Abstract
Kisspeptins, coded by the KiSS-1 gene, regulate aspects of the reproductive axis by stimulating GnRH release via the G protein coupled receptor, GPR54. Recent reports show that KiSS/GPR54 may be key mediators in photoperiod-controlled reproduction in seasonal breeders, and that KiSS-1/GPR54 are expressed in the hypothalamus, ovaries, placenta, and pancreas. This study examined the expression of KiSS-1/GPR54 mRNA and protein in ovaries of Siberian hamsters (Phodopus sungorus). Ovaries from cycling hamsters were collected during proestrus (P), estrus (E), diestrus I (DI), and diestrus II (DII). To examine KiSS-1/GPR54 during stimulated recrudescence, additional hamsters were maintained either in long day (LD 16L:8D, control) or short day (SD 8L:16D) for 14 weeks and then transferred to LD for 0–8 weeks. Staining of KiSS-1/GPR54 protein was detected by immunohistochemistry in steroidogenic cells of preantral and antral follicles, and corpora lutea. Immunostaining peaked in P and E, but decreased in the diestrus stages (p<0.05). In recrudescing ovaries, KiSS-1/GPR54 immunostaining was low after 14 wks of SD exposure (post transfer [PT] wk0), and increased during the early weeks of recrudescence. Expression of KiSS-1/GPR54 mRNA was low with short day exposure, but increased during recrudescence and was higher at PT wk8 as compared to PTwks 0 and 2 (p<0.05). The elevated KiSS-1/ GPR54 expression during P and E suggests a potential role in ovulation in Siberian hamsters. Transient increases in KiSS-1/GPR54 expression following LD stimulation are also suggestive of possible involvement in ovulation and/or restoration of ovarian function.
Keywords: Seasonal reproduction, Ovary, Kisspeptin
Introduction
KiSS-1, also known as metastin, was first identified as a tumor suppressor gene in breast cancer and melanoma cells (Lee et al. 1996). The KiSS-1 gene encodes a 145 amino acid protein which generates biologically active peptides of 10, 13, 14, and 54 amino acids in length that bind G-protein coupled receptor GPR54 (Kotani et al. 2001; Ohataki et al. 2001). KiSS-1 mRNA is predominantly expressed in distinct areas of the brain including the arcuate nucleus (ARC), the anteroventral periventricular (AVPV) nucleus, the periventricular nucleus, the anterodorsal preoptic nucleus (Gottsch et al. 2004) and to a lesser extent in the preoptic area (POA) (Estrada et al. 2006). In addition to the brain, KiSS-1/GPR54 expression has been detected in the placenta (Kotani et al. 2001), pancreas (Kotani et al., 2001; Hauge-Evans et al. 2006), ovaries (Castellano et al. 2006), oviduct (Gaytan et al. 2007) and vasculature (Mead et al. 2006; 2007). The important finding about the role of GPR54 receptor and its natural ligand KiSS-1 in the initiation of puberty via action on the hypothalamic- pituitary-gonadal (HPG) axis (de Roux et al. 2003; Seminara et al. 2003) has sparked great interest. Recently, various roles of KiSS-1/GPR54 have been extensively reviewed, such as the role of KiSS-1 in trophoblast invasion (Bilbon et al. 2004), tumor suppression (Lee et al. 1996; Ohtaki et al. 2001), reproduction (Colledge, 2008; Smith, 2007; Dungan et al. 2005; Roa and Tena-Sempere 2007; Tena-Sempere 2006; Kauffman et al. 2007) and in the cardiovascular (as a vasoconstrictor) system (Mead et al. 2007). In terms of reproduction, KiSS-1 expression in the hypothalamus (ARC, AVPV) is regulated via feedback effects of steroid hormones in both males (Navarro et al. 2004) and females (Smith et al. 2005). In mice, rats, and ewes, estrogens inhibit KiSS-1 expression in the ARC and can mediate the negative feedback effect on GnRH secretion (Smith et al. 2005, 2006 and 2007). Indeed, hypogonadotropic hypogonadism is apparent in mice lacking KiSS-1, and null mutant females do not undergo normal sexual maturation (d'Anglemont de Tassigny, et al. 2007). While the crucial and central role of the KiSS-1/GPR54 system in GnRH release, reproduction, and puberty is well recognized, its precise role in other tissues, such as the ovaries, is still emerging.
