Abstract
The case of a 39‐year‐old woman who was referred for weight gain and amenorrhoea is reported. Laboratory evaluation showed high levels of thyroid‐stimulating hormone (TSH). The patient was started on increasing doses of levothyroxine for subclinical hypothyroidism. TSH remained persistently raised and the patient became thyrotoxic. Evaluation at another laboratory showed normal levels of TSH, raising the possibility of interfering substances. TSH levels were normalised with the addition of mouse serum to the patient's sample, confirming the presence of human anti‐mouse antibodies as the interfering substance in the TSH assay.
Subclinical hypothyroidism refers to mildly increased serum thyroid‐stimulating hormone levels in the presence of normal free thyroxine (T4) and triiodothyronine (T3).1 In the US National Health and Examination Survey,2 4.3% of 16 533 people had subclinical hypothyroidism. Progression to overt hypothyroidism is reported to vary from 3% to 20%, the risks being greater in those patients with goitre or thyroid antibodies.3 Although subclinical hypothyroidism is often asymptomatic, potential risks include progression to overt hypothyroidism, cardiovascular effects, hyperlipidaemia and neuropsychiatric effects. Treatment of subclinical hypothyroidism remains controversial. It is suggested that treatment of subclinical hypothyroidism will reduce cardiovascular risk factors, improve lipid profile and minimise neurobehavioural abnormalities.4 It is recommended that patients with TSH >10 or TSH level between 5 and 10 in conjunction with goitre or positive anti‐thyroid peroxidase should be treated.1
We present a patient treated for hypothyroidism, it was later found that human anti‐mouse monoclonal antibody (HAMA) had interfered with the TSH assay.
A 39‐year‐old Hispanic woman was referred to the Division of Endocrinology, Metropolitan Hospital Center, New York, for evaluation of weight gain, increased appetite and amenorrhoea for 5 months. She denied any blurring of vision, headache, hoarseness of voice or intolerance to cold. Medical history showed hypertension, depression and schizophrenia treated for several years, and excision of an ovarian cyst. Her drugs included fosinopril, imipramine, olanzapine, haloperidol, benzatropine, fluphenazine, paroxetine and hydroxyzine. Family history was notable for breast cancer in her mother. Examination was unremarkable except for a weight of 196 pounds (89 kg). Initial laboratory evaluation showed a TSH concentration of 13.86 (range 0.35–5.50) mU/l, a total T4 concentration of 8.4 (range 3–13) μg/dl and a T3 concentration of 1.03 (range 0.6–1.18) ng/ml. Anti‐microsomal antibody titre was normal (<2 U/ml). On the basis of these results, a diagnosis of subclinical hypothyroidism was made and the patient was started on levothyroxine. She was followed up every 4–6 weeks and the thyroid function was monitored. During this period, she was given increasing doses of levothyroxine without adequate suppression of TSH. Prolactin level was raised at 135 ng/ml (range 2.8–23). Subsequently, the patient started complaining of palpitations, anxiety and tremors. Thyroid function tests showed a TSH concentration of 11.7 mU/l, with a total T4 concentration of 11.2 μg/dl and free T4 concentration of 1.72 (range 0.8–1.5) ng/dl. The levothyroxine dose at that time was 0.1 mg/day. In view of these findings, levothyroxine was slowly tapered and completely discontinued. Propranolol was temporarily prescribed for her symptoms.
The TSH concentration remained raised at 9.35 mU/ml with normal levels of free T4 and total T4. Computed tomography of the head showed a hypodense mass in the pituitary region, with erosion of the right lateral aspect of the sella. However, a magnetic resonance image of the head was normal. Thyroid scan showed normal uptake. As part of the re‐evaluation, the patient's blood sample was sent to another laboratory. Surprisingly, the TSH concentration was found to be 0.45 mU/ml, which was within the normal range. This laboratory used a different TSH assay. These findings raised the possibility of interfering substances.
