Abstract
Generalised convulsive status epilepticus is one of the most common emergencies encountered in clinical practice. This review discusses the recent understanding of this life‐threatening condition with reference to the definition, pathophysiology, evaluation, complications, refractory status and prognosis. Besides epilepsy, other neurological and medical illnesses could be associated with status epilepticus. The goals of management and pharmacological approach are outlined, considering the available evidence. Prompt recognition and timely intervention, including pre‐hospital treatment, are therapeutically beneficial. Refractory status should be managed in intensive care units under close monitoring. More evidence is needed for evolving the optimal treatment. A suitable treatment protocol would guide in avoiding the pitfalls at various points along the management pathway.
Keywords: status epilepticus, electroencephalographic monitoring, anticonvulsants, refractory status, complications
Generalised convulsive status epilepticus is one of the most common medical emergencies in clinical practice. Status epilepticus occurs not only in people with epilepsy but also in the context of other neurological disorders and systemic illness. The annual incidence in the UK is about 9000–14 000 new cases per year.1 Status epilepticus accounts for 3.5% of admissions to emergency departments in the developed nations and for 11% in a developing country.2 Prompt recognition and treatment are required to prevent associated complications. However, prospective randomised trials regarding the treatment are few. Formulation of newer anticonvulsants is limited to the oral route and hence could not be widely used in the initial management of status epilepticus. Hence, there is a substantial need to improve measures for both prevention and effective management of this life‐threatening condition. This review offers a comprehensive account of status epilepticus in adults and is based on literature search using different combinations of key words: “status epilepticus”, “treatment”, “refractory status”, “psychogenic status epilepticus”, “mechanisms”, “nonconvulsive status”, “buccal midazolam”, “lorazepam and diazepam”, “phenytoin and fosphenytoin”, “levetiracetam”, “prolactin”, “electrocardiogram”, “electroencephalographic monitoring” and “complications” in the PubMed.
Definition
The range of seizure continuum extends from isolated seizure to repetitive seizures to status epilepticus. The distinguishing feature between acute repetitive seizures and status epilepticus is the recovery of consciousness in between the episodes of seizures in acute repetitive seizures.3 There is lack of consensus on seizure duration that defines status epilepticus (table 1).4,5,6,7,8,9
Table 1 Variability in the definition of status epilepticus.
| Authors | Definition |
|---|---|
| Epilepsy Foundation of America's Working Group4 | Continuous seizure for >30 min or ⩾2 sequential seizures without full recovery of consciousness in between seizures |
| Bleck5 | Seizures lasting, or often repeated without clinical recovery, for ⩾20 min |
| Veteran Administration Cooperative Trial on Treatment of Generalised Convulsive Status Epilepticus6 | Seizure duration of 10 min |
| Pre‐Hospital Treatment of Status Epilepticus Study7 | Seizure duration of 5 min |
| Lowenstein et al8 | Continuous seizures lasting ⩾5 min or ⩾2 discrete seizures, with incomplete recovery of consciousness in between the episodes |
| Mayer et al9 | Continuous tonic–clonic or electrographic seizure activity for at least 10 min or intermittent seizure activity without recovery of consciousness for at least 30 min |
The problem lies in determining the temporal evolution of isolated seizures to status epilepticus in humans. Analysis of isolated seizures has shown that the duration of discrete episodes does not exceed 2 min.10 In status epilepticus, neuronal damage is heavily dependent on the duration.11 The duration also has a bearing on therapeutic interventions. The longer a seizure continues, the more refractory to simple therapy it becomes.12 Refractory seizures carry a high risk of therapeutic complications. In clinical practice, I prefer to initiate first‐line drugs to treat status epilepticus when seizures last ⩾5 min. However, more information is required to characterise the temporal course of seizures and its influence on treatment.
Clinical syndrome
On the basis of the seizure type, convulsive status epilepticus can be classified into tonic–clonic status epilepticus, tonic status epilepticus, clonic status epilepticus and myoclonic status epilepticus. Generalised tonic–clonic status epilepticus is the most common form. Motor events include phase of continuous muscle contraction or the tonic phase, followed by a phase of alternate contraction and relaxation of muscles or the clonic phase. At the onset, the seizures could be either generalised, with bilaterally synchronous limb movements, or focal, with movement of one or more extremities becoming secondarily generalised. Autonomic disturbances in the form of a tachycardia, cardiac arrhythmia, hypertension, high fever, salivation, vomiting and incontinence may be prominent.
Subtle status epilepticus
With continuing seizures, the motor manifestation may be subtle in the form of ocular nystagmus or brief twitching of the face, eyelids, jaw, trunk, arms, hands, legs or feet. These movements could be unilateral, or intermittent, simulating focal seizures. However, electroencephalographic (EEG) monitoring usually shows features of generalised seizures, perhaps indicating electroclinical dissociation. The clinical features may be influenced by the underlying aetiologies and treatment.
Refractory status epilepticus
Refractory status epilepticus is considered when patients fail to respond to sequential treatment with adequate dosages of benzodiazepine and phenytoin. A recent study has identified focal motor seizures at onset and non‐convulsive status epilepticus as independent risk factors.9 Another observation is the occurrence of hyponatraemia within the first 24 h.13 Treatment poses considerable challenges (see the section Treatment of refractory status epilepticus). Refractory status epilepticus carries poor prognosis compared with non‐refractory status epilepticus.13,14 A severe variant that fails to respond to aggressive treatment has been termed malignant status epilepticus.15 It typically occurs in young patients (18–50 years) in the setting of encephalitis.
Myoclonic status epilepticus
Myoclonic status epilepticus presents as a bilateral massive myoclonus along with polyspike discharges on EEG, and usually carries a good prognosis. On the other hand, status myoclonus after severe hypoxic–ischaemic insult, viral encephalitis and prion disease is associated with poor prognosis. Further discussion of this entity is beyond the scope of this review.
Non‐convulsive status epilepticus
Convulsive status epilepticus may evolve into the non‐convulsive form after treatment,16 which is characterised by abnormal mental status with unresponsiveness, ocular motor abnormalities, persistent electrographic seizures and possible response to anticonvulsants.17,18 It may also arise de novo,18 and this possibility should be kept in mind while making a diagnosis. All patients with prolonged postictal confusion or unexplained coma should undergo EEG monitoring for confirmation.
Psychogenic status epilepticus
Non‐epileptic or psychogenic status epilepticus may pose considerable diagnostic challenges.19 Diagnosis must be reached quickly, as delay in recognition leads to iatrogenic complications from aggressive treatment. Table 2 lists the differentiating features from true status epilepticus.
