Abstract
OBJECTIVE
Admission hyperglycemia has been associated with worse outcomes in ischemic stroke. We hypothesized that hyperglycemia (glucose >8.0 mmol/l) in the hyperacute phase would be independently associated with increased mortality, symptomatic intracerebral hemorrhage (SICH), and poor functional status at 90 days in stroke patients treated with intravenous tissue plasminogen activator (IV-tPA).
RESEARCH DESIGN AND METHODS
Using data from the prospective, multicenter Canadian Alteplase for Stroke Effectiveness Study (CASES), the association between admission glucose >8.0 mmol/l and mortality, SICH, and poor functional status at 90 days (modified Rankin Scale >1) was examined. Similar analyses examining glucose as a continuous measure were conducted.
RESULTS
Of 1,098 patients, 296 (27%) had admission hyperglycemia, including 18% of those without diabetes and 70% of those with diabetes. After multivariable logistic regression, admission hyperglycemia was found to be independently associated with increased risk of death (adjusted risk ratio 1.5 [95% CI 1.2–1.9]), SICH (1.69 [0.95–3.00]), and a decreased probability of a favorable outcome at 90 days (0.7 [0.5–0.9]). An incremental risk of death and SICH and unfavorable 90-day outcomes was observed with increasing admission glucose. This observation held true for patients with and without diabetes.
CONCLUSIONS
In this cohort of IV-tPA–treated stroke patients, admission hyperglycemia was independently associated with increased risk of death, SICH, and poor functional status at 90 days. Treatment trials continue to be urgently needed to determine whether this is a modifiable risk factor for poor outcome.
Admission hyperglycemia has been associated with a worse functional outcome after ischemic stroke (1–3). Poor functional outcomes and increased mortality have been described in nonthrombolyzed cohorts, and increased rates of intracerebral hemorrhage (ICH) have been found in the few studies in which patients treated with intravenous tissue plasminogen activator (IV-tPA) were studied exclusively (4–6). Baseline hyperglycemia is found more commonly in patients with preexisting diabetes but is also present in a significant proportion of nondiabetic patients (1). A causal relationship between elevated glucose and worse outcomes has not yet been proven, but current guidelines suggest that excessive hyperglycemia be treated in acute stroke patients (7). Trials of insulin therapy to treat hyperglycemia in acute stroke are ongoing (Glucose Regulation in Acute Stroke Patients [GRASP] trial), and two smaller studies showing equivocal results have been published (8,9).
The bulk of previous studies exploring the role of hyperglycemia in stroke have included only nonthrombolyzed patients. Glucose values were measured beyond the hyperacute (≤3 h) phase, and in several of these studies, ischemic and hemorrhagic strokes were pooled in the analyses (1–3,10). The few studies of admission glucose in tPA-treated ischemic stroke patients have all been relatively small (the largest included 748 patients) (4–6,11–15); some were retrospective (5,14), examined only ICH (5) or short-term (<30 days) outcomes (12,14,15), or included patients treated with IV-tPA beyond 3 h (6,14) or with intra-arterial tPA (11). We sought to determine whether, in a large national cohort of stroke patients treated with standard protocol IV-tPA, elevated admission glucose was associated with worse outcomes, particularly disability, death, and symptomatic ICH (SICH).
RESEARCH DESIGN AND METHODS
Data prospectively collected in the Canadian Alteplase for Stroke Effectiveness Study (CASES) were analyzed (16). This was a national prospective cohort study that led to the full approval of IV-tPA for acute ischemic stroke in Canada. Sixty sites across the country participated over a period of 2.5 years and, where relevant, each center obtained institutional ethics approval for data collection. If patients were incapacitated by their stroke, next of kin provided informed consent. Patients were treated at the discretion of the site neurologist according to Canadian guidelines for intravenous thrombolytic treatment in acute stroke (17).
