Abstract
OBJECTIVE
The purpose of this study was to investigate relationships between inflammatory markers and components of a metabolic syndrome cluster in adolescents.
RESEARCH DESIGN AND METHODS
This was a cross-sectional analysis of an Australian childhood cohort (n = 1,377) aged 14 years. Cluster analysis defined a “high-risk” group similar to adults with metabolic syndrome. Relevant measures were anthropometry, fasting insulin, glucose, lipids, inflammatory markers, liver function, and blood pressure.
RESULTS
Of the children, 29% fell into a high-risk metabolic cluster group compared with 2% by a pediatric metabolic syndrome definition. Relative to the “low-risk” cluster, they had higher BMI (95% CI 19.5–19.8 vs. 24.5–25.4), waist circumference (centimeters) (95% CI 71.0–71.8 vs. 83.4–85.8), insulin (units per liter) (95% CI 1.7–1.8 vs. 3.5–3.9), homeostasis model assessment (95% CI 1.7–1.8 vs. 3.5–3.9), systolic blood pressure (millimeters of mercury) (95% CI 110.8–112.1 vs. 116.7–118.9), and triglycerides (millimoles per liter) (95% CI 0.78–0.80 vs. 1.25–1.35) and lower HDL cholesterol (millimoles per liter) (95% CI 1.44–1.48 vs. 1.20–1.26). Inflammatory and liver function markers were higher in the high-risk group: C-reactive protein (CRP) (P < 0.001), uric acid (P < 0.001), alanine aminotransferase (ALT) (P < 0.001), and γ-glutamyl transferase (GGT) (P < 0.001). The highest CRP, GGT, and ALT levels were restricted to overweight children in the high-risk group.
CONCLUSIONS
Cluster analysis revealed a strikingly high proportion of 14 year olds at risk of cardiovascular disease–related metabolic disorders. Adiposity and the metabolic syndrome cluster are synergistic in the pathogenesis of inflammation. Systemic and liver inflammation in the high-risk cluster is likely to predict diabetes, cardiovascular disease, and nonalcoholic fatty liver disease.
Metabolic abnormalities such as insulin resistance have become more prevalent with the childhood obesity epidemic (1). These features, referred to as the metabolic syndrome, are also associated with inflammatory markers such as C-reactive protein (CRP) in children (2) and adults (3). Elevated CRP levels are associated with cardiovascular end points, particularly in high-risk populations such as those with type 2 diabetes (4).
Elevated liver enzyme levels in adolescents with metabolic syndrome track during childhood and are associated with worsening of the cardiovascular risk profile in young adults (5). Furthermore, γ-glutamyl transferase (GGT), a marker of oxidative stress, is related to the risk of adult cardiovascular disease (6), and both GGT and alanine aminotransferase (ALT) levels predict the risk of type 2 diabetes (7). Uric acid, another marker of inflammation, is independently associated with hypertension, cardiovascular risk, and mortality (8).
The relationship between inflammatory markers and individual components of metabolic syndrome is unclear. Salient questions are whether increased inflammation is a result of some or all of the components associated with metabolic syndrome or whether (and to what extent) inflammation contributes to or causes metabolic syndrome. For example, it has been suggested that increased inflammatory cytokines derived from adipocytes may be partly responsible for the insulin resistance seen in obese patients (9).
We aimed, first, to define the proportion of adolescents with a high risk of cardiovascular and metabolic disorders using cluster analysis rather than arbitrary definitions of metabolic syndrome. Second, we aimed to identify differences in CRP, uric acid, ALT, and GGT between the “high-risk” and “low-risk” groups. Third, we aimed to dissect out the relative importance of metabolic syndrome components for CRP, uric acid, ALT, and GGT. We used data from a cohort studied at age 14, before onset of confounding behaviors such as heavy drinking or smoking.
RESEARCH DESIGN AND METHODS
The West Australian Cohort (Raine) Study has been described elsewhere (10). A total of 2,804 pregnant women serially recruited from King Edward Memorial Hospital delivered 2,868 live births. Follow-up examinations of their children at 1, 2, 3, 5, 8, 10, and 14 years included longitudinal sociodemographic and anthropometric parameters. The age 14 follow-up involved 2,337 eligible participants; 1,695 physical assessments were conducted, including anthropometry and resting blood pressure, and 1,377 fasting blood samples were obtained. Adolescents born preterm (<37 weeks' gestation) or with congenital deformities and multiple births were excluded, resulting in 1,293 physical assessments and 1,106 fasting blood samples. Only children without missing data were used in the clusters (n = 1,094).
