Protein tyrosine kinases (PTKs) are well-established participants in signaling pathways that drive and maintain a host of normal and aberrant cellular phenotypes. The complexities associated with the regulation of PTK activation are most appropriately examined in the intracellular environment, conditions that allow PTK activity to be correlated with cellular behavior. Consequently, there has been intense interest in developing reporters that can fluorescently sense PTK activity in real time and under conditions that are consistent with living cells.[1,2] Recently, we described a strategy for the design of PTK reporters that relies upon the phosphorylation-induced disruption of noncovalent interactions between the kinase-targeted Tyr residue and a proximal pyrene moiety.[3] However, the latter, like all other PTK sensors described to date, is immediately susceptible to phosphorylation. The inability to control sensing activity precludes an assessment of intracellular kinase action as a function of the cell cycle in particular, and environmentally-stimulated behaviors in general. We report the first example of a light-regulated protein tyrosine kinase sensor.
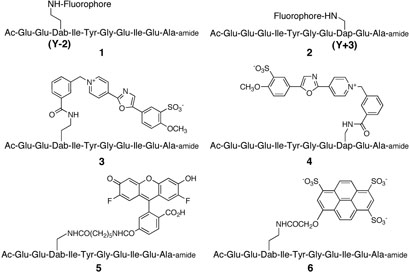
Short wavelength fluorophores, such as pyrene, are inappropriate for cell-based studies since cellular autofluorescence results in background interference. With this limitation in mind, we constructed a small peptide library containing several fluorophores positioned on the (L)-2,4-diaminobutanoic acid 1 (Dab) and the (L)-2,3-diaminopropionic acid 2 (Dap) at the Y−2 position and the Y+3 positions, respectively, of a known Src kinase substrate.[3] Oregon green,[4] cascade blue,[6] and cascade yellow[6] are relatively bright fluorophores with high quantum yields, good resistance to photobleaching and distinct spectral properties. The cascade yellow Y−2 derivative 3 exhibits a 2.7-fold enhancement in fluorescence intensity upon phosphorylation. By contrast, the corresponding cascade yellow-substituted derivative at Y+3 (i.e. 4) fails to furnish a fluorescence response. In addition, Oregon green (5) and cascade blue (6) display 2-fold enhancements at Y−2 but more modest changes at Y+3.
NOESY experiments on 3 and its phosphorylated counterpart revealed that the Tyr residue in both peptides exhibits through-space interactions with the fluorophore (Supporting Information), with significantly stronger interactions in the nonphosphorylated species. Indeed, chemical shift data demonstrates that the free Tyr phenol in 3 is significantly more shielded relative to Tyr residues in general[7,8] and the phosphoTyr moiety in phospho-3 in particular. These results suggest that the ring current of the proximal fluorophore electronically shields the Tyr aromatic ring, an interaction disrupted by phosphorylation. Tyr is known to participate in stacking interactions with and quenching by other aromatic moieties.[9,10]
The nature of the fluorophore influences the efficiency of phosphorylation (Supporting Information). The negatively charged cascade blue derivative 6 displays a low Km value (3.0 ± 0.2 μM), which may be a good barometer of enzyme affinity since the Src kinase exhibits a proclivity for negatively charged substrates. By contrast, the partially positively charged cascade yellow derivative 3 exhibits a significantly higher Km value (300 ± 10 μM). The spectral characteristics of these fluorophores should prove useful for sampling the activity of more than one protein kinase or for use in conjunction with other probes. For example, 5 (λex = 495 nm; λem = 520 nm) and 6 (λex = 400 nm; λem = 422 nm) are excited by and emit at dramatically different wavelengths. Consequently, two PTKs that phosphorylate different peptide substrates could potentially be simultaneously monitored. By contrast, 3 (λex = 400 nm; λem = 550 nm) and 6 are excited at a single wavelength, but display different emission properties.