Baseline demographic data were obtained as were pretreatment basic blood tests and data regarding timing of drug administration and stroke subtype as de-termined by the site investigator using the Oxfordshire Community Stroke Project classification. Baseline glucose was determined before IV-tPA administration, either by laboratory blood testing or from capillary blood. The particular method used was determined by the site's own usual practice and was not specifically recorded. The severity of the baseline neurological deficit was assessed with the National Institutes of Health Stroke Scale (NIHSS) before treatment. The NIHSS is a validated 11-domain systematic assessment tool that provides a quantitative measure of stroke-related neurologic deficit, such that a higher score corresponds to a more severe deficit (mild 0–15, severe >15, and maximum 42). The outcome was measured using the 7-point modified Rankin Scale (mRS) at 90 days by clinicians who were not necessarily blinded to patients' baseline glucose values. A favorable outcome was defined as an mRS of 0–1.
All patients underwent a follow-up head computed tomography (CT) scan at 24–48 h. Baseline and follow-up CT scans were centrally reviewed by a stroke neurologist and neuroradiologist blinded to clinical information, and the Alberta Stroke Program Early CT Score (ASPECTS)was applied. ASPECTS is a validated 10-point score assessing the extent of infarction in strokes of the middle cerebral artery using noncontrast CT. A lower score suggests a more extensive area of infarction (minimum score 0) (18). SICH was defined as any hemorrhage documented on a follow-up CT scan that was associated with a decline in neurological status within the first 24 h after thrombolytic treatment as judged by the site investigator, who was not blinded to clinical information. Asymptomatic ICH involved hemorrhage on a follow-up CT scan without associated clinical deterioration.
The definition of admission hyperglycemia was prespecified to be serum glucose >8.0 mmol/l in accordance with other studies (10,13,19). As a primary analysis, patients were dichotomized into those with admission hyperglycemia and those without hyperglycemia and compared with regard to baseline characteristics, rate of SICH, functional outcome at 90 days, and death. A secondary analysis examined the association of these outcomes with admission glucose as a continuous measure.
Statistical analysis
Standard descriptive statistics were used to report the data. Fisher's exact test and Student's t test were used to compare the two groups. Both binomial regression and logistic regression were used to develop models for the three outcomes of interest depending upon use. The large sample size provided sufficient power to perform multivariable analysis. For graphical descriptions, multivariable logistic regression was used to plot adjusted curves. For tabular description of risk ratios (RRs), multivariable binomial regression was used to identify predictors of outcome using a log-link function. The final models were all parsimonious models, meaning that variables that were not significant at P < 0.05 or variables that showed no evidence of confounding were eliminated from the final models. Models were developed by backwards stepwise elimination beginning with the variables listed in Table 1. Analyses were conducted using STATA 8.2 (StataCorp, College Station, TX).
Table 1.
