Abstract
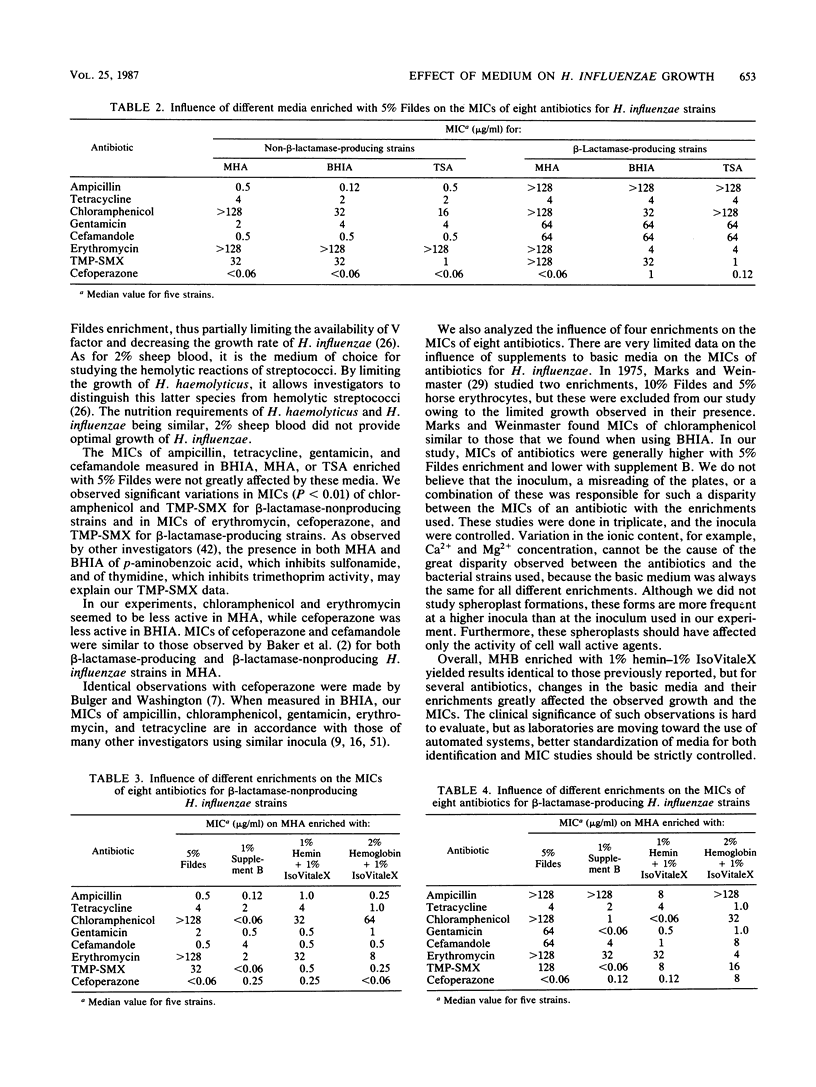
In the present study, five non-beta-lactamase- and five beta-lactamase-producing strains of Haemophilus influenzae were used to determine whether three different growth media, Mueller-Hinton broth and agar, brain heart infusion broth and agar, and tryptic soy broth and agar, and their added supplements (0.2% hemin-0.1% IsoVitaleX, 1% hemin-1% IsoVitaleX, 2% sheep blood, 10% Fildes enrichment, 5% Fildes enrichment, 1% supplement B, 5% horse erythrocytes, and 2% hemoglobin-1% IsoVitaleX) would influence the growth rate of this microorganism and the antibacterial activity of eight antibiotics, including ampicillin, tetracycline, chloramphenicol, gentamicin, cefamandole, erythromycin, trimethoprim-sulfamethoxazole (TMP-SMX), and cefoperazone. The growth curve studies were carried out with an initial inoculum of 10(4) bacteria per ml, and MICs were determined with an inoculum of 5 X 10(5) microorganisms. Mueller-Hinton broth, brain heart infusion broth, and tryptic soy broth enriched with 5% Fildes resulted in a maximal growth of more than 10(8) CFU/ml at 24 h. When 10% Fildes or 2% sheep blood was added as enrichment to Mueller-Hinton broth, a considerable reduction in the growth rate of H. influenzae strains resulted (P less than 0.01). Significant variations in MICs (P less than 0.01) were observed with chloramphenicol, TMP-SMX, erythromycin, and cefoperazone when brain heart infusion agar, Mueller-Hinton agar, or tryptic soy agar was used. Chloramphenicol, gentamicin, erythromycin, and TMP-SMX were all affected by the different enrichments added to Mueller-Hinton agar. MICs were in general higher with 5% Fildes enrichment and lower with 1% supplement B. Cefoperazone was the only drug which exhibited a lower MIC in 5% Fildes enrichment for ampicillin-resistant H. influenzae strains.