In addition to other reproductive roles, the KiSS-1/GPR54 system may mediate the association of photoperiodic signals to the HPG axis in seasonal breeders like Syrian hamsters (Revel et al. 2006, 2007), Siberian hamsters (Greives et al. 2007, Mason et al. 2007) and ewes (Smith et al. 2007). Long day seasonal breeders, including Siberian hamsters (Phodopus sungorus), restrict their reproductive function during the short winter months and resume during summer when the days are longer and resources plentiful to provide energy for successful breeding. In Siberian hamsters, 12–14 weeks of exposure to short photoperiods (SD; 8 hours of light: 16 hours of dark) induces gonadal regression and loss of ovarian function (Moffatt-Blue et al. 2006, Salverson et al. 2008), whereas the subsequent transfer to long photoperiods (LD; 16L:8D) induces rapid ovarian recrudescence within 8 weeks (Salverson et al. 2008). In many seasonally breeding species, day length (photoperiod) serves as an environmental signal which, via the release of pineal melatonin, can induce either suppression or restoration of reproductive function as a result of alterations in the hypothalamic pituitary gonadal axis (Stehle et al. 2001; Pevet, 1988; Malpaux et al. 2001). Although the mechanism of environmental signal transmission to the HPG axis is not fully understood, recent reports have suggested kisspeptin as a primary neuroendocrine switch (Revel et al. 2006, 2007). While this central role of the KiSS-1/GPR54 system has been identified, potential peripheral roles during seasonal changes in reproductive function have not been examined. Since KiSS-1/GPR54 are also expressed in the ovaries, we hypothesized that local production of these gene products may potentially play a role in return of function during long day induced recrudescence of ovaries from photo-inhibited Siberian hamsters.
In the present study, our objectives were to examine if KiSS-1 and GPR54 are expressed in Siberian hamster ovaries and if their expression is linked to photostimulated recrudescence. Specifically, we sought to (1) assess KiSS-1/GPR54 expression during the estrous cycle and (2) to elucidate if KiSS-1/GPR54 mRNA and protein expression is modulated in ovaries undergoing photo-stimulated recrudescence after 14 weeks of short day exposure using immunohistochemistry and semi-quantitative RT-PCR.
Results