Fasting blood samples from children were analyzed at Royal Perth Hospital for serum insulin, glucose, triglycerides, total cholesterol, HDL and LDL cholesterol, CRP, and uric acid. Glucose was measured by an automated Technicon Axon analyzer (Bayer Diagnostics, Sydney, Australia) using a hexokinase method. Insulin was measured by automated radioimmunoassay (Tosoh, Tokyo, Japan). Total cholesterol and triglycerides were determined enzymatically on the Cobas MIRA analyzer (Roche Diagnostics) with reagents from Trace Scientific (Melbourne, Australia). HDL cholesterol was determined on a heparin-manganese supernatant (11). HDL2 and HDL3 cholesterol were determined using single precipitation (12). LDL cholesterol was calculated using the Friedewald formula (13), valid for TG <3.5 mmol/l. For CRP, a high-sensitivity monoclonal antibody assay (Dade Behring Marburg, Marburg, Germany) with interassay precision of 2.1–2.6% for values 0.5–14 mg/l was used. Uric acid was assayed on the Technicon Axon analyser using Technicon reagents and methods (Bayer Diagnostics).
Homeostasis model assessment (HOMA) was calculated by fasting insulin (microunits per milliliter) × fasting glucose (millimoles per liter)]/22.5 (14).
Resting blood pressure readings were taken using an oscillometric sphygmomanometer (DINAMAP vital signs monitor 8100, DINAMAP XL vital signs monitor, or DINAMAP ProCare 100) after children were seated. The monitor was set to automatically record readings every 2 min. The average of the second and third readings was calculated.
Family income when children were aged 14 years (defined by annual earnings in Australian dollars [A$]: A$0–30,000, A$30,001–60,000, A$60,001–78,000, or >A$78,001) was obtained by questionnaire from the primary caregiver. Highest maternal education was obtained by questionnaire when children were aged 8 years (incomplete secondary schooling, completed secondary schooling, technical qualification or diploma, or tertiary education).
Exercise in adolescence was determined by questionnaire by the frequency of exercise causing breathlessness or sweating (monthly or less, weekly, 2–3 times weekly, 4–6 times weekly, or daily). Kilocalories per day were based on food frequency diaries.
Informed consent was obtained from parents and adolescents. Institutional ethics committees approved the study.
Statistical methods
Data were analyzed using SPSS (version 15.0; SPSS, Chicago, IL). Transformation to natural logarithms was used for variables not normally distributed. For CRP values below the lowest detectable level of the assay (0.16 mg/l), half of the detectable value (0.08 mg/l) was assigned. CRP, which could not be transformed appropriately, was analyzed as a categorical variable in tertiles and as a continuous logarithmically transformed variable.
The use of two-step cluster analysis avoided arbitrary definitions of metabolic syndrome, particularly because adult cutoffs are not applicable to children. This technique, best applied to data with natural groupings, classifies data into subsets, known as clusters. Metabolic syndrome is seen with groupings of risk factors within an individual; hence, this technique is well suited for identification of a high-risk cluster. Within a single cluster, the subjects are relatively homogeneous, sharing similar traits and being dissimilar to subjects in other clusters. The technique uses a scalable cluster analysis algorithm (15) designed specifically to handle large datasets. It preclusters subjects into subclusters before further grouping into the desired number of clusters using log-likelihood distance. The cluster groups were formed separately by sex using triglycerides, BMI, HOMA, and systolic blood pressure (SBP). This technique was previously used on a subset of children aged 8 years to define a distinct high-risk group with features consistent with metabolic syndrome (10).
The prevalence of the cluster groups was compared with the prevalence of metabolic syndrome defined by the National Cholesterol Education Program adult definition (16) and two modified pediatric definitions of metabolic syndrome (17), one from the International Diabetes Federation consensus (18).
The two cluster groups were compared using one-way ANOVA for cardiovascular risk factors including BMI, waist circumference, fasting insulin, HOMA, lipids, and SBP. The cluster groups were compared using one-way ANOVA for CRP, GGT, ALT, and uric acid.
Evaluation of effects on inflammatory markers of individual metabolic syndrome components
Linear regression was undertaken for prediction of GGT, ALT, and uric acid, adjusting for age and sex. Stepwise regression models were formed, using as independent covariates BMI, waist circumference, triglycerides, HOMA, SBP, and HDL cholesterol. A second model adjusted for socioeconomic status, puberty, kilocalories per day, and exercise. Ordinal and linear regression using categorical groups of CRP tertiles and logarithmically transformed CRP as outcomes were performed. Both regressions revealed similar results. Only linear regression results are presented.