Table 2 Differences between clinical features of true status epilepticus and psychogenic status epilepticus.
| Clinical features | True status epilepticus | Psychogenic status epilepticus |
|---|---|---|
| Sex | Occurs in both male and female patients | Observed in a higher proportion of female patients20 |
| Psychiatric history | History of abuse is as prevalent as in other medical disorders | Prevalence of abuse, traumatic life events, comorbid psychiatric disorders and treatment is higher than that observed in patients with epilepsy20 |
| Onset | Sudden | Gradual |
| Motor activity | Tonic–clonic limb jerking | Preparatory movements, body stiffening, thrashing, pelvic thrusting, back arching and head rolling |
| Progression of motor activity | Initially well‐defined or continuous episode; limb movements are usually synchronous; in prolonged status, subtle limb movements, epileptic nystagmus and focal twitching may be observed | Stopping and restarting of motor activity; out‐of‐phase, asynchronous limb movements; non‐physiological progression of activity is more common; subtle eye movements are rare |
| Vocalisation | At the start of seizure | In the middle of seizure; sobbing, crying and shouting are frequent |
| Eye open or closed | Forceful eye closure is uncommon | Eyes held shut, resisting passive lid opening |
| Ocular deviation | Upward or to one side, where present | Geotrophic eye movement; patients tend to look away from the examiner |
| Pupillary light reaction | Poorly reactive | Briskly reactive |
| Tongue biting | On the side of the tongue | On the tip of the tongue |
| Cyanosis | Frequent | Uncommon |
| Responsiveness during episode | Usually motor activity is not modified by outside stimuli; no withdrawal response to painful stimuli noted | When restrained by examiner, modification of activity with more vigorous and violent movements observed; limb withdrawal to painful stimuli more commonly observed |
| Consistency of seizure pattern | Usually stereotyped | Variable |
| Recovery | Clinical and EEG recovery is gradual; organic amnesia for the episode observed after recovery | Prompt clinical and EEG recovery; non‐organic amnesia observed |
| Episodes in sleep | Can occur | Uncommon; to exclude feigned sleep with EEG monitoring |
| Avoidance testing manoeuvres | On releasing the patient's hand over face, no attempt observed at self‐protection; (care should be taken not to cause trauma to patient) | Active resistance of hand falling over face or termination of activity |
| Induction by suggestion and saline injection in the follow‐up clinic after recovery | Controlled induction of seizure is unusual; however, caution should be exercised not to trick the patient | Creating a permissive setting to bring on a typical episode and evaluation with EEG monitoring enables better characterisation of the spell |
EEG, electroencephalographic.
To predict diagnosis of psychogenic status epilepticus, a combination of observed findings rather than a single clinical feature should be considered.20 The diagnosis may be difficult in patients with subtle writhing and in‐phase limb movements, and unresponsive behaviour.21 The issue is further complicated by the coexistence of non‐epileptic and true epileptic seizures in the same person.20 Video EEG monitoring is the gold standard of differential diagnosis.20 Absence of seizure discharges during the spell helps to confirm the diagnosis if frontal‐lobe seizures could be reliably excluded. However, movement artefacts could obscure the electrographic feature. Normal EEG background in the immediate postictal period (as opposed to slowing of background after true seizures) would provide an additional clue. The use of prolactin testing in the differential diagnosis is not established.22 In contrast with single seizures, status epilepticus does not lead to an increase in serum prolactin.23 Management strategies for psychogenic status epilepticus include disclosing diagnosis in a positive way, and treatment of abnormal illness behaviour and comorbid psychiatric problems. Anticonvulsants need to be stopped (under observation) in patients without pre‐existing epilepsy. Close liaison between emergency physicians, neurologists and psychiatrists is required. Box 1 lists the other mimics of status epilepticus.
Pathophysiology
The prevailing knowledge about seizure mechanisms is derived from animal models of focal seizures and limbic status epilepticus.24 Whether such observations remain valid for status epilepticus in humans is not known. The emergence of status epilepticus requires a pool of neurones capable of initiating and sustaining abnormal firing.24 This abnormal discharge is facilitated by loss of inhibitory synaptic transmission mediated by γ‐amino butyric acid (GABA) and sustained by excitatory transmission mediated by glutamate. Postsynaptic GABA(A) and N‐methyl‐d‐aspartate receptors have a vital role in the inhibitory and excitatory transmissions, respectively.12,25 Increased neuronal activity can also lead to loss of inhibition by accelerated internalisation of the GABA(A) receptor.26 Benzodiazepine (lorazepam and diazepam), barbiturates (phenobarbital, pentobarbital and thiopental) and propofol act through different sites on this receptor. Status epilepticus also alters specific properties of this receptor.27 This observation supports the clinical finding of barbiturate sensitivity in the setting of benzodiazepine refractoriness. The molecular mechanisms underlying this plasticity of receptor function are not known.27 Pharmacoresistance to benzodiazepine could also be regulated by N‐methyl‐d‐aspartate receptor activation.12 Neuropeptide Y and galanin serve as endogenous anticonvulsants that terminate status epilepticus.28 On the other hand, brain injury from trauma, epilepsy, infections and other causes leads to increased cortical excitability and impaired seizure termination. These changes perhaps enhance the susceptibility to develop status epilepticus.29
The abnormal neuronal discharges, together with increased cerebral and muscle blood flow and metabolism, mediate the acute pathophysiological changes. During the early phase, neuronal discharges affecting the autonomic centres produce autonomic changes mentioned earlier. With prolonged seizures, compensatory mechanisms fail, leading to various organ dysfunctions. Neurone‐specific enolase is a biological marker of neuronal injury in status epilepticus. Good correlation exists between the level of this marker and seizure duration, indicating the occurrence of neuronal damage with prolonged seizure.11 The proposed mechanisms include excitotoxicity, energy impairment, free radical generation and apoptosis.30,31
Aetiology
Box 2 lists the underlying causes of status epilepticus.
The major causes of status epilepticus in adults include epilepsy (25%), stroke (23%) and remote causes (19%).32 Status epilepticus is more common in patients with secondary generalised epilepsy than in those with idiopathic generalised epilepsy.33 The most important cause is poor drug compliance. Other factors include acute and remote symptomatic causes and previous episodes of status epilepticus. De novo status epilepticus in hospitalised patients is most often related to stroke and metabolic derangements.34 Early‐onset status epilepticus in stroke is associated with high risk of recurrence.35 Cerebral toxoplasmosis, lymphoma and anticonvulsant withdrawal are important causes in HIV infection.36 Alcohol intoxication is a major precipitant in alcohol‐dependent patients.37 Withdrawal seizure can occasionally also progress to status epilepticus.