Immunostaining of KiSS-1/GPR54 during the estrous cycle
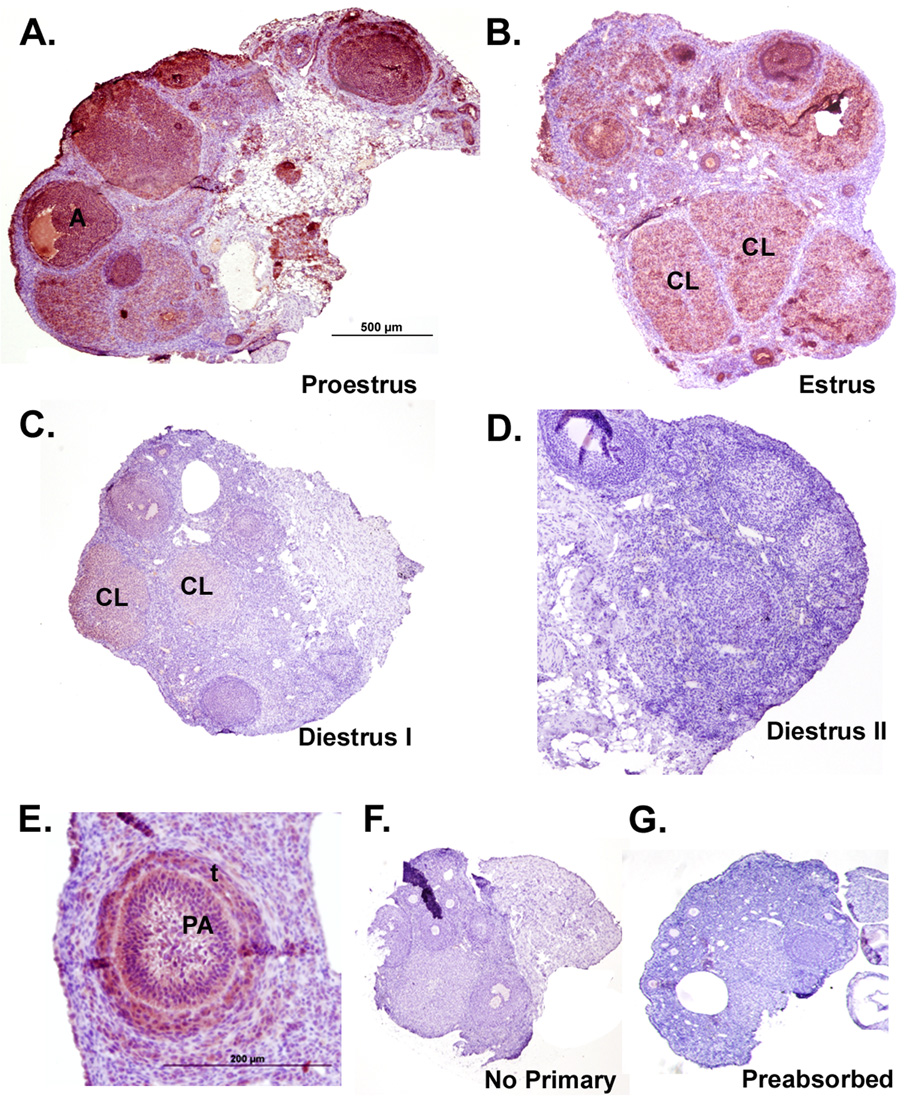
Parallel KiSS-1 and GPR54 immunostaining was observed in the ovaries during the entire estrous cycle (Figure 1, Figure 2). Cytosolic staining was observed in several cellular compartments including the surface epithelium, preantral and antral follicles, and corpora lutea, and was predominant in steroidogenic cells (Figure 1E, Figure 2E for detail). Little to no immunostaining was detected in the stroma. During proestrus (P), KiSS-1 staining was most intense in the theca and granulosa cells of secondary follicles (Figure 1A). The staining pattern remained similar during estrus (E), staining follicular and luteinized cells, but the overall intensity of immunoreactivity was reduced as compared to P (Figure 1B). The intensity of KiSS-1 immunostaining decreased substantially in diestrus I (DI) and diestrus II (DII) as compared to both P and E, and was present predominantly in the luteinized granulosa cells of the CL and some in the surface epithelial cells (Figure 1C, D). Negative controls without the KiSS-1 antibody showed no staining (Figure 1F) and preabsorption of the primary antibody with 10µg kisspeptin peptide overnight diminished virtually all staining (Figure 1G). To further confirm the specificity of kisspeptin staining we also used two additional antibodies against kisspeptin: a rabbit anti-kisspeptin-13 antibody characterized in Siberian hamster brains (Grieves et al. 2007) and a rabbit anti-kisspeptin 10 (kp10) antibody (Chemicon International) against the mouse peptide and observed similar staining patterns across the estrous cycle (data not shown).
Figure 1. Immunoreactivity of KiSS-1 protein in Siberian hamster ovaries during the estrous cycle.
Sections presented are representative of n = 6 hamsters per group. Staining is red/ink on purple background. Scale bar (A) depicts 500µm with photographs taken at 4x magnification for panels A–D. Scale bar in E represents 200µm. Tissue sections represent ovaries from the A) proestrus, B) estrus, C) diestrus I, and D) diestrus II stages of the estrous cycle. E) Detail of diffuse, cytoplasmic staining observed in both granulosa and thecal cells. F) Negative control processed without KiSS antibody. G) Preabsorbed KiSS-1 antibody used as a specificity control. PA= preantral follicle; A= antral follicle; CL=corpus luteum; t = thecal cells.
