Abstract
This article summarises the medical problems of travel to altitudes above 3000 m. These are caused by chronic hypoxia. Acute mountain sickness (AMS), a self limiting common illness is almost part of normal acclimatisation—a transient condition lasting for several days. However, in <2% of people staying above 4000 m, serious illnesses related to hypoxia develop – high altitude pulmonary oedema and cerebral oedema. These are potentially fatal but can be largely avoided by gradual ascent. Short vacations, pressure from travel companies and peer groups often encourage ascent to 4000 m more rapidly than is prudent. Sensible guidelines for ascent are outlined, clinical features, management and treatment of these conditions.
Acute mountain sickness (AMS) consists of headache in an unacclimatised person at >2500 m with anorexia, vomiting, insomnia, dizziness and fatigue.1 AMS is common and usually self‐limiting, but of note for two good reasons. Firstly, it is of major public health importance in the travel industry, in high‐altitude warfare, trekking, climbing and skiing. Secondly, in <2% of AMS cases, and rarely at <4000 m, the serious sequelae of pulmonary oedema and cerebral oedema ensue. These can be fatal. In addition, the study of mountaineers in a chronic hypobaric hypoxic environment has given valuable insights into the mechanisms and effects of lack of oxygen at sea level.
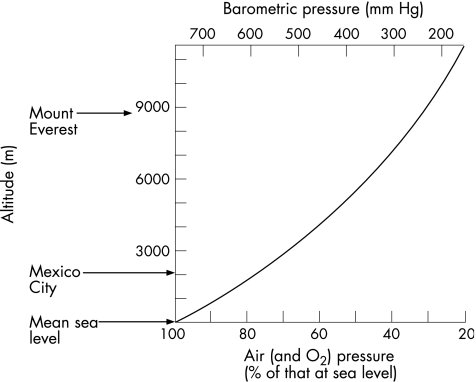
Figure 1 shows the relative lack of oxygen in the atmosphere with increasing altitude.
Figure 1 Altitude, barometric pressure, air pressure and oxygen tension.
Percentages on the horizontal axis are numerically quite close to arterial partial pressure of oxygen (pO2; in mm Hg)—a quick aide memoire. For example, on a 6000 m peak (many holiday Himalayan summits) there is 50% of sea level O2, and arterial pO2 is about 50 mm Hg. On the upper slopes of Everest (8848 m), an acclimatised man without an oxygen system is close to the physiological limits of survival.
This review outlines the clinical issues of hypoxia, reviews briefly the pathophysiology and suggests practical treatments.
Acute hypoxia, chronic hypoxia and AMS
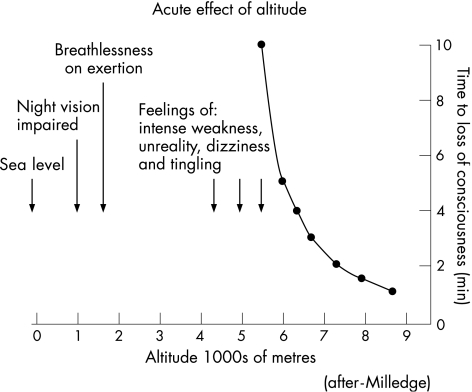
Alighting from an aircraft, road vehicle or ski lift at >3500 m causes immediate transient light‐headedness from acute hypoxia. Colour vision begins to fail. Abrupt exposure to much higher altitudes (fig 2) leads to loss of consciousness over several minutes—a problem for unwary balloonists, pilots of gliders, light aircraft and hang‐gliders, and commercial air passengers and crew when pressurisation fails.
Figure 2 Acute effects of hypoxia.
AMS is a more gradual process due to chronic hypobaric hypoxia. AMS consists of malaise and headache developing gradually over some 6–24 h. It is rare at <2500 m but nearly invariable within some hours of arrival at 4000 m—when symptoms are often very disabling. The pattern of headache is at first a tension‐like band and then often becomes generalised. AMS sometimes begins with a classical migraine. As the headache worsens, it becomes intense on moving, especially when lying down, and violent on vomiting, with relief after vomiting.
With rest at 3500 m, AMS usually improves spontaneously over several days. On recovery, although peak exercise remains limited by hypoxia, one can expect to feel fit and healthy at higher altitudes—until deterioration sets in at around 7000 m. Permanent sojourn for man (for more than several months) is impossible much above 6000 m because of chronic hypoxia. This is so for most mammals.