Because components of the metabolic cluster are strongly interrelated, we explored the relative importance of each component on inflammatory markers by dividing the data at the median of individual components of metabolic syndrome, namely, into high and low BMI, waist circumference, insulin, HOMA, triglycerides, HDL cholesterol, and SBP. In each instance, four groups were formed by combinations of high-risk or low-risk cluster with high or low individual components. CRP, GGT, ALT, and uric acid were compared among the four groups using one-way ANOVA. The majority (>90%) of the adolescents were Caucasian.
RESULTS
Cluster analysis
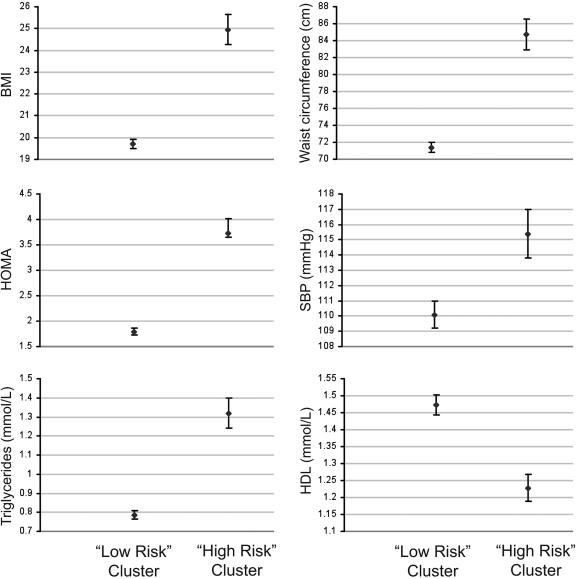
Using cluster analysis, 29.1% fell into the high-risk cluster using HOMA, SBP, triglycerides, and BMI at age 14 years as cluster components. The high- and low-risk clusters were comparable in age. The high-risk children had lower family income and lower maternal education and were more likely to be female. The high-risk group had greater weight, height, BMI, waist circumference, arm circumference, insulin, glucose, triglycerides, LDL cholesterol, total cholesterol, and SBP and lower HDL cholesterol (Fig. 1). By using an adult definition (National Cholesterol Education Program) (16) and pediatric adapted definitions from the International Diabetes Federation (18) and Cook/Ford (17) to define metabolic syndrome, prevalence was 1.8, 1.8, and 2.2%, respectively, in this cohort. All identified with the syndrome using any arbitrary cutoffs fell into the high-risk cluster.
Figure 1.
The features of the cluster groups with respect to components of the metabolic syndrome showing the 99% CIs.
Association of cluster group with inflammatory markers: inflammatory markers/liver function tests
The high-risk cluster had significantly higher CRP, ALT, GGT, and uric acid both for boys and girls separately and combined (all P < 0.001) (Table 1).
Table 1.
General characteristics of girls and boys in the “Raine cohort” at age 14 years and characteristics of the children in the high-risk and low-risk clusters
| Girls | Boys | P | Low-risk cluster | High-risk cluster | P | |
|---|---|---|---|---|---|---|
| n | 629 | 664 | 783 | 311 | ||
| General characteristics | ||||||
| Age (years) | 13.8 (13.7–13.8) | 13.8 (13.7–13.8) | 0.922 | 13.8 (13.7–13.8) | 13.8 (13.8–13.8) | 0.106 |
| Maternal education (3 df)* | 0.011† | |||||
| Income group (3 df)‡ | 0.001† | |||||
| Male/female ratio | 1.16 | 0.87 | 0.014† | |||
| Pubertal stage (boys)§ | 2.8 (2.7–2.8) | 2.8 (2.7–2.9) | 2.8 (2.7–3.0) | NS | ||
| Pubertal stage (girls)§ | 3.0 (3.0–3.1) | 3.3 (3.2–3.4) | 3.4 (3.3–3.5) | 0.032 | ||
