Abstract
OBJECTIVE
To estimate and compare associations of alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) with incident diabetes.
RESEARCH DESIGN AND METHODS
ALT and GGT were studied as determinants of diabetes in the British Women's Heart and Health Study, a cohort of 4,286 women 60–79 years old (median follow-up 7.3 years). A systematic review and a meta-analysis of 21 prospective, population-based studies of ultrasonography, which diagnosed nonalcoholic fatty liver disease (NAFLD), ALT, and GGT as determinants of diabetes, were conducted, and associations of ALT and GGT with diabetes were compared.
RESULTS
Ultrasonography-diagnosed NAFLD was associated with more than a doubling in the risk of incident diabetes (three studies). ALT and GGT both predicted diabetes. The fully adjusted hazard ratio (HR) for diabetes per increase in one unit of logged ALT was 1.83 (95% CI 1.57–2.14, I2 = 8%) and for GGT was 1.92 (1.66–2.21, I2 = 55%). To directly compare ALT and GGT as determinants of diabetes, the fully adjusted risk of diabetes in the top versus bottom fourth of the ALT and GGT distributions was estimated using data from studies that included results for both markers. For ALT, the HR was 2.02 (1.59–2.58, I2 = 27%), and for GGT the HR was 2.94 (1.98–3.88, I2 = 20%), suggesting that GGT may be a better predictor (P = 0.05).
CONCLUSIONS
Findings are consistent with the role of liver fat in diabetes pathogenesis. GGT may be a better diabetes predictor than ALT, but additional studies with directly determined liver fat content, ALT, and GGT are needed to confirm this finding.
Nonalcoholic fatty liver disease (NAFLD) is characterized by accumulation of fat in the liver, with or without inflammation, fibrosis, and cirrhosis, in the absence of substantial alcohol consumption or other causes of liver disease such as viral hepatitis. In large epidemiological studies, NAFLD and liver fat content (a continuum) are commonly revealed by elevations in alanine aminotransferase (ALT), γ-glutamyltransferase (GGT), and ultrasonography.
High rates of elevated GGT levels were noted among diabetic patients over 40 years ago (1). Cross-sectional associations between abnormal GGT and dysglycemic states were also documented in the 1980s (e.g., the study by Ford et al. [(2)]). However, cross-sectional studies cannot determine causality because in most cases it is impossible to ascertain the temporal sequence between the events studied. The first longitudinal study to examine the association of a biomarker of NAFLD with incident diabetes was published in 1998 (3). Since then, both ALT and GGT, even within the normal range, have been reported to predict incident diabetes. However, while some studies have demonstrated a stronger association between GGT and diabetes than between ALT and diabetes (4,5), other studies have reported the opposite (6).
To our knowledge, no previous publication has systematically reviewed and meta-analyzed all prospective studies of the association between NAFLD and its markers with future diabetes risk. We are also unaware of any studies that have compared the magnitude of the relative associations between ALT and GGT with diabetes risk, despite claims for one or the other being a better marker of liver fat and thus diabetes risk. We therefore examined the separate associations of ALT and GGT with incident diabetes in a population of older British women and undertook a systematic review and meta-analysis of prospective population-based studies assessing the associations of NAFLD, ALT, or GGT with diabetes. Of note, while aspartate aminotransferase has also been assessed in some studies (e.g., in the study by André et al. [(4)]), it is not as sensitive or specific to liver damage as ALT or GGT (7) and is therefore not addressed here.
RESEARCH DESIGN AND METHODS
British Women's Heart and Health Study
Full details of the selection of participants and measurements have been previously reported (8–10). A total of 4,286 women, aged 60–79 years, were randomly recruited from 23 British towns. Baseline data (self-completed questionnaire, research nurse interview, physical examination, and medical record review) were collected between 1999 and 2001. These women have been followed up for a median of 7.3 years, to September 2007, by a detailed review of their medical records conducted every 2 years and by a self-completed questionnaire. Informed consent was obtained from the women, and the approval of both the local and multicenter ethics committees was obtained for the study.
Levels of GGT and ALT were determined in fresh serum samples using an automated analyzer (Technicon Sequential Multiple Analyzer; Technicon Instruments Corporation, Tarrytown, NY). Waist and hip circumference, lipids, fasting glucose and insulin, and blood pressure were measured using standard methods as previously described. Information on smoking, physical activity, social class, and alcohol consumption was obtained from a self-completed questionnaire and nurse interview (9).
Insulin resistance was estimated according to the homeostasis model assessment (HOMA) as the product of fasting glucose (millimoles per liter) and insulin (microunits per milliliter) divided by the constant 22.5 (11). Baseline diabetes (for exclusion of these cases) was defined according to the World Health Organization criteria as any woman with a doctor's diagnosis of diabetes (based on medical records review and self-report) and/or with a fasting glucose concentration ≥7 mmol/l (12). Incident cases of diabetes were defined as either a self-report of a doctor diagnosis of diabetes or evidence of diabetes in the follow-up medical record reviews.