AMS prevention and treatment
Suggested public health guidelines2 for AMS prevention are simple, but they often conflict with the wishes of both travellers and the travel industry—a familiar issue. If travelling from sea level, move slowly, carry little and gain height slowly above 2500 m. Attempt to increase the height at which one sleeps by no more than 300–500 m each night. This allows at least 3 days before sleeping at 4000 m. Adding extra days is helpful. Once acclimatised to 4000 m, it is reasonable to continue at the same rate to 5000 m (another 2 days). Thereafter, individuals vary greatly. Few people are comfortable going to 6000 m within 10 days from sea level, but it is possible.
Almost everyone save seasoned mountaineers harbours the erroneous conviction that physical fitness must prevent AMS. The reality is that the idle, portly and sedate walker tends to have less AMS than the lean athlete with a heavy load. A recent anecdote (de Chazal, personal communication, 2005) from a correspondent is so illustrative that it is included here:
Here is my impression of altitude sickness when I climbed Kilimanjaro (5895 metres) in 2004. There were 22 people (all healthy, all British) in two groups ‐ four days up: two days down. Quite a rush.
All the men and most of the women had AMS symptoms.
Men generally had more severe problems than women.
The younger men were least likely to reach the top.
All the younger women had AMS symptoms.
The middle aged women (late 40s/early 50s) fared best of all with few or no symptoms.
The group dynamics were fascinating—and the reasons why some reached the top but others did not.
One group was fairly staid, mainly couples or relatives (e.g. my 22 year old daughter and myself), mostly in their 30s, 40s and 50s, including the two oldest (59 and 64). All but two (the oldest) reached the top.
The second group were all singles, mostly friends from a running club—all marathon runners, and young—in their 20s and 30s. They had lots of fun in the early days, singing and playing games whilst walking, and generally larking about. Not a single one reached the top.
Incidentally, my daughter and I both took Diamox. She was quite unwell with AMS. I was fine. Neither of us had headaches. We drank gallons but our water froze on summit day ...! But we both survived and reached the top.
The Lake Louise scoring system1 is used extensively as a research tool, and has some value for measuring severity.
Simple measures for treating AMS are rest, paracetamol for headaches and anti‐emetics. Sleeping propped up, if feasible, helps the headache. Acetazolamide, the carbonic anhydrase inhibitor used in glaucoma and idiopathic intracranial hypertension, is widely used in prevention. Several meta‐analyses (and numerous grants for scientific trips to high mountains) have shown its value in AMS prevention.3 Altitude sickness is listed grudgingly (unlicensed indication) in the British National Formulary as a reason for the use of a prophylactic drug widely prescribed and known universally as Diamox.
However, authorities do vary in their attitudes to recommending acetazolamide for prevention of AMS. The drug can have unpleasant unwanted effects. Tingling fingers, feet and lips, and a feeling akin to a hangover are common problems. Occasionally, rashes and blood dyscrasias occur. Stevens–Johnson syndrome, renal failure and toxic epidermal necrolysis are recorded. My own preference (and that of most people who go on expeditions frequently) is to avoid prophylactic drugs.
Rapid ascent altitude—for example, flying to 4000 m—is, however, a very reasonable indication for prophylaxis. I usually suggest a sea level treatment trial some weeks before departure—acetazolamide 125 mg tablets (half a tablet) twice daily for several days. If the traveller decides thereafter to use acetazolamide prophylactically, the usual recommended schedule is 125 mg twice daily for, say, 3 days before reaching 3500 m.
Table 1 lists other published (and accepted) suggestions. Of note is the protocol allegedly enforced by the Indian Army for physically fit jawans (infantry) on their way to the interminable high‐altitude campaign against Pakistan: 3 weeks absolute rest on arrival at 3000 m.