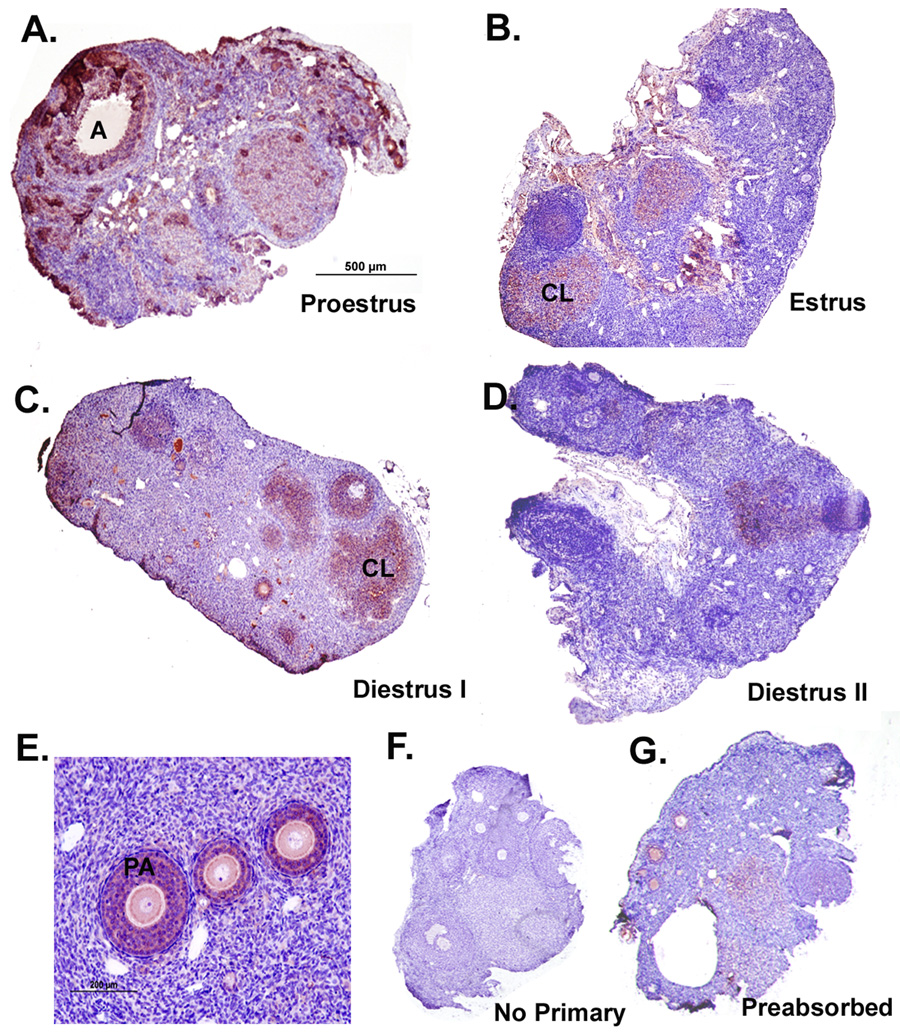
Figure 2. Immunoreactivity of GPR54 protein in Siberian hamster ovaries during the estrous cycle.
Sections presented are representative of n = 6 hamsters per group. Staining is red/ink on purple background. Scale bar (A) depicts 500µm with photographs taken at 4x magnification for panels A–D. Scale bar in E represents 200µm. Tissue sections represent ovaries from the A) proestrus, B) estrus, C) diestrus I, and D) diestrus II stages of the estrous cycle. E) Detail of diffuse, cytoplasmic staining observed in granulosa cells. F) Negative control processed without KiSS antibody. G) Preabsorbed KiSS-1 antibody used as a specificity control. PA= preantral follicle; A= antral follicle; CL=corpus luteum.
GPR54 immunostaining in preantral and antral follicles, and in CL also appeared to peak in P as compared to other stages of the cycle (Figure 2). However, in contrast to KiSS-1, GPR54 staining was primarily present in granulosa, but not theca cells (Figure 2E). Negative controls processed either without the primary antibody (Figure 2F), or with preabsorption of GPR54 peptide (Figure 2G) reduced immunoreactivity substantially.