| Exercise (proportion exercising to out of breath daily) (%)‖ | 7 | 16 | <0.001† | 14 | 9 | 0.014† |
| Anthropometry | ||||||
| Weight (kg) | 57.1 (56.1–58.1) | 58.4 (57.3–59.4) | 0.086 | 53.4 (52.9–54.0) | 69.4 (67.8–70.9) | <0.001 |
| Height (m) | 1.62 (1.62–1.63) | 1.66 (1.66–1.67) | <0.001 | 1.64 (1.64–1.64) | 1.65 (1.64–1.66) | 0.032 |
| Waist circumference (cm)¶ | 74.7 (73.9–75.6) | 76.1 (75.2–77.0) | 0.028 | 71.4 (71.0–71.8) | 84.6 (83.4–85.8) | <0.001 |
| BMI (kg/cm2)¶ | 21.7 (21.4–22.0) | 21.0 (20.7–21.3) | 0.003 | 19.6 (19.5–19.8) | 24.9 (24.5–25.4) | <0.001 |
| Arm circumference (cm) | 25.3 (25.0–25.5) | 25.3 (25.0–25.5) | 0.921 | 24.1 (24.0–24.3) | 28.2 (27.9–28.6) | <0.001 |
| Inflammatory markers/liver function tests | ||||||
| CRP (mg/l)¶ | 0.33 (0.29–0.37) | 0.33 (0.29–0.36) | 0.876 | 0.27 (0.25–0.29) | 0.65 (0.57–0.73) | <0.001 |
| CRP (girls)¶ | 0.27 (0.24–0.29) | 0.58 (0.49–0.69) | <0.001 | |||
| CRP (boys)¶ | 0.28 (0.25–0.31) | 0.73 (0.60–0.88) | <0.001 | |||
| GGT (U/l)¶ | 9.6 (9.3–9.8) | 12.1 (11.8–12.4) | <0.001 | 10.4 (10.2–10.6) | 12.1 (11.7–12.6) | <0.001 |
| GGT (girls)¶ | 9.2 (8.9–9.4) | 10.6 (10.1–11.1) | <0.001 | |||
| GGT (boys)¶ | 11.7 (11.4–12.0) | 14.2 (13.5–14.9) | <0.001 | |||
| ALT (U/l)¶ | 13.6 (13.2–14.0) | 17.6 (17.0–18.1) | <0.001 | 15.1 (14.8–15.5) | 17.1 (16.4–17.7) | <0.001 |
| ALT (girls)¶ | <0.001 | |||||
| ALT (boys)¶ | <0.001 | |||||
| Uric acid (mmol/l) | 0.27 (0.27–0.28) | 0.32 (0.32–0.33) | <0.001 | 0.29 (0.29–0.29) | 0.32 (0.31–0.33) | <0.001 |
| Uric acid (girls) | 0.26 (0.26–0.27) | 0.29 (0.28–0.30) | <0.001 | |||
| Uric acid (boys) | 0.32 (0.31–0.32) | 0.35 (0.34–0.36) | <0.001 |
Data are means (95% CI).
*Defined by education not higher than secondary schooling, completed secondary schooling, higher education including technical qualification or diploma, or tertiary education.
†χ2 testing.
‡Defined by annual earnings in Australian dollars (A$) A$0–30,000, A$30,001–60,000, A$60,001–78,000, and >A$78,001.
§Subset of adolescents correctly shown the Tanner staging pictures.
‖Defined by exercise to an out-of-breath state once a month or less, once a week, 2–3 times per week, 4–6 times a week, or every day.
¶Around geometric mean. df, degrees of freedom.
Independent predictors of CRP, GGT, ALT, and uric acid
The independent predictors of these variables using stepwise linear regression showed that for each marker a measure of adiposity explained the largest amount of variance (17–28%) and that further metabolic syndrome components were additional independent predictors. For CRP and uric acid, HDL cholesterol was an independent predictor. For ALT and GGT, HOMA was an independent predictor. For UA, SBP was an independent predictor (Table 2).
Table 2.
