Abstract
OBJECTIVE
To explore the prevalence of lipid abnormalities and their relationship with albumin excretion and microalbuminuria in adolescents with type 1 diabetes.
RESEARCH DESIGN AND METHODS
The study population comprised 895 young subjects with type 1 diabetes (490 males); median age at the baseline assessment was 14.5 years (range 10–21.1), and median diabetes duration was 4.8 years (0.2–17). A total of 2,194 nonfasting blood samples were collected longitudinally for determination of total cholesterol, LDL cholesterol, HDL cholesterol, TG, and non-HDL cholesterol. Additional annually collected data on anthropometric parameters, A1C, and albumin-to-creatinine ratio (ACR) were available.
RESULTS
Total cholesterol, LDL cholesterol, HDL cholesterol, and non-HDL cholesterol were higher in females than in males (all P < 0.001). A significant proportion of subjects presented sustained lipid abnormalities during follow-up: total cholesterol >5.2 mmol/l (18.6%), non-HDL cholesterol >3.4 mmol/l (25.9%), TG >1.7 mmol/l (20.1%), and LDL cholesterol >3.4 mmol/l (9.6%). Age and duration were significantly related to all lipid parameters (P < 0.001); A1C was independently related to all parameters (P < 0.001) except HDL cholesterol, whereas BMI SD scores were related to all parameters (P < 0.05) except total cholesterol. Total cholesterol and non-HDL cholesterol were independently related to longitudinal changes in ACR (B coefficient ± SE): 0.03 ± 0.01/1 mmol/l, P = 0.009, and 0.32 ± 0.014/1 mmol/l, P = 0.02, respectively. Overall mean total cholesterol and non-HDL cholesterol were higher in microalbuminuria positive (n = 115) than in normoalbuminuric subjects (n = 780): total cholesterol 4.7 ± 1.2 vs. 4.5 ± 0.8 mmol/l (P = 0.04) and non-HDL cholesterol 3.2 ± 1.2 vs. 2.9 ± 0.8 mmol/l (P = 0.03).
CONCLUSIONS
In this longitudinal study of adolescents with type 1 diabetes, sustained lipid abnormalities were related to age, duration, BMI, and A1C. Furthermore, ACR was related to both total cholesterol and non-HDL cholesterol, indicating a potential role in the pathogenesis of diabetic nephropathy.
Type 1 diabetes is associated with an increased risk for the development of microvascular complications and cardiovascular disease (1,2). Qualitative and quantitative lipid abnormalities are often present in subjects with type 1 diabetes and are related to glycemic control (2). The relationship between dyslipidemia and cardiovascular disease risk is well documented in adult diabetic populations, and treatment with lipid-lowering drugs has been associated with reduced cardiovascular events (2). Clinical and experimental studies have highlighted the potential role of dyslipidemia in the development of microalbuminuria and diabetic nephropathy (3). Mesangial, tubulo-interstitial, and glomerular changes in the kidney have been associated with lipid levels (3). In animal models of diabetes, treatment of hyperlipidemia with statins has been associated with reduced glomerular injury (3).
Cross-sectional studies in humans have suggested that raised lipid levels are involved in the pathogenesis and progression of renal diseases (3), and treatment of dyslipidemia can reduce albumin excretion (3). In the Steno study, subjects who developed microalbuminuria had higher cholesterol levels than subjects who did not progress (4). In addition, in this study, as in others, lower cholesterol levels predicted regression of microalbuminuria to normoalbuminuria (5). TG have also emerged as a predictor for the development and progression of renal complications (6). Based on these data, it appears that measurement of plasma lipids can add to the prognostic value of albumin excretion in the prediction of subjects at risk of diabetic nephropathy.
However, there is a lack of longitudinal studies, particularly in young people with type 1 diabetes, assessing the association between lipid abnormalities and risk for microalbuminuria. Based mainly on cross-sectional studies, dyslipidemia appears to be more common among youth with diabetes than in the general pediatric population (7,8), and its relationship with glycemic control has been repeatedly documented (7,9,10). In contrast, data on the association between lipid levels and albumin excretion are scant (11,12).