Cox proportional hazards regression models were used to examine associations of exposures with incident diabetes in those with no evidence of diabetes at baseline. In the Cox proportional hazards regression models, the participant's age was the time axis and risk was assessed from the date of baseline examination for each woman. ALT and GGT were expressed in units of SD of their respective distributions. Contributions to risk were censored at the date of diagnosis or death from any other cause or at the end of the follow-up period (30 September 2007) for those who remained alive and free of diabetes.
Systematic review and meta-analysis
PubMed and EMBASE (Excerpta Medica Database) were systematically searched by A.F. in February 2008 for all prospective population-based studies evaluating the association among NAFLD (diagnosed by any imaging technique), ALT, GGT, and incident diabetes. No language restrictions were applied. Abstracts were scanned, relevant full-text publications were obtained, and inclusion criteria were applied (i.e., prospective studies conducted in general populations). Two independent reviewers (A.F. and D.A.L.) abstracted data. Reference lists of obtained articles were hand searched for additional potential studies. Study authors were contacted for additional data or clarification when required.
Two sets of meta-analyses were undertaken to reflect the two aims of this study. In the first set, a separate meta-analysis was conducted for each NAFLD marker, maximizing data use regardless of whether they provided data on ultrasound-defined NAFLD, ALT only, GGT only, or any combination of these. In these analyses, the assumption was that all study results for ALT (GGT or ultrasound) were part of the same distribution but not that the true effect was the same in all studies; therefore, a random-effects model was used, though we did also check our assumption for this random effect using the I2 measure, which quantifies the percentage of total variation across studies that is due to heterogeneity rather than chance (13).
Because different studies presented results on different scales (e.g., risk ratios [RRs] for quantiles of ALT or GGT compared with the lowest quantile or per category or logged unit per liter), for the first meta-analysis (of all studies) a standard statistical method was used to estimate the log hazard ratio (HR) per log unit increase in ALT or GGT, together with its SE (14,15). Results are presented as the HR per unit change in ALT or GGT on a log scale.
When data were presented according to quantiles or categories of ALT or GGT, the median or mean in each group, when reported, was used. When these were not reported, the mean in each group was estimated based on the distribution of subjects across groups, as outlined by Chêne and Thompson (15). All exposure (GGT and ALT) levels were converted to the log-normal scale if not already presented as such. The log HR per 1 unit/l increase was then estimated using the method of Greenland and Longnecker (14). This allows for correlations between HRs that are related to the same reference group.
The objective of the second meta-analysis was to directly compare the associations of ALT and GGT with diabetes. We decided a priori to limit this second analysis to results of studies that provided estimates for both ALT and GGT in the same population in order to ensure the comparability of the estimates for ALT and GGT. Furthermore, only studies for which we could obtain results in which ALT and GGT had been standardized (to account for their different distributions) were included. The most common method used to standardize ALT and GGT was to present results per fourths of the ALT and GGT distributions. Therefore, we combined the RRs in the top versus the bottom fourth of the ALT and GGT. A fixed-effects model was used, thus assuming that the true effect in all studies was the same and any difference between study results was due to chance alone, and this assumption was checked using the I2 measure (13).
When possible, data were extracted separately for men and women. The effect of duration of follow-up on study results was assessed by meta-regression. Small-study effects such as publication bias were examined by using a funnel plot (16) and formally tested using the Egger test (17).
RESULTS
British Women's Heart and Health Study
Data on diabetes at baseline were available for 3,829 women (89% of 4,286), of whom 377 had diabetes and were excluded from the analysis. Of the remaining 3,452 women, 3,041 (88% of 3,452) had complete data on all covariables (Table 1). Characteristics of included women by diabetes status at the end of follow-up are presented in Table A1 (available in an online appendix at http://care.diabetesjournals.org/cgi/content/full/dc08-1870/DC1). Women with diabetes at the end of follow-up were older; had higher age-adjusted mean BMI, waist-to-hip ratio, ALT, GGT, triglycerides, glucose, and HOMA; and had lower HDL cholesterol. More of the women with incident diabetes exercised for <2 h per week, and more also had a history of smoking.
Table 1.
HRs (95% CI) of incident diabetes per SD change of natural logged ALT and GGT
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| ALT | 1.50 (1.27–1.74) | 1.51 (1.29–1.77) | 1.37 (1.15–1.64) | 1.20 (0.99–1.44) |
| GGT | 1.55 (1.34–1.79) | 1.55 (1.35–1.79) | 1.46 (1.25–1.71) | 1.24 (1.04–1.48) |
Model 1, adjusted for age; model 2, model 1 adjustment plus alcohol consumption; model 3, model 2 adjustments plus childhood and adult social class, physical activity, and smoking; model 4, model 3 adjustments plus HOMA, waist-to-hip ratio, triglycerides, HDL cholesterol, and systolic blood pressure.