Linear regression models for defining independent predictors of CRP, GGT, ALT, and uric acid
| Outcome | Variables* | Adjusted r2† | β coefficient | SEM | P |
|---|---|---|---|---|---|
| ln(CRP) | ln(BMI) | 0.25 | 2.17 | 0.37 | <0.001 |
| ln(waist) | 0.25 | 1.64 | 0.49 | 0.001 | |
| HDL cholesterol | 0.26 | −0.29 | 0.098 | 0.003 | |
| ln(GGT) | ln(BMI) | 0.22 | 0.40 | 0.05 | <0.001 |
| ln(TG) | 0.24 | 0.09 | 0.021 | <0.001 | |
| ln(HOMA) | 0.24 | 0.04 | 0.016 | 0.008 | |
| ln(ALT) | ln(waist) | 0.16 | 0.47 | 0.081 | <0.001 |
| ln(HOMA) | 0.17 | 0.05 | 0.018 | 0.006 | |
| SBP | 0.17 | 0.00 | 0.001 | 0.04 | |
| Uric acid | ln(waist) | 0.28 | 0.08 | 0.026 | 0.004 |
| SBP | 0.30 | 0.00 | 0 | <0.001 | |
| HDL cholesterol | 0.31 | −0.02 | 0.005 | <0.001 | |
| ln(BMI) | 0.31 | 0.05 | 0.019 | 0.005 | |
| Variables‡ | |||||
| CRP tertile | ln(BMI) | 0.26 | 2.34 | 0.51 | <0.001 |
| HDL cholesterol | 0.27 | −0.48 | 0.14 | 0.001 | |
| ln(waist) | 0.27 | 1.91 | 0.68 | 0.005 | |
| ln(TG) | 0.28 | −0.23 | 0.11 | 0.033 | |
| ln(GGT) | ln(BMI) | 0.21 | 0.39 | 0.06 | <0.001 |
| ln(TG) | 0.23 | 0.09 | 0.03 | 0.001 | |
| ln(HOMA) | 0.23 | 0.05 | 0.02 | 0.030 | |
| ln(ALT) | ln(waist) | 0.18 | 0.62 | 0.09 | <0.001 |
| SBP | 0.18 | 0.00 | 0.001 | 0.042 | |
| Uric acid | ln(BMI) | 0.28 | 0.07 | 0.02 | 0.008 |
| SBP | 0.29 | 0.00 | 0.0002 | <0.001 | |
| HDL cholesterol | 0.30 | −0.02 | 0.006 | 0.003 | |
| ln(waist) | 0.31 | 0.07 | 0.03 | 0.037 |
*Adjusted for age and sex.
†For progressive stepwise models.
‡Adjusted for age, sex, maternal education and family income, puberty, and exercise (kilocalories/day). TG, triglycerides.
Predictors of CRP
Importance of adiposity and cluster membership for CRP levels.
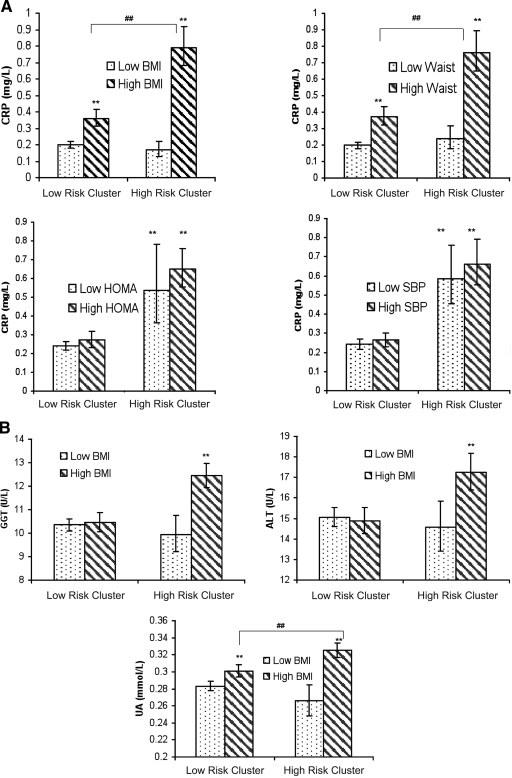
To test whether inflammation is associated with obesity without other features of the metabolic syndrome cluster, we grouped high- and low-risk children further according to the BMI median, resulting in four groups, namely 1) low-risk cluster, low BMI; 2) low-risk cluster, high BMI; 3) high-risk cluster, low BMI; and 4) high-risk cluster, high BMI. CRP was highest in those in both the high-risk cluster and high BMI groups (group 4). The overweight group in the low-risk cluster had an intermediate CRP, higher than that of their leaner counterparts but substantially lower than that of their overweight high-risk counterparts. The high-risk children with high BMI, both boys and girls, had the highest CRP.
Similarly, four groups were formed by dividing the high- and low-risk clusters according to the waist circumference median. Those with a high waist circumference in the high-risk cluster had the highest CRP (Fig. 2).
Figure 2.
A: CRP in relation to cluster membership and median splitting of BMI, waist circumference, SBP, and HOMA. ** P < 0.001 compared with the reference group (low-risk cluster and low BMI). ##P < 0.001 for difference between the two groups indicated.  , below the median level of a particular component of the metabolic syndrome (BMI, waist circumference, SBP, or HOMA);
, below the median level of a particular component of the metabolic syndrome (BMI, waist circumference, SBP, or HOMA);  , above the median level of a particular component of the metabolic syndrome (BMI, waist circumference, SBP, and HOMA). B: GGT, ALT, and uric acid in relation to cluster membership and median splitting of BMI. ** P < 0.001 compared with the reference group (low-risk cluster and low BMI). ##P < 0.001 for difference between the two groups indicated.
