Abstract
OBJECTIVE
There is a high prevalence of hypogonadism in men with type 2 diabetes. This will lead to an increase in assessments of hypogonadism. Statins could potentially decrease testosterone levels by reducing the availability of cholesterol for androgen synthesis. We compared testosterone levels and hypogonadal symptoms with statin use in a cross-sectional study of 355 men with type 2 diabetes.
RESEARCH DESIGN AND METHODS
Total testosterone, sex hormone–binding globulin (SHBG), and estradiol were measured by an enzyme-linked immunosorbent assay. Bioavailable testosterone was measured by the modified ammonium sulfate precipitation method. Free testosterone was calculated using Vermeulen's formula. Symptoms of hypogonadism were assessed using the Androgen Deficiency in the Aging Male questionnaire.
RESULTS
Statins were associated with lower total testosterone (11.9 vs. 13.4 nmol/l, P = 0.006) and a trend toward lower SHBG (29.4 vs. 35.3 nmol/l, P = 0.034) compared with no treatment. Bioavailable testosterone, free testosterone, estradiol, and hypogonadal symptoms were not affected. Subanalysis showed that atorvastatin was associated with reduced total testosterone (11.4 vs. 13.4 nmol/l, P = 0.006) and a trend toward reduced SHBG (27.6 vs. 35.3 nmol/l, P = 0.022) compared with no treatment, and there was an apparent dose-response effect with the lowest levels of total testosterone seen in men treated with ≥20 mg atorvastatin (9.6 nmol/l, P = 0.017). Simvastatin use was not associated with significant reductions in testosterone or SHBG levels.
CONCLUSIONS
Assessing androgen status using total testosterone in men with type 2 diabetes treated with statins, particularly atorvastatin, may potentially lead to diagnostic error. Levels of bioavailable testosterone or free testosterone are recommended for the assessment of hypogonadism in this group if total testosterone levels are borderline.
There is evidence that men with the metabolic syndrome, type 2 diabetes, and cardiovascular disease have a high prevalence of low circulating levels of testosterone (1). A significant proportion of these men are hypogonadal, defined as a combination of clinical symptoms and biochemical evidence of testosterone deficiency (2). The lower limit of normal serum testosterone levels, which defines the biochemical diagnosis of hypogonadism, is not fully clear. Studies have shown that about 20% of men with metabolic syndrome, diabetes, and cardiovascular disease have testosterone levels below the normal range, and there are a further 20–25% with levels in the low normal range that may also be compatible with a diagnosis of hypogonadism, depending on clinical symptoms (1,2).
The great majority of men with diabetes and cardiovascular disease are being treated with statins. Cholesterol is the substrate for testosterone biosynthesis, and theoretically, hydroxymethylglutaryl-CoA reductase inhibitors such as statins could affect serum testosterone levels. Animal studies have shown that statins can reduce testosterone production when given in high doses (3). Studies in humans, mostly involving small numbers of men, have shown various results. The majority have shown no effect of statins on testosterone levels (4–8), but some have shown reduced testosterone levels with simvastatin treatment (9–11).
Total testosterone is the most widely used biochemical test in the diagnosis of men with hypogonadism. Total testosterone comprises free testosterone (2–3%) and testosterone bound to either sex hormone–binding globulin (SHBG) (60–80%) or albumin (20–40%) (12). Free plus albumin-bound testosterone is generally regarded as the biologically active or bioavailable component. Testosterone bound to SHBG is considered to be inactive although recent research has suggested that SHBG-bound testosterone may be taken into cells by endocytosis, possibly allowing biological actions (13). Studies have demonstrated that bioavailable or free testosterone better reflects androgen status, for example, by correlations with bone mineral density (14) and erectile dysfunction (15). Laboratory measures of bioavailable and free testosterone are not routinely available, as they are time-consuming and only small numbers of tests can be analyzed simultaneously. Mathematical formulae to calculate these fractions based on total testosterone and SHBG are available but have to be validated locally before clinical use because of differences in total testosterone assays (16,17).
We have here analyzed the effect of statins on testosterone levels in a cross-sectional epidemiological study of men with type 2 diabetes. The initial study was the first to investigate the prevalence of hypogonadism in men with diabetes rather than only assessing testosterone levels (2). It showed that 14% of the men had subnormal levels of assayed bioavailable testosterone, 17% had total testosterone levels <8 nmol/l (231 ng/dl), and 42% had total testosterone levels <12 nmol/l (346 ng/dl); these are arbitrary cutoffs for levels compatible with hypogonadism taken from international guidelines (18). We have reexamined the effect of the statins on all testosterone parameters and clinical hypogonadism in this cohort compared with those men not taking statins. The clinical data were collated in years 2002–2003 when only approximately half of the men in the study were treated with statins.