During 281,081 person-years of follow-up, 112 women were diagnosed with diabetes, yielding a rate of 5.3 per 1,000 person-years (95% CI 4.4–6.4). Both ALT and GGT were associated with incident diabetes, even when adjustment was made for alcohol consumption (Table 1) and other potential confounders. When components of the metabolic syndrome were added to the model, the associations of both ALT and GGT with incident diabetes were attenuated toward the null. The 95% CI crossed the null for ALT but not for GGT. However, the magnitude of associations of ALT with diabetes and of GGT with diabetes was comparable in all four models, as was the degree of attenuation of age-adjusted estimates.
Systematic review and meta-analysis
The electronic search (crossing terms for ALT, GGT, liver steatosis, and diabetes) yielded 236 potentially relevant publications, of which 20 studied prospective population-based cohorts and assessed baseline ultrasonography-diagnosed NAFLD, ALT, and/or GGT and their associations with incident diabetes.
Ultrasound-diagnosed NAFLD
Three studies (18–20) (summarized in Table 2) assessed ultrasound-diagnosed NAFLD as a determinant of incident diabetes. No studies that used any other diagnostic test to ascertain NAFLD in its association with incident diabetes were retrieved. All three studies were conducted in Asian populations, and in all three there was evidence that ultrasound-diagnosed NAFLD was associated with diabetes risk. When estimates from these studies were meta-analyzed (using the estimate of mild vs. no NAFLD from the study by Kim et al. [(18)]), the pooled RR was 2.52 (95% CI 1.07–5.96), but there was evidence of considerable heterogeneity between studies (I2 = 90%).
Table 2.
Summary of included studies
| Exclusion criteria | Age (years) | n cases/n participants | Female sex (%) | Main results | Enzymes studied and categories | Follow-up (years) | Covariables | |
|---|---|---|---|---|---|---|---|---|
| Studies of ultrasonography-diagnosed NAFLD | ||||||||
| Kim, 2000 (ref. 18), Korea | Diabetes, positive for hepatitis B virus surface antigen, hepatitis C virus antibody, ALT or AST >3 times the upper normal limit, liver cirrhosis, and malignancy | 20–79 | 203/3,670 men; 31/1,702 women | 32 | RR of mild fatty liver vs. nonfatty liver 1.55 (95% CI 1.05–2.31); RR of moderate-to-severe fatty liver vs. nonfatty liver 1.97 (1.23–3.16) | — | 5 | Age, sex, family history of diabetes, smoking, blood pressure, fasting glucose, BMI, ALT, HDL cholesterol, and triglycerides |
| Okamoto, 2002 (ref. 19), Japan | Diabetes or fasting glucose >110 mg/dl | 43 ± 4.3 | 66/467 men; 16/373 women | 44 | OR of fatty liver vs. no fatty liver 1.83 (95% CI 0.95–3.51) | — | 10 | Age, sex, BMI, change in BMI, fasting plasma glucose, A1C, frequency of medical check-ups, alcohol intake, and family history of diabetes |
| Shibata, 2007 (ref. 20), Japan | Daily alcohol intake >20 g, impaired glucose tolerance or diabetes, medications for hypertension, dyslipidemia, liver disease, thyroid disease positive for markers of viral hepatitis, CVD, and gastrectomy | ≥40 | 109/3,189 men | 0 | HR of fatty liver vs. no fatty liver 5.5 (95% CI 3.6–8.5) | — | Mean: 4 | Age and BMI |
| Studies of liver enzymes | ||||||||
| André, 2005 (ref. 4), DESIR Study, France | Diabetes | 30–65 | 69/2,071 men; 30/2,130 women | 51 | — | ALT, AST, and GGT in quartiles | 3 | GGT: age, smoking, physical activity, and ALT; ALT: age and GGT |
| BWHHS | Diabetes | 60–79 | 112/3,041 women | 100 | — | ALT and GGT | Median: 7.3 | Age, childhood and adult social class, physical activity, smoking, alcohol intake, HOMA, waist-to-hip ratio, triglycerides, HDL cholesterol, and SBP |
| Doi, 2007 (ref. 21), Hisayama Study, Japan | Diabetes | 40–79 | 71/719 men; 64/1,085 women | 60 | — | ALT, AST, and GGT in quartiles | Mean: 9 | Age, family history of diabetes, fasting insulin, BMI, waist-to-hip ratio, total cholesterol, HDL cholesterol, triglycerides, CRP, hypertension, alcohol intake, smoking, and physical activity |
| Ford, 2008 (ref. 22), EPIC-Potsdam, Germany | Diabetes | 40–65 | 787/3,011 | 57 | — | ALT and GGT | Mean, 7; median 6.6 | Age, sex, alcohol consumption, smoking, education, sports activity, occupational activity, waist circumference, BMI, SBP, total cholesterol, HDL cholesterol, CRP, and glucose |
| Hanley, 2004 (ref. 23), IRAS, U.S. | Diabetes | 40–69 | 148/906 | 56 | — | ALT, AST, and ALP in quartiles | Mean: 5.2 | Age, sex, ethnicity, clinical center, alcohol intake, smoking, waist circumference, triglycerides, HDL cholesterol, impaired glucose tolerance, insulin sensitivity index, and acute insulin response |