, above the median level of a particular component of the metabolic syndrome (BMI, waist circumference, SBP, and HOMA). B: GGT, ALT, and uric acid in relation to cluster membership and median splitting of BMI. ** P < 0.001 compared with the reference group (low-risk cluster and low BMI). ##P < 0.001 for difference between the two groups indicated.  , below the median of BMI;
, below the median of BMI;  , above the median of BMI.
, above the median of BMI.
Relative importance of other metabolic syndrome components for CRP levels.
In contrast with BMI and waist circumference, SBP, HOMA (Fig. 2), triglycerides, and HDL cholesterol were not associated with CRP within either of the risk cluster groups.
Predictors OF ALT, GGT, and uric acid: importance of adiposity and cluster membership for aminotransferases and uric acid
ALT, GGT, and uric acid levels in the four groups defined by cluster membership and BMI median are shown (Fig. 2). High-risk children with high BMI had significantly higher ALT, GGT, and uric acid than children in the other three groups. This finding was seen in both sexes.
CONCLUSIONS
In this 14-year-old population, a strikingly high proportion, 29%, fell into a high-risk group with features of metabolic syndrome, i.e., higher BMI, insulin resistance (HOMA), SBP, and triglycerides and lower HDL cholesterol levels. The high-risk group also exhibited substantially higher levels of inflammatory markers, which are independently associated with cardiovascular disease and diabetes in adults. Importantly, only overweight adolescents within the high-risk group had raised inflammatory markers.
Cluster analysis (10) of the individual metabolic syndrome components was used to avoid definitions relying on arbitrary cutoffs, previously shown to be inconsistent for diagnosing metabolic syndrome in adolescence (19). The prevalence of metabolic syndrome by conventional cutoffs was low in this population (2%) compared with that in other adolescent Western cohorts, including the National Health and Nutrition Examination Survey, with prevalence ranging from 2 to 9% using multiple definitions. Meanwhile, the cluster approach, with the use of continuous variables, revealed almost 15 times the prevalence (29%) of children at risk. Despite the far higher prevalence of children identified at risk by cluster analysis, the high- and low-risk groups appeared to be distinct populations with wide separation of 99% CI for each of the metabolic syndrome components (Fig. 1). Therefore, this approach is powerful for public health research into cardiovascular risk in a population, as it allows identification of larger numbers at risk.
High adiposity was the strongest independent predictor of the inflammatory markers, accounting for 17–28% of the variance in CRP, uric acid, ALT, and GGT, not dissimilar to the result seen for CRP in other adolescent studies (20). However, an important point was disclosed by this study. Adiposity was exclusively associated with the highest levels of inflammatory markers when it was part of the high-risk cluster, i.e., children with increased adiposity in isolation showed levels of CRP similar to those in leaner children, best illustrated by division of the clusters according to binary groupings of their components (Fig. 2). This approach helps dissect out highly collinear data.
The NHANES study, using an arbitrary metabolic syndrome definition, showed that being overweight with metabolic syndrome was associated with higher CRP than being overweight without the syndrome (21). However, in contrast with our findings, NHANES showed that CRP was high in the few individuals with normal weight who had the syndrome. Our approach with cluster analysis probably yielded different subpopulations. In the present study, the use of cluster analysis rather than the restrictive definitions of metabolic syndrome used elsewhere to assess associations with inflammatory markers enabled us to identify a broader section of the adolescent population at risk of cardiovascular and metabolic disease.
Increased levels of GGT, CRP, and uric acid in the high-risk cluster in our study are also notable; each has been shown to be a strong predictor of adult cardiovascular disease (3,6,8), independent of the individual components of the metabolic cluster.
The association between aminotransferases and the metabolic cluster is consistent with persistent elevation of ALT and GGT in those with elevated cardiovascular risk in the Bogalusa study (5). Because GGT predicts both type 2 diabetes (7) and cardiovascular mortality in adults (6), this association further emphasizes the heightened potential for premature ill health for the 14 year olds in the metabolic cluster.
There were noteworthy differences between the markers we investigated and their relationship to metabolic syndrome components. Specifically, 1) HDL cholesterol was related to CRP and uric acid, 2) SBP was related to uric acid, and 3) HOMA was related to the aminotransferases. These results suggest that each inflammatory marker evolves via different pathways.
Aminotransferases were predicted by HOMA levels in our study. Therefore, liver inflammation is associated with adiposity and insulin resistance in adolescents, as in adults with nonalcoholic fatty liver disease (NAFLD) (22). This finding implies that a large proportion of 14 year olds (29% in the high-risk cluster) are at risk of developing NAFLD and its sequelae, bearing in mind that the incidence of NAFLD will increase as the current overweight youth reach adulthood.
