Abstract
Background: Skeletal metastases represent an underappreciated site of metastasis in patients with pancreatic cancer. Previous reports have estimated the prevalence to range from 5 percent to 20 percent. With the use of gemcitabine and novel targeted agents such as erlotinib, there has been a modest increase in survival in patients with advanced pancreatic cancer. As such, it is anticipated that previously uncommon occurrences such as skeletal metastases will become more frequent.
Patients and Methods: Retrospective chart review was conducted at two academic institutions to identify pancreatic cancer patients with skeletal metastases over a two-year period.
Results: Seven patients were identified from a database of 323 patients (2.2 percent). All patients had advanced disease and had received prior systemic therapy (range: 1-4 lines, median: 2 lines). Approximately half (57.1 percent) of the patients were symptomatic from their skeletal metastases. The most common sites of skeletal metastases were vertebrae (100 percent), hips (57.1 percent), and ribs (57.1 percent). Both blastic and lytic lesions were noted, with a predominance of blastic lesions (71.4 percent). A majority of patients (71.4 percent) received bisphosphonates as part of their care.
Discussion: Skeletal metastases are an uncommon but clinically important occurrence in patients with pancreatic cancer. Clinicians caring for patients with pancreatic cancer should be alert regarding skeletal metastases, due to the morbidity it can cause for these patients (e.g., back pain, fractures, etc.).
Introduction
The usual sites of metastases in pancreatic cancer are the liver and peritoneal cavity. Other less common sites are the lung, bone, and brain. Unusual sites such as muscle, skin, heart, pleura, stomach, umbilicus, kidney, appendix, spermatic cord, and prostate have also been reported [1-10].
Skeletal metastases from pancreatic cancer have thus far been considered an infrequent occurrence.
The first case of pancreatic cancer with skeletal metastases was described in Russian literature in 1963 [11]. Although the true incidence of skeletal metastases in patients with pancreatic cancer is not known, it is felt to be between 5 percent to 20 percent [12-13]. There appears to be some suggestion that the association is higher in patients who have a primary that is in the tail of the pancreas [13]. Both osteolytic and osteoblastic lesions have been described. Skeletal surveys using standard roentgenograms, CT scans, MRIs, and positron emission topographic (PET) scans have been used to detect the presence of skeletal metastases in pancreatic cancer [14-21]. No imaging modality appears to have a superior detection rate. However, when used in conjunction, the rates of detection may be much higher. Early detection of skeletal metastases that are asymptomatic may be achieved through the serial measurement of C-telopeptide [13].
Bone pain, pathological fractures, and hypercalcemia are possible sequelae of skeletal metastases. Unusual symptoms such as bilateral hearing loss associated with involvement of the temporal bone have been reported [22-23]. Although the pathogenesis of skeletal metastasis in pancreatic cancer remains unknown, preliminary data suggests that cytokines such as interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and parathyroid hormone-related protein (PTHrP) may play a pivotal role in the growth of pancreatic cancer in bone [13]. Extrapolating findings from other tumor types, it is conceivable that other factors that stimulate osteoclastic bone resorption such as transforming growth factor beta (TGF-β), interleukin-11 (IL-11), and matrix metalloproteinases may be involved as well [24].
Micrometastases associated with pancreatic adenocarcinoma have been found in the bone marrow of patients. It is unclear if they represent pre-clinical bone metastases [25].
Patients and Methods
A retrospective chart review was conducted at Scottsdale Clinical Research Institute, which serves as the clinical site for the Translational Genomics Research Institute, and Yale University Medical Center from July 2005 to December 2007. Patients with pancreatic cancer seen at these two institutions over a two-year period were identified, and those who had skeletal metastases were reviewed. Both their demographic and clinical details were tabulated. Only descriptive statistics were used to summarize the data.