Full text
PDF

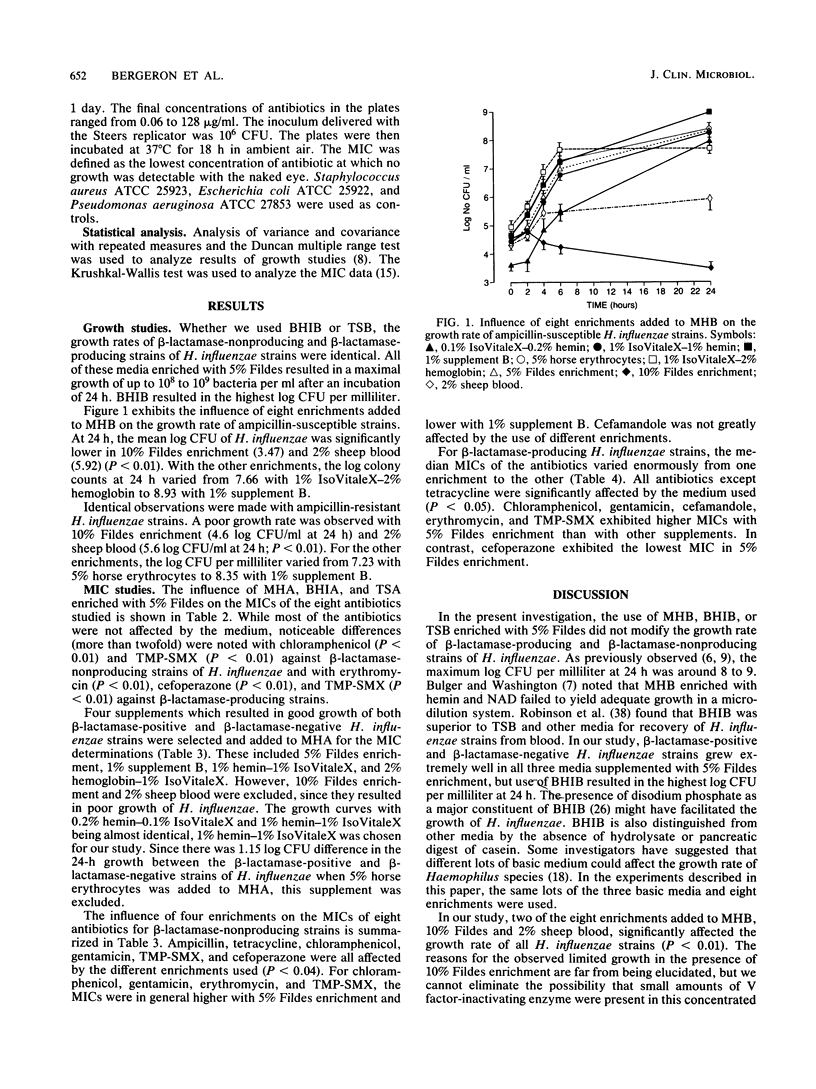


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azemun P., Stull T., Roberts M., Smith A. L. Rapid detection of chloramphenicol resistance in Haemophilus influenzae. Antimicrob Agents Chemother. 1981 Aug;20(2):168–170. doi: 10.1128/aac.20.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker C. N., Thornsberry C., Jones R. N. In vitro antimicrobial activity of cefoperazone, cefotaxime, moxalactam (LY127935), azlocillin, mezlocillin, and other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase-producing strains. Antimicrob Agents Chemother. 1980 Apr;17(4):757–761. doi: 10.1128/aac.17.4.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannatyne R. M., Cheung R. Susceptibility of Haemophilus influenzae type b to rifampin and sulfisoxazole. Antimicrob Agents Chemother. 1978 Jun;13(6):969–970. doi: 10.1128/aac.13.6.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkin R. M., Todd J. K., Roe M. H. Rapid ampicillin susceptibility testing for Haemophilus influenzae. Am J Clin Pathol. 1977 Jan;67(1):100–103. doi: 10.1093/ajcp/67.1.100. [DOI] [PubMed] [Google Scholar]
- Bergeron M. G., Claveau S., Simard P. Limited in vitro activity of cefamandole against 100 beta-lactamase- and non-beta-lactamase-producing Haemophilus influenzae strains: comparison of moxalactam, chloramphenicol, and ampicillin. Antimicrob Agents Chemother. 1981 Jan;19(1):101–105. doi: 10.1128/aac.19.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron M. G., Lavoie G. Y. Tolerance of Haemophilus influenzae to beta-lactam antibiotics. Antimicrob Agents Chemother. 1985 Aug;28(2):320–325. doi: 10.1128/aac.28.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bill N. J., Washington J. A., 2nd Comparison of in vitro activity of cephalexin, cephradine, and cefaclor. Antimicrob Agents Chemother. 1977 Mar;11(3):470–474. doi: 10.1128/aac.11.3.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulger R. R., Washington J. A., 2nd Effect of inoculum size and beta-lactamase production on in vitro activity of new cephalosporins against Haemophilus species. Antimicrob Agents Chemother. 1980 Mar;17(3):393–396. doi: 10.1128/aac.17.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabernat H. J., Delmas C., Bauriaud R., Lareng M. B. Activité bactéricide d'antibiotiques, isolés et en associations, sur des souches sensibles et résistantes de Haemophilus influenzae. Ann Microbiol (Paris) 1979 May-Jun;130 A(4):469–479. [PubMed] [Google Scholar]
- Eickhoff T. C., Ehret J. M., Baines R. D. Characterization of an ampicillin-resistant Haemophilus influenzae type B. Antimicrob Agents Chemother. 1976 Jun;9(6):889–892. doi: 10.1128/aac.9.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff T. C., Ehret J. M. In vitro comparison of cefoxitin, cefamandole, cephalexin, and cephalothin. Antimicrob Agents Chemother. 1976 Jun;9(6):994–999. doi: 10.1128/aac.9.6.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans N. M., Smith D. D. The inhibition of Haemophilus influenzae by certain agar and peptone preparations. J Med Microbiol. 1974 May;7(2):305–310. doi: 10.1099/00222615-7-2-305. [DOI] [PubMed] [Google Scholar]