RESEARCH DESIGN AND METHODS
Men, aged >30 years, with type 2 diabetes were recruited from Barnsley Hospital NHS Trust, Barnsley, U.K., in years 2002–2003. Subjects were approached during outpatient appointments at the Centre for Diabetes and Endocrinology. Most of the subjects were recruited from the retinal screening program, which is attended by virtually all patients with diabetes in the Barnsley area. Subjects were given written and verbal information regarding the study, and 355 men gave their informed consent to take part. The study population comprised patients with diabetes usually managed in primary care facilities as well as secondary care patients. All participants were of Caucasian origin.
Demography, medical history, and drug histories were collected using a questionnaire. Clinical and biochemical assessments of androgen status were made. Other measurements included blood pressure, A1C, nonfasting lipid levels, height, weight, and waist circumference. The main data from the study were published previously in Diabetes Care (2). This is a subanalysis of data relating to statin use.
Assessments
Patients were seen in the morning between 8:00 and 10:00 a.m. Symptoms of hypogonadism were assessed by completion of the Androgen Deficiency in the Aging Male (ADAM) questionnaire, which was validated to assess hypogonadism in aging men (19). Venous blood was taken, and serum samples were produced by centrifugation. Patients did not fast before having samples taken. Serum samples were then stored at −20°C for future analysis. Serum total testosterone, total estradiol, and SHBG were measured by an enzyme-linked immunosorbent assay using commercially available kits (DRG Diagnostics, Marburg, Germany). Bioavailable testosterone was determined by a modification of the ammonium sulfate precipitation method described by Tremblay and Dube (20). Free testosterone was calculated from total testosterone and SHBG by the formula of Vermeulen et al. (16). These methods of assessing bioavailable and free testosterone have been used in previous studies from our research team and have determined to be reliable for the assessment of men with diabetes and vascular disease (17).
Weight and height were recorded and used to derive BMI. Waist circumference was measured midway between the lower costal margins and the iliac crests. Blood pressure was recorded using a manual sphygmomanometer. A1C was assessed using a Menarini Analyser HA8160 (Menarini Diagnostics, Florence, Italy). Serum lipid parameters were assessed by Olympus analyzers (Olympus Diagnostics, Hamburg, Germany).
Statistical analysis
Data were analyzed using the SPSS package (SPSS, Chicago, IL). Testosterone and SHBG were log-normally distributed and were converted to a normal distribution to allow use of Student's t test for comparison of group means. With smaller groups, the normal distribution was assessed using single-sample Kolmogorov-Smirnov testing. The two-sample Kolmogorov-Smirnov test was used to compare groups when data did not fulfill the normal distribution. Results were initially considered statistically significant at P < 0.05. Because of multiple statistical testing, we performed a Bonferroni correction to minimize the chance of type 2 errors. This resulted in statistical significance being defined as P < 0.0102.
RESULTS
Statin use
In our cross-section of 355 men with type 2 diabetes, 186 were not treated with statins. Of 169 men treated with statins, 81 were treated with atorvastatin, 66 with simvastatin, 15 with pravastatin, and 7 with other statins (3 with fluvastatin, 1 with cerivastatin, and 3 with “unknown statin”). Doses were not known for some patients.
Comparison of statin-treated men with untreated men
Patients treated with statins did not significantly differ from untreated individuals in age, waist circumference, BMI, A1C, or blood pressure (Table 1). Total cholesterol and LDL cholesterol levels were lower in men taking statins, but there were no significant differences in HDL cholesterol or triglyceride levels (data not shown).
Table 1.
