Abstract
OBJECTIVE
To determine how thermoregulation of the feet is affected by diabetes and diabetic polyneuropathy in both wakefulness and sleep.
RESEARCH DESIGN AND METHODS
Normal subjects, diabetic subjects without neuropathy, diabetic subjects with small-fiber diabetic polyneuropathy, and those with advanced diabetic polyneuropathy were categorized based on neurological examination, nerve conduction studies, and quantitative sensory testing. Subjects underwent foot temperature monitoring using an iButton device attached to the foot and a second iButton for recording of ambient temperature. Socks and footwear were standardized, and subjects maintained an activity diary. Data were collected over a 32-h period and analyzed.
RESULTS
A total of 39 normal subjects, 28 patients with diabetes but without diabetic polyneuropathy, 14 patients with isolated small-fiber diabetic polyneuropathy, and 27 patients with more advanced diabetic polyneuropathy participated. No consistent differences in foot temperature regulation between the four groups were identified during wakefulness. During sleep, however, multiple metrics revealed significant abnormalities in the diabetic patients. These included reduced mean foot temperature (P < 0.001), reduced maximal temperature (P < 0.001), increased rate of cooling (P < 0.001), as well as increased frequency of variation (P = 0.005), supporting that patients with diabetic polyneuropathy and even those with only diabetes but no diabetic polyneuropathy have impaired nocturnal thermoregulation.
CONCLUSIONS
Nocturnal foot thermoregulation is impaired in patients with diabetes and diabetic polyneuropathy. Because neurons are highly temperature sensitive and because foot warming is part of the normal biology of sleep onset and maintenance, these findings suggest new potentially treatable mechanisms of diabetes-associated nocturnal pain and sleep disturbance.
Thermoregulation is impaired in diabetes and diabetic polyneuropathy (1,2). Distal sudomotor (3,4) and microvascular dysfunction (5,6) are believed to contribute to this impairment, mainly through neuropathic mechanisms. Because the feet are affected early in diabetic polyneuropathy, they are therefore likely to exhibit early thermoregulatory abnormalities. Characterizing these changes may be of particular importance for two reasons. First, it could provide a novel approach for the detection of early neuropathy, since small unmyelinated fibers specifically play an important role in thermoregulation (7,8) and are often the earliest nerve fibers affected in diabetic polyneuropathy (9). In addition, disruptions in normal temperature control may be central to the development of neuropathic pain and sleep disturbance, because neuronal activity itself is highly temperature sensitive (10) and distal extremity warming is critical to sleep onset and maintenance (11–13).
Over the past several years, we have adopted a novel approach to the study of real-time changes in foot temperature during daily activity, using a small temperature-measuring and recording device that can be affixed directly to the foot (14,15). Our initial work demonstrated what appeared to be differences in temperature control in patients with neuropathic processes versus normal subjects, but the data were limited to only a small cohort of individuals with heterogeneous disorders (14). Thus, in this study, we characterize changes in foot thermoregulation during daily activity and during sleep in patients with isolated small-fiber diabetic polyneuropathy and more advanced diabetic polyneuropathy by comparing their results with data from healthy individuals and from patients with diabetes but no neuropathy, hypothesizing that diabetic polyneuropathy patients would demonstrate evidence of impaired thermoregulation.
RESEARCH DESIGN AND METHODS
All patients and normal subjects were recruited either through advertisement or by approaching the patient in the clinic or the electromyography (EMG) laboratory. All patients were reimbursed for their participation, and the study was approved by the Beth Israel Deaconess Medical Center institutional review board.
Patients were prescreened to identify their eligibility for the study. Inclusion criteria included the following: Either healthy subjects or subjects with established type 1 or type 2 diabetes (age 18–80 years). Exclusion criteria included the following: 1) Inability to refrain from smoking during a 32-h time period; 2) known peripheral vascular disease or findings suggestive of peripheral vascular disease on examination (reduced or unobtainable pulses in the feet or a reduced popliteal blood pressure relative to that of the arm); 3) presence of a medical disorder or drug therapy known to be associated with neuropathy; and 4) being wheelchair bound or otherwise severely physically disabled.
On the day of the study, after signing the informed consent, all patients underwent an additional brief review of their medical history, including their diabetes history; any patients who were now identified as meeting one of the exclusion criteria were excluded. Unless the patient expressed a preference or there was a history of trauma or other potential confounding, the side to be studied was chosen arbitrarily.