EEG in the evaluation of status epilepticus
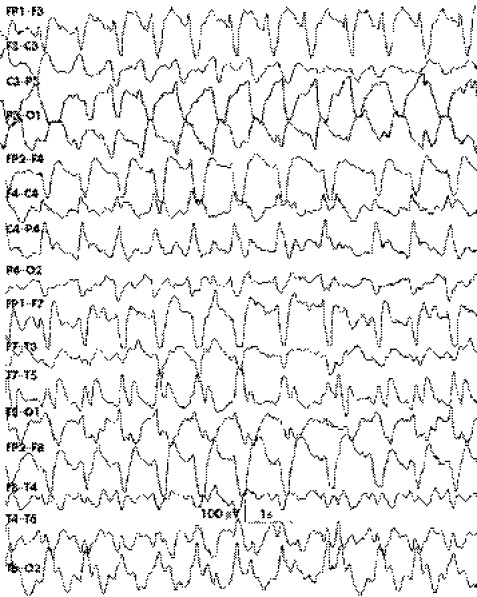
Digital video EEG monitoring aids in the differentiation of psychogenic status epilepticus from true status epilepticus. Recent technological advances include automated seizure‐detection software and user‐friendly online quantitative analysis.38 Five phases of EEG pattern have been described in the temporal evolution of true status epilepticus.39 These include intermittent discrete seizure discharges with interictal background slowing, waxing and waning of seizure discharges, continuous discharges with or without intervening flat periods (fig 1), and periodic epileptiform discharges. However, these sequential patterns may not be observed in all cases and in all situations.
Figure 1 Continuous generalised seizure discharges viewed on a longitudinal bipolar montage in an adult with status epilepticus.
Continuous monitoring serves as a useful guide for titration of anticonvulsants and determining prognosis in patients who have paralysis and are on mechanical ventilators. Relapse of status epilepticus during dose tapering may require intense seizure and EEG background suppression for some period.40 EEG monitoring is also required to diagnose electrographic or non‐convulsive status epilepticus (see section on Non‐convulsive status epilepticus) reliably.
Management of generalised convulsive status epilepticus
Boxes 3A,B show an algorithm for management of generalised convulsive status epilepticus. The principles underlying successful treatment are discussed.
Box 1: Mimics of generalised convulsive status epilepticus
Psychogenic status epilepticus
Decerebrate spasms
Tetanus
Malignant hyperthermia
Malignant neuroleptic syndrome
Paroxysmal dyskinesia
Acute chorea, ballismus, dystonia
Box 2: Aetiologies of status epilepticus
Acute symptomatic causes (seizure occurring within 1 week of onset of underlying aetiologies)
Stroke, including cerebral sinovenous thrombosis
Subtherapeutic anticonvulsant levels: abrupt drug withdrawal
Alcohol related: alcohol withdrawal or intoxication
-
Toxicity:
-
-
Drugs—recreational, such as cocaine; prescription, such as aminophylline, imipramine; or acute drug overdose, such as isoniazid
-
-
Environmental exposure—carbon monoxide, lead, camphor, organophosphates
-
-
Hypoxia
Metabolic disturbances: dyselectrolytaemia, hypoglycaemia, uraemia or other metabolic disorders
-
Infections or infestations:
-
-
Meningoencephalitis, granuloma
-
-
Others: infections not primarily affecting the brain
-
-
Pregnancy related: eclampsia
Trauma: head injury, post‐intracranial surgery
Tumours: primary or metastatic brain tumours
Multiple causes: seizures occurring with several concomitant conditions
Remote symptomatic causes (seizures occurring more than 1 week after brain insult)
Stroke
Head injury
Brain infections or infestations: seizures occurring as sequelae
Post‐encephalopathy sequelae: toxic or metabolic encephalopathy
Progressive neurological diseases: slow viral diseases, degenerative diseases, autoimmune diseases, disorders due to inborn errors of metabolism
Developmental brain malformation, severe neonatal encephalopathy
Cryptogenic aetiology
Seizure with no identifiable acute or remote cause
Diagnosis
The crucial step is distinguishing status epilepticus from psychogenic events (table 2). This avoids potentially dangerous and wrong therapeutic strategies. Once status epilepticus is promptly recognised, vital signs should be monitored. Priority should be given for immediate and continued stabilisation of vital parameters. Optimal airway patency must be achieved by proper head positioning. If the assessment shows an airway compromise or a risk of aspiration, rapid sequence intubation must be attempted.41 Supplemental oxygen must be provided to combat hypoxaemia. Electrocardiography helps to identify ischaemic patterns, arrhythmia and arrhythmogenic disorders such as Brugada syndrome, which may rarely present with status epilepticus.42 Cardiac monitoring may also provide clues to metabolic derangements apart from being pertinent in intravenous phenytoin (or fosphenytoin) treatment. High incidence of cardiac dysfunction in patients with status epilepticus also underscores the need for close monitoring.43,44 After establishing vascular access, blood must be drawn for glucose, urea, creatinine, electrolytes, bicarbonate, magnesium, calcium, liver function tests, creatine kinase, complete haemogram, arterial blood gases, toxicological screening and anticonvulsants. Blood pressure should be maintained with intravenous fluids. EEG monitoring should be considered in the management of psychogenic status epilepticus, prolonged postictal coma and refractory status epilepticus. Serum anticonvulsant levels are useful for documenting withdrawal seizures and optimising treatment with intravenous fosphenytoin, phenytoin and phenobarbital. Box 3 outlines the other pertinent diagnostic and supportive measures. The management algorithm should include “reconsider the diagnosis” option at various points along the pathway.
Early control of seizures
One of the major goals in the treatment of status epilepticus is the preservation of neuronal function. This requires early cessation of seizure. In addition, the risk of development of refractory status epilepticus increases if status epilepticus persists longer.
First‐line drugs: lorazepam or diazepam?
Four randomised trials of anticonvulsants in patients with status epilepticus showed that both lorazepam and diazepam are effective as initial treatment.6,7,45,46 Intravenous lorazepam given by trained paramedics in prehospital settings was also found to be therapeutically beneficial.7 Seizure cessation on admission to emergency department outweighed the presumed side effects of treatment. Owing to the ease of administration, longer effective duration of action and better side effect profile,47 I prefer lorazepam to diazepam for the initial treatment. This choice is also favoured by others.48,49,50 These drugs may be repeated after 10 min if necessary. Intravenous clonazepam (1 mg) is another alternative benzodiazepine.
Alternative delivery options
In pre‐hospital settings and remote clinical set‐ups, where intravenous access is a problem, it is important to explore alternative routes of drug delivery in the management of continuing or serial seizures. Recently, two randomised trials compared buccal midazolam with rectal diazepam for the treatment of continuing seizures in children and adolescents.51,52 Buccal midazolam was found to be equally or more effective than rectal diazepam in terminating seizures without increased adverse cardiorespiratory events. Other drug delivery options include intranasal, intramuscular and rectal midazolam.53,54,55 These rescue options could prevent emergence of established and refractory status from incipient seizures. Owing to the ease of administration, large surface area of absorption, social acceptance and predictable response in patients who have constipation and use wheelchairs, the buccal route of delivery is a better option.56 Table 3 outlines the drug treatment.