Table 1 Prevention of acute mountain sickness.
| Drug/treatment | Dose | Duration | Comment |
|---|---|---|---|
| Acetazolamide | 125 mg twice daily | 5 days before 3500 m | Parasthaesiae frequent, serious problems rare. Try at sea level |
| Dexamethasone | 2 mg three times daily | 3 days before 3500 m | Heartburn especially common at altitude |
| Ginkgo biloba4 | 120 mg twice daily | 5 days before 3500 m | Preparations vary, little compelling evidence |
| Bed rest at 3000 m | 3 weeks | Said to be used by the Indian Army, and very effective |
Case report (my own symptoms and my wife's)
AMS can wreck a holiday. Lhasa airport is at 3600 m. Whenever I fly there I feel listless for several days. I try to stay three nights in Lhasa before leaving by road for the highlands of 4000–5000 m. Once at 5000 m I am tired, have a slight headache and dislike walking for more than 1 h. I would not consider climbing a 5800–6000 m peak (often no more than a walk on the Tibetan plateau) for at least 10 days. I have tried taking Diamox. I dislike the tingling and it makes me feel odd.
My wife has been to Lhasa once. After 12 h she felt dreadful, with severe headaches and nausea. She rarely left our hotel room for 5 days. She was indignant that her medical adviser (husband) had not suggested Diamox, and opted anyway for all future holidays at sea level.
Dexamethasone seems overzealous for prophylaxis, but it does help and is sometimes used; 2 mg dexamathasone three times a day in oral doses leaves plenty in reserve for possible cerebral oedema.
Treatment of severe AMS is discussed with pulmonary and cerebral oedema.
High‐altitude pulmonary and cerebral oedema
In stark contrast to AMS—an unpleasant but transient problem—oedema of the lungs and brain at altitude is serious and potentially fatal.
Until 1960, severe breathlessness and even respiratory deaths at high altitude were mentioned from time to time, but the distinct syndrome of hypoxia‐related, pulmonary oedema remained unrecognised. A seminal article5 by the well‐known American climber Dr Charles Houston described sudden pulmonary oedema in a skier in Aspen, Colorado, breaking the rule that a single case report is of little value. Pulmonary oedema usually follows AMS. Breathlessness develops when at rest (everyone puffs more than usual on exercise at altitude), a dry cough crackles in the chest, and a dusky blue tinge develops around the face and lips. Later, but less commonly, copious frothy sputum develops. By this point the patient is already gravely ill.
Case report 1
A 30‐year‐old man struggled on foot to the Everest base camp at 5200 m, anxious to keep up with a climbing team. He had had AMS at >4000 m and had never been well on the 2‐week approach march through the foothills. At 5200 m he became breathless at rest over a couple of days. It was obvious that he had to pause to catch his breath when he spoke or ate. He was blue and unduly breathless, with a few basal crackles. He was persuaded to descend and made a complete recovery without drugs when he reached 4000 m in a matter of hours.
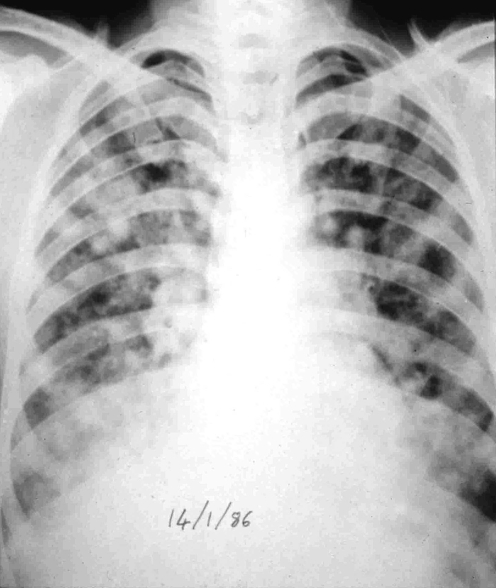
Investigation of these cases shows fluffy shadowing on chest x ray (figure 1), often in the upper zone and more on the right side than on the left, a normal heart size, absence of raised jugular venous pressure and dependent oedema, but high pulmonary artery and pulmonary wedge pressure.
Figure 1 Chest x ray: high‐altitude pulmonary oedema (Charles Clarke Collection).
A cerebral form of high‐altitude illness6 was described in 1975 by Charles Houston, along with the British doctor John Dickinson, working in Kathmandu. Now known as high‐altitude cerebral oedema, the problem seems at first to be severe, persistent AMS. Unsteadiness and change in behaviour develop. Headache persists and can be extreme, and postural— patients prefer to sleep propped up.