Mean sex hormone levels, measures of obesity, and cholesterol levels in men treated with any statin, atorvastatin, and simvastatin compared with untreated men
| No statin | Statin | P value | Atorvastatin | P value | Simvastatin | P value | |
|---|---|---|---|---|---|---|---|
| Total testosterone (nmol/l) | 13.4 | 11.9 | 0.006 | 11.4 | 0.006 | 12.47 | 0.13 |
| Bioavailable testosterone (nmol/l) | 4.14 | 3.90 | 0.133 | 3.82 | 0.151 | 3.96 | 0.292 |
| Free testosterone (nmol/l) | 0.284 | 0.263 | 0.228 | 0.259 | 0.308 | 0.266 | 0.125 |
| SHBG (nmol/l) | 35.3 | 29.4 | 0.034 | 27.6 | 0.022 | 31.8 | 0.392 |
| Estradiol (pmol/l) | 23.2 | 24.0 | 0.763 | 24.7 | 0.193 | 24.0 | 0.705 |
| Waist circumference (cm) | 109.6 | 109.8 | 0.919 | 111.9 | 0.26 | 108.3 | 0.513 |
| BMI (kg/m2) | 32.19 | 32.47 | 0.646 | 33.52 | 0.108 | 31.25 | 0.252 |
| Total cholesterol (mmol/l) | 5.04 | 4.58 | <0.001 | 4.64 | 0.001 | 4.50 | <0.001 |
| ADAM score | 4.15 | 4.26 | 0.26 | 4.08 | 0.809 | 4.3 | 0.675 |
P values given for Student's t test comparing treated patients with the untreated group. Significant results (P < 0.01) are shown in boldface. Total testosterone was significantly lower in the statin and atorvastatin groups but not in the simvastatin group. Bioavailable and free testosterone levels were not significantly lower in any group. Both statins were associated with lower cholesterol levels, but none of the groups were significantly different in terms of obesity.
Total testosterone levels were significantly lower in men treated with statins, and there was a trend toward lower SHBG levels, but this did not reach statistical significance (Table 1). Bioavailable testosterone, calculated free testosterone, and serum estrogen levels were not significantly different between the groups. The symptom score from the ADAM questionnaire was not significantly altered in those men receiving statin therapy.
Comparison of simvastatin- or atorvastatin-treated men with untreated men
There were sufficient numbers of men treated with simvastatin and atorvastatin to consider these groups more closely (Tables 1 and 2). These groups were not significantly different from untreated men in terms of BMI, waist circumference, blood pressure, A1C, or age. Total cholesterol and LDL cholesterol levels were lower in both groups compared with those in untreated men but were not significantly different between simvastatin- and atorvastatin-treated groups. Men treated with simvastatin did not have significantly different total, bioavailable, or free testosterone, estradiol, or SHBG levels than untreated men (Fig. 1 and Table 1). In contrast, men treated with atorvastatin had an average total testosterone level 1.96 nmol/l (56 ng/dl) less than that in untreated men. There was a trend toward lower SHBG levels in this group (35.3 vs. 27.6 nmol/l, P = 0.022). Estradiol and free and bioavailable testosterone were not significantly affected. Further analysis revealed an apparent dose-response relationship, with the lowest testosterone levels seen in men taking higher doses of atorvastatin (Table 3). The average total testosterone level of men taking ≥20 mg atorvastatin was 3.78 nmol/l (108 ng/dl) less than that of untreated men (Table 3), but this value did not reach statistical significance (P = 0.017). SHBG was also reduced without reaching significance (P = 0.043), but free and bioavailable testosterone levels were unaltered. No significant differences were seen in sex hormone levels when the simvastatin-treated men were split into similar groups (data not shown). There were no significant differences in age, BMI, waist circumference, blood pressure, or A1C in any of the atorvastatin- or simvastatin-treated subgroups.
Table 2.
Characteristics of patients untreated with statins and those treated with atorvastatin and simvastatin
| No statin | Atorvastatin | P value | Simvastatin | P value | |
|---|---|---|---|---|---|
| n | 169 | 81 | 66 | ||
| Age (years) | 58.3 | 57.9 | 0.75 | 60.7 | 0.1 |
| Waist circumference (cm) | 109.6 | 111.9 | 0.26 | 108.3 | 0.51 |
| BMI (kg/m2) | 32.2 | 33.5 | 0.11 | 31.3 | 0.25 |
| A1C (%) | 7.2 | 7.2 | 0.82 | 7.14 | 0.6 |
| Systolic blood pressure (mmHg) | 145.1 | 140.7 | 0.09 | 142.3 | 0.33 |
| Diastolic blood pressure (mmHg) | 82.8 | 81.36 | 0.33 | 81.4 | 0.38 |
| Total cholesterol (mmol/l) | 5.04 | 4.64 | 0.001 | 4.5 | <0.001 |
| HDL cholesterol (mmol/l) | 1.16 | 1.08 | 0.03 | 1.16 | 0.93 |
| LDL cholesterol (mmol/l) | 2.9 | 2.34 | <0.001 | 2.27 | <0.001 |
| Triglycerides (mmol/l) | 2.44 | 2.84 | 0.19 | 2.54 | 0.78 |
P values given for Student's t test. Significant results (P < 0.01) are shown in boldface. There were no significant differences in age, anthropomorphic data, glycemic control, or blood pressure. Total and LDL cholesterol are lower in the statin-treated group, reflecting the primary action of the drugs.