- Farooki Z. Q., Henry J. G., Green E. W. Hemophilus influenzae pericarditis associated with meningitis. Clin Pediatr (Phila) 1974 Jul;13(7):609–610. doi: 10.1177/000992287401300712. [DOI] [PubMed] [Google Scholar]
- Golberg R., Washington J. A., 2nd The taxonomy and antimicrobial susceptibility of Haemophilus species in clinical specimens. Am J Clin Pathol. 1978 Dec;70(6):899–904. doi: 10.1093/ajcp/70.6.899. [DOI] [PubMed] [Google Scholar]
- Gordon R. C., Thompson T. R., Stevens L. I., Carlson W. H. In vitro susceptibility of Haemophilus influenzae to eight antibiotics. Antimicrob Agents Chemother. 1974 Jul;6(1):114–115. doi: 10.1128/aac.6.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granoff D. M., Sargent E., Jolivette D. Haemophilus influenzae type b osteomyelitis. Am J Dis Child. 1978 May;132(5):488–490. doi: 10.1001/archpedi.1978.02120300048009. [DOI] [PubMed] [Google Scholar]
- Gray B. M., Hubbell C. A., Dillon H. C., Jr Manner and meaning of susceptibility testing of ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Jun;11(6):1021–1026. doi: 10.1128/aac.11.6.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunberg E., Cleeland R., Beskid G., DeLorenzo W. F. In vivo synergy between 6 beta-amidinopenicillanic acid derivatives and other antibiotics. Antimicrob Agents Chemother. 1976 Apr;9(4):589–594. doi: 10.1128/aac.9.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Alexander G. A. Comparative activities of selected beta-lactam antibiotics against Haemophilus influenzae. Antimicrob Agents Chemother. 1978 Feb;13(2):342–343. doi: 10.1128/aac.13.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Jones P. M. Simplified medium for ampicillin susceptibility testing of Haemophilus influenzae. Antimicrob Agents Chemother. 1975 Feb;7(2):186–190. doi: 10.1128/aac.7.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H., Lee J. C. Microdilution technique for antimicrobial susceptibility testing of Haemophilus influenzae. Antimicrob Agents Chemother. 1975 Nov;8(5):610–611. doi: 10.1128/aac.8.5.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kammer R. B., Preston D. A., Turner J. R., Hawley L. C. Rapid detection of ampicillin-resistant Haemophilus influenzae and their susceptibility to sixteen antibiotics. Antimicrob Agents Chemother. 1975 Jul;8(1):91–94. doi: 10.1128/aac.8.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976 Mar;93(1):9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- Kirven L. A., Thornsberry C. In vitro susceptibility of Haemophilus influenzae to trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 1974 Dec;6(6):869–870. doi: 10.1128/aac.6.6.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks M. I. In vitro activity of clindamycin and other antimicrobials against gram-positive bacteria and Hemophilus influenzae. Can Med Assoc J. 1975 Jan 25;112(2):170–173. [PMC free article] [PubMed] [Google Scholar]