Table 3 Pharmacotherapy of generalised convulsive and refractory status epilepticus.
| Drug | Timing | Route | Loading dose | Maximal rate of administration | Maintenance dose | Parameters to monitor |
|---|---|---|---|---|---|---|
| Generalised convulsive status epilepticus | ||||||
| Lorazepam | Within the first 5–10 min of admission or as prehospital treatment | IV bolus | 0.1 mg/kg | 2 mg/min | – | Respiration; blood pressure; consciousness level |
| Diazepam | Within the first 5–10 min, as an alternative to lorazepam; or as pre‐hospital treatment | IV bolus, rectal administration if IV access is not available | 0.2 mg/kg (IV); 10–30 mg (rectal) | 5 mg/min | – | Same as for lorazepam |
| Phenytoin | 10–45 min | IV infusion | 20 mg/kg | 50 mg/min; maximal loading dose 30 mg/kg | 5 mg/kg/day in three divided doses IV | Blood pressure; ECG; watch for purple glove syndrome; avoid glucose‐containing fluids for dilution |
| Fosphenytoin | As an alternative to phenytoin | IV infusion | 20 mg/kg PE | 150 mg/min | 5 mg/kg/day PE IV or IM | Same as for phenytoin; except can be given with glucose‐containing fluids |
| Refractory status epilepticus | ||||||
| Phenobarbital | 45–60 min | IV infusion | 10–20 mg/kg | 100 mg/min | 1–4 mg/kg/day | Cardiorespiratory monitoring |
| Valproate | 45–60 min | IV infusion | 20–25 mg/kg | 3 mg/kg/min | 2 mg/kg/h | Liver function test, ammonia, serum amylase, lipase, blood pressure, platelet count |
| Midazolam | >60 minPre‐hospital treatment | IV bolus or infusion; IM or rectal; buccal; intranasal | Initial bolus: 0.15–0.2 mg/kg; 5–10 mg (IM or rectal); 10 mg or 0.5 mg/kg (buccal); 10 mg (intranasal) | Repeat dose 0.2 mg/kg boluses every 5 min until seizures stop (maximum loading dose 2 mg/kg) | Continuous infusion 0.1–0.4 mg/kg/h, dose titration to seizure or EEG suppression or change in vital signs | Cardiorespiratory monitoring, tachyphylaxis with long‐term infusion |
| Propofol | >60 min | IV bolus or infusion | Initial bolus 1 mg/kg | Repeat 1–2 mg/kg boluses every 5 min until seizures stop (maximum loading dose: 10 mg/kg) | Continuous infusion 1–12 mg/kg/h, for dose titration as for midazolam | Arterial blood gases, blood pressure, (propofol infusion syndrome) lipids, rebound seizures with rapid withdrawal |
| Pentobarbital | >60 min | IV bolus or infusion | Initial bolus 5 mg/kg | Repeat 5 mg/kg boluses until seizures stop (maximum bolus rate: 50 mg/min), dose titration to change in blood pressure | Continuous infusion: 0.5–10 mg/kg/h, for dose titration as for midazolam | Cardiorespiratory monitoring |
| Thiopental | >60 min | IV bolus or infusion | Initial bolus 100–250 mg over 20 s | Repeat 50 mg boluses every 2–3 min until seizures stop, dose titration to change in blood pressure | Continuous infusion 3–5 mg/kg/h, for dose titration as for midazolam | Cardiorespiratory monitoring, prolonged coma, laryngospasm, spasm at injection site, avoid plastic‐giving sets and exposure to air |
| Isoflurane | >60 min | Inhalation | End tidal concentration of 0.8–2% | Dose tailored to cardiorespiratory monitoring | For dose titration as for midazolam | Cardiorespiratory monitoring, administration requires anaesthetic system with a scavenging apparatus |
ECG, electrocardiogram, EEG, electroencephalographic; IM, intramuscular; IV, intravenous; PE, phenytoin equivalent.
Prevention of seizure recurrence
After treatment with lorazepam, phenytoin or its prodrug, fosphenytoin, is given as a second‐line drug in patients at risk of recurring seizures. Phenytoin (or fosphenytoin) is also used if seizure persists despite benzodiazepine injection.
Second‐line drugs: phenytoin or fosphenytoin?
Fosphenytoin offers certain pharmacokinetic advantages over phenytoin:
It can be infused using standard intravenous solutions, whereas phenytoin should not be given in dextrose‐containing fluids (because of drug precipitation)
It can be given intramuscularly
Rate of infusion is three times as fast with fosphenytoin
However, a randomised study showed no distinct advantages over phenytoin in terms of reduction of adverse events or length of hospital stay.57 Purple glove syndrome (triad of oedema, discolouration and pain distal to injection site) after phenytoin use is an uncommon and a mild adverse effect.58 Phenytoin has an equivalent onset and duration of action. Besides being a cost‐effective agent,59,60 the drug has been in clinical usage for a long time. After administration of an equivalent dose of fosphenytoin, the maximal plasma (phenytoin) concentration achieved is 15% lower.61 Hence, I continue to use phenytoin rather than opting for formulary conversion to fosphenytoin. The maintenance dose of phenytoin should be continued. Patients with epilepsy should also continue the prescription drugs (anticonvulsants prescribed before status epilepticus) after initial seizure cessation to prevent emergence of withdrawal seizures.
Treatment of underlying and precipitating causes
Treatable precipitants and causes, including missed anticonvulsants, infections, fever, hypoglycaemia, electrolyte imbalance, organ dysfunction, drug poisoning and withdrawal, alcohol misuse and withdrawal, immunosuppressive responsive inflammatory disorders, stroke, trauma and hypertensive encephalopathy, must be actively pursued and treated. Patients with epilepsy need to be evaluated for these causes before attributing emergence of status epilepticus to missed drugs. Those taking medications irregularly should be counselled regarding drug compliance before discharge from hospital. Meningoencephalitis should be treated with appropriate antimicrobials. Guidelines to prevent central pontine myelinolysis should be adhered to during correction of severe hyponatraemia. Porphyric status epilepticus should be treated with parenteral magnesium sulphate or intravenous benzodiazepine for immediate control of seizures. High carbohydrate and haematin intake reduces porphyrin production. As conventional anticonvulsants exacerbate the disease, newer drugs such as levetiracetam and gabapentin could be used for preventing seizure recurrence.62,63 Intoxication with isoniazid should be treated with pyridoxine, whereas tricyclic antidepressants and other antimuscarinic agents require physostigmine. Pyridoxine can also be given for suspected pyridoxine‐dependent seizures. Nerve agent‐induced status epilepticus requires benzodiazepine, atropine and pralidoxime. Phenytoin (fosphenytoin) provides little benefit in this setting64 and in the secondary prophylaxis of seizures resulting from alcohol withdrawals.37 Aggressive treatment with anticonvulsants and plasma exchange are useful for thrombotic thrombocytopenic purpura complicated by status epilepticus.65
Box 3A: Diagnosis and early management of seizures
Diagnosis
Diagnose ongoing clinical status epilepticus and exclude psychogenic seizures
Ensure adequate airway, breathing and circulation
Initiate cardiorespiratory monitoring, laboratory tests and evaluation for underlying causes
Give glucose with thiamine if hypoglycaemia is suspected or detected by a bedside glucometer
Treat hyperthermia or fever with appropriate measures
Reconsider the diagnosis at various points along the management pathway described below
Pre‐hospital management
Give lorazepam if venous access is available
Consider buccal, nasal, intramuscular or rectal midazolam, or rectal diazepam if no vascular access is available
First‐line drugs (for ⩾5 min of persisting seizures)
Prefer intravenous lorazepam (or diazepam)
Second‐line drugs (for continuing or risk of recurring seizures after giving first‐line drug)
Give intravenous phenytoin (or fosphenytoin) under cardiac monitoring
Treatment of cerebral sinovenous thrombosis includes antioedema measures and heparin. For eclamptic seizures, parenteral magnesium sulphate could be used initially.66 Benzodiazepine can also be given without depressant effect on the fetus.