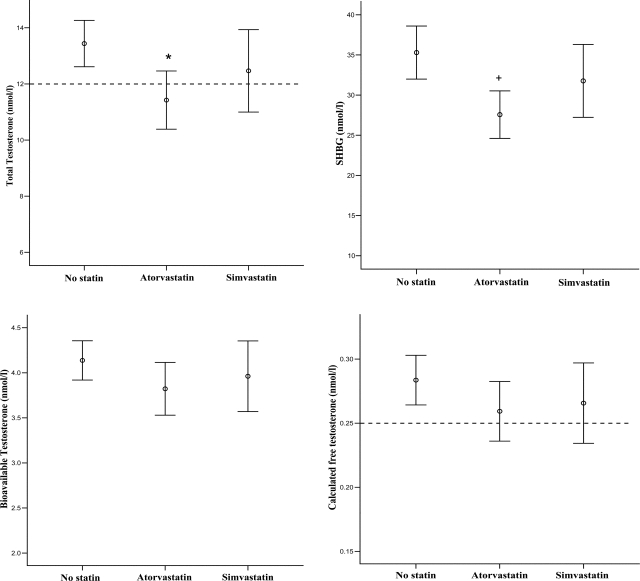
Figure 1.
Mean testosterone levels in untreated men and those treated with atorvastatin or simvastatin; 95% CIs are shown. International guidelines suggest that testosterone replacement is invariably warranted when the total testosterone level is <8 nmol/l (231 ng/dl) or the free testosterone level is <0.18 nmol/l. Treatment may also benefit men with a total testosterone level between 8 and 12 nmol/l (346 ng/dl) or a free testosterone between 0.18 and 0.25 nmol/l. *P < 0.01; + P < 0.05.
Table 3.
Average testosterone, SHBG, and measures of obesity in men treated with atorvastatin split into two groups: those treated with 10 mg and those treated with 20 mg or more
| No statin | Atorvastatin |
||||
|---|---|---|---|---|---|
| 10 mg | P value | ≥20 mg | P value | ||
| n | 186 | 38 | 14 | ||
| Total testosterone (nmol/l) | 13.4 | 11.9 | 0.102 | 9.63 | 0.017 |
| Bioavailable testosterone (nmol/l) | 4.14 | 3.8 | 0.197 | 3.71 | 0.598 |
| Free testosterone (nmol/l) | 0.284 | 0.257 | 0.129 | 0.249 | 0.538 |
| SHBG (nmol/l) | 35.3 | 30.1 | 0.322 | 21.7 | 0.043 |
| Waist circumference (cm) | 109.8 | 111.4 | 0.5 | 115 | 0.829 |
| BMI (kg/m2) | 32.47 | 33.19 | 0.348 | 34.87 | 0.0.755 |
| Total cholesterol (mmol/l) | 5.04 | 4.55 | 0.003 | 4.82 | 0.224 |
The 10-mg atorvastatin group was compared with the no statin group with Student's t test; significant results are shown in boldface. The ≥20-mg atorvastatin group was compared with the no statin group using a two-sample Kolmogorov-Smirnov test. There is a trend toward lower total testosterone and SHBG levels in men taking the higher doses of atorvastatin, but this does not reach statistical significance.
CONCLUSIONS
This is the first study to analyze fully testosterone levels and hypogonadal symptoms in comparison with statin use in a population receiving routine medical management. Those treated with atorvastatin had lower levels of total testosterone with a trend toward lower SHBG compared with untreated men, whereas those treated with simvastatin were not significantly affected. The effects were particularly evident in those men taking higher doses of atorvastatin. Statin treatment did not significantly affect the biologically active fractions of testosterone, symptoms of hypogonadism, or estradiol levels.