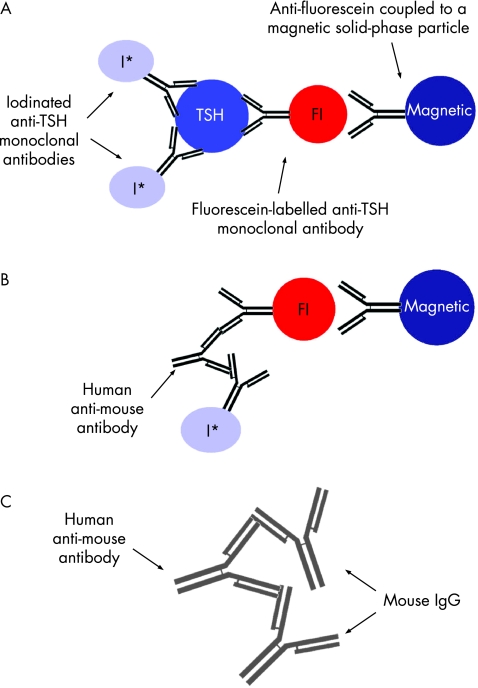
The patient's TSH levels had been initially measured by an automated chemiluminescence system. This assay is a two‐site sandwich immunoassay using direct chemiluminescence technology, which uses constant amounts of two antibodies (fig 1). The first antibody is a monoclonal mouse anti‐TSH antibody labelled with acridinium ester. The second antibody is a polyclonal sheep anti‐TSH antibody, which is covalently coupled to paramagnetic particles. A direct relationship exists between the amount of TSH present in the patient's sample and the amount of relative chemiluminescence units detected by the system. With this technique, the patient's TSH level was found to be raised on many occasions. We then tested for the presence of HAMA by treating the patient's serum with normal mouse IgG in increasing concentrations. The TSH concentration was normalised to 1.14 mU/l, with a concentration of mouse IgG at 2 μg/ml, confirming the presence of human anti‐mouse antibodies (table 1).
Figure 1 Automated chemiluminescence system using a two‐site sandwich immunoassay. (A) Normal assay showing radiolabelled sandwiches containing thyroid‐stimulating hormone (TSH), which sediment in a magnetic field. (B) Assay showing interference of human anti‐mouse antibodies by creating a radiolabelled sandwich. (C) Prevention of interference: adding immunoglobulin (Ig) G forms unlabelled sandwiches, which are not detected by the assay. Fl, fluorescein‐labelled anti‐TSH sheep monoclonal antibodies. I*, I‐125‐labelled anti‐TSH mouse monoclonal antibodies. Some clipart used with permission from Madlantern Arts.
Table 1 Interference study showing normalisation of thyroid stimulating hormone after addition of mouse immunoglobulin G.
| TSH test number | Dose of mouse IgG (μg/ml) | TSH concentration (0.35–5.5 mU/ml) |
|---|---|---|
| 1 | 0.000334* | 19.1 |
| 2 | 1 | 17.4 |
| 3 | 2 | 1.40 |
| 4 | 4 | 1.14 |
IgG, immunoglobulin G; TSH, thyroid stimulating hormone.