Phenytoin loading may be pursued with a lower dose (10 mg/kg intravenously) owing to reduced protein binding in pregnancy. Subsequently, 5 mg/kg can be given as a second dose.
Treatment of refractory status epilepticus
Continuing seizures after treatment with two agents—lorazepam followed by phenytoin (fosphenytoin)—require admission to an intensive care unit in view of necessity of assisted ventilation and close monitoring. Assessment with continuous EEG is also mandatory. A psychogenic seizure must be excluded in an apparently refractory status epilepticus.19 If refractory status epilepticus is confirmed by electrographic monitoring, intravenous phenobarbital can be chosen. However, if the seizure persists longer (after giving the second drug), continuous intravenous drugs could be a better option. There is no unanimously accepted guideline in this setting.67,68,69 In the absence of randomised therapeutic trials, a firm recommendation is difficult to evolve. My preference is continuous treatment with intravenous midazolam or propofol. These agents are more often used nowadays and are found to be relatively safe in retrospective studies.70,71,72 If these measures fail, the next step includes induction of pharmacological coma with intravenous pentobarbital or thiopental. Alternative lines of treatment include continuous infusion of diazepam or lorazepam,73 and use of inhalational anaesthetics such as isoflurane and desflurane.74 Less commonly used options are intramuscular paraldehyde; administration of lidocaine, clomethiazole, althesin, ketamine, verapamil75; repetitive transcranial magnetic stimulation; vagal nerve stimulation76; and surgery.77,78
The electrographic end point of treatment is not satisfactorily defined. Burst suppression pattern on EEG results in less incidence of breakthrough seizures.72 However, this benefit is achieved at the expense of side effects such as hypotension. The alternative target is seizure suppression. Treatment failure, breakthrough, and post‐treatment seizures and other complications need to be monitored and treated. Parenteral anticonvulsants should be continued for at least 24 h after seizure cessation. During attempts at tapering of continuous intravenous drugs, oral or nasogastric administration of anticonvulsants should be started to avoid precipitation of withdrawal seizures.
Box 3B: Suggested treatment protocol for status epilepticus
Treatment for refractory status epilepticus (when first‐line and second‐line drugs fail)
Transfer patient to intensive care unit
Intubate if not intubated earlier
Institute continuous electroencephalographic (EEG) monitoring.
Start intravenous phenobarbital
Initiate continuous intravenous midazolam (or propofol) when phenobarbital fails or seizures persist longer after giving phenytoin (bypassing phenobarbital)
Try continuous pentobarbital or thiopental if the above measures are unsuccessful
Titrate dose to seizure suppression or suppression burst on EEG (if blood pressure permits)
Maintain the patient in a seizure‐free state for 24 h
Taper infusion gradually over the next 24 h
Resume prior infusion rate if seizure recurs
Maintain the second‐line drug, prescription drugs (may add new oral drugs in effective doses) during infusion tapering
Continue clinical and EEG monitoring (throughout the treatment course) in case of treatment failure, breakthrough and withdrawal seizures
Treatment of underlying causes
Identify the underlying causes (epilepsy related, acute symptomatic or remote symptomatic)
Determine the drug levels in patients with missed anticonvulsants
Pursue the treatable precipitants and causes
Management of complications
Monitor and treat the complications related to seizures, underlying cause, drugs and intensive care management
Role of intravenous valproate
Intravenous valproate can be considered to be a therapeutic option for the group of patients with cardiorespiratory impairment and “do not ventilate” status,48 and myoclonic status epilepticus. It seems to be safe, easy to use and non‐sedating.79,80,81,82 However, more clinical experience is needed to establish its place in the treatment protocols and to know whether it could be substituted for phenytoin or phenobarbital.
Newer anticonvulsants
Topiramate has been reported to be given nasogastrically along with conventional anticonvulsants for treating refractory status epilepticus in two small case series.83,84 Preliminary observation indicated that it might be successful in achieving seizure control. In another study, levetiracetam was coadministered for treating status epilepticus in four patients.85 Therapeutic response was favourable in two patients and could not be determined in the remaining two. These agents could also aid in the prophylaxis of breakthrough and withdrawal seizures.48 Once a parenteral formulation becomes available, therapeutic role of these newer anticonvulsants can be better characterised.
Complications
Complications may arise because of status epilepticus itself, drug treatment, underlying causes and intensive care management. If the core temperature exceeds 40°C, patients should be cooled. Presence of myoglobinuria or marked rise in serum creatine phosphokinase level may necessitate saline diuresis or urinary alkalinisation. Cerebral oedema may occur as a result of seizures or underlying aetiologies. However, cerebral oedema primarily due to seizure does not require aggressive therapeutic measures. Pathological changes identical to those in mesial temporal sclerosis have been documented in an autopsy series of patients dying from status epilepticus.86 Non‐neurological complications are often encountered in patients admitted to intensive care units.87 These include nosocomial and ventilator‐associated pneumonia, atelectasis, adult respiratory distress syndrome, neurogenic pulmonary oedema, pulmonary embolism, hypovolaemia, myocardial dysfunction, hypertension, arrhythmias, stress ulcer, gastrointestinal bleed, constipation, diarrhoea, paralytic ileus, renal dysfunction, urinary tract infection and vascular catheter‐related sepsis. These complications could be life threatening and should be treated and prevented from recurring. Pain, agitation, anxiety and sleep deprivation are other problems that need to be considered in the intensive care set‐up.
Pitfalls in management
Misdiagnosis, especially of psychogenic status epilepticus, leads to therapeutic failure. Other common pitfalls in the management include inadequate anticonvulsant dosage, delay in switching to another drug, wrong route of administration (intramuscular phenytoin), delay or failure in initiating maintenance anticonvulsants, missing and not treating the precipitating and underlying causes and complications, and delay in providing cardiorespiratory supports such as intubation and vasopressor administration.