This study demonstrates that treatment with statins in this group can be a confounding factor in the assessment of hypogonadism. Total testosterone is the primary test used in the diagnosis of hypogonadism, and the effect of atorvastatin could potentially lead to misdiagnosis of the condition in men with normal free and bioavailable testosterone levels. Alternatively, it is possible that reductions in total testosterone without changes in bioactive testosterone fractions have clinical effects in view of findings suggesting that SHBG-bound testosterone may be biologically active (13). The ADAM questionnaire failed to detect an increase in hypogonadal symptoms in groups with lower total testosterone levels, but the questionnaire does not correlate closely with testosterone levels, and men with diabetes have a high level of false-positive hypogonadal symptoms (2) such as erectile dysfunction owing to vascular and neuropathic factors, medications, and depression.
The findings suggest that atorvastatin has significant biological effects on testosterone. Atorvastatin is a more potent statin than simvastatin, thus causing a greater reduction in the total cholesterol and hence total testosterone levels. No patients in our study were treated with other potent statins such as rosuvastatin so we are unable to confirm that atorvastatin reduces testosterone and SHBG levels secondary to more potent effects on cholesterol levels. Indeed, cholesterol and other lipid fraction levels were not significantly different between simvastatin- and atorvastatin-treated groups.
In a normal physiological state, luteinizing hormone (LH) promotes uptake of cholesterol by the testis and stimulates testosterone synthesis. A reduction in testosterone is sensed by the hypothalamic-pituitary axis and leads to greater LH release, which completes a negative feedback loop and maintains testosterone levels. The apparent failure of the hypothalamic-pituitary-testicular axis to respond and maintain testosterone levels in the statin-treated patients in our study may be explained by the hypogonadal-obesity-adipocytokine hypothesis (1). The majority of the patients in our study were overweight or obese. In this population, greater production of adipocytokines such as tumor necrosis factor-α, interleukin-6, and leptin and increased estradiol from metabolism of testosterone by aromatase in adipose tissue inhibit LH release from the pituitary gland, which leads to lower circulating testosterone levels. In our study, gonadotrophin levels were only tested in men with total testosterone levels <12 mmol/l (2), leaving us unable to test this hypothesis. The most likely reason that bioavailable and free testosterone levels are unaltered despite lower total testosterone is a homeostatic mechanism via reduced SHBG production.
An alternative hypothesis is that atorvastatin causes a primary reduction in SHBG with consequent reductions in total testosterone. There was a consistent trend toward low SHBG levels in all groups with reduced levels of total testosterone, although these low levels did not reach statistical significance. SHBG is produced in the liver, the primary site of action of the statins, but the mechanism by which statins could alter SHBG is unknown. SHBG levels are known to be modulated by a number of factors including downregulation by insulin resistance and upregulation by estrogens (21). Insulin resistance was not measured, but glycemic control was similar in all groups. Estrogen levels did not vary among groups.
We are aware of one clinical trial assessing the effects of atorvastatin on testosterone levels in men. It was small and of 3 months' duration. The results showed a nonsignificant fall in total testosterone and no change in SHBG (4). These data are insufficient to confirm or refute our findings relating to atorvastatin, and it may be that the study allowed insufficient time for changes to develop. There are more published data concerning the effects of simvastatin on testosterone. Some trials have shown that simvastatin reduces serum testosterone levels (9–11), whereas others have shown no effect (6–8). Our data suggest that any effect of simvastatin in reducing serum testosterone levels is not clinically significant in men with type 2 diabetes. We are not aware of any previous evidence for an effect of any statin on SHBG levels.
International guidelines have suggested that symptomatic men with total testosterone levels <8 nmol/l (231 ng/dl) are hypogonadal, that men with total testosterone levels >12 nmol/l (346 ng/dl) do not have hypogonadism, and that men with levels between 8 and 12 nmol/l need consideration for treatment depending on their clinical picture (18). An Endocrine Society Clinical Practice Guideline suggested a diagnostic cutoff value of testosterone of <10.4 nmol/l (300 ng/dl) for hypogonadism. Our findings are important in this context because statin treatment was associated with lower total testosterone levels of 11.9 nmol/l (340 ng/dl) (versus 13.4 nmol/l [384 ng/dl]). Men treated with ≥20 mg/day atorvastatin had an average total testosterone level of only 9.6 nmol/l (275 ng/dl), which is 3.8 nmol/l (109 ng/dl) lower than that of men not treated with statins. Thus, statin treatment may lead to reductions in total testosterone levels to <12 or 10.4 nmol/l in many men but will not significantly alter bioavailable or free testosterone levels or hypogonadal symptoms. We recommend a low threshold for measuring or calculating bioavailable or free testosterone in men receiving statin therapy.
