Abstract
OBJECTIVE
Increased urinary albumin excretion rates have been linked to nephropathy and macrovascular disease. We here describe the baseline prevalence and effect of Diabetes Prevention Program (DPP) interventions on the development and reversal of elevated albumin excretion.
RESEARCH DESIGN AND METHODS
Urine albumin-to-creatinine ratios (ACRs) were calculated from untimed urine collections. Analyses compared participants by treatment group, diabetes and hypertension status, and use of ACE inhibitors or angiotensin II receptor blockers (ARBs).
RESULTS
Elevated ACR levels (≥30 mg/g creatinine) were present at baseline in 198 (6.2%) of 3,188 participants: placebo 5.3%, metformin 6.5%, and intensive lifestyle (ILS) 6.8%. Of the 2,802 with ACR measurements at baseline and at the end of the study, the percentage with elevated levels declined (incident and regression) from 6.2 to 6.1%, with no significant differences between the groups even with adjustment for ACE inhibitor and ARB use. The odds of developing an elevated ACR were 59% higher for a participant who developed diabetes compared with one who did not.
CONCLUSIONS
At entry into the DPP, an elevated ACR was present in 6.2%. Despite the marked decrease in progression to diabetes and the improvement in insulin resistance and other cardiovascular risk markers in the ILS and metformin groups, there was no improvement in ACR, on average, in those two groups. However, the frequency of an elevated ACR was higher in participants who developed diabetes. An increased ACR may have multiple causes, thus obscuring the improvements that might have been expected with the reduction in insulin resistance seen in the DPP.
Increased urinary albumin excretion rates (AERs) have been linked to the development of diabetic nephropathy and macrovascular disease in patients with type 1 and type 2 diabetes (1,2). The development of increased AER is associated not only with hyperglycemia but also with blood pressure elevations (3–6). Because of difficulties in precisely timing the onset of type 2 diabetes, the duration and degree of glucose intolerance necessary for the development of elevations of AER have been addressed in large, cross-sectional, and longitudinal studies. In cross-sectional studies of Pima Indians, microalbuminuria was found in 8% of those with normal glucose tolerance, 15% of those with impaired glucose tolerance (IGT), and 47% of those with type 2 diabetes (7). These studies have also shown that microalbuminuria was correlated with insulin resistance (8), rising glucose levels (9,10), and the presence of the metabolic syndrome (11).
The Diabetes Prevention Program (DPP) was a randomized, prospective, clinical trial that tested strategies to prevent or delay the development of type 2 diabetes in overweight or obese participants aged ≥25 years with elevated fasting glucose and IGT (12,13). We have previously reported that 28% of the 3,819 participants initially entered into the study had hypertension, that the mean urine albumin was 14 mg/g creatinine, and that the albumin-to-creatinine ratio (ACR) had a weak (r = 0.09) but statistically significant correlation with systolic blood pressure (SBP) at baseline (14). Both lifestyle modification and metformin treatment resulted in significant decreases in the development of diabetes during the DPP (13). We now analyze the development of elevations in ACR as a function of time and treatment group during the DPP.
RESEARCH DESIGN AND METHODS
Full details of the protocol, recruitment, and outcomes have been published (5,6). The current report includes 3,188 of the 3,234 participants entering the study who had urine ACR measurements before randomization. This number does not include participants from the troglitazone arm, which was discontinued.
Inclusion and exclusion criteria have been published previously (12,13). Pertinent to the current analysis, the following exclusions should be noted: serum creatinine ≥1.4 mg/dl (124 μmol/l) for men or ≥1.3 mg/dl (115 μmol/l) for women; urine protein ≥2+ on one occasion (dipstick) in the absence of infection or vaginal contamination; and in individuals who were or would become 80 years of age during the study, a direct measure of creatinine clearance <75 ml/min, based on a 24-h urine collection.