Baseline patient characteristics
| Baseline glucose ≤8.0 mmol/l | Baseline glucose >8.0 mmol/l | P | |
|---|---|---|---|
| n | 802 | 296 | |
| Age (years) | 69.7 ± 13.6 | 71.6 ± 12.0 | 0.03 |
| Sex (female) | 370 (46.4) | 125 (42.7) | 0.3 |
| Caucasian | 699 (92.6) | 232 (86.2) | 0.003 |
| Vascular risk factors | |||
| Hypertension | 369 (48.1) | 156 (57.4) | 0.009 |
| Diabetes | 50 (6.5) | 116 (42.6) | <0.001 |
| Atrial fibrillation | 160 (20.9) | 74 (27.2) | 0.035 |
| Dyslipidemia | 141 (18.4) | 59 (21.7) | 0.25 |
| Current cigarette use | 130 (16.9) | 29 (10.7) | 0.014 |
| Ischemic heart disease | 165 (21.5) | 89 (32.7) | <0.001 |
| Valvular heart disease | 30 (3.9) | 9 (3.3) | 0.85 |
| Congestive heart failure | 41 (5.3) | 31 (11.4) | 0.001 |
| Prior stroke/transient ischemic attack | 170 (22.2) | 72 (26.5) | 0.16 |
| Pretreatment systolic blood pressure (mmHg) | 151 ± 21.6 | 153 ± 21.8 | 0.18 |
| Pretreatment ASPECTS | 8 | 8 | 0.64 |
| Baseline NIHSS | 14 | 15 | 0.14 |
| Protocol violations, all causes | 113 (14.1) | 39 (13.2) | 0.77 |
| Onset-to-treatment time (min) | 150 ± 37.2 | 150 ± 38.9 | 0.92 |
| Oxfordshire Community Stroke Project stroke subtype | 0.8 | ||
| Total anterior circulation | 198 (26.4) | 82 (31.1) | |
| Partial anterior circulation | 481 (64.1) | 155 (58.7) | |
| Posterior circulation | 22 (2.9) | 13 (4.9) | |
| Lacunar | 49 (6.5) | 14 (5.3) |
Data are means ± SD, median, or n (%). Not all percentages are calculated from the total number of patients because certain clinical variables were not available for all patients.
RESULTS
Baseline characteristics
Of 1,135 patients enrolled in CASES, 1,098 had adequate admission glucose data for inclusion in the current study. Of these patients, median age was 73 years, 45% were women, 96% had an anterior circulation stroke, and 16% had a known prior history of diabetes. A glucose value of 8.0 mmol/l corresponded to the 75th percentile and, overall, 296 (27%) patients had admission hyperglycemia. Hyperglycemia was present in 70% of those with diabetes and 18% of those without diabetes. Hyperglycemic patients were older; were more likely to be non-Caucasian; were more likely to have atrial fibrillation, hypertension, congestive heart failure, coronary artery disease, and known diabetes; and were less likely to be current smokers. The greater prevalence of comorbid illness in the hyperglycemic group is probably explained by older age and the higher proportion of diabetes. There were no differences in sex, stroke subtype, or baseline stroke severity between groups (Table 1). No relationship was observed between baseline serum glucose and baseline NIHSS score.
SICH, 90-day outcome, and death
Rates of ICH, 90-day outcomes, and mortality are shown in Table 2. A total of 49 (4.5%) patients had SICH. Among hyperglycemic patients, this proportion was 6.8% compared with 3.6% for nonhyperglycemic patients (P = 0.03), resulting in an unadjusted RR of 1.87 (95% CI 1.07–3.25) for SICH in patients with baseline glucose >8.0 mmol/l (Table 2). Multivariable regression attenuated this relationship somewhat, yielding an adjusted RR of 1.69 (95% CI 0.95–3.00).
Table 2.
Outcome at 90 days and SICH in hyperglycemic (baseline glucose >8 mmol/l) and nonhyperglycemic patients with unadjusted and adjusted RRs (after multivariate logistic regression)
| Outcome variable | Baseline glucose ≤8.0 mmol/l | Baseline glucose >8.0 mmol/l | P | RR (95% CI) |
|
|---|---|---|---|---|---|
| Unadjusted | Adjusted | ||||
| n | 802 | 296 | |||
| ICH | |||||
| All | 213 (26.6) | 105 (35.5) | 0.007 | ||
| Symptomatic | 29 (3.6) | 20 (6.8) | 0.03 | 1.87 (1.07–3.25) | 1.69 (0.95–3.00) |
| Outcome at 90 days | |||||
| Excellent outcome (mRS 0–1) | 316 (40) | 80 (27.7) | <0.001 | 0.69 (0.56–0.85) | 0.7 (0.5–0.9) |
| Death from all causes (mRS 6) | 148 (18.7) | 89 (30.8) | <0.001 | 1.64 (1.31–2.06) | 1.5 (1.2–1.9) |
Data are n (%). Not all percentages are calculated from the total number of patients because certain outcome variables were not available for all patients.