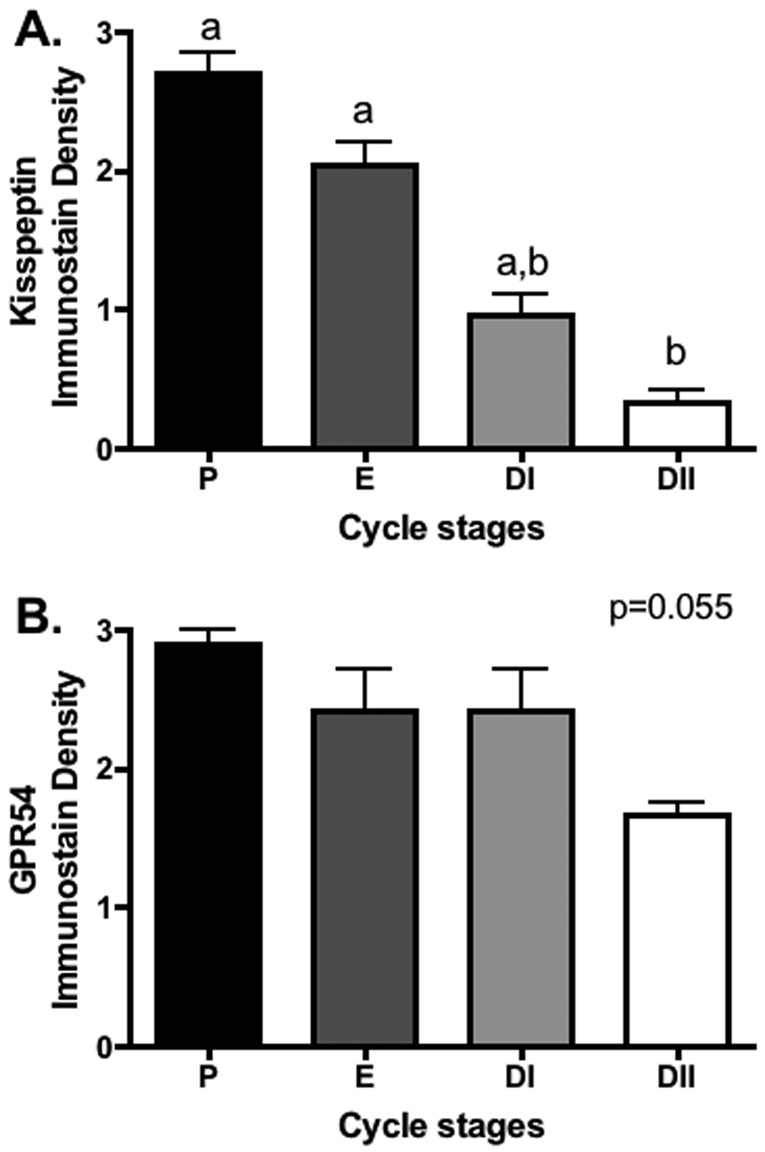
Relative quantification (0–3 scale) of staining intensity during the estrus cycle shows that KiSS-1 immunoreactivity peaked in P and E, with significant decreases noted when ovaries advanced to the DII stage (p<0.05; Figure 3A). GPR54 staining intensity followed a similar pattern with apparent peaks during P and E, however no significant differences were observed (p=0.055; Figure 3B).
Figure 3.
Quantification of the relative extent of KiSS-1 and GPR54 immunostaining (mean + S.E.M.) in Siberian hamsters housed in proestrus (P), estrus (E), diestrus I (DI), and diestrus II (DII) stages of the estrous cycle. Groups with different letters are significantly different (P < 0.05).