Standardized interviewer-administered questionnaires were used to obtain self-reported data on personal medical history, medications, diet, and other factors. Overall, adiposity was assessed by BMI. All anthropometric measures reflected the average of two measurements. Blood pressure was measured with a standard mercury manometer with the participant seated in a chair for 5 minutes before the first of two measures separated by 30 s. The mean of the two readings were used in the analyses. Hypertension is defined as meeting any of three criteria: SBP ≥140 mmHg, diastolic blood pressure (DBP) ≥90 mmHg, or taking medications that lower blood pressure. Further details have been published elsewhere (5,6,12,13).
Laboratory methods
All of the analytical measurements were performed at the Central Biochemistry Laboratory (Northwest Lipid Research Laboratories, University of Washington, Seattle, WA) as described previously (12,13). Pertinent to the current analyses, creatinine concentrations in the serum and urine were measured by a variation of the Jaffe method and urine albumin concentration was measured by a fluoroimmunoassay. Albuminuria was assessed using a spot urine test of albumin and creatinine. The ACR was used to define categories of albuminuria: normal (<30 mg/g creatinine), microalbuminuria (30-<300 mg/g creatinine), and macroalbuminuria (≥300 mg/g creatinine). In this report, the term “elevated ACR” indicates the combined categories of microalbuminuria and macroalbuminuria.
Statistical analyses
For this analysis, participants were followed for an average of 3.4 years with the end of study assessment ranging from 2.4 to 5.4 years, a period 4 months longer than that reported previously (13) to maximize the available data that were collected during the masked phase of the DPP. Nominal (unadjusted) P values and confidence intervals are reported. Logistic regression was used to compare the prevalence of elevated ACR at baseline and end of study. Wilcoxon's signed-rank test was used to assess whether the paired ACR levels changed between baseline and the end of study within groups, whereas the Kruskal-Wallis test was used to compare the ACR levels at the end of the study among the three treatment groups.
RESULTS
Baseline assessments (n = 3,188)
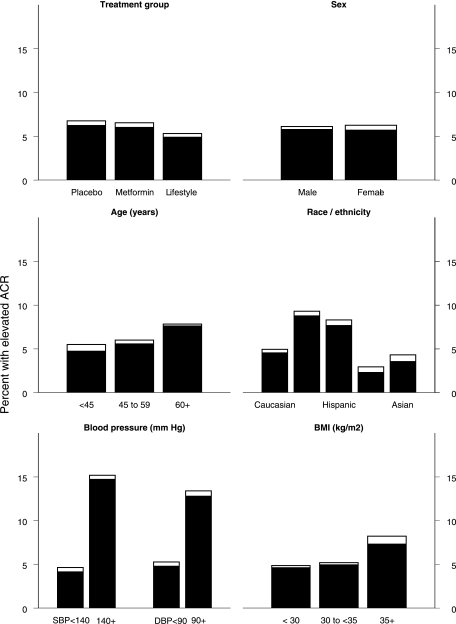
The baseline prevalence of elevated albuminuria by baseline characteristics and treatment group are displayed in Fig. 1. Elevated ACR levels were present in 198 participants (6.2%), with similar percentages in the three groups: placebo 5.3%, metformin 6.5%, and intensive lifestyle (ILS) 6.8%. Only 14 participants in the entire study had an ACR ≥300 mg/g creatinine at baseline. ACE inhibitors or angiotensin II receptor blockers (ARBs) were used at baseline in 8.2, 9.9, and 8.6% of the participants in the placebo, metformin, and ILS groups, respectively. At baseline there were no treatment group differences, including systolic and diastolic blood pressure, the presence of hypertension, mean urine ACR, or serum creatinine.
Figure 1.
Prevalence of elevated ACR levels at baseline by subgroups. The height of the bars represents the percentage of subjects with an elevated ACR (≥30 mg/g), ■ represents microalbuminuria (ACR between 30 and <300), and □ represents macroalbuminuria (ACR ≥300). The prevalence of albuminuria differed among subgroups for age, race/ethnicity, SBP, DBP, and BMI (P < 0.05).
When the baseline ACR measurements were broken down by quartiles (≤3.7, >3.7–5.5, >5.5–9.7, and >9.7 mg/g), those with higher ACR levels had higher BMIs, greater waist circumferences, higher fasting insulin level, higher SBP and DBP levels, and greater frequencies of hypertension (Table 1).