Therefore, the aim of the present study was to assess the prevalence of lipid abnormalities, their determinants, and their relationship with albumin excretion and the development of microalbuminuria in a large population of young people with type 1 diabetes, followed longitudinally during puberty.
RESEARCH DESIGN AND METHODS
Study population: Nephropathy Family Study cohort
The Nephropathy Family Study (NFS) was established between 2000 and 2005 as part of the Juvenile Diabetes Research Foundation/Wellcome Trust Diabetes Inflammation Laboratory: Genetic Resource Investigating Diabetes (GRID) study (http://www.childhood-diabetes.org.uk/grid.shtml).A total of 1,066 subjects, aged 10–16 years, who had developed type 1 diabetes before the age of 16 years, were recruited throughout four English regions (East Anglia, Birmingham, Bristol, and Oxford). Subjects with insulin-treated diabetes secondary to other pathologies were excluded. Similarly, children with chronic renal disease or other chronic diseases likely to affect renal function were excluded. The median duration of follow-up is currently 2.3 years (interquartile range 1.0–3.4).
The longitudinal study schedule comprised annual collection of three consecutive early-morning urine specimens for centralized measurement of albumin-to-creatinine ratio (ACR) and blood samples for measurements of A1C and lipids.
Ethical approval was obtained from the regional ethics committee, and written informed consent was obtained from the parents together with assent from the children.
Methods
Nonfasting blood samples, collected longitudinally between February 2001 and January 2006, were analyzed for determination of total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides (TG), and non-HDL cholesterol.
To exclude lipid abnormalities related to untreated diabetes, samples collected within the first month of diagnosis were excluded. In addition, patients on treatment with statins were also excluded from the analysis.
A total of 2,194 lipid measurements were available from 895 subjects; the mean ± SD number of measurements per patient was 2.5 ± 1.0 and the median 3 (range 1–5). A total of 183 subjects had only one measurement, 254 had two measurements, 333 had three measurements, 121 had four measurements, and only 4 subjects had five measurements.
A1C
Samples were analyzed centrally on a TOSOH G7 analyzer, using high-performance liquid chromatography and absorbance change detection and Diabetes Control and Complications Trial (DCCT)-aligned methods. The normal range for A1C was 4.9–6.3%, and the coefficient of variation (CV) was 4.8 and 6.6% at a level of 5.5 and 10.1%, respectively.
Lipids
All samples were assayed centrally. Measurements of total cholesterol, HDL cholesterol, and TG were performed enzymatically on a Dimension RXL system (Dade Behring) using reagents and calibrants supplied by the manufacturer. Between-run CVs were for total cholesterol 1.3% at 3.2 mmol/l and 1.2% at 7.5 mmol/l, for TG 3.2% at 1.0 mmol/l and 1.1% at 2.2 mmol/l, and for HDL cholesterol 3.3% at 0.6 mmol/l and 2.1% at 1.5 mmol/l.
LDL cholesterol was calculated with Friedwald's formula: LDL = total cholesterol − HDL cholesterol − TG/2.2.
Because our samples were collected in nonfasting conditions, non-HDL cholesterol (total cholesterol minus HDL cholesterol) was also assessed.
Urinary albumin and creatinine
All urine samples were stored at −70°C before the centralized analysis in a single reference laboratory. Albumin was measured by a double antibody enzyme-linked immunosorbent assay method. The within and in-between assay CVs were 6 and 12%, respectively. Creatinine was measured using a modified Jaffe method (Unimate 7, Roche Diagnostic Systems, Basel, Switzerland) on a Cobas Mira (Roche Diagnostic Systems) automated spectrophotometer. The CV was 2% at 2.2 mmol/l.
Calculations
BMI was calculated as weight/height2. SD scores for BMI were calculated using the British 1990 Growth Reference and Cole's LMS method.
ACR was summarized as the geometric mean of three consecutive early-morning urine samples during each annual assessment. The most recent ACR measurements in relation to the time when each lipid assessment was done (within ±3 months) was used for the analyses of the relationship with lipid levels. A total of 1,991 ACR measurements were available from 870 subjects.