Testosterone levels are frequently reduced in men with type 2 diabetes (2,22). It has now been confirmed that reductions in total testosterone are accompanied by similar changes in free and bioavailable testosterone and a high prevalence of hypogonadal symptoms (2). Furthermore, short-term studies in small numbers of hypogonadal diabetic men have shown improvements in glycemic control, central obesity, and serum leptin during testosterone replacement therapy (23,24). Other short-term studies have shown beneficial effects on further cardiovascular risk factors including total cholesterol levels (25). In this context, the assessment of hypogonadism in men with type 2 diabetes is likely to increase in frequency. Assessment of the clinical syndrome of hypogonadism can be challenging in this group because of the confounding effects of vascular disease, psychological factors, and medications on symptoms such as erectile dysfunction. It is therefore especially important to adequately assess biochemical testosterone status. This assessment is complicated by low SHBG levels, which have long been associated with insulin resistance, leading to suggestions that low testosterone levels in type 2 diabetes are due to low SHBG rather than to reductions in bioactive testosterone fractions. This suggestion has been refuted by studies showing low free and bioavailable testosterone levels in men with type 2 diabetes (2) and meta-analysis data suggesting relatively small changes in SHBG in men with diabetes (22). The realization that statin treatment may reduce total testosterone and SHBG levels in this patient group serves to refocus attention to a subgroup who are particularly likely to have low SHBG levels with the potential for a misdiagnosis of hypogonadism.
In summary, this large data set is the first to suggest a significant effect of statins in lowering total testosterone and SHBG levels in a population of men with type 2 diabetes. The findings have important implications for the diagnosis of hypogonadism in men receiving statin treatment. The opportunity to conduct similar studies of men with type 2 diabetes, comparing men treated with statins to untreated men, has now probably passed owing to the almost ubiquitous use of statins in this group. Limitations of this study are its observational nature and the resultant inability to prove causality. Statin-treated men did not differ from other men in terms of age, blood pressure, obesity, or glycemic control, but unidentified confounders cannot be excluded. Therefore, our findings require confirmation in appropriately powered randomized controlled trials. Further longitudinal or interventional studies are also needed to assess the effects of statins on androgen status in various patient groups and could include assessment of gonadotrophins and prolactin, which were not measured here. Researchers should also investigate mechanisms that lead to changes in SHBG and total testosterone in this context.
Acknowledgments
This study was funded by Barnsley Hospital Charitable fund for Endocrinology and grants from the Barnsley Research Alliance.
No potential conflicts of interest relevant to this article were reported.
Thanks are owed to Tracey Young from Sheffield University and Trent Research and Development Support Unit for her help with statistical analysis and to Emma Goodwin, Bernadette Hardware, and Hazel Aldred from Barnsley Hospital Research and Development for help in patient assessments.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Jones TH: Testosterone associations with erectile dysfunction, diabetes and the metabolic syndrome. Eur Urol Suppl 6: 847– 857, 2007 [Google Scholar]
- 2. Kapoor D, Aldred H, Clark S, Channer KS, Jones TH: Clinical and biochemical assessment of hypogonadism in men with type 2 diabetes: correlations with bioavailable testosterone and visceral adiposity. Diabetes Care 30: 911– 917, 2007 [DOI] [PubMed] [Google Scholar]
- 3. MacDonald JS, Gerson RJ, Kornbrust DJ, Kloss MW, Prahalada S, Berry PH, Alberts AW, Bokelman DL: Preclinical evaluation of lovastatin. Am J Cardiol 62: 16J– 27J, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Santini SA, Carrozza C, Lulli P, Zuppi C, CarloTonolo G, Musumeci S: Atorvastatin treatment does not affect gonadal and adrenal hormones in type 2 diabetes patients with mild to moderate hypercholesterolemia. J Atheroscler Thromb 10: 160– 164, 200 [DOI] [PubMed] [Google Scholar]
- 5. Nakaya N, Homma Y, Tamachi H, Shigematsu H, Hata Y, Goto Y: The effect of CS-514 on serum lipids and apolipoproteins in hypercholesterolemic subjects. JAMA 257: 3088– 3093, 1987 [PubMed] [Google Scholar]