Physical examination
All patients underwent examination of distal strength and muscle bulk, deep tendon reflexes, and lower-extremity sensory perception (including pinprick, light touch, and joint position sense). Standard 10-g monofilament testing was also performed. Peripheral pulses (dorsalis pedis and posterior tibial) were assessed. Both brachial and popliteal blood pressures were measured.
Nerve conduction studies
All patients had a unilateral peroneal motor conduction study and F responses and bilateral sural sensory studies at a limb temperature of at least 32°C, with a Medelec Synergy T2–EMG Monitoring System or Medelec Synergy N2–EMG Monitoring System (Oxford Instruments Medical, Surrey, U.K.). Motor and sensory amplitudes, conduction velocities, and latencies were recorded. Determination of normality was made by comparing the results obtained for individual patients to the established Beth Israel Deaconess Electromyography Laboratory reference values.
Quantitative sensory testing
Quantitative sensory testing (QST) was performed with a TSA-II NeuroSensory Analyzer (Medoc, Durham, NC) on the same side as the peroneal motor study. Sensory thresholds were measured using cold and warm stimuli and compared with an age-matched normal population value. Vibration testing on the foot was also performed.
Neuropathy scales
The Michigan Neuropathy Screening Instrument (16) and Utah Early Neuropathy Scale (17) were completed.
Group assignment
Based on these data, patients were assigned to one of four groups. Any normal subjects found to have abnormalities on examination were excluded from the study; otherwise, they were included in the normal subject category. Subjects with diabetes who were found to be normal on all the above measures were placed in the diabetic normal category. Subjects with diabetes found to have abnormalities involving only small fibers—reduced pin prick on examination or reduced heat and cold thresholds on QST, but normal large-fiber function, based on normal examination of vibration (by both examination and QST), reflexes, and normal nerve conduction studies—were placed in the small-fiber diabetic polyneuropathy category. Subjects with diabetes with evidence of large-fiber involvement on one or more of these tests, including on nerve conduction studies, were placed in the large-fiber diabetic polyneuropathy category.
Ambulatory temperature measurement
Temperature measurement was performed using iButton Temperature monitors (Maxim Integrated Products, Sunnyvale, CA). Two separate iButtons were used. Model no. DS1921H-F5, with a range of 15–46°C and resolution of 0.125°C, was used to measure foot temperature. Model no. DS1921G-F5, with a range of −40 to +85°C and a resolution of ±0.5°C from −30 to +70°C, was used to measure ambient temperature. During this study, a third device with higher resolution and greater memory also became available (model no. DS1922L-F50) and was used instead for both sets of measurements; however, to maintain consistent data analysis, we kept the parameters of recording identical for this additional device. Studies were conducted for a minimum of 32 h.
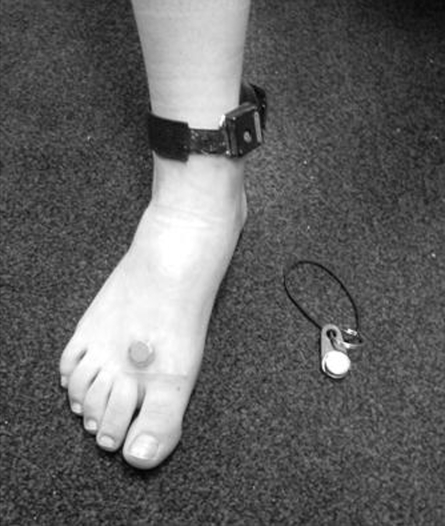
The foot iButton was affixed to the web space between the first and second toes (Fig. 1) using medical-grade adhesive tape (3M Transpore Surgical Tape, no. 1527-1, 3M Health Care, St. Paul, MN). The ambient iButton was affixed via a key ring apparatus (Fig. 1) to external clothing. Subjects were instructed to keep the device attached to the foot throughout the study period, except while bathing. During sleep, we requested that the ambient temperature device be placed by the bed.
Figure 1.
Setup for measuring ambulatory foot temperature. One iButton is attached to the foot; the other is attached to a key ring apparatus that can be affixed to external clothing. The actigraphy monitor is attached to the ankle as well.
Footwear
We provided individuals with identical socks (Banda Men's/Women's No-Elastic Acrylic Crew Socks, #99158, FootSmart, Norcross, GA) and requested that during the study they wear simple noninsulated footwear, while going about their normal daily activities. Subjects were instructed to wear the socks to bed as well.