Table 1.
Baseline characteristics by ACR quartiles
| ACR quartiles |
||||||
|---|---|---|---|---|---|---|
| Overall (mg/g) | ≤3.7 mg/g | 3.7 to ≤5.5 mg/g | 5.5 to ≤9.7 mg/g | >9.7 mg/g | P | |
| n | 3,188 | 772 | 818 | 798 | 800 | |
| Sex (% female) | 2,158 (68) | 453 (59) | 536 (66) | 595 (75) | 574 (72) | <0.001 |
| Age (years) | 50.6 ± 10.7 | 50.0 ± 10.6 | 50.3 ± 10.4 | 50.6 ± 10.5 | 51.6 ± 11.1 | 0.02 |
| BMI (kg/m2) | 32.5 ± 8.5 | 31.8 ± 7.2 | 32.4 ± 7.1 | 32.3 ± 7.1 | 33.5 ± 7.2 | <0.001 |
| Waist circumference (cm) | 103 ± 19 | 102 ± 16 | 103 ± 16 | 103 ± 16 | 106 ± 16 | <0.001 |
| Fasting glucose (mg/dl) | 106 ± 11 | 106 ± 9 | 106 ± 9 | 106 ± 9 | 107 ± 9 | 0.12 |
| 120-min glucose (mg/dl) | 165 ± 24 | 165 ± 20 | 164 ± 20 | 164 ± 20 | 166 ± 20 | 0.10 |
| A1C (%) | 5.98 ± 0.65 | 5.94 ± 0.55 | 5.99 ± 0.54 | 6.00 ± 0.54 | 6.00 ± 0.54 | 0.04 |
| Fasting insulin (μU/ml) | 23.7 ± 2.1 | 22.1 ± 1.8 | 23.6 ± 1.8 | 23.8 ± 1.8 | 25.6 ± 1.8 | <0.001 |
| Systolic blood pressure (mmHg) | 124 ± 19 | 120 ± 16 | 122 ± 16 | 124 ± 16 | 128 ± 16 | <0.001 |
| Diastolic blood pressure (mmHg) | 79 ± 13 | 77 ± 11 | 78 ± 10 | 79 ± 10 | 81 ± 10 | <0.001 |
Data are n (%) for categories and means ± SD for continuous variables except for fasting insulin represented as geometric mean. All variables except female sex and age are adjusted for baseline age, sex, and race/ethnicity.
Paired baseline and end of study assessments (n = 2,802)
Of the participants with baseline evaluations, 2,802 had measurements performed at the end of the study. In these 2,802 participants the total number with elevated ACR levels did not change significantly, going from 174 (6.2%) to 171 (6.1%) participants, after a mean of 3.4 years in the study. These numbers comprise both a regression to normal from prior elevated levels plus incident cases (Table 2). The net change in individuals moving from normal to elevated ACR (numbers worsening minus numbers regressing) were 9 (placebo), 0 (metformin) and −12 (ILS). Overall, there were more improvements in the ILS group and more individuals who worsened in the placebo group, although this difference was not statistically significant (P = 0.07) (Table 2). Despite the significant decrease in the incidence of diabetes among the ILS and metformin groups compared with the placebo group, there were only minimal and not statistically significant differences in the frequency of elevated ACR levels between the groups (placebo 6.3%, metformin 6.7%, and ILS 5.4%) at the end of the study. The median ACR levels in all three groups did not change significantly and the changes did not differ significantly among the treatment groups: placebo 0.10 mg/g creatinine, metformin 0.12 mg/g creatinine, and ILS 0.06 mg/g creatinine. Although at the end of the study, the frequency of SBP ≥140 mmHg was lower in the ILS group (10.1%) than in the other two groups (placebo 12.2% and metformin 12.6%), this difference was not significantly different. The differences in frequencies of DBP ≥90 mmHg at the end of the study approached significance (P = 0.056) among the three groups: ILS 5.7%, metformin 8.5%, and placebo 6.7%.
Table 2.