- 6. Azzarito C, Boiardi L, Zini M, Agosti A, Dotti C, Biagi R, Portioli I: Long-term therapy with high-dose simvastatin does not affect adrenocortical and gonadal hormones in hypercholesterolemic patients. Metabolism 41: 148– 153, 1992 [DOI] [PubMed] [Google Scholar]
- 7. Dobs AS, Miller S, Neri G, Weiss S, Tate AC, Shapiro DR, Musliner TA: Effects of simvastatin and pravastatin on gonadal function in male hypercholesterolemic patients. Metabolism 49: 115– 121, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Travia D, Tosi F, Negri C, Faccini G, Moghetti P, Muggeo M: Sustained therapy with 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors does not impair steroidogenesis by adrenals and gonads. J Clin Endocrinol Metab 80: 836– 840, 1995 [DOI] [PubMed] [Google Scholar]
- 9. Dobs AS, Schrott H, Davidson MH, Bays H, Stein EA, Kush D, Wu M, Mitchel Y, Illingworth RD: Effects of high-dose simvastatin on adrenal and gonadal steroidogenesis in men with hypercholesterolemia. Metabolism 49: 1234– 1238, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Hyyppa MT, Kronholm E, Virtanen A, Leino A, Jula A: Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology 28: 181– 194, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Rossato M, Guarneri G, Lavagnini T, Padovan D, Foresta C: Simvastatin influences testicular steroidogenesis in human. Horm Metab Res 25: 503– 505, 1993 [DOI] [PubMed] [Google Scholar]
- 12. Rommerts FFG: Testosterone: an overview of biosynthesis, transport, metabolism and non-genomic actions. In Testosterone. Action, Deficiency, Substitution. Behre HM: Ed. Cambridge, Cambridge University Press, 2004, p. 1– 38 [Google Scholar]
- 13. Hammes A, Andreassen TK, Spoelgen R, Raila J, Hubner N, Schulz H, Metzger J, Schweigert FJ, Luppa PB, Nykjaer A, Willnow TE: Role of endocytosis in cellular uptake of sex steroids. Cell 122: 751– 762, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Lorentzon M, Swanson C, Andersson N, Mellstrom D, Ohlsson C: Free testosterone is a positive, whereas free estradiol is a negative, predictor of cortical bone size in young Swedish men: the GOOD study. J Bone Miner Res 20: 1334– 1341, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Kapoor D, Clarke S, Channer KS, Jones TH: Erectile dysfunction is associated with low bioactive testosterone levels and visceral adiposity in men with type 2 diabetes. Int J Androl 30: 500– 507, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Vermeulen A, Stoica T, Verdonck L: The apparent free testosterone concentration, an index of androgenicity. J Clin Endocrinol Metab 33: 759– 767, 1971 [DOI] [PubMed] [Google Scholar]
- 17. Morris PD, Malkin CJ, Channer KS, Jones TH: A mathematical comparison of techniques to predict biologically available testosterone in a cohort of 1072 men. Eur J Endocrinol 151: 241– 249, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Nieschlag E, Swerdloff R, Behre HM, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morley JE, Schulman C, Wang C, Weidner W, Wu FC: Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, and EAU recommendations Int J Androl 28: 125– 127, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Morley JE, Charlton E, Patrick P, Kaiser FE, Cadeau P, McCready D, Perry HM, 3rd: Validation of a screening questionnaire for androgen deficiency in aging males. Metabolism 49: 1239– 1242, 2000 [DOI] [PubMed] [Google Scholar]
- 20. Tremblay RR, Dube JY: Plasma concentrations of free and non-TeBG bound testosterone in women on oral contraceptives. Contraception 10: 599– 605, 1974 [DOI] [PubMed] [Google Scholar]
- 21. Hampl R, Starka L: Sex hormone-binding globulin in endocrine regulation (minireview). Endocr Regul 30: 57– 65, 1996 [PubMed] [Google Scholar]
- 22. Ding EL, Song Y, Malik VS, Liu S: Sex differences of endogenous sex hormones and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA 295: 1288– 1299, 2006 [DOI] [PubMed] [Google Scholar]
- 23. Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH: The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol 156: 595– 602, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Kapoor D, Goodwin E, Channer KS, Jones TH: Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur J Endocrinol 154: 899– 906, 2006 [DOI] [PubMed] [Google Scholar]
- 25. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH: The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab 89: 3313– 3318, 2004 [DOI] [PubMed] [Google Scholar]