| Lee, 2003 (ref. 24), Korea | Liver disease and diabetes | 25–55 | 83/4,088 men | 0 | — | ALT, GGT, and AST in 6 categories (≤0.9, 10–19, 20–29, 30–39, 40–49, and >49 units/l) | 4 | Age, BMI, smoking, physical activity, family history of diabetes, fasting glucose, and alcohol intake |
| Lee, 2003 (ref. 32), CARDIA Study, U.S. | Diabetes | 18–33 | 157/4,812 | 53 | — | GGT in 25th, 50th, 75th, and 90th percentiles | 15 | Age, sex, race, study center, alcohol intake, BMI, smoking, physical activity, fasting glucose, and insulin |
| Lee, 2004 (ref. 33), Finland | Diabetes | 25–64 | 212/9,771 men; 176/10,387 women | 52 | — | GGT in 25th, 50th, 75th, and 90th percentiles | Mean 12.7 | Age, BMI, smoking, physical activity, alcohol intake, physical activity, and fasting glucose |
| Meisinger, 2005 (ref. 34), MONICA, Augsburg, Germany | Diabetes | 25–64 | 172/1,851 men; 109/1,836 women | 50 | — | GGT in 25, 50, 75, and 87.5 percentiles | Mean 14.7 | Age, hypertension, dyslipidemia, parental history of diabetes, regular smoking, alcohol intake, physical activity, and BMI |
| Monami, 2008 (ref. 25), FIBAR Study, Italy | Diabetes, liver disease other than NAFLD, metastatic cancer, severe heart failure, recent cholelithiasis, alcohol consumption >2 drinks a day, and hepatotoxic medication | 40–75 | 36/2,662 | 57 | — | ALT, AST, and GGT per increase in 10 units/l | Mean ± SD 3.3 ± 1 | Age, sex, alcohol consumption, smoking, and fasting glucose |
| Nakanishi, 2003, 2004 (refs. 26,27), Japan | Hepatitis treatment, ALT >3 times the upper limit of the reference range, CVD, and diabetes | 35–59 | 276/3,260 men | 0 | — | ALT, AST, GGT, and ALP in quintiles | 7 | Age, family history of diabetes, BMI, alcohol intake, smoking, physical activity, WBC, and all other liver enzymes |
| Nannipieri, 2005 (ref. 28), Mexico City Diabetes Study, Mexico | Type 1 diabetes, liver enzymes >3 SD above the mean, and alcohol intake >250 g/week | 35–64 | 94/1,233 | 60 | — | ALT, AST, GGT, and ALP in thirds | 7 | Age, BMI, waist, fasting insulin, and alcohol intake |
| Ohlson, 1988 (ref. 31), Sweden | Diabetes | 54 | 47/766 men | 0 | — | ALT and AST in quintiles | 13.5 | Glucose, BMI, bilirubin, SBP, uric acid, and family history |
| Perry, 1998 (ref. 3), British Regional Heart Study, U.K. | Diabetes | 40–59 | 194/7,458 men | 0 | — | GGT in quintiles | Mean: 12.8 | Age, BMI, physical activity, alcohol intake, smoking, preexisting CHD, SBP, heart rate, HDL cholesterol, uric acid, FEV1, hematocrit, and glucose |
| Sattar, 2004 (ref. 29), WOSCOPS, Scotland | Diabetes, CHD, ALT >70 or AST >60 units/l, and all moderately hypercholesterolemic men | Mean 55 | 139/6,595 | 0 | — | ALT in quartiles | 4.9 | Age, BMI, smoking, SBP, HDL cholesterol ratio, pravastatin treatment, triglycerides, alcohol intake, fasting glucose |
| Schindhelm, 2005 (ref. 30), Hoorn Study, the Netherlands | Diabetes | 50–75 | 123/1,289 | N/A | — | ALT in tertiles | 6 | Age, sex, follow-up duration, waist circumference, BMI, alcohol intake, fasting insulin, and 2-h postload glucose |
| Vozarova, 2002 (ref. 6), U.S. | Diabetes, impaired glucose tolerance, smoking, clinical or lab evidence of acute or chronic infection, and liver enzymes >3 times the upper limit | 18–50 | 63/370 | 38 | — | ALT, AST, GGT, and ALP in percentiles | Mean 6.9 | Age, sex, % body fat, acute insulin response, and M-low |
| Wannamethee, 2005 (ref. 5), British Regional Heart Study, U.K. | Diabetes and ALT or GGT >3 times the upper limit of the reference range | 60–79 | 100/3,500 | 0 | — | ALT, AST, and GGT in quartiles | Mean 5 | Age, social class, physical activity, smoking, alcohol intake, preexisting CHD or stroke, statins, BMI, and HOMA-IR |
Data for age are means ± SD or range. Data for follow-up are n unless otherwise indicated. ALP, alkaline phosphatase; AST, aspartate aminotransferase; CARDIA Study, Coronary Artery Risk Development in Young Adults Study; CHD, cardiovascular heart disease; CRP, C-reactive protein; CVD, cardiovascular disease; EPIC-Potsdam Study, European Prospective Investigation Into Cancer and Nutrition–Potsdam Study; FEV1, forced expiratory volume in 1 s; FIBAR Study, Firenze Bagno a Ripoli Study; HOMA-IR, HOMA of insulin resistance; IRAS, Insulin Resistance Atherosclerosis Study; M-low, glucose disposal during a low-dose insulin infusion; MONICA Study, Monitoring of Trends and Determinants in Cardiovascular Disease Study; N/A, not available; SBP, systolic blood pressure; WBC, white blood cell; WOSCOPS, West of Scotland Coronary Prevention Study.