Prognosis
Mortality from status epilepticus ranges from 3% to 50% in different studies.88 The risk factors for mortality include refractory seizures, acute symptomatic aetiologies (eg, hypoxia or central nervous system infections) and old age (>70 years).89 Cardiovascular changes during the stress of status epilepticus, medical complications and overtreatment may also have a role in the overall mortality.43,44 Coma with intercurrent electrographic status and multisystem failure also tend to have poor clinical outcome.90 Morbidity associated with status epilepticus includes epilepsy, cognitive impairment, incoordination, motor weakness, dysphasia, dysarthria and visual field defects.91
Key references
Shorvon S. Status epilepticus: its clinical features and treatment in children and adults. Cambridge: Cambridge University Press, 1999.
Macdonald RL, DeJong RN. Status epilepticus in adults and children: new developments in pathogenesis and treatment. Epilepsia 1999;40(Suppl 1).
Claassen J, Hirsch LJ, Emerson RG, et al. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 2002;43:146–53.
Walker M. Status epilepticus: an evidence based guide. BMJ 2005;331:673–7.
Pang T, Hirsch LJ. Treatment of convulsive and nonconvulsive status epilepticus. Curr Treat Options Neurol 2005;7:247–59.
Conclusion
Status epilepticus is a common medical problem in the emergency department. It requires early recognition and prompt differentiation from psychogenic seizures. A team approach, including emergency physicians, neurologists, anaesthetists, psychiatrists, and nursing and other paramedical staff, is necessary for proper evaluation and management. Prehospital treatment is a useful strategy for terminating incipient seizures. Treatment according to protocol may avoid management pitfalls. Well‐designed studies on management of refractory status epilepticus and neuroprotection are needed for improving the clinical outcome.
Self‐assessment questions (true (T)/false (F)); answers at the end of references
Psychogenic seizure can present as refractory status epilepticus.
Non‐convulsive status epilepticus requires electroencephalographic monitoring for confirmation.
Serum prolactin is always increased in generalised convulsive status epilepticus.
Status epilepticus due to acute symptomatic causes usually carries good prognosis.
The first‐line drug to treat status epilepticus is intravenous lorazepam.
Abbreviations
EEG - electroencephalographic
GABA - γ‐amino butyric acid
Answers
(1) T (2) T (3) F (4) F (5) T
Footnotes
Competing interest: None.
References
- 1.Shorvon S D. The management of status epilepticus. J Neurol Neurosurg Psychiatry 200170II22–II27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meena A K, Prasad V S, Kaul S.et al Neurological intensive care in India—disease spectrum and outcome. Neurol India 200048S1–S7. [PubMed] [Google Scholar]
- 3.Pellock J M. Management of acute seizure episodes. Epilepsia 199839S28–S35. [Google Scholar]
- 4.Dodson W E, DeLorenzo R J, Pedley T A.et al The treatment of convulsive status epilepticus: recommendations of Epilepsy Foundation of America's Working Group on Status Epilepticus. JAMA 1993270854–859. [PubMed] [Google Scholar]
- 5.Bleck T P. Convulsive disorders: status epilepticus. Clin Neuropharmacol 199114191–198. [DOI] [PubMed] [Google Scholar]
- 6.Treiman D M, Meyers P D, Walton N Y.et al A comparison of four treatments for generalized convulsive status epilepticus. Veterans Affairs Status Epilepticus Cooperative Study Group. N Engl J Med 1998339792–798. [DOI] [PubMed] [Google Scholar]
- 7.Alldredge B K, Gelb A M, Isaacs S M.et al A comparison of lorazepam, diazepam, and placebo for the treatment of out‐of‐hospital status epilepticus. N Engl J Med 2001345631–637. [DOI] [PubMed] [Google Scholar]
- 8.Lowenstein D H, Bleck T, Macdonald R L. It's time to revise the definition of status epilepticus. Epilepsia 199940120–122. [DOI] [PubMed] [Google Scholar]
- 9.Mayer S A, Claassen J, Lokin J.et al Refractory status epilepticus: frequency, risk factors and impact on outcome. Arch Neurol 200259205–210. [DOI] [PubMed] [Google Scholar]
- 10.Theodore W H, Porter R J, Albert P.et al The secondarily generalized tonic‐clonic seizure: a videotape analysis. Neurology 1994441403–1407. [DOI] [PubMed] [Google Scholar]
- 11.DeGiorgio C M, Heck C N, Rabinowicz A L.et al Serum neuron‐specific enolase in the major subtypes of status epilepticus. Neurology 199952746–749. [DOI] [PubMed] [Google Scholar]
- 12.Rice A C, DeLorenzo R J. N‐methyl‐D‐aspartate receptor activation regulates refractoriness of status epilepticus to diazepam. Neuroscience 199993117–123. [DOI] [PubMed] [Google Scholar]
- 13.Holtkamp M, Othman J, Buchheim K.et al Predictors and prognosis of refractory status epilepticus treated in a neurological intensive care unit. J Neurol Neurosurg Psychiatry 200576534–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossetti A O, Logroscino G, Bromfield E B. Refractory status epilepticus: effect of treatment aggressiveness on prognosis. Arch Neurol 2005621698–1702. [DOI] [PubMed] [Google Scholar]
- 15.Holtkamp M, Othman J, Buchheim K.et al A “malignant” variant of status epilepticus. Arch Neurol 2005621428–1431. [DOI] [PubMed] [Google Scholar]
- 16.DeLorenzo R J, Waterhouse E J, Towne A R.et al Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia 199839833–840. [DOI] [PubMed] [Google Scholar]
- 17.Husain A M, Horn G J, Jacobson M P. Non‐convulsive status epilepticus: usefulness of clinical features in selecting patients for urgent EEG. J Neurol Neurosurg Psychiatry 200374189–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan P W. Nonconvulsive status epilepticus in the emergency room. Epilepsia 199637643–650. [DOI] [PubMed] [Google Scholar]
- 19.Walker M C, Howard R S, Smith S J.et al Diagnosis and treatment of status epilepticus on a neurological intensive care unit. Q J Med 199689913–920. [DOI] [PubMed] [Google Scholar]
- 20.Cragar D E, Berry D T, Fakhoury T A.et al A review of diagnostic techniques in the differential diagnosis of epileptic and nonepileptic seizures. Neuropsychol Rev 20021231–64. [DOI] [PubMed] [Google Scholar]
- 21.Leis A A, Ross M A, Summers A K. Psychogenic seizures: ictal characteristics and diagnostic pitfalls. Neurology 19924295–99. [DOI] [PubMed] [Google Scholar]