Change in classification between normal and elevated ACR from baseline to end of study by treatment group
| Baseline | End-of-study status | Placebo | Metformin | ILS |
|---|---|---|---|---|
| Normal ACR | 890 (95) | 869 (93) | 869 (93) | |
| Developed elevated ACR | 33 (4) | 35 (4) | 28 (3) | |
| Remained without elevated ACR | 857 (96) | 834 (96) | 841 (95) | |
| Elevated ACR | 50 (5) | 62 (7) | 62 (7) | |
| Resolved elevated ACR | 24 (48.0) | 35 (56) | 40 (64) | |
| Remained with elevated ACR | 26 (52) | 27 (44) | 22 (35) | |
| Total | 940 | 931 | 931 | |
| Stable status | 883 (93.9) | 861 (92.5) | 863 (92.7) | |
| Worsened albuminuria | 33 (3.5) | 35 (3.8) | 28 (3.0) | |
| Improved albuminuria | 24 (2.6) | 35 (3.8) | 40 (4.3) | |
| Net increase in elevated ACR | 9 (1.0) | 0 (0.0) | −12 (−1.3)* |
Data are n (%). Elevated ACR is defined as ACR ≥30 mg/g.
*Ptrend = 0.07 for test of linear trend between treatment group (placebo to metformin to ILS) and change in category (worsened to stable to improved).
The frequency of ACE inhibitor or ARB use increased in all three groups, from 8.3 to 23% in the placebo group, from 9.6 to 23.3% in the metformin group, and from 8.7 to 17.9% in the ILS group. The increase in the ILS group was significantly less than that in the other two groups (P = 0.023), possibly because of the slightly lower frequency of hypertension at the end of the study in this group. We performed detailed analyses to determine incident new cases of elevated ACR levels versus regression to normal, with and without use of ACE inhibitors or ARBs, because the use of these drugs at baseline might have prevented the detection of a possible elevated level and the institution of therapy with these drugs might either cause regression to normal of preexisting elevated levels or prevent the development of abnormal levels. For example, of the 931 ILS participants who had a baseline and end-of-study assessment for ACR, 62 (6.7%) had elevated levels at baseline. However, there were an additional 71 (7.6%) participants who were taking ACE inhibitors or ARBs. Thus, the prevalence of elevated ACR at baseline, when unmasked by concurrent use of ACE inhibitors or ARBs, could have been between 6.7 and 14.3%. Among the 869 ILS participants who did not have elevated ACR levels at baseline, 28 (3.2%) had elevated ACR levels at the end of study examination and an additional 70 (8.1%) participants initiated treatment with ACE inhibitors or ARBs after baseline, so the incidence of an increased ACR ranged from 3.0 to 11.3%. Conversely, 40 of the 62 participants (64.5%) with elevated ACR levels at baseline no longer had elevated levels at the end of study; however, 18 of these 40 were taking ACE inhibitors or ARBs so the resolution of an elevated ACR level ranges from 35.5 to 64.5%. When the overall prevalence (new incidence cases and reversal of elevated ACR levels) for the three groups was analyzed in this way, the estimates of elevated ACR levels at the end of the study were not significantly different among the three treatment groups, even adjusted for ACE inhibitor and ARB use. In addition, the treatment assignments had no significant effect on log ACR at the end of the study whether or not adjustments were made for the baseline covariates: log-transformed ACR, systolic and diastolic blood pressures, use of ACE inhibitors/ARBs, age, sex, and race/ethnicity.
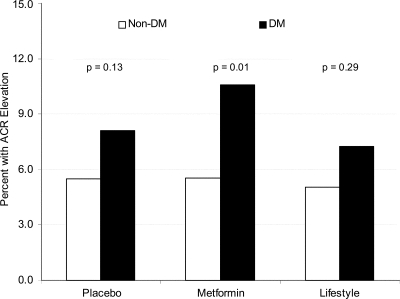
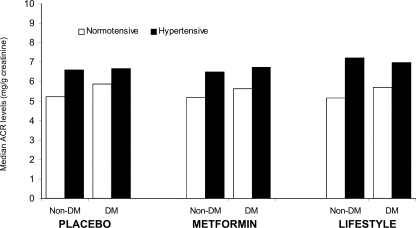
The odds of developing an elevated ACR level were 59% higher for a participant who developed diabetes compared with one who did not, and there was no difference among the three treatment groups in this regard (Fig. 2). Participants in the placebo group who developed diabetes experienced a significantly greater change in ACR compared with those without diabetes (P = 0.036; median 0.02 vs. 0.34 mg/g creatinine) (Fig. 3), although these changes were so small as to be of little clinical importance. The presence of hypertension also increased the median ACR in diabetic and nondiabetic participants in each treatment group (Fig. 3).