Microalbuminuria was defined as an ACR between 3.5 and 35 mg/mmol in males and between 4.0 and 47 mg/mmol in females in two of three consecutive early-morning urine collections during an annual assessment (13). Persistent microalbuminuria was defined as an ACR within the microalbuminuric range based on two of three urines or two of two urines each year for at least 2 consecutive years. Transient microalbuminuria was defined as the presence of microalbuminuria for 1 year with subsequent regression to normal.
Lipid levels were categorized, based on the National Cholesterol Education Programme (14) and American Heart Association (15) guidelines, as follow: high total cholesterol: >5.2 mmol/l, borderline total cholesterol: 4.4–5.2 mmol/l; high LDL cholesterol: >3.4 mmol/l, borderline LDL cholesterol: 2.9–3.4 mmol/l; low HDL cholesterol: <0.9 mmol/l; high TG: >1.7 mmol/l; high non-HDL cholesterol: >3.4 mmol/l.
Statistical analysis
Data are summarized as means ± SD or median (range) for continuous variables and as cell frequencies and percentages for categorical variables. Non–normally distributed variables (ACR and TG) were log transformed before analysis. Comparisons between different groups were performed by unpaired t tests. Comparisons across categories were made using χ2 or Fisher's exact test. Correlations between variables of interest were performed by Pearson correlation. General linear models were used to assess longitudinal associations between variables, which are expressed as B coefficient ± SE.
RESULTS
The baseline characteristics of the study population are shown in Table 1. Age, duration of diabetes, and age at diagnosis were similar between male (n = 490) and female (n = 405) subjects. No significant differences were found in glycemic control between sexes, whereas BMI SD scores were significantly higher in females than in males. Levels of total cholesterol, LDL cholesterol, HDL cholesterol, and non-HDL cholesterol were higher in females than in males (all P < 0.001), whereas TG levels were comparable. These sex differences in lipid levels persisted after adjustment for BMI (Table 1). Similar sex differences in lipid parameters persisted during the subsequent follow-up visits (data not shown).
Table 1.
Baseline characteristics
| All | Male | Female | |
|---|---|---|---|
| n | 895 | 490 | 405 |
| Age at diagnosis (years) | 9.6 (0.2–16.5) | 9.8 (0.2–16.5) | 9.5 (0.2–16.2) |
| Age at first assessment (years) | 14.5 (0.1–21.1) | 14.6 (10.0–21) | 14.5 (10.1–21.1) |
| Duration at first assessment (years) | 4.8 (0.2–17.0) | 4.8 (0.2–16.9) | 5.0 (0.2–17.0) |
| Age at microalbuminuria onset (years) | 15.6 (6.0–20.7) | 15.3 (11.2–20.7) | 15.9 (6.0–18.7) |
| BMI SD score | 0.79 ± 0.97 | 0.70 ± 0.97 | 0.87 ± 0.97 |
| A1C (%) | 9.3 ± 1.9 | 9.2 ± 1.8 | 9.4 ± 1.9 |
| Total cholesterol (mmol/l) | 4.5 ± 0.9 | 4.3 ± 0.8 | 4.7 ± 1.0* |
| HDL cholesterol (mmol/l) | 1.6 ± 0.4 | 1.6 ± 0.4 | 1.7 ± 0.4* |
| LDL cholesterol (mmol/l) | 2.3 ± 0.7 | 2.2 ± 0.7 | 2.5 ± 0.8* |
| TG (mmol/l) | 1.0 (0.2–8.5) | 1.0 (0.2–7.3) | 1.0 (0.3–8.5) |
| Non-HDL cholesterol (mmol/l) | 2.9 ± 0.9 | 2.7 ± 0.8 | 3.1 ± 1.0* |
Data are medians (range) and means ± SD.
*P < 0.01 for females vs. males.