Disorganised heel–toe walking, severe limb ataxia and papilloedema develop. Various false‐localising signs (eg, double vision from sixth nerve palsy) may occur. Coma follows, by which time death is likely.
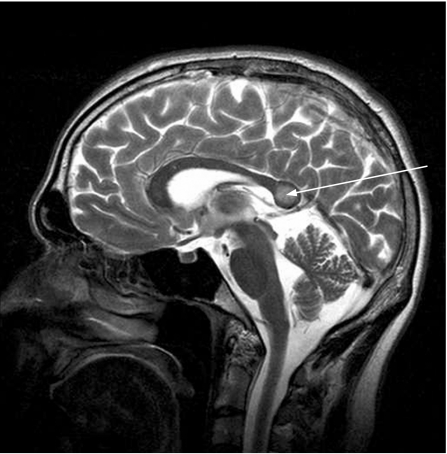
Magnetic resonance imaging has shown white matter abnormalities, especially in the splenium of the corpus callosum (figure 2).
Figure 2 Oedema of the splenium of the corpus callosum (T2W MRI; courtesy of Dr Sui Wong, 2004).
Case report 2
A journalist and a doctor (myself) had travelled by jeep across the Tibetan plateau to the Cho Oyu base camp at 5000 m. We had come from 1500 m over 3 days and had had moderate AMS—mainly lassitude. After a night at 5000 m we both became distinctly unwell, with increasing headache, excruciating on lying down, and slightly unsteady while walking. The journalist had no papilloedema, but he could not stand heel to toe. A single oral dose of 8 mg dexamethasone cured the headache and restored walking to normal. I was distinctly unsteady myself. The next day we had both recovered and were able to walk up over a 5400 m pass as planned, but in the secure knowledge that we could drop rapidly to <4000 m the other side. We had both had mild cerebral oedema and had taken a distinct risk going higher.
Case report 3
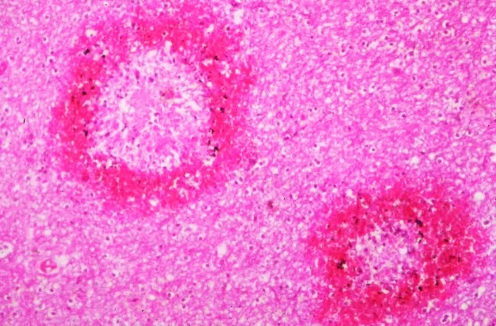
A male trekker to the base camp of Everest flew in to 2750 m and walked up >4000 m, disregarding AMS symptoms. Over 3 days at 4270 m he became unsteady, developed a severe headache and became drowsy. He was airlifted back to Kathmandu in a coma, but was dead on arrival at hospital. At autopsy, the brain was swollen and haemorrhagic (figures 3 and 4). A prominent infarct in the brain stem was the probable cause of death.
Figure 3 Brainstem infarction (courtesy of the late Professor Donald Heath).
Figure 4 Ring haemorrhages of the brain in high‐altitude cerebral oedema (courtesy of the late Professor Donald Heath).
Extreme altitude oedemas of sudden onset
Our early knowledge of high‐altitude oedemas came largely from patients who seemed not to acclimatise—those who failed to heed advice about gradual ascent, those who disregarded AMS symptoms, whose AMS seemed simply not to resolve, or who were, quite simply, unlucky. However, it is now quite clear that sudden, devastating altitude‐related oedemas also occur unpredictably at extreme altitude, usually at >7000 m in climbers who have been well acclimatised. These dramatic cases, especially on peaks that excite the media, often receive intense publicity.
Case report 1
A highly experienced well‐acclimatised mountaineer was attempting to climb Kangchenjunga (8598 m) with his wife in winter. Above 7000 m, he became breathless, coughed bloody sputum and became gravely ill. He soon developed features entirely suggestive of pulmonary and cerebral oedema. Descent was attempted. He became weaker and died high on the mountain within 24 h of the onset.