Monitoring foot movement
We also monitored foot movement to determine how foot activity affected thermoregulation and as an additional marker for sleep onset and activity level. We used a commercially available actigraphy monitor (Actiwatch 16, #198-0101-02, Mini Mitter/Respironics, Bend, OR). The device was loosely affixed to the ankle (Fig. 1). The internal chronometer on the actigraphy device was synchronized with the two iButtons.
Diaries
In addition to wearing the measurement devices, subjects were requested to maintain simple paper diaries for the entire 32-h period. Subjects recorded the date, general activity, start and end time of the activity, and whether they were outside, inside, or both during the particular activity. Subjects also marked down the exact times the devices were removed for bathing and replaced.
Data analysis
The data from both iButtons and from the actigraph were downloaded into a spreadsheet program. Data points from before and after the device was affixed to the foot were removed, as were those points associated with removal of the iButton from the foot, for example, during bathing. Sleep and wakefulness were also identified on the tracings. The sleep and waking data were analyzed separately.
Because the findings in our earlier study suggested obvious differences between patients with and without polyneuropathy, our goal here was to find a quantifiable property for detecting differences that could discriminate between subject groups. Also, because nocturnal ambient temperature measurements did not accurately reflect the temperature experienced by the foot at night, we analyzed the sleep data by looking at foot temperature in isolation. For waking measurements, however, we assessed foot temperature accounting for ambient temperature.
A variety of statistically appropriate analyses were performed, from simple (e.g., mean and SD) to complex (e.g., fractal dimension [(18)], slopewise comparisons [(19)], and other signal analysis paradigms). These are described individually in results. Appropriate multiple group tests (one-way ANOVA and Kruskal-Wallis) were used to compare the four groups to determine if there was a difference between them and, if positive, a Tukey's honestly significance difference test or individual Mann-Whitney U tests with Bonferroni's correction were used to determine where the difference lay, depending on the normality of the data. Outlying values (defined nonparametrically as any value >1.5 times the interquartile range from the nearest quartile boundary) were excluded for all calculations (this generally amounted to no more than two to three subjects' data across all four groups). Significance was set at P < 0.05, two-tailed for all analyses. Values are provided as means ± SD, except where otherwise specified.
RESULTS
General overview
Using our screening testing and categorization methods, we identified 39 normal subjects (43.5 ± 14.8 years of age), 28 diabetic normal subjects (46.8 ± 14.6 years of age), 14 diabetic small-fiber diabetic polyneuropathy patients (55.9 ± 11.0 years of age), and 27 with more severe diabetic polyneuropathy with large-fiber involvement (61.4 ± 9.8 years of age), on whom we also obtained at least 32 h of reliable iButton data. The Utah Early Neuropathy Scale scores, specifically designed to discriminate between large- and small-fiber diabetic polyneuropathy, perhaps best summarize the success of our categorization. Diabetic normal subjects all scored 0, small-fiber diabetic polyneuropathy patients had a score of 4.0 ± 2.0, and large-fiber diabetic polyneuropathy patients had a score of 8.0 ± 6.5 (median ± interquartile difference). The relatively limited number of small-fiber diabetic polyneuropathy patients was because a number of individuals who by history and examination seemed likely to have small-fiber neuropathy were found to have large-fiber impairment as well on additional testing (most often on nerve conduction studies). Despite our efforts at age-matching subjects, age did vary significantly between groups (P = 0.011). Thus, in each of the analyses below, age was treated as a covariate where possible.
Additionally, A1C values (when available within 3 months of participation of the study) were similar among all three groups of diabetic patients (7.8 ± 0.8% for diabetic normal subjects, 7.0 ± 0.6% for small-fiber neuropathy patients, and 7.1 ± 1.3% for large-fiber patients). Eighty-four percent of diabetic normal subjects were treated with an oral hypoglycemic or insulin, compared with 86% of patients with small-fiber diabetic polyneuropathy and 96% of patients with large-fiber diabetic polyneuropathy.
Waking data analysis
Our next goal was to determine whether foot movement affected the waking foot temperature data in any consistent fashion. Fortunately, foot temperature showed only a very weak and nonsignificant relationship with foot actigraphy in any of the four groups, with Spearmen's ρ <0.10 and nonsignificant for each of the four groups (P = 0.152); thus, this potential covariate was effectively eliminated.