Uric acid is associated with the prevalence of the metabolic syndrome (8). However, our results indicate that uric acid has different associations with other inflammatory markers. Uric acid was independently associated with SBP and HDL cholesterol. The relation between uric acid and blood pressure is consistent with that in studies showing elevated uric acid with hypertension in adults, in youth (23), and in women with preeclampsia, for which hypertension is a hallmark. In contrast, an association between uric acid and HDL cholesterol, independent of adiposity, has not previously been reported in youth, although it was hinted at by the independent association between uric acid and the triglyceride–to–HDL cholesterol ratio in 352 middle-aged men (24). In our study, not only did HDL cholesterol independently predict uric acid, but also the highest levels of uric acid occurred in the high-risk cluster with low HDL cholesterol (data not shown). The explanation for this association is unclear, but, if causal, it may be related to the anti-inflammatory effects of HDL cholesterol (25).
Caution is needed in postulating causation from a cross-sectional study. It is possible that in using stepwise regression for identifying predictors of the markers, the variables selected may be surrogates, appearing in the model when measured with less error or in place of unmeasured variables. However, lack of adult confounders in our young population may be advantageous in clarifying causal pathways. We believe that the metabolic cluster in our adolescents signals disease processes early in evolution before other downstream effects. Discrepancies between adolescent and adult studies may relate to secondary effects of advanced vascular disease and pathological changes concomitant with aging or complicating effects of lifestyle factors such as smoking. Our study helps in understanding the critical early steps in the pathogenesis of inflammation and the metabolic syndrome. The biomarkers studied are on pathways of inflammation, along with cytokines such as interleukin-6 and tumor necrosis factor-α, previously shown to be closely related to adiposity. Longitudinal data will be available for a high proportion of adolescents with future follow-ups and will be of considerable interest in unraveling the evolution of cardiovascular risk during the transition to adulthood.
In summary, cluster analysis demonstrates that approximately 30% of 14-year-old predominantly Caucasian Australian children exhibit early features of the metabolic syndrome accompanied by a range of inflammatory markers. In addition to emphasizing the magnitude of the problem in a proximate adolescent population, the findings extend current understanding in several ways. The results suggest that adiposity in isolation is insufficient to cause inflammation but requires the synergistic effect of other features of the metabolic syndrome cluster, particularly insulin resistance and dyslipidemia. Furthermore, different associations of metabolic syndrome components with inflammatory markers suggest that the metabolic syndrome is likely to be a heterogeneous condition produced via different pathways, with implications for adult pathological conditions such as diabetes, NAFLD, and hypertension. We previously reported the effect of perinatal factors and early weight gain on cardiovascular risk in 8 year olds from this cohort (10). The results from these 14 year-olds highlight the need for effective childhood intervention to prevent obesity, diabetes, adult cardiovascular disease, and NAFLD.
Acknowledgments
We acknowledge the support of the Raine Medical Foundation, Healthway, Western Australia, the Telethon Institute of Child Research (University of Western Australia), and the Australian National Health and Medical Research Council (NHMRC). R.C.H. is supported by the NHMRC Centre for Training in Clinical Cardiovascular and Cerebrovascular Research (Royal Perth Hospital) and by Athelstan and Amy Saw scholarships.
No potential conflicts of interest relevant to this article were reported.