Figure 2.
Prevalence of elevated ACR (≥30 mg/g) at end of study by treatment group and diabetes (DM) status. P value indicates the difference in rates between the diabetic and nondiabetic treatment groups.
Figure 3.
Median ACR (milligrams per gram) by diabetes (DM) and hypertension status at end of study.
CONCLUSIONS
In participants entering the DPP, the frequency of an elevated ACR level was 5.8%, a proportion considerably lower than that found in other comparable populations. In the Third National Health and Nutrition Examination Survey (1988–1994), microalbuminuria was present overall in 7.8% of women and 5.0% of men but in those with the metabolic syndrome, microalbuminuria was found in 12% of men and 13% of women (11). As mentioned previously, 15% of Pima Indians with IGT have microalbuminuria (7). In other studies in subjects with IGT, 9.9% of Australians (15), 14 (16) and 24% (17) of Japanese, 11.8% of Koreans (18), and 19% of Indians had microalbuminuria (19).
The reasons that our participants had such low rates of elevated ACR levels are not clear. Blood pressure was particularly well controlled (mean SBP 123.7 ± 14.7 and mean DBP 78.3 ± 9.3 mmHg). These blood pressures are substantially lower than those found in the patients with microalbuminuria in the Australian Diabetes, Obesity, and Lifestyle (AusDiab) Study (151 ± 23/78 ± 13 mmHg) (15). Furthermore, entry exclusion criteria (creatinine ≥1.4 mg/dl in men, ≥1.3 mg/dl in women, creatinine clearance <75 ml/min in subjects aged >80 years, and ≥2+ proteinuria on dipstick) may have removed many with or at high risk for developing elevated ACR. Another issue is the use of ACE inhibitors or ARBs, which may lower urinary albumin excretion; these drugs were used in 6.7, 8.3, and 6.1% in the ILS, metformin, and placebo groups, respectively. As these drugs were often used for hypertension treatment without knowing baseline albuminuria status, the frequency of elevated ACR levels could have been as high as 13.0, 14.4, and 11.1% in the ILS, metformin, and placebo groups, respectively, making these percentages more in line with the frequencies found in other studies.
Elevated ACR correlated with insulin resistance in the DPP, and this has also been shown in other studies of individuals with normal and IGT (8). Therefore, it would have been expected that the interventions with ILS and metformin, which decreased the development of diabetes and decreased the degree of insulin resistance (20,21), would similarly decrease albumin excretion and the frequency of microalbuminuria. Furthermore, metformin has previously been shown to decrease urinary albumin excretion in patients with type 2 diabetes (22). However, this was not the case in the DPP cohorts. One possible reason is that although this is the largest study to have addressed this issue, we still did not have enough power to detect such changes, as there were relatively small numbers who had microalbuminuria at baseline and there was only a short time to detect incident cases of microalbuminuria.
The presence of micro- and macroalbuminuria in patients with IGT and diabetes has been thought to be a marker of increased cardiovascular risk (2,23,24). However, despite the improvement in insulin resistance and other cardiovascular risk markers in the ILS and metformin groups (21,22), there was no improvement in ACR in those two groups.
Obesity has been associated with glomerular hypertrophy, increased urinary albumin excretion, and even decreased glomerular filtration rate in the absence of diabetes in some patients (25). It may be that the increased ACR found in the DPP participants has multiple causes, including insulin resistance, endothelial dysfunction, early diabetic nephropathy related to hyperglycemia, hypertensive nephropathy, and focal sclerosis related to obesity. We did not adjust for antihypertensive drug use other than ACE inhibitors and ARBs, and this may also be a shortcoming. Thus, the modest changes in insulin resistance and weight loss that occurred with active intervention in the DPP over the relatively short period of time of 3.4 years may be only one set of factors that need to be corrected to affect the kidney disease in this patient population.