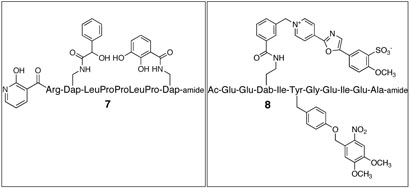
Src kinase activity is regulated via intramolecular interactions between the catalytic core and appended SH2 and SH3 domains.[11] Furthermore, ligands that target SH2 or SH3 domains are known to activate Src and related PTKs.[12] We recently described a series of moderate to high affinity SH3 ligands.[13] In the presence of SH3 ligand 7, Src kinase activity is enhanced 2.7-fold in terms of Vmax (4.8 ± 0.6 vs. 1.8 ± 0.1 μmol/min-mg) and nearly 4-fold in terms Vmax/Km.
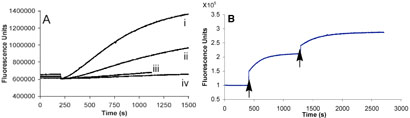
We examined the ability of 3 to fluorescently report Src kinase activity in cell lysates. Hek 293 cells were transiently transfected with a plasmid encoding wild type Src kinase. Cells were lysed, the lysate added to a buffer solution of 3, and ATP subsequently introduced to initiate the reaction. Kinase activity is visualized as a smooth enzyme-catalyzed progress curve [Figure 1A (ii)]. In addition, the SH3 domain ligand 7 accelerates the initial rate by 2.4-fold (i), which is consistent with the effect produced by 7 on pure enzyme. By contrast, lysates from non-transfected cells display negligible PTK activity (iv), with only minor activity in the presence of ligand 7 (iii). The latter suggests that endogenous PTK(s) capable of phosphorylating 3 are present, but their intracellular concentration is highly diluted upon addition of lysis buffer.
Figure 1.
A. (i) Reporter 3, SH3 domain ligand 7, and lysate from 293 cells overexpressing Src kinase, (ii) reporter 3 and lysate from 293 cells overexpressing Src kinase, (iii) reporter 3, SH3 domain ligand 7, and lysate from nontransfected 293 cells, (iv) reporter 3 and lysate from nontransfected 293 cells, B. Light-driven conversion of caged reporter 8 to active reporter 3. In the absence of photolysis (t = 0 − 400 sec), no detectable Src kinase activity is observed. Limited photolysis (arrow) at t = 400 sec (8 sec, Hg arc lamp) generates active sensor which produces a fluorescent response. Subsequent photolysis (arrow) unleashes an additional packet of active sensor.
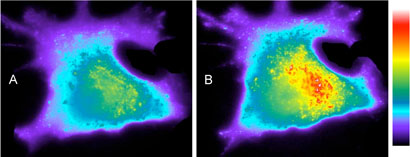
As noted above, a limitation associated with PTK reporters is their unregulated vulnerability to phosphorylation. PTKs may be constitutively active or may display periods of intermittent activity as a function of some cellular event. The ability to control when the reporter is functional provides a means to define an explicit start point and sample period. With this in mind, we prepared peptide 8, which contains a tyrosine residue that is covalently modified with a photolabile nitrobenzyl substituent. As expected, compound 8 fails to serve as a Src kinase substrate (Figure 1B). Upon photolysis, the nitrobenzyl moiety is lost and the liberated Tyr suffers phosphorylation, which elicits a time-dependent enhancement in fluorescent intensity. In addition to affording exquisite temporal control over sensing activity, limited intermittent photolysis allows the active reporter to be unleashed as a series of discrete packets over time (Figure 1B and Supporting Information). The latter permits multiple sampling experiments in an investigator-controlled chronological fashion. Finally, compound 8 was microinjected into A549 cells, a human lung carcinoma cell line expressing high levels of activated Src kinase. 15 Photolysis triggers a time-dependent increase in fluorescent intensity (Figure 2).
Figure 2.
Light-induced time-dependent change in fluorescence intensity in A549 cells microinjected with the caged tyrosine kinase sensor 8 (estimated final intracellular concentration of 2 – 20 μM). Following microinjection, cells were exposed to UV from a 100 W Hg-Arc lamp through the objective for 8 sec to activate the tyrosine kinase sensor. Immediately after UV treatment (A), images were collected at 1 min intervals (Supporting Information for movie). Final image B was obtained at 25 min following activation of the sensor. Fluorescence intensity measurements are relative to control cells containing the caged sensor.