- 22.Chen D K, So Y T, Fisher R S. Use of serum prolactin in diagnosing epileptic seizures: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 200565668–675. [DOI] [PubMed] [Google Scholar]
- 23.Tomson T, Lindbom U, Nilsson B Y.et al Serum prolactin during status epilepticus. J Neurol Neurosurg Psychiatry 1989521435–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noe K H, Manno E M. Mechanisms underlying status epilepticus. Drugs Today (Barc) 200541257–266. [DOI] [PubMed] [Google Scholar]
- 25.Naylor D E, Liu H, Wasterlain C G. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005257724–7733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodkin H P, Yeh J L, Kapur J. Status epilepticus increases the intracellular accumulation of GABAA receptors. J Neurosci 2005255511–5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Macdonald R L, Kapur J. Acute cellular alterations in the hippocampus after status epilepticus. Epilepsia 199940S9–20. [DOI] [PubMed] [Google Scholar]
- 28.Kapur J. Status epilepticus in epileptogenesis. Curr Opin Neurol 199912191–195. [DOI] [PubMed] [Google Scholar]
- 29.Rafiq A, Gong Q Z, Lyeth B G.et al Induction of prolonged electrographic seizures in vitro has a defined threshold and is all or none: implications for diagnosis of status epilepticus. Epilepsia 2003441034–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujikawa D G. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 200573–11. [DOI] [PubMed] [Google Scholar]
- 31.Niquet J, Liu H, Wasterlain C G. Programmed neuronal necrosis and status epilepticus. Epilepsia 20054643–48. [DOI] [PubMed] [Google Scholar]
- 32.DeLorenzo R J, Hauser W A, Towne A R.et al A prospective population based epidemiological study of status epilepticus in Richmond, Virginia. Neurology 1996461029–1035. [DOI] [PubMed] [Google Scholar]
- 33.Shorvon S, Walker M. Status epilepticus in idiopathic generalized epilepsy. Epilepsia 20054673–79. [DOI] [PubMed] [Google Scholar]
- 34.Delanty N, French J A, Labar D R.et al Status epilepticus arising de novo in hospitalized patients: an analysis of 41 patients. Seizure 200110116–119. [DOI] [PubMed] [Google Scholar]
- 35.Velioglu S K, Ozmenoglu M, Boz C.et al Status epilepticus after stroke. Stroke 2001321169–1172. [DOI] [PubMed] [Google Scholar]
- 36.Lee K C, Garcia P A, Alldredge B K. Clinical features of status epilepticus in patients with HIV infection. Neurology 200565314–316. [DOI] [PubMed] [Google Scholar]
- 37.Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol‐dependent patients: epidemiology, pathophysiology and management. CNS Drugs 2003171013–1030. [DOI] [PubMed] [Google Scholar]
- 38.Claassen J, Mayer S A. Continuous electroencephalographic monitoring in neurocritical care. Curr Neurol Neurosci Rep 20022534–540. [DOI] [PubMed] [Google Scholar]
- 39.Treiman D, Walton N, Kendrick C. A progressive sequence of electroencephalographic changes during generalised convulsive status epilepticus. Epilepsy Res 1990549–60. [DOI] [PubMed] [Google Scholar]
- 40.Krishnamurthy K B, Drislane F W. Depth of EEG suppression and outcome in barbiturate anesthetic treatment for refractory status epilepticus. Epilepsia 199940759–762. [DOI] [PubMed] [Google Scholar]
- 41.Roppolo L P, Walters K. Airway management in neurological emergencies. Neurocrit Care 20041405–414. [DOI] [PubMed] [Google Scholar]
- 42.Huang C C, Chen T W, Lin F C.et al Status epilepticus as an initial presentation of Brugada syndrome: a case report. Kaohsiung J Med Sci 200521387–391. [DOI] [PubMed] [Google Scholar]
- 43.Boggs J G, Painter J A, DeLorenzo R J. Analysis of electrocardiographic changes in status epilepticus. Epilepsy Res 19931487–94. [DOI] [PubMed] [Google Scholar]
- 44.Manno E M, Pfeifer E A, Cascino G D.et al Cardiac pathology in status epilepticus. Ann Neurol 200558954–957. [DOI] [PubMed] [Google Scholar]
- 45.Appleton R, Sweeney A, Choonara I.et al Lorazepam versus diazepam in the acute treatment of epileptic seizures and status epilepticus. Dev Med Child Neurol 199537682–688. [DOI] [PubMed] [Google Scholar]
- 46.Leppik I E, Derivan A T, Homan R W.et al Double‐blind study of lorazepam and diazepam in status epilepticus. JAMA 19832491452–1454. [PubMed] [Google Scholar]
- 47.Treiman D M. Pharmacokinetics and clinical use of benzodiazepines in the management of status epilepticus. Epilepsia 198930S4–10. [DOI] [PubMed] [Google Scholar]
- 48.Pang T, Hirsch L J. Treatment of convulsive and nonconvulsive status epilepticus. Curr Treat Options Neurol 20057247–259. [DOI] [PubMed] [Google Scholar]
- 49.Walker M. Status epilepticus: an evidence based guide. BMJ 2005331673–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prasad K, Al‐Roomi K, Krishnan P.et al Anticonvulsant therapy for status epilepticus. Cochrane Database Syst Rev 20054CD003723. [DOI] [PubMed] [Google Scholar]
- 51.McIntyre J, Robertson S, Norris E.et al Safety and efficacy of buccal midazolam versus rectal diazepam for emergency treatment of seizures in children: a randomised controlled trial. Lancet 2005366205–210. [DOI] [PubMed] [Google Scholar]
- 52.Scott R C, Besag F M, Neville B G. Buccal midazolam and rectal diazepam for treatment of prolonged seizures in childhood and adolescence: a randomized trial. Lancet 1999353623–626. [DOI] [PubMed] [Google Scholar]
- 53.Towne A R, DeLorenzo R. Use of intramuscular midazolam for status epilepticus. J Emerg Med 199917323–328. [DOI] [PubMed] [Google Scholar]
- 54.Jeannet P Y, Roulet E, Maeder‐Ingvar M.et al Home and hospital treatment of acute seizures in children with nasal midazolam. Eur J Pediatr Neurol 1999373–77. [DOI] [PubMed] [Google Scholar]
- 55.Scheepers M, Scheepers B, Clarke M.et al Is intranasal midazolam an effective rescue medication in adolescents and adults with severe epilepsy? Seizure 20009417–422. [DOI] [PubMed] [Google Scholar]
- 56.Body R, Ijaz M. Buccal midazolam as an alternative to rectal diazepam for prolonged seizures in childhood and adolescence. Emerg Med J 200522364–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coplin W M, Rhoney D H, Rebuck J A.et al Randomized evaluation of adverse events and length‐of‐stay with routine emergency department use of phenytoin or fosphenytoin. Neurol Res 200224842–848. [DOI] [PubMed] [Google Scholar]