- Marks M. I., Weinmaster G. Influences of media and inocula on the in vitro susceptibility of Haemophilus influenzae to co-trimoxazole, ampicillin, penicillin, and chloramphenicol. Antimicrob Agents Chemother. 1975 Dec;8(6):657–663. doi: 10.1128/aac.8.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan J. E., Jr, Terry P. M., Nahmias A. J. Susceptibility of Haemophilus influenzae isolates from blood and cerebrospinal fluid to ampicillin, chloramphenicol, and trimethoprim-sulfamethoxazole. Antimicrob Agents Chemother. 1976 Jan;9(1):137–139. doi: 10.1128/aac.9.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLinn S. E., Nelson J. D., Haltalin K. C. Antimicrobial susceptibility of Hemophilus influenzae. Pediatrics. 1970 May;45(5):827–838. [PubMed] [Google Scholar]
- Medeiros A. A., O'Brien T. F. Ampicillin-resistant Haemophilus influenzae type B possessing a TEM-type beta-lactamase but little permeability barrier to ampicillin. Lancet. 1975 Mar 29;1(7909):716–719. doi: 10.1016/s0140-6736(75)91630-x. [DOI] [PubMed] [Google Scholar]
- Norrby R., Brorsson J. E., Seeberg S. Comparative study of the in vitro antibacterial activity of cefoxitin, cefuroxine, and cephaloridine. Antimicrob Agents Chemother. 1976 Mar;9(3):506–510. doi: 10.1128/aac.9.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf G. D., Steinberg E. A., Underman A. E., Wilkins J., Leedom J. M., Mathies A. W., Jr, Wehrle P. F. Comparative trial of carbenicillin and ampicillin therapy for purulent meningitis. Antimicrob Agents Chemother. 1977 Mar;11(3):420–426. doi: 10.1128/aac.11.3.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overturf G. D., Wilkins J., Leedom J. M., Ivler D., Mathies A. W. Susceptibility of Hemophilus influenzae, type b, to ampicillin at Los Angeles County/University of Southern California Medical Center. A reappraisal after ten years. J Pediatr. 1975 Aug;87(2):297–300. doi: 10.1016/s0022-3476(75)80606-8. [DOI] [PubMed] [Google Scholar]
- Piot P., van DYCK E., Pattyn S. R. Sensibilitê d' "Haemophilus influenzae" ă 5 antibiotiques et dêtection rapide de sa rêsistance a l'ampicilline. Pathol Biol (Paris) 1977 Feb;25(2):83–87. [PubMed] [Google Scholar]
- Ryan R., Tilton R. C. Rapid method for determining the minimum inhibitory concentration of ampicillin for Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Jan;11(1):114–117. doi: 10.1128/aac.11.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr In vitro activity of rosamicin against Neisseria and Haemophilus, including penicillinase-producing strains. Antimicrob Agents Chemother. 1977 Aug;12(2):293–294. doi: 10.1128/aac.12.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R., Rodriguez W., Khan W. N. Resistance of H. influenzae to ampicillin. J Pediatr. 1978 Feb;92(2):343–344. doi: 10.1016/s0022-3476(78)80049-3. [DOI] [PubMed] [Google Scholar]