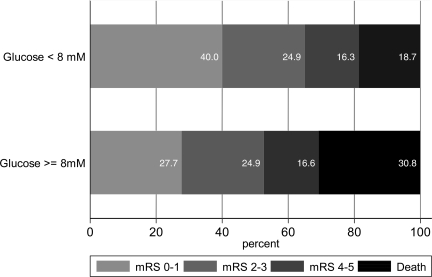
Clinical outcomes at 90 days, unadjusted for confounders, are shown in Fig. 1. Only 80 (27.7%) hyperglycemic patients experienced a favorable functional outcome (mRS 0–1) compared with 316 (40%) nonhyperglycemic patients (P = 0.0002). The proportion of patients who were dead at the 3 month follow-up was also significantly higher among patients with admission glucose >8.0 mmol/l (30.8 vs. 18.7%, P < 0.0001). Unadjusted risk ratios for favorable outcome and death among hyperglycemic patients were 0.69 (95% CI 0.56–0.85) and 1.64 (1.31–2.06), respectively.
Figure 1.
Patient outcome at the 90-day follow-up by baseline glucose (unadjusted for other predictors of outcome). mRS 0–1, excellent outcome; mRS 2–3, moderate disability; mRS 4–5, severe disability; and mRS 6, dead.
After multivariable regression, adjusted risk ratios were 0.7 (95% CI 0.5–0.9) for favorable outcome and 1.5 (1.2–1.9) for death. Multivariable analysis showed that independent predictors of 90-day outcome and death were age, baseline NIHSS, baseline ASPECTS, and admission glucose. Each of these had a comparable magnitude of association with outcome. When dichotomized as age >80 years, baseline NIHSS >15, and ASPECTS >7, unadjusted RRs for favorable functional outcome were 0.64 (95% CI 0.52–0.80), 0.40 (0.32–0.48), and 1.53 (1.26–1.86), respectively. The negative association between admission glucose and functional outcome and death persisted even after exclusion of patients with SICH, a known independent predictor of death and disability after thrombolysis (4,20). These relationships were not different for patients with and without diabetes (no evidence of an interaction effect).
Effect of stroke subtype and severity
No heterogeneity was found with regard to the association between baseline hyperglycemia and a favorable clinical outcome when analyzed by Oxfordshire Community Stroke Project stroke subtype (χ2 test, P = 0.80) or when mild strokes (NIHSS ≤5) and moderate-to-severe strokes were compared (χ2 test, P = 0.29), suggesting a deleterious effect of hyperglycemia on outcome regardless of stroke subtype or severity.
Admission glucose as a continuous measure
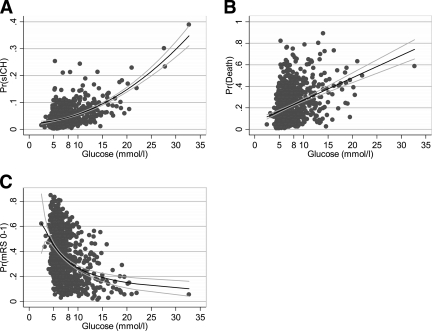
With glucose as a continuous variable, the predicted probability of SICH adjusted for age, atrial fibrillation, onset-to-treatment time, and sex was calculated and represented as a fractional polynomial estimate of best fit (Fig. 2A). An increasing probability of SICH was seen with increasing admission glucose values. Similarly, the predicted probability of death at 90 days according to admission glucose, adjusted for age, baseline NIHSS, ASPECTS, onset-to-treatment time, and sex was calculated and represented using a linear least-squares line of best fit (Fig. 2B). An increasing risk of death was found with increasing baseline glucose. Finally, the predicted probability of a favorable outcome at 90 days, adjusted for age, baseline NIHSS, ASPECTS, congestive heart failure, atrial fibrillation, and diabetes was determined, yielding an inverse relationship between admission glucose levels and the probability of achieving an mRS of 0–1 (Fig. 2C). The predicted probability of a favorable outcome was also determined for only those patients with baseline glucose <8 mmol/l. Even among these nonhyperglycemic patients, a linear decline in the probability of a good outcome was seen with increasing glucose levels.