A number of comparative summary analytical metrics were then assessed on the foot temperature traces for each patient, including mean foot temperature, maximum and minimum foot temperature, within-subject SD of foot temperature, positive and negative rates of change in foot temperature, and the correlation between foot and ambient temperature. All of these were negative, except for the negative rate of change that showed a significant difference (P = 0.013), an effect due to the difference in values for the diabetic non-neuropathy group and the normal subject group. In addition, no significant differences between subjects were identified using a variety of more sophisticated approaches to time series analysis, either looking at the foot temperature in isolation or in relation to the ambient temperature. These included Euclidean distance (19), slopewise comparisons (19), fractal dimension (18), wavelet analysis (20), and several measurements of entropy (21).
Asleep data analysis
Unlike the waking data analysis, all of the metrics used to analyze the sleeping data showed significant differences between the four groups (Table 1 provides the basic comparisons and Table 2 the post hoc analyses). Mean foot temperature was reduced for all three diabetic groups, including the diabetic normal subjects, compared with the normal subjects. This tendency toward cooling, or the presence of impaired warming and reduced thermoregulatory control, was supported by several other metrics. For example, we determined the highest and lowest temperatures attained and held for at least two consecutive time points by each subject. This constraint was imposed to ensure that the maximum value attained was sustained for a minimum of 2 min and unlikely to be the result of noise. Like the mean foot temperature, the maximum temperature was reduced in the diabetes patients, both those with and those without neuropathy. However, the positive and negative rates of change also were found to be different between the groups (P = 0.041 positive, P = 0.0011 negative). The negative rate of change was most impressive and was considerably larger for the advanced diabetic polyneuropathy group versus the other groups, supporting that in this group, feet tended to cool rapidly. Similarly, the SD of sleeping foot temperatures revealed a difference between groups (P = 0.006), with the largest disparity occurring between the nondiabetic normal and large-fiber diabetic groups (P = 0.046), again supporting an increased tendency for foot temperature variability in the diabetic polyneuropathy patients.
Table 1.
Asleep data statistics
| Normal | Diabetic normal | Small fiber diabetic polyneuropathy | Advanced diabetic polyneuropathy | Significance | |
|---|---|---|---|---|---|
| n | 25 | 18 | 14 | 25 | |
| Average foot temperature* | 34.8 ± 0.75 | 33.6 ± 0.60 | 33.2 ± 0.89 | 33.9 ± 1.11 | <0.001 |
| Maximum foot temperature* | 35.9 ± 0.31 | 35.1 ± 0.38 | 34.8 ± 0.53 | 35.2 ± 0.34 | <0.001 |
| Minimum foot temperature* | 32.1 ± 1.78 | 31.4 ± 2.94 | 31.2 ± 1.31 | 29.5 ± 2.53 | 0.025 |
| SD of foot temperature | 0.89 ± 0.45 | 1.02 ± 0.65 | 0.86 ± 0.21 | 1.27 ± 0.66 | 0.006 |
| Positive rate of change | 0.06 ± 0.03 | 0.08 ± 0.04 | 0.07 ± 0.03 | 0.08 ± 0.04 | 0.041 |
| Negative rate of change | −0.21 ± 0.05 | −0.25 ± 0.07 | −0.22 ± 0.06 | −0.28 ± 0.07 | 0.001 |
| Wavelet energy* | 0.38 ± 0.22 | 0.60 ± 0.55 | 0.54 ± 0.41 | 0.75 ± 0.59 | 0.005 |
Data are means ± SD.
*Given the nonparametric distribution of these data, significance was computed via individual Mann-Whitney U tests with Bonferroni's correction; differences are presented as median values rather than means. Boldface represents statistical significance.
Table 2.