- Sinai R., Hammerberg S., Marks M. I., Pai C. H. In vitro susceptibility of Haemophilus influenzae to sulfamethoxazole-trimethoprim and cefaclor, cephalexin, and cephradine. Antimicrob Agents Chemother. 1978 May;13(5):861–864. doi: 10.1128/aac.13.5.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strausbaugh L. J., Mandaleris C. D., Sande M. A. Cefamandole and ampicillin therapy in experimental Haemophilus influenzae meningitis. J Infect Dis. 1977 Feb;135(2):210–216. doi: 10.1093/infdis/135.2.210. [DOI] [PubMed] [Google Scholar]
- Sykes R. B., Griffiths A., Ryan D. M. Comparative acitivity of ampicillin and cefuroxime against three types of Haemophilus influenzae. Antimicrob Agents Chemother. 1977 Apr;11(4):599–604. doi: 10.1128/aac.11.4.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syriopoulou V. P., Scheifele D. W., Sack C. M., Smith A. L. Effect of inoculum size on the susceptibility of Haemophilus influenzae b to beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Oct;16(4):510–513. doi: 10.1128/aac.16.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Jones R. N. In vitro antimicrobial activity of piperacillin and seven other beta-lactam antibiotics against Neisseria gonorrhoeae and Haemophilus influenzae, including beta-lactamase producing strains. J Antimicrob Chemother. 1979 Mar;5(2):137–142. doi: 10.1093/jac/5.2.137. [DOI] [PubMed] [Google Scholar]
- Thornsberry C., Baker C. N., Kirven L. A., Swenson J. M. Susceptibility of ampicillin-resistant Haemophilus influenzae to seven penicillins. Antimicrob Agents Chemother. 1976 Jan;9(1):70–73. doi: 10.1128/aac.9.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Kirven L. A. Antimicrobial susceptibility of Haemophilus influenzae. Antimicrob Agents Chemother. 1974 Nov;6(5):620–624. doi: 10.1128/aac.6.5.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk D. C. A comparison of chloramphenicol and ampicillin as bactericidal agents for Haemophilus influenzae type B. J Med Microbiol. 1977 Feb;10(1):127–131. doi: 10.1099/00222615-10-1-127. [DOI] [PubMed] [Google Scholar]
- Washington J. A., 2nd, Snyder R. J., Kohner P. C. Spurious ampicillin resistance by testing Haemophilus influenzae with agar containing supplement C. Antimicrob Agents Chemother. 1976 Jan;9(1):199–200. doi: 10.1128/aac.9.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. D., Andrews J. Sensitivity of Haemophilus influenzae to antibiotics. Br Med J. 1974 Jan 26;1(5899):134–137. doi: 10.1136/bmj.1.5899.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yourassowsky E., Schoutens E. In vitro bacteriostatic and bactericidal activities of 7 cephalosporin antibiotics on Haemophilus influenzae. Int J Clin Pharmacol Biopharm. 1975 Dec;12(4):433–436. [PubMed] [Google Scholar]