Results
We identified seven cases (among 323 reviewed, prevalence 2.2 percent) of patients with pancreatic cancer who developed skeletal metastases during the course of their disease (Table 1). The demographics were representative of patients with advanced pancreatic cancer (age: median 59 years, range 57-70; males 4 [57.1 percent]/females 3 [48.3 percent]; race: Caucasian 7 [100 percent]). All patients had advanced disease (stage III: 2 [28.6 percent], stage IV: 5 [71.4 percent]) at the time of their initial diagnoses. The time to development of skeletal metastases was variable and ranged from two to 17.3 months (median: 5.5 months). Patients with stage III disease at initial diagnosis apparently had a longer time to development of skeletal metastases (range: 17.3-32 months), compared to patients with stage IV disease at initial diagnosis (range: 2-8.5 months). In this case series, four of seven patients (57.1 percent) had symptomatic disease. The most common site of skeletal metastasis was the vertebrae (seven of seven patients [100 percent]). Sites such as the hips and ribs were somewhat less prevalent (four of seven cases each [57.1 percent]); whereas, sites such as the upper and lower extremities, skull, and face were much less common. Both blastic (Figures 1 and 2) and lytic lesions were seen in these patients. However, there appeared to be a predominance of blastic lesions (five of seven [71.4 percent]) in these patients. All patients had at least one other site of metastatic disease (range: 1-3, median: 2) with the liver being most common extra-skeletal site (six of seven [85.7 percent]). A majority of patients (five of seven [71.4 percent]) had received bisphosphonates as part of their care. All patients had received prior systemic chemotherapy (range: 1-4 lines, median: 2 lines).
Figure 1.
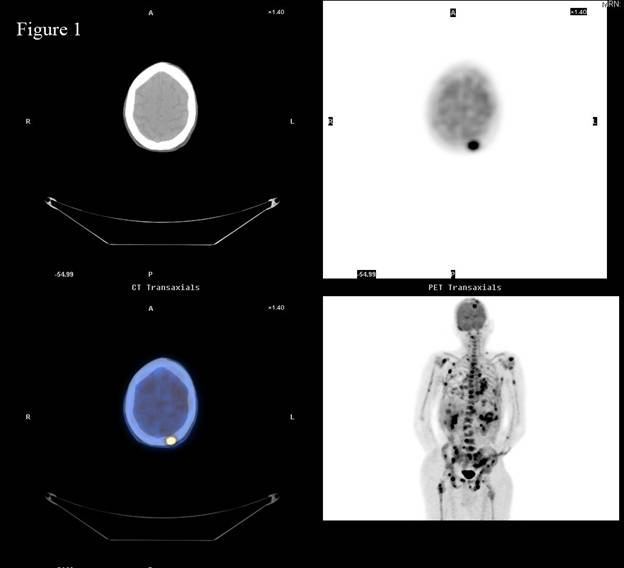
Positron Emission Tomography/Computed Tomography (PET/CT) scan of patient with metastatic pancreatic adenocarcinoma reveals multiple areas of skeletal involvement most notably left frontal calvarium, spine, ribs, bilateral humeri, and pelvis.
Figure 2.
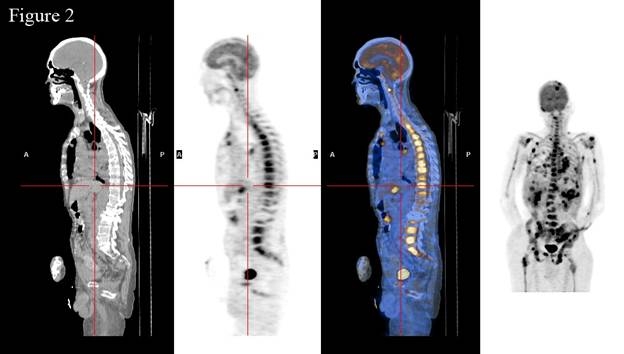
Extensive involvement of spine by skeletal metastases as visualized by PET/CT scan (sagittal view).