Categories of lipid levels at baseline and during follow-up
A high prevalence of high and borderline lipid levels was found at the baseline visit, and these abnormalities persisted during follow-up (see Fig. A1 in the online appendix available at http://care.diabetesjournals.org/cgi/content/full/dc08-1641/DC1). Mean frequency of lipid abnormalities during follow-up was as follows: total cholesterol 18.6%, TG 20.1%, non-HDL cholesterol 25.9%, LDL cholesterol 9.6%, low HDL cholesterol 2.5%, borderline total cholesterol 34.8%, and borderline LDL cholesterol 12.7%.
Lipids and A1C
There was a significant association between mean lipid levels, except HDL cholesterol, and mean A1C (total cholesterol, r = 0.35; LDL cholesterol, r = 0.21; TG, r = 0.40; non-HDL cholesterol, r = 0.36; all P < 0.001). These associations were significantly stronger in girls than in boys (total cholesterol, r = 0.45 vs. 0.25, P < 0.01; LDL cholesterol, r = 0.31 vs. 0.10, P < 0.01; TG, r = 0.41 vs. 0.26, P < 0.05).
Longitudinal predictors of lipid levels
A longitudinal evaluation of factors associated with lipid levels was performed in the 712 subjects with more than one lipid assessment, with age, sex, duration of diabetes, A1C, and BMI SD score as the independent variables. In a covariate model, age was significantly related to all lipid parameters; A1C was independently related to all parameters except HDL cholesterol, whereas BMI SD score was related to all parameters except total cholesterol (Table 2). Similar results were obtained when duration was included as a covariate instead of age (data not shown).
Table 2.
Independent predictors of lipid levels during follow-up
| B ± SE | P | |
|---|---|---|
| Total cholesterol | ||
| Age (years) | 0.042 ± 0.014 | 0.004 |
| A1C (%) | 0.161 ± 0.016 | <0.001 |
| BMI SD score | 0.077 ± 0.043 | NS |
| Log TG | ||
| Age (years) | 0.018 ± 0.005 | <0.001 |
| A1C (%) | 0.045 ± 0.005 | <0.001 |
| BMI SD score | 0.062 ± 0.014 | <0.001 |
| HDL cholesterol | ||
| Age (years) | −0.038 ± 0.006 | <0.001 |
| A1C (%) | 0.001 ± 0.007 | NS |
| BMI SD score | −0.06 ± 0.019 | 0.001 |
| LDL cholesterol | ||
| Age (years) | 0.050 ± 0.011 | 0.013 |
| A1C (%) | 0.072 ± 0.013 | <0.001 |
| BMI SD score | 0.079 ± 0.03 | 0.025 |
| Non-HDL cholesterol | ||
| Age (years) | 0.080 ± 0.013 | <0.001 |
| A1C (%) | 0.160 ± 0.015 | <0.001 |
| BMI SD score | 0.138 ± 0.040 | 0.001 |
Data are from 712 subjects with more than one lipid measurement and are adjusted for repeated measurements and sex.
The analysis was then repeated separately for males and females, with similar findings, except for the relationship between lipid levels and age, which persisted in males but was no longer significant in females (data not shown).
Changes in lipids and albumin excretion
We examined whether lipid parameters predicted trends in albumin excretion during follow-up. Table 3 shows the results of this analysis, before and after adjusting for age, sex, duration, BMI SD score, and A1C. Total cholesterol and non-HDL cholesterol were independently related to changes in log ACR during follow-up.
Table 3.
Relationship between lipid parameters and ACR
| B ± SE* | P * | B ± SE† | P † | |
|---|---|---|---|---|
| Total cholesterol | 0.041 ± 0.011 | <0.001 | 0.033 ± 0.013 | 0.009 |
| Log TG | 0.12 ± 0.033 | <0.001 | 0.072 ± 0.037 | NS |
| HDL cholesterol | −0.006 ± 0.026 | NS | 0.03 ± 0.007 | NS |
| LDL cholesterol | 0.007 ± 0.015 | NS | −0.002 ± 0.016 | NS |
| Non-HDL cholesterol | 0.47 ± 0.012 | <0.001 | 0.32 ± 0.014 | 0.02 |
The dependent variable is log ACR. Regression coefficients B are for each 1 mmol/l increase in lipid levels.