Expression of KiSS-1/GPR54 mRNA: Recrudescing ovaries
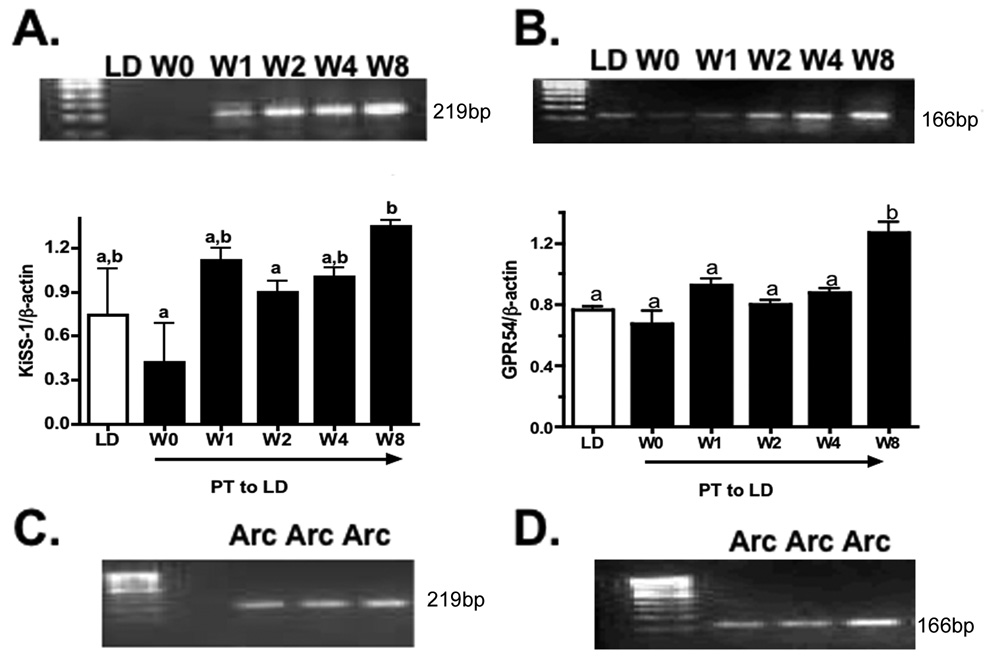
A single KiSS-1 mRNA band was detected in the recrudescing ovaries (Figure 4A). KiSS-1 mRNA expression in the ovaries from PT week 0 (14 weeks of SD exposure) did not differ from LD control ovaries (p>0.05) but photostimulation induced increases during recrudescence. Kiss-1 mRNA was significantly higher at PT week 8 as compared to PT weeks 0 and 2(Figure 4A; p<0.05). GPR54 mRNA expression in PT week 0 ovaries was not different than LD control (Figure 4B), but increased during recrudescence and was significantly higher at PT week 8 compared to all other groups (Figure 4B; p<0.05). To establish that the observed bands indeed represented KiSS-1 and GPR54, bands were extracted, column purified, and sequenced. KiSS-1 showed an 88 – 83% homology with Mus musculus (NM178260.13), Macaca mulatta (XM001098284.1), (EU365872) and Homo sapiens (AY117143.1), and 99% with Phodopus sungorus metastin mRNA. Sequence analysis of the GPR54 band showed 89% to human (NM032551.3) and 92% (NM053244.3) homology to the mouse GPR54 gene. As an additional positive control, we assessed KiSS-1 and GPR54 mRNA expression in the arcuate nucleus of Siberian hamsters, and bands identical to those from ovarian tissue were observed (Figure 4C, D).
Figure 4. KiSS-1 and GPR54 mRNA expression in recrudescing Siberian hamster ovaries.
Semi-quantitative RT-PCR expression of A) KiSS-1 and B) GPR54 mRNA in Siberian hamsters exposed to long day (LD), or post-transfer (PT) from short to long day photoperiod for 0, 1, 2, 4, and 8 weeks. Mean ± S.E.M. relative levels mRNA expression for KiSS-1 and GPR54 shown in respective graphs. β-actin was used as a control gene for all RT-PCR reactions. Columns with different letters on bar graphs indicate p<0.05. C) Kiss-1 and D) GPR54 expression in the arcuate nucleus of Siberian hamsters, used as a positive control.