Discussion
With the advent of the use of novel agents such as erlotinib in conjunction with more widely used agents such as gemcitabine, modest improvements of survival in patients with metastatic pancreatic adenocarcinoma have been achieved [26,27]. Despite this, it appears there has not been a considerable increase in the prevalence of patients with pancreatic cancer developing skeletal metastases. In our study, the incidence may be low, as only symptomatic bone metastases were detected. It is possible that many more clinically silent metastases would have been detected if we perform a cross-sectional imaging. Most patients in our review had restaging preformed (CT scan) every eight to nine weeks, and no incidental bone disease was noticed in the radiological reports. PET/CT may be a useful tool in detecting bone metastases as found in two of our patients. A rigorous imaging follow-up in all patients likely would reveal many more metastases. Nevertheless, it remains to be a clinically significant problem that can be particularly complicated by the fact that symptomatic presentation mistakenly might be thought to be a result of the underlying primary malignancy (e.g., back pain).
The patients identified in this case series are representative of patients with advanced pancreatic cancer in their demographic characteristics. Survival from the time of diagnosis of skeletal metastases can be quite variable in this group of patients (range: 0.3-9 months), and as such, the case for the benefit of therapies such as bisphosphonates would have to be on a case-by-case basis. It would be evident that bisphosphonates likely would benefit those patients who would have a better performance status, stage III vs. stage IV disease at the time of diagnosis of skeletal metastases, given that these have been shown to be factors associated with prolonged overall survival. Of note, none of the patients in this case series experienced a skeletal metastatic complication such as cord compression or pathological fracture. This possibly could be attributed to the frequent use of bisphosphonates and the short duration of survival, which potentially would have not allowed such events to occur. Larger studies examining this question would need to be conducted to draw any firm conclusions.
Interestingly, the spine (in the form of vertebral metastases) was the most common site of skeletal metastasis. Given this, back pain can be a manifestation of pancreatic cancer itself but clinicians should be alert that it can occasionally be a presenting symptom of skeletal metastases. Early diagnosis of skeletal metastases in such patients may be paramount toward reducing morbidity through early and effective interventions such as the use of bisphosphonates [28].
We agree that conclusions are limited because of the retrospective nature of the study and exact incidence cannot be given. However, at the same time, this study sends an important message: Clinicians treating patients with pancreatic cancer should consider evaluating bone metastases in these patients, as palliative radiation or even surgery can improve the quality of life and may even prolong survival in patients with a single metastatic site.
Conclusions
The incidence of bone metastases secondary to adenocarcinoma of the exocrine pancreas is unknown, since radiological studies of the bones during life, routine bone scintigrams, or extensive examination of the skeleton at autopsy are rarely undertaken in the absence of specific clinical indications. Symptom-producing bone metastases are relatively uncommon; a review of the literature suggests that the vast majority are osteolytic in nature with only a few isolated case reports of purely blastic deposits. Our study suggests that osteoblastic bone metastases are more common than generally recognized. We described the clinical, radiological, and pathological findings in these seven cases in order to emphasize that the pancreas is a potential source of bone metastases, especially blastic. Clinicians caring for these patients should be aware of this rare site of metatstases, and they also should consider the pancreas as a possible primary site in patients who present initially with osteoblastic bone deposits of unknown origin.
Table 1. Characteristics of Pancreatic Cancer Patients with Skeletal Metastases.
| Patient A | Patient B | Patient C | Patient D | Patient E | Patient F | Patient G | |
| Age | 58 | 70 | 62 | 53 | 74 | 59 | 57 |
| Sex | Female | Male | Female | Male | Female | Male | Male |
| Stage at Initial Diagnosis of Cancer | III | IV | IV | III | IV | IV | IV |
| Symptomatic vs. Asymptomatic | Symptomatic | Symptomatic | Asymptomatic | Asymptomatic | Symptomatic | Asymptomatic | Symptomatic |
| Bone Metastases: Sites of bone metastases | |||||||
| Vertebrae | + | + | + | + | + | + | + |
| Hips | + | + | + | + | |||
| Ribs | + | + | + | + | |||
| Upper Extremities | + | ||||||
| Lower Extremeties | + | + | |||||
| Skull | + | ||||||
| Nature of Lesions | Blastic | Blastic | Blastic | Blastic | Unknown | Lytic | Blastic |
| Bisphosponate Use | Yes | Yes | Yes | Yes | Yes | No | No |
| Time Between Initial Diagnosis and Development of Metastases | 17.3 months | 8.5 months | 2.3 months | 32 months | 4.25 months | 5.5 months | 2 months |
| Survival Since Diagnosis of Bone Metastases | 2 months | Alive | 6.6 months | 9 months | 1.75 months | 3.5 months | 0.3 months |
| Survival Since Initial Diagnosis of Cancer | 19.3 months | Alive | 8.9 months | 41 months | 6 months | 9 months | 4.25 months |
| Number of Other Metastatic Sites | 2 | 1 | 2 | 3 | 1 | 1 | 3 |
| Sites of Other Metastases | |||||||
| Liver | + | + | + | + | + | + | |
| Peritoneum | + | + | |||||
| Lungs | + | + | |||||
| Lymph nodes | + | ||||||
| Kidney | + | ||||||
| Number of Lines of Systemic Chemotherapy | 4 | 2 | 2 | 2 | 1 | 1 | 1 |
Acknowledgments
The authors thank Susan McCall for editorial assistance.