ALT and GGT
Study characteristics of the 18 prospective population-based studies of the associations of ALT and/or GGT with incident diabetes are also summarized in Table 2. Ten studies (4–6,21–28) (including the current analysis of the British Women's Heart and Health Study [BWHHS]) assessed both ALT and GGT as predictors of diabetes, an additional three studies looked at ALT only (29–31), and another four studies looked at the association of GGT with diabetes risk (3,32–34). Age-adjusted and fully adjusted study results examining (natural logged) ALT and GGT as determinants of incident diabetes were pooled (separately).
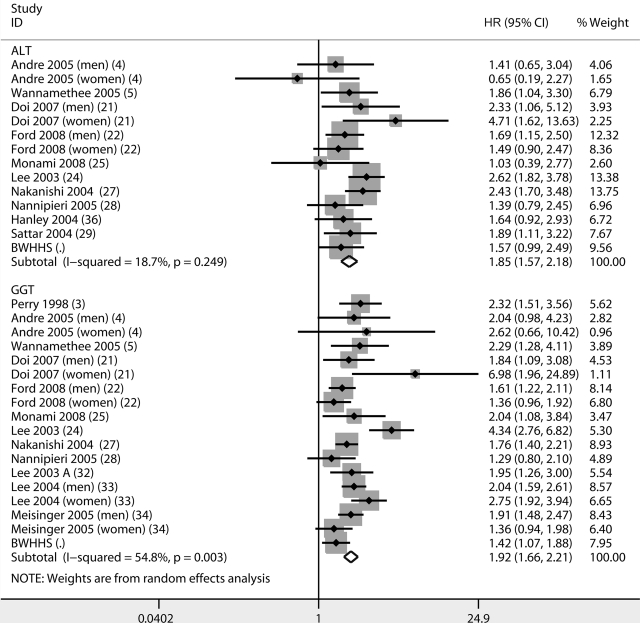
A change in one logged unit in ALT was associated with an HR of 3.05 (95% CI 2.59–3.59, I2 = 26%, 13 comparisons) and in GGT 2.56 (2.31–2.84, I2 = 32%, 17 comparisons) (online appendix Fig. A1). The meta-analysis of fully adjusted results for both ALT and GGT are presented in Fig. 1. The fully adjusted HR for diabetes per increase in one unit of logged ALT was 1.85 (1.57–2.18, I2 = 19, 14 comparisons), and in one unit of logged GGT the HR was 1.92 (1.66–2.21, I2 = 55%, 18 comparisons).
Figure 1.
Meta-analysis of fully adjusted results of ALT and GGT as a determinant of incident diabetes.
No strong evidence was found for the presence of a small-studies effect for ALT or GGT. There was also no strong evidence of an association between duration of follow-up and study results (all P >0.69).