Post hoc tests on asleep data
| Normal vs. diabetic normal |
Normal vs. small-fiber diabetic polyneuropathy |
Normal vs. advanced diabetic polyneuropathy |
Diabetic normal vs. small-fiber diabetic polyneuropathy |
Diabetic normal vs. large-fiber diabetic polyneuropathy |
Small fiber diabetic polyneuropathy vs. large-fiber diabetic polyneuropathy |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean difference | Significance | Mean difference | Significance | Mean difference | Significance | Mean difference | Significance | Mean difference | Significance | Mean difference | Significance | |
| Mean foot* temperature | 1.19 | 0.016 | 1.59 | 0.002 | 0.89 | 0.006 | 0.40 | 1.00 | −0.30 | 1.00 | −0.07 | 1.00 |
| Maximum* foot temperature | 0.75 | 0.016 | 1.13 | <0.001 | 0.63 | 0.048 | 0.38 | 0.064 | −0.13 | 1.00 | −0.50 | 0.005 |
| Minimum* foot temperature | 0.75 | 1.00 | 0.94 | 1.00 | 2.63 | 0.416 | 0.19 | 1.00 | 1.89 | 1.00 | 1.69 | 1.00 |
| SD of foot temperature | −0.13 | 0.904 | 0.02 | 1.00 | −0.43 | 0.046 | 0.15 | 0.900 | −0.30 | 0.900 | −0.45 | 0.482 |
| Positive rate of change | −0.02 3 | 0.422 | −0.01 | 0.711 | −0.02 | 0.043 | 0.00 | 0.982 | −0.01 | 0.808 | −0.01 | 0.593 |
| Negative rate of change | 0.04 | 0.267 | 0.01 | 0.956 | 0.07 | 0.002 | −0.03 | 0.668 | 0.03 | 0.419 | 0.06 | 0.042 |
| Wavelet energy | −0.60 | 0.084 | −0.39 | 0.461 | −0.79 | 0.007 | 0.21 | 0.879 | −0.18 | 0.899 | −0.40 | 0.482 |
For all differences, the values for the second group are subtracted from the first group.
*Given the nonparametric distribution of these data, significance was computed via individual Mann-Whitney U tests with Bonferroni's correction; differences are presented as median values rather than means. Boldface indicates represents statistical significance.
Advanced metrics
As with the waking temperature data, we tested a variety of other metrics that might be sensitive to time-dependent changes in temperature, including measures of entropy, fractal dimension, and wavelet analysis. Although the first two measures did not reveal significant differences between the groups, wavelet analysis, a signal processing technique used to estimate the amount of energy occurring at different frequency bands, did show a significant difference. Differences were again most substantial between the large-fiber and normal subject groups (P = 0.007) at high frequencies, with the diabetic polyneuropathy patients showing an elevation in these components, indicating that their foot temperature fluctuated more rapidly.
CONCLUSIONS
These data show that foot temperature regulation during sleep in patients with diabetes is different from that of normal subjects. For example, diabetic subjects with and without diabetic polyneuropathy had lower mean and maximum foot temperatures than normal subjects. They also differed from normal subjects in that they had more rapid variation in foot temperature than normal; this was reflected in differences in the SD of foot temperature, the rate of change in the foot temperature, as well as in a more complex measure of embedded frequencies, the wavelet analysis. These findings are consistent with our hypothesis that diabetes and diabetic polyneuropathy impair vasomotor control in the feet and therefore foot thermoregulation.
Our initial reason for undertaking this study was to identify whether differences in ambulatory foot temperature regulation could serve as a novel noninvasive test for small-fiber diabetic polyneuropathy. Unfortunately, we were not able to identify a parameter of foot temperature regulation that discriminated diabetic patients with small-fiber diabetic polyneuropathy from diabetic patients with no diabetic polyneuropathy or from normal control subjects. The fact that thermoregulatory differences were most consistently found in patients with large-fiber diabetic polyneuropathy but not purely small-fiber diabetic polyneuropathy likely reflects the greater severity of diabetic polyneuropathy in the former group rather than necessarily suggesting that large fibers are more important for thermoregulation.
Our finding that even the diabetic patients without evidence of polyneuropathy had reduced foot temperature during sleep compared with normal subjects was somewhat unexpected. Indeed, a receiver operating characteristic curve analysis revealed that by selecting a cutoff mean temperature of 34.2°C, the mean foot temperature during sleep alone would have a 87% sensitivity and a 72% specificity for the diagnosis of diabetes, with an overall accuracy of 81%. In contrast, a similar analysis comparing the small-fiber diabetic polyneuropathy subjects to the diabetic normal subjects revealed an accuracy of only 60%, or little better than chance. This reduction in foot temperature while sleeping may reflect an impaired ability to vasodilate peripherally, as normally occurs in sleep (11–13). Although impaired vasodilation might be anticipated in early diabetic polyneuropathy due to dysfunction of normal thermoregulatory pathways (4), it may appear surprising that this was also identified in the patients without clinical diabetic polyneuropathy. One simple explanation for this is that at least some of these patients may have had very mild diabetic polyneuropathy that was otherwise undetectable with our standard assessment tools. Work has shown, however, that even diabetic individuals without any evidence of polyneuropathy have cutaneous blood flow patterns that differ from healthy control subjects (5). Such differences in cutaneous blood flow could affect their thermoregulatory function and may offer a reasonable explanation for our findings.