In summary, the PTK reporters described in this study contain an array of bright, photochemically stable, fluorophores that display easily differentiated photophysical properties. The change in fluorescence intensity, upon phosphorylation, is consistent with the phosphate-driven disruption of stacking interactions between the fluorophore and the Tyr moiety. Finally, the corresponding caged derivative affords, for the first time, the opportunity to control PTK activity sensing by delivering the active reporter at single or multiple time points over the course of the experiment.
Supplementary Material
Chart 1.
General structures (1 – 2) and the cascade yellow (3 – 4), Oregon green (5), and cascade blue (6) derivatives of the PTK reporters.
Chart 2.
The SH3 domain ligand 7, which activates Src kinase activity, and the caged tyrosine kinase reporter 8.
ACKNOWLEDGMENT
Financial Support from NIH (CA79954) and Panomics, Inc. is gratefully acknowledged. We thank Michael Cammer for technical assistance with the microscopy experiments and image processing.
Footnotes
Supporting Information Available
Experimental details of peptide synthesis and characterization, and enzyme and cell-based assays.
REFERENCES
- 1.Chen CA, Yeh RH, Yan X, Lawrence DS. Biochim Biophys Acta. 2004;1697:39–51. doi: 10.1016/j.bbapap.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 2.Rothman DM, Shults MD, Imperiali B. Trends Cell Biol. 2005;15:502–510. doi: 10.1016/j.tcb.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Cahill SM, Blumenstein M, Lawrence DS. J Am Chem Soc. 2006;128:1808–1809. doi: 10.1021/ja0577692. [DOI] [PubMed] [Google Scholar]
- 4.Sun W-C, Gee KR, Klaubert DH, Haugland RP. J Org Chem. 1997;62:6469–6475. [Google Scholar]
- 5.Whitaker JE, Haugland RP, Moore PL, Hewitt PC, Reese M. Anal Biochem. 1991;198:119–130. doi: 10.1016/0003-2697(91)90515-u. [DOI] [PubMed] [Google Scholar]
- 6.Anderson MT, Baumgarth N, Haugland RP, Gerstein RM, Tjioe T, Herzenberg LA. Cytometry. 1998;33:435–444. doi: 10.1002/(sici)1097-0320(19981201)33:4<435::aid-cyto7>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 7.Bundi A, Wuthrich K. Biopolymers. 1979;18:285–298. [Google Scholar]
- 8.Bienkiewicz EA, Lumb KJ. J Biomol NMR. 1999;15:203–206. doi: 10.1023/a:1008375029746. [DOI] [PubMed] [Google Scholar]
- 9.Kraft BJ, Masuda S, Kikuchi J, Dragnea V, Tollin G, Zaleski JM, Bauer CE. Biochemistry. 2003;42:6726–6734. doi: 10.1021/bi030055o. [DOI] [PubMed] [Google Scholar]
- 10.Mayer R, Toulme F, Montenay-Garestier T, Helene C. J Biol Chem. 1979;254:75–82. [PubMed] [Google Scholar]
- 11.Boggon TJ, Eck MJ. Oncogene. 2004;23:7918–7927. doi: 10.1038/sj.onc.1208081. [DOI] [PubMed] [Google Scholar]
- 12.Moarefi I, LaFevre-Bernt M, Sicheri F, Huse M, Lee CH, Kuriyan J, Miller WT. Nature. 1997;385:650–653. doi: 10.1038/385650a0. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Lawrence DS. Chem Biol. 2005;12:905–912. doi: 10.1016/j.chembiol.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Veldhuyzen WF, Nguyen Q, McMaster G, Lawrence DS. J Am Chem Soc. 2003;125:13358–13359. doi: 10.1021/ja037801x. [DOI] [PubMed] [Google Scholar]
- 15.Zheng R, Yano S, Matsumori Y, Nakataki E, Muguruma H, Yoshizumi M, Sone S. Clin. Exp. Metastasis. 2005;22:195–204. doi: 10.1007/s10585-005-7768-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.