Comparing associations of ALT and GGT with incident diabetes
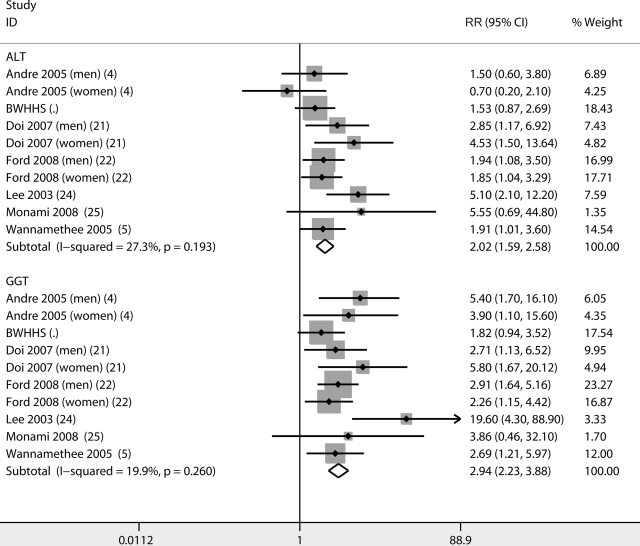
Seven studies (including the BWHHS) contributed to the analysis of ALT and GGT categorized in fourths as predictors of diabetes (4,5,21,22,24,25). Additional studies assessed both ALT and GGT; however, one used fifths (26,27), another used tenths (6), and a final study (28) used fourths but did not present results in full and therefore could not be included in this analysis. Age-adjusted pooled analyses of the included studies are presented in online appendix Figure A2. The RR in the top versus the bottom quartile of ALT was 4.42 (95% CI 3.61–5.42, I2 = 42%), and for GGT it was 5.87 (4.63–7.44, I2 = 21%). The fully adjusted RR for ALT was 2.02 (1.59–2.58, I2 = 27%) and for GGT 2.94 (1.98–3.88, I2 = 20%) (Fig. 2). According to a formal statistical test for heterogeneity between estimates for ALT and GGT, there was some evidence suggesting that pooled estimates for GGT were larger than those for ALT (age-adjusted results, P = 0.08, and fully adjusted results, P = 0.05).
Figure 2.
Meta-analysis of fully adjusted results (RR in top versus bottom fourth) of ALT and GGT as determinants of incident diabetes.
However, repeating the analysis after excluding results for women in the DESIR (Data From the Epidemiological Study on the Insulin Resistance Syndrome) cohort (4), which was the only study that showed a potential protective effect of ALT, resulted in greater pooled estimates for GGT (HR 2.91 [95% CI 2.19–3.85]) than for ALT (2.12 [1.66–2.72]) but no strong evidence for heterogeneity between the estimates (P ≥0.10 for both age-adjusted and fully adjusted results).
CONCLUSIONS
Ultrasound-diagnosed NAFLD, ALT, and GGT all predicted diabetes risk. NAFLD defined by ultrasound was associated with more than a doubling of the risk of incident diabetes. In a meta-analysis of fully adjusted results (albeit variably adjusted) of all population-based, systematically identified prospective studies, a 1 unit/l increase of natural logged ALT was associated with an 85% increase in diabetes risk and a 1 unit/l increase of natural logged GGT was associated with a 92% increase in diabetes risk.
A second meta-analysis that assessed the association of both ALT and GGT (using fourths of their distribution) with incident diabetes in the same populations was performed. Belonging to the top fourth of the GGT distribution was associated with a 194% increase in the risk of diabetes compared with risk increase associated with belonging to the bottom fourth. For ALT, the corresponding increase in the risk of diabetes was 102%, with formal evidence of heterogeneity between these two estimates (both based on fully adjusted models).
There are two possible explanations for the potentially stronger association of GGT with diabetes compared with that of ALT. First, both ALT and GGT are biomarkers of liver fat, but GGT may simply be the better marker. In light of the paucity of relevant evidence as to which liver enzyme better reflects liver fat content, this explanation cannot be ruled out. However, GGT is present on the surface of most cell types and is highly active in organs other than the liver, such as the kidney and pancreas (35). Therefore, ALT is considered a more liver-specific marker than GGT. GGT is the enzyme responsible for the extracellular catabolism of antioxidant glutathione (36) and may be linked to greater oxidative stress (37). Because oxidative stress has been implicated in insulin resistance, diabetes, and cardiovascular disease (37,38), GGT's potentially stronger association with diabetes may reflect its associations with several different processes relevant to diabetes pathogenesis. This speculative suggestion requires more data.
Although a stronger association of GGT (compared with that of ALT) with diabetes is biologically plausible, our results should be interpreted with caution. The exclusion of a single observation substantially reduced the strength of evidence that the associations of ALT and GGT with incident diabetes were different. In addition, some limitations should be noted. Only 6 of the 10 studies that presented results for both ALT and GGT could be included in the meta-analyses of the risk estimates in the top fourth of the ALT and GGT distributions versus the bottom fourth (despite our best efforts to contact and obtain relevant information from authors, results in the form necessary to include the additional three studies were not forthcoming). An additional limitation is that two of the studies included in the systematic review could not be included in the meta-analysis of all studies as a result of missing information. Attempts were made to obtain additional results from study authors, but this was not always possible. Limitations of the BWHHS include the lack of oral glucose tolerance testing at baseline, the use of self-reported medical diagnoses of diabetes at follow-up, a less robust measure of insulin resistance (HOMA), and the inclusion of women only. The latter is also the study's strength because 6 of the 18 studies do not include any women.