Supplementary Material
Acknowledgments
Funding was provided by the National Institutes of Health through the National Institute of Diabetes and Digestive and Kidney Diseases (Grant 5U01DK048489), the National Center on Minority Health and Health Disparities, the National Institute of Child Health and Human Development, the Office of Women's Health, and the National Institute on Aging. In addition, the Indian Health Service, the Centers for Disease Control and Prevention, the American Diabetes Association, and two pharmaceutical companies, Bristol-Myers Squibb and Parke-Davis, contributed support. The General Clinical Research Center Program, National Center for Research Resources, supported many of the clinical centers. Support to the clinical centers and the Coordinating Center was provided by the National Institute of Diabetes and Digestive and Kidney Diseases through a Cooperative Agreement, except for the Southwestern American Indian Centers, which were supported directly by the National Institute of Diabetes and Digestive and Kidney Diseases and the Indian Health Service. This research was also supported, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases. The Diabetes Prevention Program was principally supported by the National Institute of Diabetes and Digestive and Kidney Diseases and other components of the National Institutes of Health and the Centers for Disease Control and Prevention.
No potential conflicts of interest relevant to this article were reported.
APPENDIX
Writing Group: Mark E. Molitch, MD (chair), Elizabeth Barrett-Connor, MD, Jill Crandall, MD, Samia Das, MS, MBA, RD, LD, Ronald Goldberg, MD, Steven Haffner, MD, MPH, Hermes Florez, MD, William C. Knowler, MD, DrPH, Trevor Orchard, MD, Robert Ratner, MD, and Marinella Temprosa, MSA. A complete listing of the authorship of the Diabetes Prevention Program Research Group is available in an online appendix.
Footnotes
Representatives of the National Institutes of Health and the Centers for Disease Control and Prevention participated in the design of the study and in the reporting of the results. Although additional funding was provided by a variety of other sources, as listed in the acknowledgments, these sources had no role in the design or conduct of the study or in the reporting of results.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1. Gross JL, Canani LH, de Azevedo MJ, Caramori ML, Silveiro SP, Zelmanovitz T: Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28: 176– 188, 2005 [DOI] [PubMed] [Google Scholar]
- 2. Dinneen SF, Gerstein HC: The association of microalbuminuria and mortality in non-insulin-dependent diabetes mellitus. Arch Intern Med 157: 1413– 1418, 1997 [PubMed] [Google Scholar]
- 3. UK Prospective Diabetes Study (UKPDS) Group: Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837– 853, 1998 [PubMed] [Google Scholar]
- 4. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW: 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359: 1577– 1589, 2008 [DOI] [PubMed] [Google Scholar]
- 5. UK Prospective Diabetes Study Group: Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 317: 703– 713, 1998 [PMC free article] [PubMed] [Google Scholar]
- 6. Holman RR, Paul SK, Bethel MA, Neil HAW, Mathews DR: Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med 2: 1565– 1576 [DOI] [PubMed] [Google Scholar]
- 7. Nelson RG, Kunzelman CL, Pettitt DJ, Saad MF, Bennett PH, Knowler WC: Albuminuria in type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in Pima Indians. Diabetologia 32: 870– 876, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Mykkäkenen L, Zaccaro DJ, Wagenknecht W, Robbins DC, Gabriel M, Haffner SM: Microalbuminuria is associated with insulin resistance in nondiabetic subjects: ;The Insulin Resistance Atherosclerosis Study. Diabetes 47: 793– 800, 1998 [DOI] [PubMed] [Google Scholar]
- 9. Sosenko JM, Hu D, Welty T, Howard BV, Lee E, Robbins DC: Albuminuria in recent-onset type 2 diabetes: the Strong Heart Study Diabetes Care 25: 1078– 1084, 2002 [DOI] [PubMed] [Google Scholar]