*Unadjusted values.
†Adjusted values for age, sex, duration, BMI SD score, and A1C.
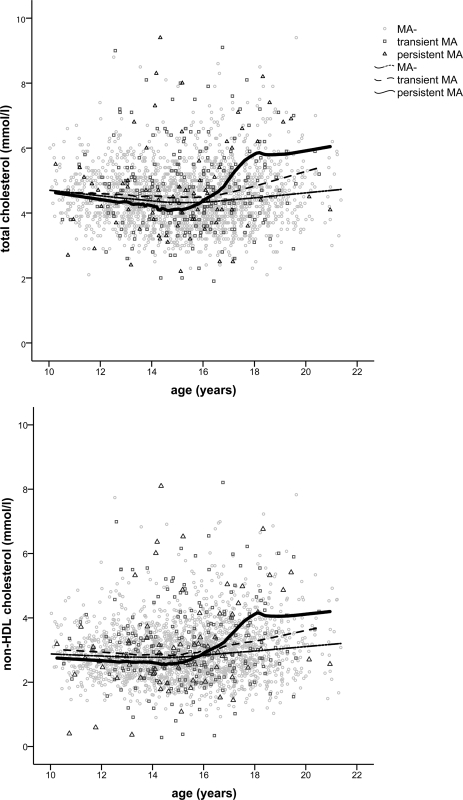
During follow-up, 115 (13%) subjects developed microalbuminuria (28 persistent and 87 transient microalbuminuria). Age-related changes in total cholesterol and non-HDL cholesterol, and specifically the rise in their levels after the age of 15–16 years, were particularly marked in subjects with persistent microalbuminuria when compared with individuals with transient microalbuminuria and normoalbuminuria (Fig. 1).
Figure 1.
Longitudinal changes in total cholesterol and non-HDL cholesterol with age in subjects with normoalbuminuria (MA−) and in individuals with transient and persistent microalbuminuria (MA).
Mean concentrations of total cholesterol (4.7 ± 1.2 vs. 4.5 ± 0.8 mmol/l, P = 0.04) and non-HDL cholesterol (3.2 ± 1.2 vs. 2.9 ± 0.8 mmol/l, P = 0.03) were higher in subjects developing microalbuminuria when compared with individuals with normoalbuminuria (see Table A1 in the online appendix). However, these differences disappeared after adjusting for A1C. Microalbuminuria-positive subjects also presented a high percentage of abnormal lipid levels, specifically total cholesterol and LDL cholesterol, when compared with normoalbuminuric subjects.
CONCLUSIONS
In the present study, we found a high prevalence of lipid abnormalities in an adolescent population with type 1 diabetes, diagnosed during childhood and followed longitudinally during puberty. Lipid levels were significantly influenced by age, duration of diabetes, BMI, and glycemic control. In addition, we found that total cholesterol and non-HDL cholesterol were significantly related to albumin excretion during the study period.
In our study, the mean frequency of high and borderline total cholesterol during follow-up was 18.6 and 34.8%, respectively. A large proportion of subjects had high non-HDL cholesterol (25.9%), whereas the frequency of low HDL cholesterol was not particularly high (2.5%), similar to findings from previous studies (9). A high proportion of subjects had abnormal levels of TG and LDL cholesterol, even though these parameters are less reliable, given that blood samples were collected in nonfasting conditions.
Few data are available on lipid levels in young people with type 1 diabetes, and the majority of studies have been cross-sectional (7,8,16,17), with only a few being longitudinal with short-term follow-up or a retrospective design (9,18,19). In the SEARCH study (16), one of five children with type 1 diabetes presented total cholesterol >5.2 mmol/l, similar to our results. Data from the Oxford Regional Prospective Study showed that 15.3% subjects had total cholesterol >5.2 mmol/l and 17.9% TG above 1.7 mmol/l (12). Similar data have been reported in a study from the U.S. where 15.2% of children had high total cholesterol (7) and from a German study where 28.6% of patients had dyslipidemia (8). Therefore, in line with these studies, we confirmed a high prevalence of dyslipidemia in youth with type 1 diabetes, and this is potentially clinically significant, given the well-known relationship of dyslipidemia with cardiovascular events (1) and the fact that lipid levels frequently track from childhood to adulthood (20).