In conclusion, NAFLD and associated elevations in liver enzymes are associated with incident diabetes above and beyond commonly measured diabetes risk factors. Results suggest that the association of GGT with diabetes risk may be of greater magnitude than that of ALT with diabetes, but further studies are needed to confirm this. In the meantime, raised levels of both enzymes in the context of other clinical features of insulin resistance (obesity, hypertriglyceridemia, raised fasting glucose, etc.) give good insight into excess hepatic fat and thus elevated diabetes risk.
Supplementary Material
Acknowledgments
We thank the U.K. Department of Health Policy Research Programme for core support to the BWHHS and the British Heart Foundation for additional funding. A.F. receives support from the University of Bristol Overseas Research Student Award Scheme, and D.L. is funded by a U.K. Department of Health career scientist award.
No potential conflicts of interest relevant to this article were reported.
We thank the following authors for their assistance with our work: Ele Ferranni, CNR (National Research Council) Institute of Clinical Physiology; Duk-Hee Lee, Kyungpook National University; Matteo Monami, U.O. Geriatria Universitaria; and Matthias Schulze, German Institute of Human Nutrition. The BWHHS is codirected by Shah Ebrahim, Debbie Lawlor, Peter Whincup, and Goya Wannamethee. We thank Carol Bedford, Alison Emerton, Nicola Frecknall, Karen Jones, Mark Taylor, Simone Watson, and Katherine Wornell, together with Rita Patel, for collecting and entering data; all of the general practitioners and their staff who have supported data collection; and the women who have participated in the study.
Footnotes
The views expressed in this study are those of the authors and not necessarily those of any funding body. No funding body influenced the analysis or its interpretation.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Whitfield JB: Gamma glutamyl transferase. Crit Rev Clin Lab Sci 38: 263– 355, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Trell E, Kristenson H, Peterson B, Fex G, Henningsen NC, Berntorp K, Hood B: Two-hour glucose and insulin responses after a standardized oral glucose load in relation to serum gamma-glutamyl transferase and alcohol consumption. Acta Diabetol Lat 18: 311– 317, 1981 [DOI] [PubMed] [Google Scholar]
- 3. Perry IJ, Wannamethee SG, Shaper AG: Prospective study of serum γ-glutamyltransferase and risk of NIDDM. Diabetes Care 21: 732– 737, 1998 [DOI] [PubMed] [Google Scholar]
- 4. André P, Balkau B, Born C, Royer B, Wilpart E, Charles MA, Eschwège E: Hepatic markers and development of type 2 diabetes in middle aged men and women: a three-year follow-up study: the D.E.S.I.R. Study (Data from an Epidemiological Study on the Insulin Resistance syndrome). Diabetes Metab 31: 542– 550, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Wannamethee SG, Shaper AG, Lennon L, Whincup PH: Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care 28: 2913– 2918, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, Tataranni PA: High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes 51: 1889– 1895, 2002 [DOI] [PubMed] [Google Scholar]
- 7. Pratt DS, Kaplan MM: Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med 342: 1266– 1271, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Lawlor DA, Ebrahim S, Davey Smith G: Socioeconomic position in childhood and adulthood and insulin resistance: cross sectional survey using data from British women's heart and health study. BMJ 2:805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lawlor DA, Bedford C, Taylor M, Ebrahim S: Geographical variation in cardiovascular disease, risk factors, and their control in older women: British Women's Heart and Health Study. J Epidemiol Community Health 57: 134– 140, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawlor DA, Davey Smith G, Ebrahim S: Life course influences on insulin resistance: findings from the British Women's Heart and Health Study. Diabetes Care 26: 97– 103, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 12. World Health Organisation: Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation: Part 1: Diagnosis and Classification of Diabetes Mellitus. Geneva, World Health Org., 1999 [Google Scholar]
- 13. Higgins JP, Thompson SG, Deeks JJ, Altman DG: Measuring inconsistency in meta-analyses. BMJ 327: 557– 560, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenland S, Longnecker MP: Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135: 1301– 1309, 1992 [DOI] [PubMed] [Google Scholar]
- 15. Chêne G, Thompson SG: Methods for summarizing the risk associations of quantitative variables in epidemiologic studies in a consistent form. Am J Epidemiol 144: 610– 621, 1996 [DOI] [PubMed] [Google Scholar]