Figure 2.
A: Probability of SICH by baseline glucose level. Quadratic polynomial line of best fit with range and 95% CIs adjusted for age, baseline NIHSS score, sex, onset-to-treatment time, and atrial fibrillation. For each increase of 1 mmol/l of serum glucose, the relative risk of SICH rises by 10%. B: Probability of death by baseline glucose level. The line and 95% CIs are based upon a linear regression of predicted probability of death adjusted for age, baseline NIHSS score, sex, onset-to-treatment time, and baseline ASPECTS score. For every 1 mmol/l rise in the baseline serum glucose, the probability of death at 90 days increases by an absolute risk of 2%. C: Relationship between glucose and good outcome. The line and 95% CIs are derived from a fractional polynomial regression of baseline serum glucose and the predicted probability of good outcome adjusted for age, baseline NIHSS score, baseline ASPECTS, sex, and onset-to-treatment time. For every increase of 1 mmol/l of baseline serum glucose, the relative risk of a good outcome falls by 12%.
CONCLUSIONS
This study further confirms the relationship between admission hyperglycemia and SICH, death, and poor functional outcome in ischemic stroke patients treated with IV-tPA. Our findings bolster and expand on those of previous studies, the majority of which examined smaller numbers of nonthrombolyzed stroke patients and some of which analyzed hemorrhagic and ischemic strokes together (1–3,10).
Published studies of baseline hyperglycemia in stroke patients treated with thrombolysis have been few, were generally small, and often did not report 3-month outcomes (4,6,12–15). However, all have suggested an increased risk of poor outcome with elevated glucose, despite using different cutoff levels to define hyperglycemia (from 7.7 to 10 mmol/l) (4,6,12–15). One study included 312 tPA-treated patients from the original National Institute of Neurological Disorders and Stroke IV-tPA trial and analyzed these together with 312 placebo-treated patients (4). This study showed that regardless of treatment assignment, as admission glucose level increased, the odds for a favorable outcome progressively decreased and the odds of SICH increased (4). A recent post hoc analysis of 748 patients from the European Cooperative Acute Stroke Study (ECASS-II), a trial of IV-tPA given within 6 h of stroke onset, examined the prognostic value of hyperglycemia at baseline and 24 h (6). Patients were classified into four groups: isolated baseline hyperglycemia, isolated 24-h hyperglycemia, hyperglycemia persisting at both time points, and persistent normoglycemia. Thrombolyzed (n = 384) and nonthrombolyzed (n = 364) patients were analyzed together. Interestingly, isolated baseline hyperglycemia was not found to independently predict poor outcome or ICH. In this study, the strongest predictor of poor outcome, death, and ICH was persistent hyperglycemia at baseline and 24 h, although this observation was only true in patients without known diabetes. The prognostic utility of hyperglycemia exclusively in those patients treated with IV-tPA cannot be determined because this subgroup was not analyzed separately. Two other studies of tPA-treated patients, one with intravenous treatment and the other with intra-arterial treatment, also showed that elevated glucose was an independent predictor of ICH (5,11).
What remains unclear is whether hyperglycemia is merely an epiphenomenon of underlying stroke severity or if it is itself directly harmful to ischemic brain tissue. After adjustment for clinical stroke severity, a dose-response relationship between baseline glucose and unfavorable outcome is still suggested by our results and those of others (1,4,6,10). There is ample animal literature suggesting plausible mechanisms by which glucose may exert a deleterious effect on ischemic brain, including cellular acidosis caused by anaerobic glycolysis, enhanced free radical production, increased blood-brain barrier permeability, impaired mitochondrial function, influx of intracellular Ca2+, and cellular edema (21).