An overall increase in lipid parameters with age was found in the present study, and this was particularly evident in male subjects. However, our study shows also a small but identifiable fall in cholesterol around the age of 15–16 years, followed by an increase thereafter. In healthy adolescents, there is a decline of ∼10–20% in cholesterol levels during puberty (21). This decline has been constantly reported in boys, whereas in girls, the picture has been more controversial, since some studies have not shown any pubertal decline in total cholesterol (22). However, an influence of age and puberty on lipid levels has not always been reported in children and adolescents with type 1 diabetes, and this is probably related to differences in the age range across different studies (9,10,12). Lipid levels, except TG, were higher in type 1 diabetic girls than in boys. This is in line with previous data (8,23), and it might be related to different degrees of insulin resistance between the two sexes or to a direct effect of the hormonal status on one or more enzymes implicated in lipoprotein metabolism (23).
Glycemic control significantly influenced changes in lipid levels during follow-up. The only parameter not related to A1C was HDL cholesterol, similarly to previous findings in adults (24). The lack of a relationship between A1C and HDL cholesterol could be due to opposite effects of glycemia on different HDL subclasses, which cannot be detected by simply assessing total HDL cholesterol (24). A strong relationship between other lipid parameters and A1C was detected in the DCCT as well as in studies more specifically targeting children and adolescents with type 1 diabetes (7,9,10). The adverse effect of glycemic control could be due to glycation of lipoproteins, with consequent reduction of their catabolism, and to stimulation of transfer of cholesteryl esters from HDL to apolipoprotein B–containing lipoproteins (2). The strong relationship between lipid levels and A1C underlines the role of good management of diabetes in controlling dyslipidemia. This is confirmed by data from the DCCT, where intensive treatment was associated with a significant reduction in lipid levels (24). However, it is important to acknowledge that, despite attempts to improve glycemic control, the present study and previous studies indicate that the prevalence of lipid abnormalities is high and persistent over time in youth with type 1 diabetes (8,9), therefore suggesting the possible need of additional interventions with lipid-lowering drugs.
In the present study, BMI was another important determinant of lipid levels. Previously, a similar association between overweight and an adverse lipid profile was documented in subjects with type 1 diabetes (7,9). In the DCCT cohort, excessive weight gain was related to dyslipidemia and declines in A1C were associated with improvements in lipid levels only in subjects with the least weight gain during the interventional period (25). These observations have been related to a state of insulin resistance/hyperinsulinemia associated with increased body weight (23).
The relationship between microalbuminuria and dyslipidemia has not been extensively investigated in young people with type 1 diabetes. In adult populations, increased total cholesterol and/or TG have been associated with microalbuminuria (6), although associations with lipid abnormalities were found to be more marked in patients with macroalbuminuria (6,23). With respect to the pediatric populations with diabetes, data from the Oxford Regional Prospective Study showed that the prevalence of microalbuminuria increased across tertiles of total cholesterol (12), and a recent German study has shown a predictive value of both LDL cholesterol and TG on the development of persistent microalbuminuria (11). In the present study, we examined lipid levels in relation to changes in albumin excretion, as a continuous variable, and the development of microalbuminuria. Increased total cholesterol and non-HDL cholesterol levels were independently related to ACR during follow-up, even after adjusting for glycemic control and other confounding factors. In addition, the changes in lipid levels with age in subjects with persistent microalbuminuria were remarkable when compared with those with transient microalbuminuria or normoalbuminuria. Both total cholesterol and non-HDL cholesterol showed a marked increase from the age of about 15 years in individuals developing persistent microalbuminuria. It is interesting that in our study population the mean age at microalbuminuria onset was 15 years, therefore providing further support for a potential relationship between lipid levels and microalbuminuria. Overall, lipid levels were higher in subjects developing microalbuminuria when compared with normoalbuminuric subjects. However, these differences were probably related to the worse glycemic control in subjects with microalbuminuria, since they disappeared when adjusting for A1C.