- 10. Meigs JB, D'Agostino RB, Sr, Nathan DM, Rifai N, Wilson PWF: Longitudinal association of glycemia and microalbuminuria: the Framingham Offspring Study. Diabetes Care 25: 977– 983, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Palaniappan L, Carenthon M, Fortmann SP: Association between microalbuminuria and the metabolic syndrome: NHANES III. Am J Hypertens 16: 952– 958, 2003 [DOI] [PubMed] [Google Scholar]
- 12. The Diabetes Prevention Program: Design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 22: 623– 634, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Diabetes Prevention Program Research Group: Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 346: 393– 403, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Diabetes Prevention Program: Hypertension, insulin, and proinsulin in participants with impaired glucose tolerance. Hypertension 40: 679– 686, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tapp RJ, Dip G, Shaw JE, Zimmet PZ, Balkau B, Chadban SJ, Tonkin AM, Welborn TA, Atkins RC: AusDiab Study Group: Albuminuria is evident in the early stages of diabetes onset: results from the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Am J Kidney Dis 44: 792– 798, 2004 [PubMed] [Google Scholar]
- 16. Suzuki H, Fukushima M, Usami M, Ikeda M, Taniguchi A, Nakai Y, Matssura T, Yasuda K, Hosokawa M, Selno Y, Yamada Y: IGT with fasting hyperglycemia is more strongly associated with microalbuminuria than IGT without fasting hyperglycemia. Diabetes Res Clin Pract 64: 213– 219, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Tomura S, Kawada K, Saito K, Lin YL, Endou K, Hirano C, Yanagi H, Tsuchiya S, Shiba K: Prevalence of microalbuminuria and relationship to the risk of cardiovascular disease in the Japanese population. Am J Nephrol 19: 13– 20, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Kim YI, Kim CH, Choi CS, Chung YE, Lee MS, Lee SI, Park JY, Hong SK, Lee KU: Microalbuminuria is associated with the insulin resistance syndrome independent of hypertension and type 2 diabetes in the Korean population. Diabetes Res Clin Pract 52: 145– 152, 2001 [DOI] [PubMed] [Google Scholar]
- 19. Yajnik CS, Naik SS, Raut KN, Khade AD, Bhat DS, Nagarkar VD, Deshpande JA, Shelgikar KM: Urinary albumin excretion rate (AER) in newly-diagnosed type 2 Indian diabetic patients is associated with central obesity and hyperglycaemia. Diabetes Res Clin Pract 17: 55– 60, 1992 [DOI] [PubMed] [Google Scholar]
- 20. Orchard TJ, Temprosa M, Goldberg R, Haffner S, Ratner R, Marcovina S, Fowler S: Diabetes Prevention Program Research Group: The effect of metformin and intensive lifestyle intervention on the metabolic syndrome: the Diabetes Prevention Program randomized trial. Ann Intern Med 142: 611– 619, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Diabetes Prevention Program Research Group: Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program: effects of lifestyle intervention and metformin. Diabetes 54: 2404– 2414, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Amador-Licona N, Guízar-Mendoza J, Vargas E, Sánchez-Camargo G, Zamora-Mata L: The short-term effect of a switch from glibenclamide to metformin on blood pressure and microalbuminuria in patients with type 2 diabetes mellitus. Arch Med Res 31: 571– 575, 2000 [DOI] [PubMed] [Google Scholar]
- 23. Haffner SM, Gonzales C, Valdez RA, Mykkänen L, Hazuda HP, Mitchell BD, Monterrosa A, Stern MP: Is microalbuminuria part of the prediabetic state? The Mexico City Diabetes Study. Diabetologia 36: 1002– 1006, 1993 [DOI] [PubMed] [Google Scholar]
- 24. Valmadrid CT, Klein R, Moss SE, Klein BEK: The risk of cardiovascular disease mortality associated with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch Intern Med 160: 1093– 1100, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Kambham N, Markowitz GS, Valeri AM, Lin J, D'Agati VD: Obesity-related glomerulopathy: an emerging epidemic. Kidney Int 59: 1498– 1509, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.