However, noncausal explanations have also been proposed. Baseline hyperglycemia may represent an acute stress response from activation of the hypothalamic-pituitary-adrenal axis causing a rise in cortisol and catecholamines and, therefore, may simply be indicative of underlying stroke severity. Furthermore, it may also be a result of injury or irritation of brain areas involved in glucose regulation, a theory supported by the association of hyperglycemia with strokes involving the insula (22). Finally, hyperglycemia may reflect previously undiagnosed diabetes.
The argument for causality is also further mitigated by the equivocal results of trials targeting aggressive glucose-lowering therapy in the acute phase after stroke (8,9). No evidence to date supports the concept that ensuring strict poststroke normoglycemia improves outcome. The UK Glucose Insulin in Stroke Trial (GIST-UK) was both stopped early and underpowered but suggested no difference in clinical outcome between patients randomly assigned to glucose-potassium-insulin infusion to maintain glucose levels at 4–7 mmol/l over the first 24 h and patients given saline without glucose-lowering interventions (9). The lack of benefit may be related to the relatively late initiation of therapy after stroke (median 14 h) and the modest mean reduction in glucose achieved in the treatment arm (0.57 mmol/l).
Our prospective cohort study is the largest yet to report on the effect of admission glucose exclusively in stroke patients treated uniformly with IV-tPA. Our results confirm the notion that poststroke hyperglycemia is a predictor of death, worse functional outcome, and SICH in thrombolyzed patients. This association remains true regardless of stroke subtype or severity and in patients with and without diabetes. We acknowledge that our work has several limitations. First, it is an observational study. Nonetheless, the data were prospectively collected from a large number of representative patients drawn from 60 hospitals across Canada. Second, our results are based on only a single admission glucose value. Contrary to data for acute coronary syndromes arguing that dynamic changes in glucose are not prognostically significant (23), it has recently been suggested that changes in glucose over the first 24 h among stroke patients may provide additional prognostic value (6). However, use of a single baseline glucose value presumably underestimates potential harm, because patients with the highest admission glucose levels would most likely have been treated earlier and more aggressively with glucose-lowering therapies. Also, because thrombolytic treatment is necessarily rapid, a single admission glucose level has greater clinical utility for guiding acute treatment decisions. Third, we did not collect A1C values and so we do not have any measures of chronic dysglycemia. However, in acute coronary syndromes, A1C levels convey little short-term prognostic value with respect to mortality (24). Fourth, the method of blood glucose determination was not uniform, with either capillary blood or laboratory glucose measurements used at different sites. Both methods have been shown to provide comparable results in critically ill patients (25). Fifth, we have no information regarding glycemic management during hospitalization or after discharge. Last, baseline differences between both groups may be potential confounders that cannot be entirely accounted for using statistical adjustments.
Although it remains unclear whether correcting elevated glucose in the acute phase after ischemic stroke is beneficial, it is apparent that admission hyperglycemia rapidly identifies patients at higher risk for poor outcomes in whom glucose levels should be closely monitored.
Acknowledgments
This study was funded cooperatively by the Canadian Stroke Consortium, the Canadian Stroke Network, and Hoffmann-La Roche Canada.
S.R.M. was supported by a Health Scholar Salary Award from the Alberta Heritage Foundation for Medical Research. A.M.B. was funded in part by the Alberta Heritage Foundation for Medical Research and the Heart and Stroke Foundation of Alberta, NWT & Nunavut. M.D.H. received salary support from the Alberta Heritage Foundation for Medical Research and the Heart and Stroke Foundation of Alberta, NWT & Nunavut. No other potential conflicts of interest relevant to this article were reported.
Statistical analyses were conducted by Michael D. Hill, MD, MSc, FRCPC, Department of Clinical Neurosciences/Medicine/Community Health Sciences, Hotchkiss Brain Institute, University of Calgary Foothills Medical Centre.