Abbreviations
- PET
positron emission topographic
- VEGF
vascular endothelial growth factor
- PTHrP
parathyroid hormone-related protein
- TGF-β
transforming growth factor beta
- IL-11
interleukin-11
References
- Wafflart E, Gibaud H, Lerat F, et al. Muscular metastases of cancer of the pancreas. J Chir. 1996;133(4):167–170. [PubMed] [Google Scholar]
- Otegbayo JA, Oluswasola OA, Akere A, et al. Pancreatic carcinoma presenting as cutaneous nodules in a diabetic Nigerian male. West Afr J Med. 2005;24(2):180. [PubMed] [Google Scholar]
- Robinson BW, Lewis RR. Myocardial metastases from carcinoma of pancreas presenting as acute myocardial infarction. JR Soc Med. 1982;75(7):560–562. [PMC free article] [PubMed] [Google Scholar]
- Turiaf J, Battesti JP, Basset F, et al. Metastatic pleurisy in pancreatic cancer with the presence of considerable quantities of amylase in the pleural effusion and a major paraneoplastic peripheral neurologic syndrome. Ann Med Interne. 1969;120(6):449–458. [PubMed] [Google Scholar]
- Takamori H, Kanemitsu K, Tsuji T, et al. Metastatic gastric tumor secondary to pancreatic adenocarcinoma. J Gastroenterol. 2005;40(2):209–212. doi: 10.1007/s00535-004-1524-5. [DOI] [PubMed] [Google Scholar]
- Crescentini F, Deutsch F, Sobrado CW, et al. Umbilical mass as the sole presenting symptom of pancreatic cancer: a case report. Rev Hosp Clin Fac Med Sao Paulo. 2004;59(4):198–202. doi: 10.1590/s0041-87812004000400008. [DOI] [PubMed] [Google Scholar]
- Martino L, Martino F, Coluccio A, et al. Renal metastases from pancreatic adenocarcinoma. Arch Ital Urol Androl. 2004;76(1):37–39. [PubMed] [Google Scholar]
- Filik L, Ozdal-Kuran S, Cicek B, et al. Appendicular metastases from pancreatic adenocarcinoma. Int J Gastrointest Cancer. 2003;34(1):55–58. doi: 10.1385/IJGC:34:1:55. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay D, Kapadia CR, Da Costa PE, et al. Pancreatic carcinoma: report of two cases presenting with unusual metastases. Indian J Gastroenterol. 2005;24(2):75–76. [PubMed] [Google Scholar]
- Merseburger AS, Muller CC, Merseburger Schonborn CT, et al. A rare case of isolated prostate metastasis from primary pancreatic cancer. Urologe A. 2005;44(5):527–529. doi: 10.1007/s00120-005-0783-y. [DOI] [PubMed] [Google Scholar]
- Lipko AA. Features of the course of lung and bone metastases of pancreatic cancer. Vopr Onkol. 1963;92:80–85. [PubMed] [Google Scholar]
- Hatfield DR, Leland FH, Maruyama Y. Skeletal metastases in pancreatic carcinoma: Study by Isotopic bone scanning. Oncology. 1976;33(1):44–47. doi: 10.1159/000225100. [DOI] [PubMed] [Google Scholar]
- Iguchi H, Yasuda M, Matsuo T, Samii T, Funakoshi A. Clinical features and management of pancreatic cancer with bone metastases. Nippon Shokakibyo Gakkai Zasshi. 2004;101(8):872–878. [PubMed] [Google Scholar]
- Tamm E, Charnsangavej C. Pancreatic Cancer: Current concepts in imaging for diagnosis and staging. Cancer J. 2001;7:298–311. [PubMed] [Google Scholar]
- Gillison EW, Grainger RG, Fernandez D. Osteoblastic metastases in carcinoma of Pancreas. Br J Radiol. 1970;43(515):818–820. doi: 10.1259/0007-1285-43-515-818. [DOI] [PubMed] [Google Scholar]