We are grateful to all the families who took part in this study and the Raine Study team, which includes data collectors, cohort managers, data managers, clerical staff, research scientists, and volunteers.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Wang Y, Beydoun MA: The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev 29: 6– 28, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Ford ES, Ajani UA, Mokdad AH: The metabolic syndrome and concentrations of C-reactive protein among U.S. youth. Diabetes Care 28: 878– 881, 2005 [DOI] [PubMed] [Google Scholar]
- 3. Rutter MK, Meigs JB, Sullivan LM, D'Agostino RB, Sr, Wilson PW: C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the Framingham Offspring Study. Circulation 110: 380– 385, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Bonora E, Targher G, Formentini G, Calcaterra F, Lombardi S, Marini F, Zenari L, Saggiani F, Poli M, Perbellini S, Raffaelli A, Gemma L, Santi L, Bonadonna RC, Muggeo M: The metabolic syndrome is an independent predictor of cardiovascular disease in type 2 diabetic subjects: prospective data from the Verona Diabetes Complications Study. Diabet Med 21: 52– 58, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS: Persistent elevation of liver function enzymes within the reference range is associated with increased cardiovascular risk in young adults: the Bogalusa Heart Study. Metabolism 56: 792– 798, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Ruttmann E, Brant LJ, Concin H, Diem G, Rapp K, Ulmer H: γ-Glutamyltransferase as a risk factor for cardiovascular disease mortality: an epidemiological investigation in a cohort of 163,944 Austrian adults. Circulation 112: 2130– 2137, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Andre P, Balkau B, Born C, Charles MA, Eschwege E: Three-year increase of gamma-glutamyltransferase level and development of type 2 diabetes in middle-aged men and women: the D.E.S.I.R. cohort. Diabetologia 49: 2599– 2603, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fang J, Alderman MH: Serum uric acid and cardiovascular mortality: the NHANES I Epidemiologic Follow-up Study. JAMA 283: 2404– 2410, 2000 [DOI] [PubMed] [Google Scholar]
- 9. Greenfield JR, Campbell LV: Relationship between inflammation, insulin resistance and type 2 diabetes: ‘cause or effect’? Curr Diabetes Rev 2: 195– 211, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Huang RC, Burke V, Newnham JP, Stanley FJ, Kendall GE, Landau LI, Oddy WH, Blake KV, Palmer LJ, Beilin LJ: Perinatal and childhood origins of cardiovascular disease. Int J Obes (Lond) 31: 236– 244, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Warnick GR, Albers JJ: A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res 19: 65– 76, 1978 [PubMed] [Google Scholar]
- 12. Gidez LI, Miller GJ, Burstein M, Slagle S, Eder HA: Separation and quantitation of subclasses of human plasma high density lipoproteins by a simple precipitation procedure. J Lipid Res 23: 1206– 1223, 1982 [PubMed] [Google Scholar]
- 13. Friedewald WT, Levy RI, Fredrickson DS: Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18: 499– 502, 1972 [PubMed] [Google Scholar]
- 14. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC: Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412– 419, 1985 [DOI] [PubMed] [Google Scholar]
- 15. Zhang TRR, Livny M: BIRCH: An efficient data clustering method for very large databases. In Proceedings of the ACM SIGMOD Conference on Management of Data, Montreal, QC, Canada. New York, ACM Press, 1996, p. 103– 114 [Google Scholar]
- 16. Executive summary of the Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 285: 2486– 2497, 2001 [DOI] [PubMed] [Google Scholar]
- 17. Cook S, Auinger P, Li C, Ford ES: Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J Pediatr 152: 165– 170, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Zimmet P, Alberti G, Kaufman F, Tajima N, Silink M, Arslanian S, Wong G, Bennett P, Shaw J, Caprio S: The metabolic syndrome in children and adolescents. Lancet 369: 2059– 2061, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Goodman E, Daniels SR, Meigs JB, Dolan LM: Instability in the diagnosis of metabolic syndrome in adolescents. Circulation 115: 2316– 2322, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Patel DA, Srinivasan SR, Xu JH, Li S, Chen W, Berenson GS: Distribution and metabolic syndrome correlates of plasma C-reactive protein in biracial (black-white) younger adults: the Bogalusa Heart Study. Metabolism 55: 699– 705, 2006 [DOI] [PubMed] [Google Scholar]
- 21. de Ferranti SD, Gauvreau K, Ludwig DS, Newburger JW, Rifai N: Inflammation and changes in metabolic syndrome abnormalities in US Adolescents: findings from the 1988–1994 and 1999–2000 National Health and Nutrition Examination Surveys. Clin Chem 52: 1325– 1330, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Bugianesi E, McCullough AJ, Marchesini G: Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology 42: 987– 1000, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Alper AB, Jr, Chen W, Yau L, Srinivasan SR, Berenson GS, Hamm LL: Childhood uric acid predicts adult blood pressure: the Bogalusa Heart Study. Hypertension 45: 34– 38, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Desai MY, Santos RD, Dalal D, Carvalho JA, Martin DR, Flynn JA, Nasir K, Blumenthal RS: Relation of serum uric acid with metabolic risk factors in asymptomatic middle-aged Brazilian men. Am J Cardiol 95: 865– 868, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Birjmohun RS, van Leuven SI, Levels JH, van 't Veer C, Kuivenhoven JA, Meijers JC, Levi M, Kastelein JJ, van der Poll T, Stroes ES: High-density lipoprotein attenuates inflammation and coagulation response on endotoxin challenge in humans. Arterioscler Thromb Vasc Biol 27: 1153– 1158, 2007 [DOI] [PubMed] [Google Scholar]