- 58.Burneo J G, Anandan J V, Barkley G L. A prospective study of the incidence of the purple glove syndrome. Epilepsia 2001421156–1159. [DOI] [PubMed] [Google Scholar]
- 59.Rudis M I, Touchette D R, Swadron S P.et al Cost‐effectiveness of oral phenytoin, intravenous phenytoin, and intravenous fosphenytoin in the emergency department. Ann Emerg Med 200443386–397. [DOI] [PubMed] [Google Scholar]
- 60.Swadron S P, Rudis M I, Azimian K.et al A comparison of phenytoin‐loading techniques in the emergency department. Acad Emerg Med 200411244–252. [DOI] [PubMed] [Google Scholar]
- 61.Browne T R, Kugler A R, Eldon M A. Pharmacology and pharmacokinetics of fosphenytoin. Neurology 199646S3–S7. [DOI] [PubMed] [Google Scholar]
- 62.Zaatreh M M. Levetiracetam in porphyric status epilepticus: a case report. Clin Neuropharmacol 200528243–244. [DOI] [PubMed] [Google Scholar]
- 63.Pandey C K, Singh N, Bose N.et al Gabapentin and propofol for treatment of status epilepticus in acute intermittent porphyria. J Postgrad Med 200349285. [PubMed] [Google Scholar]
- 64.Holstege C P, Dobmeier S G. Nerve agent toxicity and treatment. Curr Treat Options Neurol 2005791–98. [DOI] [PubMed] [Google Scholar]
- 65.Beydoun A, Vanderzant C, Kutluay E.et al Full neurologic recovery after fulminant thrombotic thrombocytopenic purpura with status epilepticus. Seizure 200413549–552. [DOI] [PubMed] [Google Scholar]
- 66.Lucas M J, Leveno K J, Cunningham F G. A comparison of magnesium sulphate with phenytoin for the prevention of eclampsia. N Engl J Med 199533201–205. [DOI] [PubMed] [Google Scholar]
- 67.Holtkamp M, Masuhr F, Harms L.et al The management of refractory generalised convulsive and complex partial status epilepticus in three European countries: a survey among epileptologists and critical care neurologists. J Neurol Neurosurg Psychiatry 2003741095–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Claassen J, Hirsch L J, Mayer S A. Treatment of status epilepticus: a survey of neurologists. J Neurol Sci 200321137–41. [DOI] [PubMed] [Google Scholar]
- 69.Walker M C, Smith S J, Shorvon S D. The intensive care treatment of convulsive status epilepticus in the UK. Results of a national survey and recommendations. Anaesthesia 199550130–135. [DOI] [PubMed] [Google Scholar]
- 70.Prasad A, Worrall B B, Bertram E H.et al Propofol and midazolam in the treatment of refractory status epilepticus. Epilepsia 200142380–386. [DOI] [PubMed] [Google Scholar]
- 71.Rossetti A O, Reichhart M D, Schaller M D.et al Propofol treatment of refractory status epilepticus: a study of 31 episodes. Epilepsia 200445757–763. [DOI] [PubMed] [Google Scholar]
- 72.Claassen J, Hirsch L J, Emerson R G.et al Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia 200243146–153. [DOI] [PubMed] [Google Scholar]
- 73.Labar D R, Ali A, Root J. High dose intravenous lorazepam for the treatment of refractory status epilepticus. Neurology 1994441400–1403. [DOI] [PubMed] [Google Scholar]
- 74.Mirsattari S M, Sharpe M D, Young G B. Treatment of refractory status epilepticus with inhalational anesthetic agents isoflurane and desflurane. Arch Neurol 2004611254–1259. [DOI] [PubMed] [Google Scholar]
- 75.Iannetti P, Spalice A, Parisi P. Calcium‐channel blocker verapamil administration in prolonged and refractory status epilepticus. Epilepsia 200546967–969. [DOI] [PubMed] [Google Scholar]
- 76.Patwardhan R V, Dellabadia J, Jr, Rashidi M.et al Control of refractory status epilepticus precipitated by anticonvulsant withdrawal using left vagal nerve stimulation: a case report. Surg Neurol 200564170–173. [DOI] [PubMed] [Google Scholar]
- 77.Duane D C, Ng Y T, Rekate H L.et al Treatment of refractory status epilepticus with hemispherectomy. Epilepsia 2004451001–1004. [DOI] [PubMed] [Google Scholar]
- 78.Ma X, Liporace J, O'Connor M J.et al Neurosurgical treatment of medically intractable status epilepticus. Epilepsy Res 20014633–38. [DOI] [PubMed] [Google Scholar]
- 79.Limdi N A, Shimpi A V, Faught E.et al Efficacy of rapid IV administration of valproic acid for status epilepticus. Neurology 200564353–355. [DOI] [PubMed] [Google Scholar]
- 80.Peters C N, Pohlmann‐Eden B. Intravenous valproate as an innovative therapy in seizure emergency situations including status epilepticus—experience in 102 adult patients. Seizure 200514164–169. [DOI] [PubMed] [Google Scholar]
- 81.Hodges B M, Mazur J E. Intravenous valproate in status epilepticus. Ann Pharmacother 2001351465–1470. [DOI] [PubMed] [Google Scholar]
- 82.Venkataraman V, Wheless J W. Safety of rapid intravenous infusion of valproate loading doses in epilepsy patients. Epilepsy Res 199935147–153. [DOI] [PubMed] [Google Scholar]
- 83.Towne A R, Garnett L K, Waterhouse E J.et al The use of topiramate in refractory status epilepticus. Neurology 200360332–334. [DOI] [PubMed] [Google Scholar]
- 84.Bensalem M K, Fakhoury T A. Topiramate and status epilepticus: report of three cases. Epilepsy Behav 20034757–760. [DOI] [PubMed] [Google Scholar]
- 85.Rossetti A O, Bromfield E B. Levetiracetam in the treatment of status epilepticus in adults: a study of 13 episodes. Eur Neurol 20055434–38. [DOI] [PubMed] [Google Scholar]
- 86.DeGiorgio C M, Tomiyasu U, Gott P S.et al Hippocampal pyramidal cell loss in human status epilepticus. Epilepsia 19923323–27. [DOI] [PubMed] [Google Scholar]
- 87.Howard R S, Radcliffe J, Hirsch N P. General medical care on the neuromedical intensive care unit. J Neurol Neurosurg Psychiatry 20037410–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DeLorenzo R J, Towne A R, Pellock J M.et al Status epilepticus in children, adults and the elderly. Epilepsia 199233515–525. [DOI] [PubMed] [Google Scholar]
- 89.Towne A, Pellock J, Ko D.et al Determinants of mortality in status epilepticus. Epilepsia 19943527–34. [DOI] [PubMed] [Google Scholar]
- 90.Scholtes F B, Renier W O, Meinardi H. Generalised convulsive status epilepticus: causes, therapy and outcome in 346 patients. Epilepsia 1994351104–1112. [DOI] [PubMed] [Google Scholar]
- 91.Cascino G D, Hesdorffer D, Logroscino G.et al Morbidity of nonfebrile status epilepticus in Rochester, Minnesota, 1965–1984. Epilepsia 199839829–834. [DOI] [PubMed] [Google Scholar]