Abstract
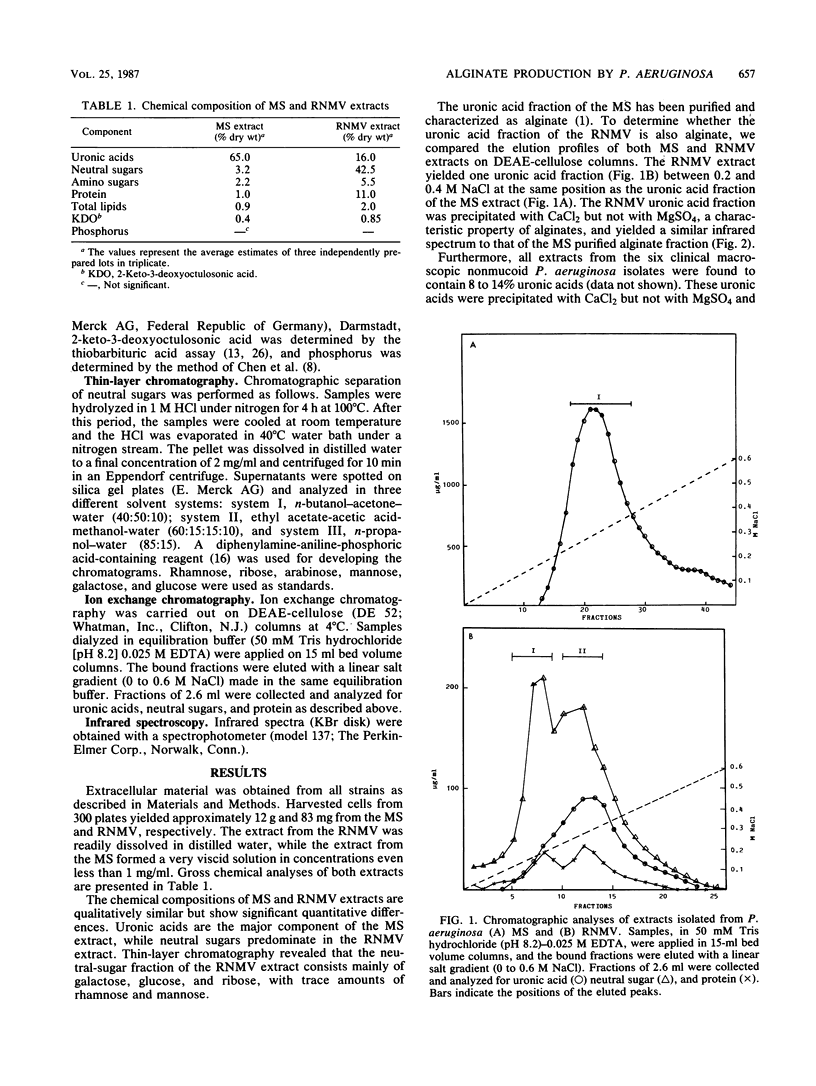
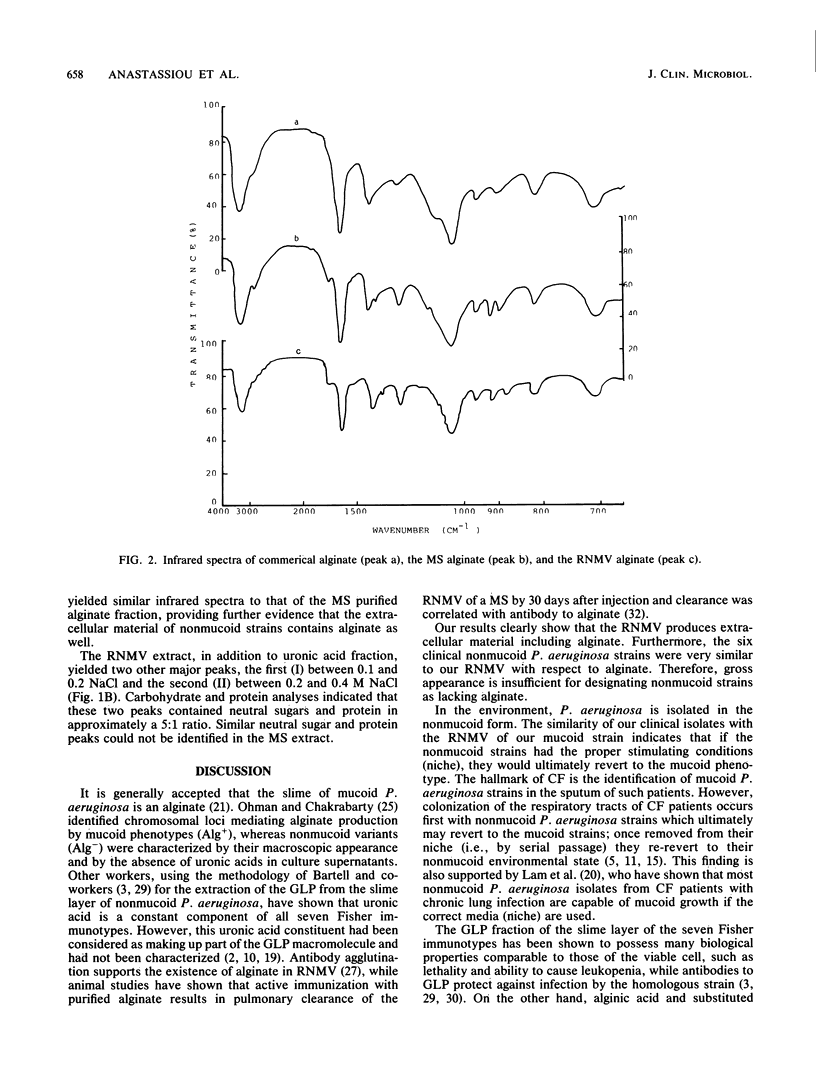
The slime material from a revertant nonmucoid variant, derived by serial passage of a heavily mucoid Pseudomonas aeruginosa strain isolated from a patient with bacteremia, was found to contain 16% uronic acids, 48.5% carbohydrates, 11% protein, and 2% lipids. Chromatographic analysis by ion exchange chromatography revealed that this extracellular material consisted of three fractions, one uronic acid fraction with properties similar to those of the alginate fraction of the parental strain and two other fractions consisting of neutral sugars and proteins in approximately a 5:1 ratio. In addition, the slime material from six other clinical macroscopic nonmucoid P. aeruginosa strains was found to contain alginate. These results demonstrate that alginate production in various amounts is a property shared by all P. aeruginosa strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastassiou E. D., Frangides C., Dimitracopoulos G. Nonfatal bacteremia caused by a mucoid, alginate-producing strain of Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 1986 Nov;5(4):277–283. doi: 10.1016/0732-8893(86)90029-5. [DOI] [PubMed] [Google Scholar]
- Arsenis G., Dimitracopoulos G. Chemical composition of the extracellular slime glycolipoprotein of Pseudomonas aeruginosa and its relation to gentamicin resistance. J Med Microbiol. 1986 May;21(3):199–202. doi: 10.1099/00222615-21-3-199. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Bartell P. F., Orr T. E., Chudio B. Purification and Chemical Composition of the Protective Slime Antigen of Pseudomonas aeruginosa. Infect Immun. 1970 Nov;2(5):543–548. doi: 10.1128/iai.2.5.543-548.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergan T., Hoiby N. Epidemiological markers for Pseudomonas aeruginosa. 6. Relationship between concomitant non-mucoid and mucoid strains from the respiratory tract in cystic fibrosis. Acta Pathol Microbiol Scand Suppl. 1975 Dec;83(6):553–560. [PubMed] [Google Scholar]