Discussion
Heterophile antibodies that interfere with the TSH assay have been described in the past. Hedenborg et al5 first reported falsely raised serum TSH levels due to the presence of IgG directed against rabbit immunoglobulin. Subsequently, Brennan et al6 reported falsely raised TSH levels secondary to endogenous anti‐mouse immunoglobulin. Since the report by Brennan et al, there have been occasional reports of HAMA interfering with TSH assays.7 HAMA, which is the most commonly encountered heterophile antibody, may be present in the serum of up to 10% of patients.8 The incidence increases in people who have received or have been treated with radiolabelled mouse monoclonal antibodies. It is now believed, on circumstantial evidence, that these heterophile antibodies are natural antibodies in normal people, although they could also represent autoantibodies.9
We could attempt to block or reduce the effect of HAMA interference by preincubating the patient's serum sample for 1 h at room temperature with increasing amounts of non‐immune mouse serum, between 10 and 100 ml/l (μl/ml). After this absorption procedure, the assay is carried out, taking into account the dilution factor used. Other approaches include the use of commercially available HAMA‐blocking reagents to counteract heterophile antibody interferences in the clinical laboratory, as well as heterophile‐blocking reagents, heterophilic blocking tubes, and non‐specific antibody‐blocking tubes (Scantibodies Laboratory Santee, California USA). In addition, some commercial kits detect HAMA‐positive patient samples (HAMA‐ELISA medac, from MEDAC; ImmuSTRIP, from Immunomedics; ETI‐HAMAK immunoenzymometric assay, from Sorin Biomedica; and IDeaL HAMA ELISA, from ALPCO).7
In our patient, TSH levels were measured by an automated chemiluminescence system. Interference of the assay due to the presence of HAMA gave a falsely raised TSH level and led to the patient being started on levothyroxine. Later, the TSH level was found to be normal at a different laboratory, raising the possibility of circulating heterophile antibodies. This was confirmed by the TSH value being normalised after the addition of mouse immunoglobulin to the serum to neutralise the circulating heterophile antibodies.
Both clinicians and laboratory staff should be aware of this type of interference in routine immunoassays, as monoclonal antibody assays are being increasingly used to guide treatment of various conditions.
Learning points
human anti‐mouse antibody (HAMA) is not uncommon and may be found in up to 10% of patients. These may be naturally occurring in many patients and the incidence tends to increase with exposure to monoclonal antibodies.
Abnormal increase in thyroid‐stimulating hormone (TSH) levels may be seen secondary to HAMA, depending on the type of assay used to detect TSH.
Heterophilic antibody interference should be considered if there is any discrepancy between clinical presentation and laboratory values to prevent extensive investigation.
Both clinicians and laboratory staff should be aware of this type of interference in routine immunoassays, as monoclonal antibody assays are being increasingly used to guide treatment of various conditions.
Abbreviations
TSH - thyroid‐stimulating hormone
IgG - immuno‐globulin G
HAMA - human anti‐mouse antibody
Footnotes
Competing interests: None declared.
References
- 1.Baskin H J, Cobin R H, Duick D S.et al AACE thyroid guidelines. Endocr Pract 20028457–469. [PubMed] [Google Scholar]
- 2.Hollowell J G, Staehling N W, Flanders W D.et al Serum TSH, T4 and thyroid antibodies in the United States population(1988–1994): National Health and Examination Survey (NHANES 3). J Clin Endocrinol Metab 200287489–499. [DOI] [PubMed] [Google Scholar]
- 3.Cooper D S. Clinical practice: subclinical hypothyroidism. N Engl J Med 2001345260–265. [DOI] [PubMed] [Google Scholar]
- 4.McDermott M T, Ridway E C. Subclinical hypothyroidism is mild thyroid failure and should be treated. J Clin Endocrinol Metab 2001864585–4590. [DOI] [PubMed] [Google Scholar]
- 5.Hedenborg G, Pettersson T, Carlstrom A. Heterophilic antibodies causing falsely raised thyroid stimulating hormone result. Lancet 19792755. [DOI] [PubMed] [Google Scholar]
- 6.Brennan M D, Klee G G, Preissner C M.et al Heterophilic serum antibodies: a cause for falsely elevated serum thyrotropin levels. Mayo Clin Proc 198762894–898. [DOI] [PubMed] [Google Scholar]
- 7.Despres N, Grant A M. Antobody interference in thyroid assays: a potential for clinical misinformation. Clinical Chemistry 199844440–454. [PubMed] [Google Scholar]
- 8.Klee G G. Human anti‐mouse antibodies. CAP Today, 1997 [DOI] [PubMed]
- 9.Levison S S, Miller J J. Towards a better understanding of heterophile (and the like) antibody interference with modern immunoassays: Clinica Chemica Acta20023251–15. [DOI] [PubMed] [Google Scholar]