Case report 2
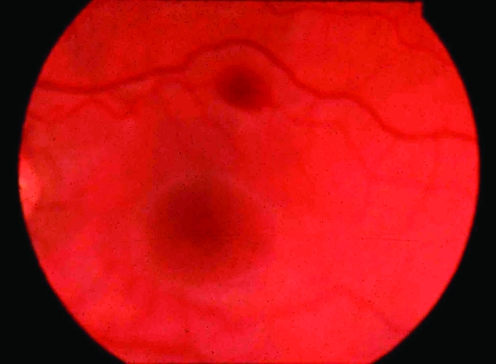
A Sherpa had been carrying loads daily for a fortnight on the southwest face of Everest in 1975. He suddenly became ataxic with a severe headache at 7600 m. He was unable to stand and was lowered down the face. He had severe papilloedema (figure 5). He recovered over 24 h with descent and dexamethasone.
Figure 5 Swollen optic disc, retinal oedema and venous tortuosity (Charles Clarke, 1975).
Case report 3
A well‐acclimatised man on an Everest expedition in 1982 suddenly developed a left hemiparesis while leading on a steep snow pitch at 7200 m. He had severe papilloedema but no headache. He recovered within a week without steroids after descending with help to 5000 m.
Early history, mechanisms and pathophysiology
The headaches of AMS were recorded in Chinese literature over 2000 years ago. In the early 20th century, Thomas Ravenhill, a British doctor working in South America, recognised respiratory and neurological problems at altitude. The study of the mechanisms of AMS was first pursued intensively7 by Angelo Mosso (Professor of Physiology, Turin) in the later decades of the 19th century. For example, Mosso used an ingenious scalp manometer, resting on a gutta percha membrane over a previous traumatic skull defect. He also recorded pulmonary oedema at autopsy after the death of a young doctor high on Mont Blanc.
The precise reasons why one feels so ill with AMS remain unknown. Mechanisms8 are complex, multifactorial, and bedevilled by difficulty in distinguishing what is a normal, physiological response to hypoxia—the acclimatisation process—and what is pathological and so damaging to the brain and lungs.
Acclimatisation has been studied intensively in the field and in the controlled environment of the hypobaric chamber. The immediate response to hypoxia is hyperventilation (via the carotid bodies). Respiratory alkalosis follows, and changes in regional blood flow—for example, cerebral blood flow— with increases of some 30% during the first week of residence at 5000 m. Pulmonary hypertension develops, with arterial values nearing systemic levels. These may not in themselves be a cause of symptoms, but they are an indication of the magnitude of the changes taking place.
Three underlying elements in the production of symptoms are:
An underlying genetic susceptibility: Some people seem very prone to AMS and altitude oedemas. Tentative associations are variation in angiotensin‐converting enzyme genes and polymorphisms of pulmonary surfactant protein A.
Cellular responses to hypoxia: For example, mitochondrial acclimatisation, slowing of axonal transport.
Microvascular changes: Overperfusion of the brain and lungs.
One unifying hypothesis that is attractive is that hypoxia leads to overperfusion of microvascular beds, endothelial leakage and hence oedema. Precise mediators are likely to be activation of vascular endothelial growth factor by hypoxia‐inducible factor and possibly nitric oxide.
In the lungs the overperfusion is patchy. Exercise makes it worse, and so does respiratory infection. In the brain, headache follows stimulation of the trigeminovascular system—the cause of most headaches—and there is also overperfusion. The posterior arterial circulation is more susceptible than the anterior, possibly because its noradrenoceptor profiles differ. Brain oedema follows.
Management of pulmonary and cerebral oedema
To generalise, both pulmonary and cerebral oedema have a distinct tendency to steal up on the patient and the doctor (if present), whereas AMS is a source of misery and much complaint obvious to all. Management decisions about these potentially serious medical conditions are often taken in difficult settings. A climb or an adventurous journey is a task in progress. Furthermore, there may be external pressures from a fit, determined team, and the lure of a summit. The physical environment may be distinctly hostile—dangerous terrain or movement impossible at night. Cold, bad weather, one's own fatigue and mountaineering competence may all be or become factors to consider when handing the situation.
The axioms are easy:
Diagnose, or suspect high‐altitude oedemas
Avoid going higher
Avoid exercise
Descend as soon as feasible
Use drugs, oxygen and portable pressure chambers if available.
Diagnosis sounds simple enough when written down. A windy cold tent is noisy and cramped. Chest auscultation can be difficult and neurological examination impractical. It is often hard to separate pulmonary from cerebral oedema—patients with breathlessness and hypoxia may be confused and drowsy in any event. In practice, the distinction is of little importance because the principles of management are much the same.