- Brown M. R., Foster J. H., Clamp J. R. Composition of Pseudomonas aeruginosa slime. Biochem J. 1969 May;112(4):521–525. doi: 10.1042/bj1120521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S. C., Bass J. A., Shetlar M. R. Composition of the protective antigen from the slime layer of Pseudomonas aeruginosa. Tex Rep Biol Med. 1971 Fall;29(3):265–272. [PubMed] [Google Scholar]
- Dimitracopoulos G., Bartell P. F. Phage-related surface modifications of Pseudomonas aeruginosa: effects on the biological activity of viable cells. Infect Immun. 1979 Jan;23(1):87–93. doi: 10.1128/iai.23.1.87-93.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doggett R. G., Harrison G. M., Carter R. E. Mucoid Pseudomonas aeruginosa in patients with chronic illnesses. Lancet. 1971 Jan 30;1(7692):236–237. doi: 10.1016/s0140-6736(71)90973-1. [DOI] [PubMed] [Google Scholar]
- Doggett R. G. Incidence of mucoid Pseudomonas aeruginosa from clinical sources. Appl Microbiol. 1969 Nov;18(5):936–937. doi: 10.1128/am.18.5.936-937.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellwood D. C. The distribution of 2-keto-3-deoxy-octonic acid in bacterial walls. J Gen Microbiol. 1970 Mar;60(3):373–380. doi: 10.1099/00221287-60-3-373. [DOI] [PubMed] [Google Scholar]
- Govan J. R. Mucoid strains of Pseudomonas aeruginosa: the influence of culture medium on the stability of mucus production. J Med Microbiol. 1975 Nov;8(4):513–522. doi: 10.1099/00222615-8-4-513. [DOI] [PubMed] [Google Scholar]
- HAYNES W. C. Pseudomonas aeruginosa--its characterization and identification. J Gen Microbiol. 1951 Nov;5(5 Suppl):939–950. doi: 10.1099/00221287-5-5-939. [DOI] [PubMed] [Google Scholar]