Footnotes
None of the sponsors had any role in the collection, analysis, or interpretation of the data.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein H: Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke 32: 2426– 2432, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Diener HC, Lees KR, Lyden P, Grotta J, Davalos A, Davis SM, Shuaib A, Ashwood T, Wasiewski W, Alderfer V, Hårdemark HG, Rodichok L: NXY-059 for the treatment of acute stroke: pooled analysis of the SAINT I and II trials. Stroke 39: 1751– 1758, 2008 [DOI] [PubMed] [Google Scholar]
- 3. Stead LG, Gilmore RM, Bellolio MF, Mishra S, Bhagra A, Vaidyanathan L, Decker WW, Brown RD, Jr: Hyperglycemia as an independent predictor of worse outcome in non-diabetic patients presenting with acute ischemic stroke. Neurocrit Care. 21 March 2008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4. Bruno A, Levine SR, Frankel MR, Brott TG, Lin Y, Tilley BC, Lyden PD, Broderick JP, Kwiatkowski TG, Fineberg S: Admission glucose level and clinical outcomes in the NINDS rt-PA Stroke Trial. Neurology 59: 669– 674, 2002 [DOI] [PubMed] [Google Scholar]
- 5. Demchuk AM, Morgenstern LB, Krieger DW, Linda Chi T, Hu W, Wein TH, Hardy RJ, Grotta JC, Buchan A: Serum glucose level and diabetes predict tissue plasminogen activator-related intracerebral hemorrhage in acute ischemic stroke. Stroke 30: 34– 39, 1999 [DOI] [PubMed] [Google Scholar]
- 6. Yong M, Kaste M: Dynamic of hyperglycemia as a predictor of stroke outcome in the ECASS-II trial. Stroke 39: 2749– 2755, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Adams HP, Jr, del Zoppo G, Alberts MJ, Bhatt DL, Brass L, Furlan A, Grubb RL, Higashida RT, Jauch EC, Kidwell C, Lyden PD, Morgenstern LB, Qureshi AI, Rosenwasser RH, Scott PA, Wijdicks E: Guidelines for the early management of adults with ischemic stroke: a guideline from the American Heart Association/American Stroke Association Stroke Council, Clinical Cardiology Council, Cardiovascular Radiology and Intervention Council, and the Atherosclerotic Peripheral Vascular Disease and Quality of Care Outcomes in Research Interdisciplinary Working Groups: The American Academy of Neurology affirms the value of this guideline as an educational tool for neurologists. Circulation 115: e478– e534, 2007 [DOI] [PubMed] [Google Scholar]
- 8. Bruno A, Kent TA, Coull BM, Shankar RR, Saha C, Becker KJ, Kissela BM, Williams L: Treatment of hyperglycemia in ischemic stroke (THIS): a randomized pilot trial. Stroke 39: 384– 389, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Gray CS, Hildreth AJ, Sandercock PA, O'Connell JE, Johnston DE, Cartlidge NE, Bamford JM, James OF, Alberti K: Glucose-potassium-insulin infusions in the management of post-stroke hyperglycaemia: the UK Glucose Insulin in Stroke Trial (GIST-UK). Lancet Neurol 6: 397– 406, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Uyttenboogaart M, Koch MW, Stewart RE, Vroomen PC, Luijckx GJ, Keyser JD: Moderate hyperglycaemia is associated with favourable outcome in acute lacunar stroke. Brain 130: 1626– 1630, 2007 [DOI] [PubMed] [Google Scholar]
- 11. Kase CS, Furlan AJ, Wechlser L: Cerebral hemorrhage after intra-arterial thrombolysis for ischemic stroke: the PROACT II trial. Neurology 57: 1603– 1610, 2001 [DOI] [PubMed] [Google Scholar]