- 16. Sterne JA, Egger M: Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol 54: 1046– 1055, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Egger M, Smith GD, Schneider M, Minder C: Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629– 634, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kim CH, Park JY, Lee KU, Kim JH, Kim HK: Fatty liver is an independent risk factor for the development of type 2 diabetes in Korean adults. Diabet Med 2: 476– 481, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Okamoto M, Takeda Y, Yoda Y, Kobayashi K, Fujino MA, Yamagata Z: The association of fatty liver and diabetes risk. J Epidemiol 13: 15– 21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shibata M, Kihara Y, Taguchi M, Tashiro M, Otsuki M: Nonalcoholic fatty liver disease is a risk factor for type 2 diabetes in middle-aged Japanese men. Diabetes Care 30: 2940– 2944, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Doi Y, Kubo M, Yonemoto K, Ninomiya T, Iwase M, Tanizaki Y, Shikata K, Iida M, Kiyohara Y: Liver enzymes as a predictor for incident diabetes in a Japanese population: the Hisayama Study. Obesity Res 15: 1841– 1850, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Ford ES, Schulze MB, Bergmann MM, Thamer C, Joost HG, Boeing H: Liver enzymes and incident diabetes: findings from the European Prospective Investigation Into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes Care 31: 1138– 1143, 2008 [DOI] [PubMed] [Google Scholar]
- 23. Hanley AJG, Williams K, Festa A, Wagenknecht LE, D'Agostino RB, Jr, Kempf J, Zinman B, Haffner SM: Elevations in markers of liver injury and risk of type 2 diabetes: the Insulin Resistance Atherosclerosis Study. Diabetes 53: 2623– 2632, 2004 [DOI] [PubMed] [Google Scholar]
- 24. Lee DH, Ha MH, Kim JH, Christiani DC, Gross MD, Steffes M, Blomhoff R, Jacobs DR, Jr: Gamma-glutamyltransferase and diabetes: a 4 year follow-up study. Diabetologia 46: 359– 364, 2003 [DOI] [PubMed] [Google Scholar]
- 25. Monami M, Bardini G, Lamanna C, Pala L, Cresci B, Francesconi P, Buiatti E, Rotella CM, Mannucci E: Liver enzymes and risk of diabetes and cardiovascular disease: results of the Firenze Bagno a Ripoli (FIBAR) study. Metabolism 57: 387– 392, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Nakanishi N, Nishina K, Li W, Sato M, Suzuki K, Tatara K: Serum gamma-glutamyltransferase and development of impaired fasting glucose or type 2 diabetes in middle-aged Japanese men. J Intern Med 254: 287– 295, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Nakanishi N, Suzuki K, Tatara K: Serum γ-glutamyltransferase and risk of metabolic syndrome and type 2 diabetes in middle-aged Japanese men. Diabetes Care 27: 1427– 1432, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Nannipieri M, Gonzales C, Baldi S, Posadas R, Williams K, Haffner SM, Stern MP, Ferrannini E: Liver enzymes, the metabolic syndrome, and incident diabetes: the Mexico City Diabetes Study. Diabetes Care 28: 1757– 1762, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Sattar N, Scherbakova O, Ford I, O'Reilly DS, Stanley A, Forrest E, MacFarlane PW, Packard CJ, Cobbe SM, Shepherd J: Elevated alanine aminotransferase predicts new-onset type 2 diabetes independently of classical risk factors, metabolic syndrome, and C-reactive protein in the West of Scotland Coronary Prevention Study. Diabetes 53: 2855– 2860, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Schindhelm RK, Dekker JM, Nijpels G, Heine RJ, Diamant M: No independent association of alanine aminotransferase with risk of future type 2 diabetes in the Hoorn Study. Diabetes Care 2: 2812, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Ohlson LO, Larsson B, Björntorp P, Eriksson H, Svärdsudd K, Welin L, Tibblin G, Wilhelmsen L: Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia 31: 798– 805, 1988 [DOI] [PubMed] [Google Scholar]
- 32. Lee DH, Jacobs DR, Jr, Gross M, Kiefe CI, Roseman J, Lewis CE, Steffes M: Gamma-glutamyltransferase is a predictor of incident diabetes and hypertension: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Clin Chem 49: 1358– 1366, 2003 [DOI] [PubMed] [Google Scholar]
- 33. Lee DH, Silventoinen K, Jacobs DR, Jr, Jousilahti P, Tuomileto J: Gamma-glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab 89: 5410– 5414, 2004 [DOI] [PubMed] [Google Scholar]
- 34. Meisinger C, Lowel H, Heier M, Schneider A, Thorand B: Serum gamma-glutamyltransferase and risk of type 2 diabetes mellitus in men and women from the general population. J Intern Med 258: 527– 535, 2005 [DOI] [PubMed] [Google Scholar]
- 35. Hanigan MH, Frierson HF, Jr: Immunohistochemical detection of gamma-glutamyl transpeptidase in normal human tissue. J Histochem Cytochem 44: 1101– 1108, 1996 [DOI] [PubMed] [Google Scholar]
- 36. Turgut O, Yilmaz A, Yalta K, Karadas F, Birhan Yilmaz M: Gamma-glutamyltransferase is a promising biomarker for cardiovascular risk. Med Hypotheses 67: 1060– 1064, 2006 [DOI] [PubMed] [Google Scholar]
- 37. Ceriello A, Motz E: Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler Thromb Vasc Biol 24: 816– 823, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Evans JL, Goldfine ID, Maddux BA, Grodsky GM: Are oxidative stress-activated signaling pathways mediators of insulin resistance and β-cell dysfunction? Diabetes 52: 1– 8, 2003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.