zhong W, Ruvoen-Clouet N, et al. Noroviruses Bind to Human ABO, Lewis, and Secretor Histo-Blood Group Antigens: Identification of 4 Distinct Strain-Specific Patterns. J Infect Dis. 2003;188:19–31. doi: 10.1086/375742. [DOI] [PubMed] [Google Scholar]
- 22.Huang P, Farkas T, Zhong W, Tan M, Thornton S, et al. Norovirus and histo-blood group antigens: demonstration of a wide spectrum of strain specificities and classification of two major binding groups among multiple binding patterns. J Virol. 2005;79:6714–6722. doi: 10.1128/JVI.79.11.6714-6722.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. Norwalk virus infection associates with secretor status genotyped from sera. J Med Virol. 2005;77:116–120. doi: 10.1002/jmv.20423. [DOI] [PubMed] [Google Scholar]
- 24.Hutson AM, Atmar RL, Graham DY, Estes MK. Norwalk virus infection and disease is associated with ABO histo-blood group type. J Infect Dis. 2002;185:1335–1337. doi: 10.1086/339883. [DOI] [PubMed] [Google Scholar]
- 25.Hutson AM, Atmar RL, Marcus DM, Estes MK. Norwalk virus-like particle hemagglutination by binding to h histo-blood group antigens. J Virol. 2003;77:405–415. doi: 10.1128/JVI.77.1.405-415.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrington PR, Lindesmith L, Yount B, Moe CL, Baric RS. Binding of Norwalk virus-like particles to ABH histo-blood group antigens is blocked by antisera from infected human volunteers or experimentally vaccinated mice. J Virol. 2002;76:12335–12343. doi: 10.1128/JVI.76.23.12335-12343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrington PR, Vinje J, Moe CL, Baric RS. Norovirus Capture with Histo-Blood Group Antigens Reveals Novel Virus-Ligand Interactions. J Virol. 2004;78:3035–3045. doi: 10.1128/JVI.78.6.3035-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lindesmith L, Moe C, Marionneau S, Ruvoen N, Jiang X, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med. 2003;9:548–553. doi: 10.1038/nm860. [DOI] [PubMed] [Google Scholar]
- 29.Marionneau S, Ruvoen N, Le Moullac-Vaidye B, Clement M, Cailleau-Thomas A, et al. Norwalk virus binds to histo-blood group antigens present on gastroduodenal epithelial cells of secretor individuals. Gastroenterology. 2002;122:1967–1977. doi: 10.1053/gast.2002.33661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan M, Jiang X. Norovirus and its histo-blood group antigen receptors: an answer to a historical puzzle. Trends Microbiol. 2005;13:285–293. doi: 10.1016/j.tim.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 31.Tan M, Jiang X. Norovirus-host interaction: implications for disease control and prevention. Expert Rev Mol Med. 2007;9:1–22. doi: 10.1017/S1462399407000348. [DOI] [PubMed] [Google Scholar]
- 32.Shirato H, Ogawa S, Ito H, Sato T, Kameyama A, et al. Noroviruses distinguish between type 1 and type 2 histo-blood group antigens for binding. J Virol. 2008;82:10756–10767. doi: 10.1128/JVI.00802-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ruvoen-Clouet N, Ganiere JP, Andre-Fontaine G, Blanchard D, Le Pendu J. Binding of rabbit hemorrhagic disease virus to antigens of the ABH histo-blood group family. J Virol. 2000;74:11950–11954. doi: 10.1128/jvi.74.24.11950-11954.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Farkas T, Sestak K, Jiang X. Tulane virus specifically binds to type B histo-blood group antigen. Scientific Program and Abstracts of 27th Annual Meeting for American Society for Virology. 2008:271. [Google Scholar]
- 35.Tan M, Xia M, Cao S, Huang P, Farkas T, et al. Elucidation of strain-specific interaction of a GII-4 norovirus with HBGA receptors by site-directed mutagenesis study. Virology. 2008;379:324–334. doi: 10.1016/j.virol.2008.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tan M, Fang P, Chachiyo T, Xia M, Huang P, et al. Noroviral P particle: structure, function and applications in virus-host interaction. Virology. 2008;382:115–123. doi: 10.1016/j.virol.2008.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Briscoe AD. Six opsins from the butterfly Papilio glaucus: molecular phylogenetic evidence for paralogous origins of red-sensitive visual pigments in insects. J Mol Evol. 2000;51:110–121. doi: 10.1007/s002390010071. [DOI] [PubMed] [Google Scholar]
- 38.Stewart CB, Schilling JW, Wilson AC. Adaptive evolution in the stomach lysozymes of foregut fermenters. Nature. 1987;330:401–404. doi: 10.1038/330401a0. [DOI] [PubMed] [Google Scholar]
- 39.Sumiyama K, Kitano T, Noda R, Ferrell RE, Saitou N. Gene diversity of chimpanzee ABO blood group genes elucidated from exon 7 sequences. Gene. 2000;259:75–79. doi: 10.1016/s0378-1119(00)00440-6. [DOI] [PubMed] [Google Scholar]
- 40.Fukami-Kobayashi K, Tateno Y, Nishikawa K. Parallel evolution of ligand specificity between LacI/GalR family repressors and periplasmic sugar-binding proteins. Mol Biol Evol. 2003;20:267–277. doi: 10.1093/molbev/msg038. [DOI] [PubMed] [Google Scholar]
- 41.Lindesmith LC, Donaldson EF, Lobue AD, Cannon JL, Zheng DP, et al. Mechanisms of GII.4 norovirus persistence in human populations. PLoS Med. 2008;5:e31. doi: 10.1371/journal.pmed.0050031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan M, Zhong W, Song D, Thornton S, Jiang X. E. coli-expressed recombinant norovirus capsid proteins maintain authentic antigenicity and receptor binding capability. J Med Virol. 2004;74:641–649. doi: 10.1002/jmv.20228. [DOI] [PubMed] [Google Scholar]
- 43.Stuart AD, Brown TD. Alpha2,6-linked sialic acid acts as a receptor for Feline calicivirus. J Gen Virol. 2007;88:177–186. doi: 10.1099/vir.0.82158-0. [DOI] [PubMed] [Google Scholar]
- 44.Siebenga JJ, Vennema H, Renckens B, de Bruin E, van der Veer B, et al. Epochal evolution of GGII.4 norovirus capsid proteins from 1995 to 2006. J Virol. 2007;81:9932–9941. doi: 10.1128/JVI.00674-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan M, Huang P, Meller J, Zhong W, Farkas T, et al. Mutations within the P2 Domain of Norovirus Capsid Affect Binding to Human Histo-Blood Group Antigens: Evidence for a Binding Pocket. J Virol. 2003;77:12562–12571. doi: 10.1128/JVI.77.23.12562-12571.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]