I view with suspicion anyone with apparent AMS whose condition does not improve within several days, who becomes breathless at rest (watch them talking or eating), who is unsteady on his or her feet or whose behaviour changes towards the unreasonable.
Once diagnosed and separated from uncomplicated AMS, the situation is already serious and descent is the best treatment. Time and again, reports of deaths at altitude indicate that there has been a delay in reaching a lower altitude. Descent means more oxygen—and it is surprising how much difference a drop of several hundred metres makes. Portable hyperbaric chambers also achieve this, by providing a higher barometric pressure and thus mimicking a drop in altitude of hundreds (or more) of metres. Table 2 outlines the various treatments for severe forms of altitude illness.
Table 2 Treatments for severe forms of altitude illness.
| Manouevre/drug | Regimen | Comment |
|---|---|---|
| Descent | 250 m often helpful | Essential, if possible |
| Dexamethasone | 8 mg+4 mg every 6 h (iv, im, oral) | Often helpful in minutes, used primarily for brain oedema |
| Nifedipine | 10 mg + 20 mg sustained release every 12 h | Mainly for pulmonary oedema |
| Oxygen | 2–4 l/min by mask or cannula | Always helpful, calming |
| Portable chamber (figure 6) | 2–4 psi for 2–3 h | Never truly portable/claustrophobia |
im, intramuscular; iv, intravenous.
Figure 6 Portable hyperbaric chamber in use at 4000 m in Tibet (Charles Clarke, 1997).
Other issues at high altitude
Retinal haemorrhages
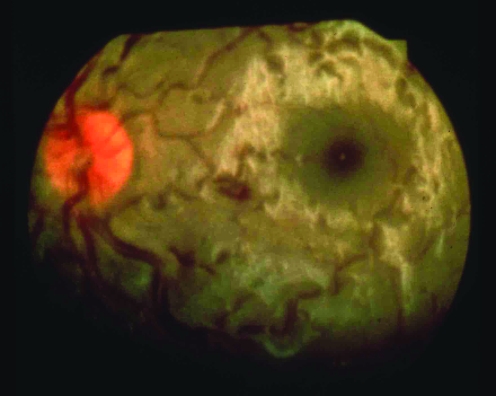
Symptomless retinal haemorrhages9 are common at >5000 m (figure 7). They rarely cause visual loss and resolve spontaneously.
Figure 7 Symptomless subhyaloid retinal haemorrhage at 5500 m (Charles Clarke, 1975).
Cerebral infarction
Stroke and transient ischaemic attack occur more commonly than one might expect at altitude; dehydration and hypoxia‐induced polycythaemia are presumably involved.
Seizures and “funny turns”
Seizures seem to be occasionally provoked by hypoxia, but there are cases where fits have occurred on several separate occasions at 4000 m. Fainting can occur with AMS, and migraine sometimes develops. Thromboembolic transient ischaemic attack‐like events have been recorded at altitude. With these various possibilities, it is hardly surprising that blackouts and funny turns are common on expeditions. I have diagnosed non‐epileptic episodes on more than one occasion.
The old and young
Age is no bar to going high, provided one is well. The age record for the summit of Everest (8848 m) is now >70 years. Children develop AMS as much as anyone, and are sometimes pressured into going high by ambitious parents.10
Deterioration
Above 7000 m, hypoxia takes a steady toll. Exhaustion, lack of appetite, vagueness and lackadaisical behaviour are common. This becomes evident over a few days at 7600 m. Stays longer than several days at >7000 m should be avoided.
High‐altitude native populations and chronic mountain sickness
Residents of the valleys of Nepal and the Tibetan plateau do not experience AMS at their usual altitudes—they are acclimatised. However, as they go higher they develop AMS like anyone else. There is a tendency to believe that Sherpas and highlanders are more resilient than visitors. The modern reality of close proximity to one's workmates—for example, sharing a tent—suggests that highlanders have much the same symptoms as lowlanders. Symptoms in any way suggestive of serious forms of AMS should not be disregarded.
Chronic mountain sickness11 refers to the polycythaemia, lethargy and headaches common in South American populations, and are now reported from Asia.
Further reading
Ward MP, Milledge JS, West JB. High altitude medicine and physiology. 2nd edn. London: Chapman & Hall, 1995.