In this longitudinal study of young people with type 1 diabetes, we found that lipid levels varied with age and were higher in females than in males. Lipid levels were independently related to BMI, and all parameters, except HDL cholesterol, were also influenced by glycemic control. A significant number of subjects presented high and borderline lipid levels that persisted over time. Total cholesterol and non-HDL cholesterol were closely related to albumin excretion during follow-up, suggesting a potential role in the pathogenesis of diabetic nephropathy. These results highlight the need of screening for dyslipidemia in adolescents with type 1 diabetes to identify early subjects at risk for complications, who need more intensive follow-up and perhaps other therapeutic interventions.
Supplementary Material
Acknowledgments
The Nephropathy Family Study (NFS) is funded by the Juvenile Diabetes Research Foundation (JDRF) and the Wellcome Trust. We acknowledge the study field workers, pediatricians, physicians, and diabetes nurse specialists involved in the NFS and the National Institute for Health Research (NIHR) Cambridge Comprehensive Biomedical Research Centre. This study was also supported by an ESPE Research Fellowship, sponsored by Novo Nordisk A/S (M.L.M.).
No potential conflicts of interest relevant to this article were reported.
The results of this study were presented in poster form at the European Society for Pediatric Endocrinology 47th annual meeting, September 2008, Istanbul, Turkey.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Libby P, Nathan DM, Abraham K, et al. : Report of the National Heart, Lung, and Blood Institute: National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of Type 1 Diabetes Mellitus. Circulation 2005; 111: 3489– 3493 [DOI] [PubMed] [Google Scholar]
- 2. Durrington PN: Diabetic dyslipidaemia. Baillieres Best Pract Res Clin Endocrinol Metab 1999; 13: 265– 278 [DOI] [PubMed] [Google Scholar]
- 3. Bonnet F, Cooper ME: Potential influence of lipids in diabetic nephropathy: insights from experimental data and clinical studies. Diabetes Metab 2000; 26: 254– 264 [PubMed] [Google Scholar]
- 4. Hovind P, Tarnow L, Rossing P, et al. : Predictors for the development of microalbuminuria and macroalbuminuria in patients with type 1 diabetes: inception cohort study. BMJ 2004; 328: 1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Perkins BA, Ficociello LH, Silva KH, et al. : Regression of microalbuminuria in type 1 diabetes. N Engl J Med 2003; 348: 2285– 2293 [DOI] [PubMed] [Google Scholar]
- 6. Mattock MB, Cronin N, Cavallo-Perin P, et al. : Plasma lipids and urinary albumin excretion rate in type 1 diabetes mellitus: the EURODIAB IDDM Complications Study. Diabet Med 2001; 18: 59– 67 [DOI] [PubMed] [Google Scholar]
- 7. Maahs DM, Maniatis AK, Nadeau K, et al. : Total cholesterol and high-density lipoprotein levels in pediatric subjects with type 1 diabetes mellitus. J Pediatr 2005; 147: 544– 546 [DOI] [PubMed] [Google Scholar]
- 8. Schwab KO, Doerfer J, Hecker W, et al. : Spectrum and prevalence of atherogenic risk factors in 27,358 children, adolescents, and young adults with type 1 diabetes: cross-sectional data from the German diabetes documentation and quality management system (DPV). Diabetes Care 2006; 29: 218– 225 [DOI] [PubMed] [Google Scholar]
- 9. Maahs DM, Wadwa RP, McFann K, et al. : Longitudinal lipid screening and use of lipid-lowering medications in pediatric type 1 diabetes. J Pediatr 2007; 150: 146– 150 [DOI] [PubMed] [Google Scholar]