- 12. Els T, Klisch J, Orszagh M, Hetzel A, Schulte-Mönting J, Schumacher M, Lücking C: Hyperglycemia in patients with focal cerebral ischemia after intravenous thrombolysis: influence on clinical outcome and infarct size. Cerebrovasc Dis 13: 89– 94, 2002 [DOI] [PubMed] [Google Scholar]
- 13. Alvarez-Sabín J, Molina CA, Montaner J, Arenillas JF, Huertas R, Ribo M, Codina A, Quintana M: Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke 34: 1235– 1241, 2003 [DOI] [PubMed] [Google Scholar]
- 14. Leigh R, Zaidat OO, Suri MF, Lynch G, Sundararajan S, Sunshine H, Tarr R, Selman W, Landis DM, Suarez J: Predictors of hyperacute clinical worsening in ischemic stroke patients receiving thrombolytic therapy. Stroke 35: 1903– 1907, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Saposnik G, Young B, Silver B, Di Legge S, Webster F, Beletsky V, Jain V, Nilanont Y, Hachinski V: Lack of improvement in patients with acute stroke after treatment with thrombolytic therapy: predictors and association with outcome. JAMA 292: 1839– 1844, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Hill MD, Buchan A: Thrombolysis for acute ischemic stroke: results of the Canadian Alteplase for Stroke Effectiveness Study. CMAJ 172: 1307– 1312, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Norris JW BA, Cote R, Hachinski V, Phillips SJ, Shuaib A, Silver F, Simard D, Teal P: Canadian guidelines for intravenous thrombolytic treatment in acute stroke: a consensus statement of the Canadian Stroke Consortium. Can J Neurol Sci 25: 257– 259, 1998 [DOI] [PubMed] [Google Scholar]
- 18. Barber PA, Demchuk AM, Zhang J, Buchan A: Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy: ASPECTS Study Group: Alberta Stroke Programme Early CT Score. Lancet 355: 1670– 1674, 2000 [DOI] [PubMed] [Google Scholar]
- 19. Weir GJ, Murray GD, Dyker AG, Lees K: Is hyperglycaemia an independent predictor of poor outcome after acute stroke: results of a long-term follow-up study. BMJ 314: 1303– 1306, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dzialowski I, Pexman JH, Barber PA, Demchuk AM, Buchan AM, Hill M: CASES Investigators: Asymptomatic hemorrhage after thrombolysis may not be benign: prognosis by hemorrhage type in the Canadian Alteplase for Stroke Effectiveness Study registry. Stroke 38: 75– 79, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Dietrich WD, Alonso O, Busto R: Moderate hyperglycemia worsens acute blood-brain barrier injury after forebrain ischemia in rats. Stroke 24: 111– 116, 1993 [DOI] [PubMed] [Google Scholar]
- 22. Allport LE, Butcher KS, Baird TA, MacGregor L, Desmond PM, Tress BM, Colman P, Davis S: Insular cortical ischemia is independently associated with acute stress hyperglycemia. Stroke 35: 1886– 1891, 2004 [DOI] [PubMed] [Google Scholar]
- 23. Kosiborod M, Inzucchi SE, Krumholz HM, Xiao L, Jones PG, Fiske S, Masoudi FA, Marso SP, Spertus J: Glucometrics in patients hospitalized with acute myocardial infarction: defining the optimal outcomes-based measure of risk. Circulation 117: 1018– 1027, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Hadjadj S, Coisne D, Mauco G, Ragot S, Duengler F, Sosner P, Torremocha F, Herpin D, Marechaud R: Prognostic value of admission plasma glucose and HbA in acute myocardial infarction. Diabet Med 21: 305– 310, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Lacara T, Domagtoy C, Lickliter D, Quattrocchi K, Snipes L, Kuszaj J, Prasnikar M: Comparison of point-of-care and laboratory glucose analysis in critically ill patients Am J Crit Care 16: 336– 346, 2007 [PubMed] [Google Scholar]