Clarke C. High altitude medicine. Travel Med Infect Dis 2005;3:189–97.
Hackett PH, Roach CR. High‐altitude illnesses. N Engl J Med 2001;345:107–14.
Schoene RB. Unraveling the mechanism of high altitude pulmonary edema. High Altitude Med Biol 2004;5:125–35.
Existing medical conditions and return to altitude after severe altitude sickness
Most general medical conditions are well tolerated at altitude.
Asthma, when there is an evident allergic basis, tends to improve at >3000 m.
Blood pressure tends to fall. Controlled hypertension is rarely a cause of problems.
Control of diabetes or epilepsy rarely becomes an issue.
After undergoing successful bypass surgery, people, including those with mild angina, have climbed to high altitudes. Obviously cardiac failure and substantial chronic respiratory impairment are contraindications.
Many climbers seem undeterred by an episode of severe AMS, or even altitude oedema, despite frequently having been warned by the medical profession never to venture high again. One episode of altitude oedema is a signal for caution in the future, but not a definite reason for never setting foot on a high mountain again; many successful high‐altitude climbers have had one or more episodes of serious altitude‐related illness. Recurrent altitude oedema is a relative rarity, and usually poses a risk (for a mountaineer) lower than the substantial dangers of high‐altitude terrain. For the holiday maker who has experienced a similar event, the notion that a serious environmental illness might recur is sufficient to deter all but the keenest traveller from venturing high again.
Abbreviations
AMS - acute mountain sickness
AMS - acute mountain sickness
Self assessment Questions
1 What is a reasonable rate of ascent from sea level to 5000 m, assuming one has to fly in to 3600 m?
2 Discuss the use of acetazolamide in prevention of AMS.
3 What are the severe forms of altitude sickness?
4 Are retinal haemorrhages at altitude suggestive of severe altitude sickness?
5 Can people with epilepsy, high blood pressure or diabetes mellitus go above 5000 m?
Answers
1 300–500 m height gain per day.
2 Acetazolamide is used widely in prevention of AMS. A dose of 125 mg twice daily for 3 days before reaching 3500 m. Side effects are common.
3 High‐altitude pulmonary and cerebral oedema.
4 No, they are common during acclimatisation and do not herald brain oedema.
5 Yes, provided control is reasonable.
Footnotes
Competing interests: None declared.
References
- 1.Roach R C, Bärtsch P, Oelz O.et al The Lake Louise AMS scoring system. In: Sutton JR, Houston CS, Coates G, eds. Hypoxia & molecular medicine. Burlington, Vermontt: Charles S Houston, 1993272–274.
- 2.British Mountaineering Council Information Service Information Sheets www.thebmc.co.uk (accessed 14 Sep 2006)
- 3.Ried L D, Carter K A, Ellsworth A. Acetazolamide or dexamethasone for prevention of acute mountain sickness: a meta‐analysis. J Wilderness Med 1994534–48. [Google Scholar]
- 4.Gertsch J H, Basnyat B, Johnson E W.et al Randomised, double blind, placebo controlled comparison of ginkgo biloba and acetazolamide for prevention of acute mountain sickness among Himalayan trekkers: the prevention of high altitude illness trial (PHAIT). BMJ 2004328797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houston C S. High altitude pulmonary oedema. N Engl J Med 1960293478–480. [DOI] [PubMed] [Google Scholar]
- 6.Houston C S, Dickinson J. Cerebral form of high altitude illness. Lancet 19752758–761. [DOI] [PubMed] [Google Scholar]
- 7.Mosso A.Life of man in the high Alps. London: Fisher Unwin, 1898
- 8.Schoene R B. Unraveling the mechanism of high altitude pulmonary edema. High Altitude Med Biol 20045125–135. [DOI] [PubMed] [Google Scholar]
- 9.Clarke C, Duff J. Mountain sickness, retinal haemorrhages and acclimatisation on Everest in 1975. BMJ 19762495–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollard A J.et al Children at altitude: an international consensus statement. High Altitude Med Biol 20012389–403. [DOI] [PubMed] [Google Scholar]
- 11.Ge R ‐ L, Helun G. Current concept of chronic mountain sickness: pulmonary hypertension–related high‐altitude heart disease. Wilderness Environ Med 200112190–194. [DOI] [PubMed] [Google Scholar]