- 10. Polak M, Souchon PF, Benali K, et al. : Type 1 diabetic children have abnormal lipid profiles during pubertal years. Pediatr Diabetes 2000; 1: 74– 81 [DOI] [PubMed] [Google Scholar]
- 11. Raile K, Galler A, Hofer S, et al. : Diabetic nephropathy in 27,805 children, adolescents, and adults with type 1 diabetes: effect of diabetes duration, A1C, hypertension, dyslipidemia, diabetes onset, and sex. Diabetes Care 2007; 30: 2523– 2528 [DOI] [PubMed] [Google Scholar]
- 12. Abraha A, Schultz C, Konopelska-Bahu T, et al. : Glycaemic control and familial factors determine hyperlipidaemia in early childhood diabetes: Oxford Regional Prospective Study of Childhood Diabetes. Diabet Med 1999; 16: 598– 604 [DOI] [PubMed] [Google Scholar]
- 13. Schultz CJ, Konopelska-Bahu T, Dalton RN, et al. : Microalbuminuria prevalence varies with age, sex, and puberty in children with type 1 diabetes followed from diagnosis in a longitudinal study. Oxford Regional Prospective Study Group. Diabetes Care 1999; 22: 495– 502 [DOI] [PubMed] [Google Scholar]
- 14. National Cholesterol Education Program (NCEP). Highlights of the report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents. Pediatrics 1992; 89: 495– 501 [PubMed] [Google Scholar]
- 15. Kavey RE, Daniels SR, Lauer RM, et al. : American Heart Association guidelines for primary prevention of atherosclerotic cardiovascular disease beginning in childhood. J Pediatr 2003; 142: 368– 372 [DOI] [PubMed] [Google Scholar]
- 16. Kershnar AK, Daniels SR, Imperatore G, et al. : Lipid abnormalities are prevalent in youth with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth Study. J Pediatr 2006; 149: 314– 319 [DOI] [PubMed] [Google Scholar]
- 17. Cruickshanks KJ, Orchard TJ, Becker DJ: The cardiovascular risk profile of adolescents with insulin-dependent diabetes mellitus. Diabetes Care 1985; 8: 118– 124 [DOI] [PubMed] [Google Scholar]
- 18. Lopes-Virella MF, Wohltmann HJ, Mayfield RK, et al. : Effect of metabolic control on lipid, lipoprotein, and apolipoprotein levels in 55 insulin-dependent diabetic patients: a longitudinal study. Diabetes 1983; 32: 20– 25 [DOI] [PubMed] [Google Scholar]
- 19. Attia N, Touzani A, Lahrichi M, et al. : Response of apolipoprotein AIV and lipoproteins to glycaemic control in young people with insulin-dependent diabetes mellitus. Diabet Med 1997; 14: 242– 247 [DOI] [PubMed] [Google Scholar]
- 20. Lauer RM, Lee J, Clarke WR: Factors affecting the relationship between childhood and adult cholesterol levels: the Muscatine Study. Pediatrics 1988; 82: 309– 318 [PubMed] [Google Scholar]
- 21. Berenson GS, Srinivasan SR, Cresanta JL, et al. : Dynamic changes of serum lipoproteins in children during adolescence and sexual maturation. Am J Epidemiol 1981; 113: 157– 170 [DOI] [PubMed] [Google Scholar]
- 22. Twisk JW, Kemper HC, Mellenbergh GJ: Longitudinal development of lipoprotein levels in males and females aged 12–28 years: the Amsterdam Growth and Health Study. Int J Epidemiol 1995; 24: 69– 77 [DOI] [PubMed] [Google Scholar]
- 23. Jenkins AJ, Lyons TJ, Zheng D, et al. : Lipoproteins in the DCCT/EDIC cohort: associations with diabetic nephropathy. Kidney Int 2003; 64: 817– 828 [DOI] [PubMed] [Google Scholar]
- 24. Effect of intensive diabetes management on macrovascular events and risk factors in the Diabetes Control and Complications Trial. Am J Cardiol 1995; 75: 894– 903 [DOI] [PubMed] [Google Scholar]
- 25. Purnell JQ, Hokanson JE, Marcovina SM, et al. : Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the Diabetes Control and Complications Trial. JAMA 1998; 280: 140– 146 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.