Although we identified foot temperature dysregulation in diabetic patients during sleep, we were unable to do so while awake. It is likely that any difference in foot temperature during wakefulness between the groups was so slight as to be completely obscured by the variations in ambient temperature. In contrast, the relatively static environment of the foot during sleep allowed subtle differences in the intrinsic thermoregulatory mechanisms to become apparent.
One limitation of this study is the relatively restricted number of small-fiber diabetic polyneuropathy patients. Unfortunately, this group was especially difficult to recruit, since many individuals with apparent isolated involvement of the small fibers on screening also had evidence for large-fiber dysfunction on examination or by nerve conduction studies. In addition, the iButton devices themselves were limited by their temperature sensitivity. Although a second more sensitive device became available during the study, the data obtained with those devices needed to be reduced to that obtained from the earlier device so as to avoid inconsistencies in the analysis. It is possible that a more sensitive device would have revealed additional thermoregulatory differences. Third, we did not obtain skin biopsies on these patients, which would have helped solidify our diagnosis of small-fiber diabetic polyneuropathy; when the study was first planned, however, this test was not readily available and thus was not included. Nonetheless, we believe our clinical criteria were likely sufficient to adequately diagnose small-fiber involvement. Finally, as with any home-monitoring technique, compliance is uncertain, although this technique does require relatively little effort on the patient's part, outside of wearing the socks and iButtons as indicated and recording diary entries accurately.
In conclusion, using iButton technology, we have demonstrated in an ambulatory setting that there are consistent differences in distal thermoregulation during sleep in diabetic patients compared with nondiabetic control subjects, with diabetic subjects exhibiting consistently cooler foot temperatures. Diabetic patients with large-fiber polyneuropathy also show an increased variability and more rapid changes in foot temperature, suggesting that intact peripheral nerve function is important for distal thermoregulation. Although ambulatory foot temperature monitoring may not provide a practical way of diagnosing early small-fiber diabetic polyneuropathy, our data document interesting and previously unreported findings that may have direct clinical implications. For example, reduced foot temperature decreases the excitability of neurons, potentially resulting in discomfort via gating mechanisms (22). In addition, the ability to autoregulate and effectively warm the feet is part of the normal biology of sleep onset and maintenance (11–13). Therefore, impairment of normal foot thermoregulation could play an important role in diabetic polyneuropathy-associated sleep disturbance. Indeed, some have even advocated the potential value of external warming to help treat insomnia (23,24). Our findings suggest that such an approach may be especially worthy of further study in diabetic patients with sleep difficulties.
Acknowledgments
This study was funded by National Institutes of Health Grant R21 DK071178.
No potential conflicts of interest relevant to this article were reported.
The authors thank Catherine Stamoulis for her helpful input into the biostatistical analysis.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Scott AR, Bennett T, Macdonald IA: Diabetes mellitus and thermoregulation. Can J Physiol Pharmacol 197; 65: 1365– 1376 [DOI] [PubMed] [Google Scholar]
- 2. Scott AR, MacDonald IA, Bennett T, Tattersall RB: Abnormal thermoregulation in diabetic autonomic neuropathy. Diabetes 1988; 37: 961– 968 [DOI] [PubMed] [Google Scholar]
- 3. Papanas N, Papatheodorou K, Christakidis D, Papazoglou D, Giassakis G, Piperidou H, Monastiriotis C, Maltezos E: Evaluation of a new indicator test for sudomotor function (Neuropad) in the diagnosis of peripheral neuropathy in type 2 diabetic patients. Exp Clin Endocrinol Diabetes 2005; 113: 195– 198 [DOI] [PubMed] [Google Scholar]