- Koepp L. H., Orr T., Bartell P. F. Polysaccharide of the slime glycolipoprotein of Pseudomonas aeruginosa. Infect Immun. 1981 Sep;33(3):788–794. doi: 10.1128/iai.33.3.788-794.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINKER A., JONES R. S. A POLYSACCHARIDE RESEMBLING ALGINIC ACID FROM A PSEUDOMONAS MICRO-ORGANISM. Nature. 1964 Oct 10;204:187–188. doi: 10.1038/204187a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lam J., Chan R., Lam K., Costerton J. W. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect Immun. 1980 May;28(2):546–556. doi: 10.1128/iai.28.2.546-556.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian F. A., Jarman T. R., Righelato R. C. Biosynthesis of exopolysaccharide by Pseudomonas aeruginosa. J Bacteriol. 1978 May;134(2):418–422. doi: 10.1128/jb.134.2.418-422.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohman D. E., Chakrabarty A. M. Genetic mapping of chromosomal determinants for the production of the exopolysaccharide alginate in a Pseudomonas aeruginosa cystic fibrosis isolate. Infect Immun. 1981 Jul;33(1):142–148. doi: 10.1128/iai.33.1.142-148.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. IV. The composition of the cell walls of some Gram-positive and Gram-negative bacteria. Biochim Biophys Acta. 1953 Apr;10(4):512–523. doi: 10.1016/0006-3002(53)90296-0. [DOI] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. Glycolipoprotein from Pseudomonas aeruginosa as a protective antigen against P. aeruginosa infection in mice. Infect Immun. 1977 Nov;18(2):304–309. doi: 10.1128/iai.18.2.304-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Woods D. E., Bryan L. E. Studies on the ability of alginate to act as a protective immunogen against infection with Pseudomonas aeruginosa in animals. J Infect Dis. 1985 Apr;151(4):581–588. doi: 10.1093/infdis/151.4.581. [DOI] [PubMed] [Google Scholar]
- Zierdt C. H., Williams R. L. Serotyping of Pseudomonas aeruginosa isolates from patients with cystic fibrosis of the pancreas. J Clin Microbiol. 1975 Jun;1(6):521–526. doi: 10.1128/jcm.1.6.521-526.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