- Hatfield DR, Leland FH, Maruyama Y. Skeletal metastases in Pancreatic Carcinoma: Study by Isotopic bone scanning. Oncology. 1976;33(1):44–47. doi: 10.1159/000225100. [DOI] [PubMed] [Google Scholar]
- Joffe N, Antonioli DA. Osteoblatic bone metastases secondary to Adenocarcinoma of the Pancreas. Clin Radiol. 1978;29(1):41–46. doi: 10.1016/s0009-9260(78)80162-7. [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto Y, Yokoe K, et al. Contribution of Whole body FDG-PET to the detection of distant metastases in pancreatic cancer. Ann Nucl Med. 2005;19(6):491–497. doi: 10.1007/BF02985577. [DOI] [PubMed] [Google Scholar]
- Sperti C, Pasquali C, Chierichetti F, et al. 18-Fluorodeoxyglucose positron emission tomography in predicting survival in patients with pancreatic carcinoma. J Gastrointest Surg. 2003;7:953–959. doi: 10.1016/j.gassur.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Lytras D, Connor S, Bosonnet L, et al. Positron Emmision Tomography does not add to the Computer Tomography for the diagnosis and staging of Pancreatic Cancer. Dig Surg. 2005;22(1-2):55–61. doi: 10.1159/000085347. [DOI] [PubMed] [Google Scholar]
- Lyons JD, Alibazoglu B, Harris JE, Ali A, Hollinger EF. Pelvic metastases from pancreatic carcinoma: a pattern observed on bone scan. Clin Nucl Med. 2001;26(3):230–231. doi: 10.1097/00003072-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Wu CH, Kaga K, Ohira Y, Suzuki J. Histopathology of temporal bone metastases from Pancreatic Adenocarcinoma: A case showing bilateral hearing loss and Bechterew’s phenomenon. Otolaryngol Head Neck Surg. 2000;122(4):613–615. doi: 10.1067/mhn.2000.101816. [DOI] [PubMed] [Google Scholar]
- Ohira Y, Kaga K, Kodama A. A case of bilateral sudden hearing loss and vertigo caused by bilateral temporal metastases from Pancreatic Carcinoma-comparison of clinical findings and temporal bone pathological findings. Nippon Jibiinkoka Gakkai Kaiho. 1991;94(1):9–15. doi: 10.3950/jibiinkoka.94.9. [DOI] [PubMed] [Google Scholar]
- Kakonen SM, Mundy GR. Mechanisms of Osteolytic bone metastases in breast carcinoma. Cancer. 2003;97(3):834–839. doi: 10.1002/cncr.11132. [DOI] [PubMed] [Google Scholar]
- Schmitz-Winnenthal FH, Volk C, et al. High frequencies of functional tumor-reactive T cells in bone marrow and blood of pancreatic cancer patients. Cancer Res. 2005;65(21):10079–10087. doi: 10.1158/0008-5472.CAN-05-1098. [DOI] [PubMed] [Google Scholar]
- Burris HA, Moore MJ, Anderson J, et al. Improvement in survival and clinical cancer. A Randomized benefit with Gemcitabine as first line therapy for patients with advanced pancreas trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared to gemcitabine alone in patients with advanced pancreatic cancer. A Phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25(15):1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- Clezarin P, Ebetino FH, Fournier PG. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65(12):4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]