- 4. Sun PC, Lin HD, Jao SH, Chan RC, Kao MJ, Cheng CK: Thermoregulatory sudomotor dysfunction and diabetic neuropathy develop in parallel in at-risk feet. Diabet Med 2008; 25: 413– 418 [DOI] [PubMed] [Google Scholar]
- 5. Arora S, Smakowski P, Frykberg RG, Simeone LR, Freeman R, LoGerfo FW, Veves A: Differences in foot and forearm skin microcirculation in diabetic patients with and without neuropathy. Diabetes Care 1998; 21: 1339– 1344 [DOI] [PubMed] [Google Scholar]
- 6. Quattrini C, Harris ND, Malik RA, Tesfaye S: Impaired skin microvascular reactivity in painful diabetic neuropathy. Diabetes Care 2007; 30: 655– 659 [DOI] [PubMed] [Google Scholar]
- 7. Pfutzner A, Forst T, Engelbach M, Margin T, Goitom K, Lobig M, Beyer J, Kunt T: The influence of isolated small nerve fibre dysfunction on microvascular control in patients with diabetes mellitus. Diabet Med 2001; 18: 489– 494 [DOI] [PubMed] [Google Scholar]
- 8. Low VA, Sandroni P, Fealey RD, Low PA: Detection of small-fiber neuropathy by sudomotor testing. Muscle Nerve 2006; 34: 57– 61 [DOI] [PubMed] [Google Scholar]
- 9. Sumner C, Sheth S, Griffin J, Cornblath D, Polydefkis M: The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology 2003; 60: 108– 111 [DOI] [PubMed] [Google Scholar]
- 10. Rutkove SB: Effects of temperature on neuromuscular electrophysiology. Muscle Nerve 2001; 24: 867– 882 [DOI] [PubMed] [Google Scholar]
- 11. Krauchi K, Cajochen C, Werth E, Wirz-Justice A: Warm feet promote the rapid onset of sleep. Nature 1999; 401: 36– 37 [DOI] [PubMed] [Google Scholar]
- 12. Rogers NL, Bowes J, Lushington K, Dawson D: Thermoregulatory changes around the time of sleep onset. Physiol Behav 2007; 90: 643– 647 [DOI] [PubMed] [Google Scholar]
- 13. Burgess HJ, Holmes AL, Dawson D: The relationship between slow-wave activity, body temperature, and cardiac activity during nighttime sleep. Sleep 2001; 24: 343– 349 [DOI] [PubMed] [Google Scholar]
- 14. Kang P, Hoffman S, Krismitsos E, Rutkove S: Ambulatory foot temperature measurement: a new technique in polyneuropathy evaluation. Muscle Nerve 2003; 27: 737– 742 [DOI] [PubMed] [Google Scholar]
- 15. Rutkove SB, Nie R, Mitsa T, Nardin RA: A methodology for the real-time measurement of distal extremity temperature. Physiol Meas 2007; 28: 1421– 1428 [DOI] [PubMed] [Google Scholar]
- 16. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA: A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care 1994; 17: 1281– 1289 [DOI] [PubMed] [Google Scholar]
- 17. Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, McArthur J: The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci 2005; 228: 65– 69 [DOI] [PubMed] [Google Scholar]
- 18. Leduc A, Prairie Y, Bergeron Y: Fractal dimension estimates of a fragmented landscape: sources of variability. Landscape Ecology 1994; 9: 279– 286 [Google Scholar]
- 19. Altiparmak F, Ferhatosmanoglu H, Erdal S, Trost D: Information mining over heterogeneous and high-dimensional time-series data in clinical trials databases. IEEE Trans Inf Technol Biomed 2006; 10: 254– 263 [DOI] [PubMed] [Google Scholar]
- 20. Pavlov AN, Makarov VA, Mosekilde E, Sosnovtseva OV: Application of wavelet-based tools to study the dynamics of biological processes. Brief Bioinform 2006; 7: 375– 389 [DOI] [PubMed] [Google Scholar]
- 21. Davison M: Extended entropies and disorder. Adv Complex Systems 2005; 8: 125– 128 [Google Scholar]
- 22. Wall P: The gate control theory of pain mechanisms: a re-examination and a restatement. Brain 1978; 101: 1– 18 [DOI] [PubMed] [Google Scholar]
- 23. Fronczek R, Raymann R, Overeem S, Romeijn N, Van Dijk JG, Lammers GJ, Van Someren E: Manipulation of skin temperature improves nocturnal sleep in narcolepsy. J Neurol Neurosurg Psychiatry 2008; 79: 1354– 1357 [DOI] [PubMed] [Google Scholar]
- 24. Raymann RJ, Swaab DF, Van Someren EJ: Cutaneous warming promotes sleep onset. Am J Physiol Regul Integr Comp Physiol 2005; 288: R1589– R1597 [DOI] [PubMed] [Google Scholar]