Immunostaining of KiSS-1/GPR54 protein: Recrudescing ovaries
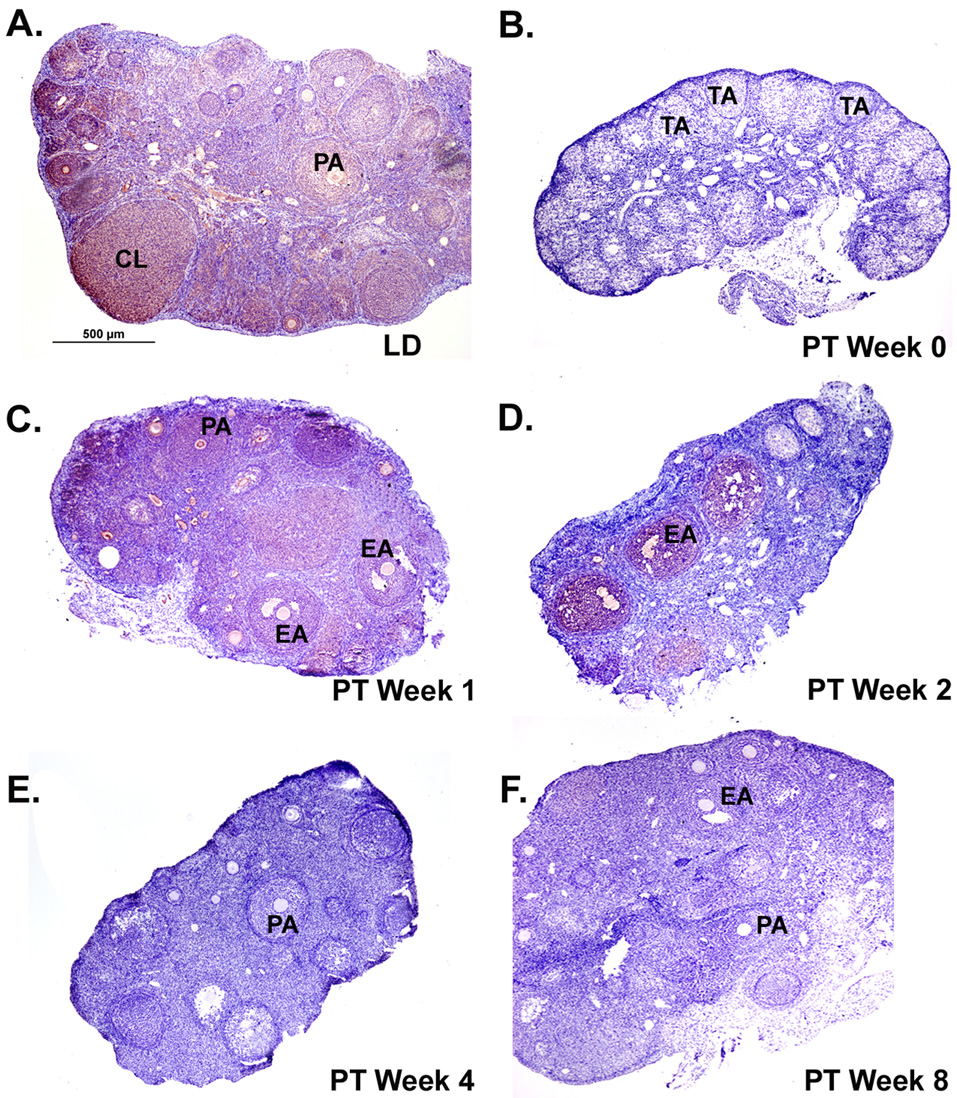
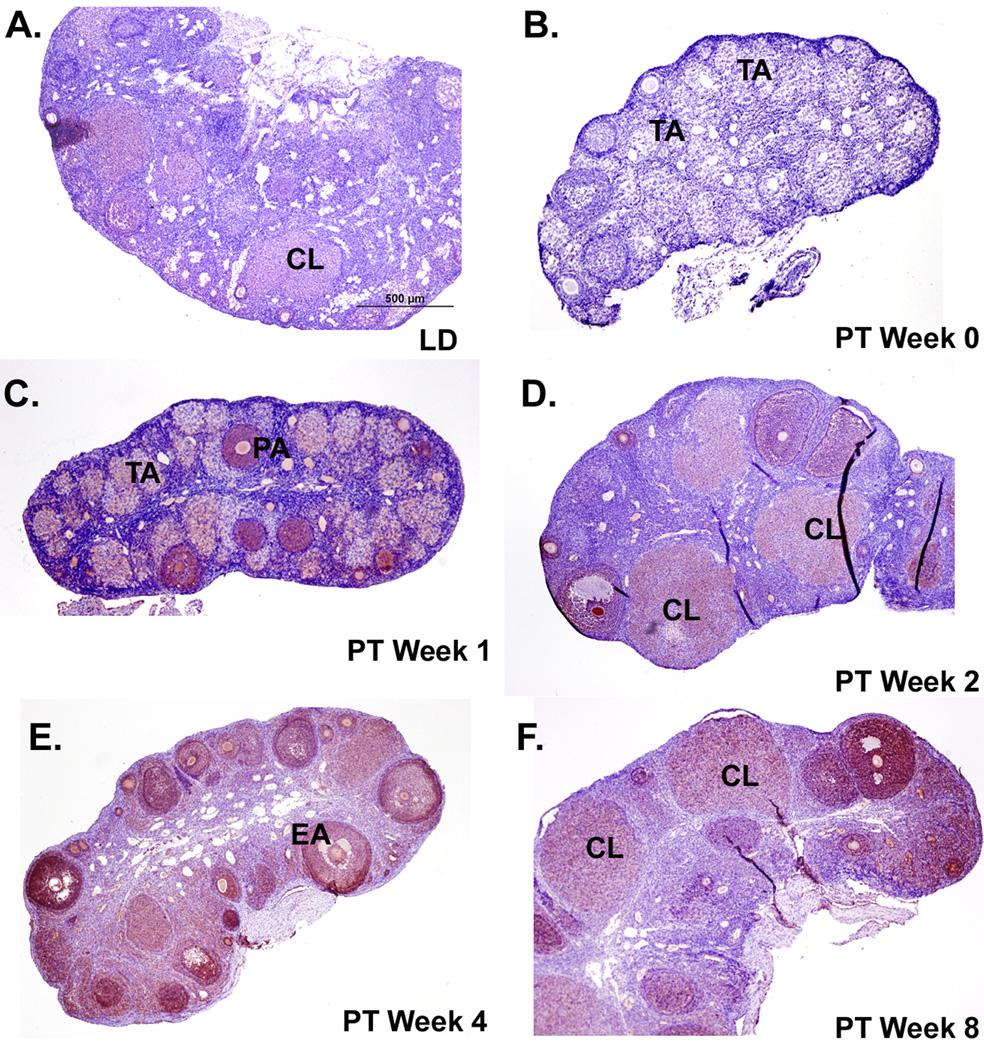
The overall intensity of KiSS-1 immunoreactivity was lower in the recrudescing ovaries from LD and PT weeks 0 to 8 (Figure 5) as compared to the staining in cycling ovaries during the P and E phases, but was similar to the diestrus stages in the estrous cycle experiment (Figure 1). Ovaries in the recrudescence experiment were collected when the hamsters were in diestrus phase (see Materials and Methods section). Due to this low intensity, KiSS-1 immunoexpression was not reliably quantifiable, although qualitatively it appeared reduced at PT week 0 as compared to LD and PT weeks 1 and 2 (Figure 5). GPR54 staining was the lowest in PT week 0 compared to LD and all recrudescing groups and appeared to increase slightly with recrudescence (Figure 6). Staining for both Kiss-1 and GPR54 appeared in granulosa cells of preantral, early antral, and antral follicles, along with CL; however staining was low and diffuse in the terminal atretic follicles (Figure 5 and Figure 6) that characterize SD regressed ovaries (Moffatt-Blue et al., 2006).
Figure 5. Immunoreactivity of KiSS-1 protein in Siberian hamster ovaries during photostimulated recrudescence.
Sections presented are representative of n = 6 hamsters per time-point. Staining is red/ink on purple background. Scale bar (A) depicts 500µm with photographs taken at 4x magnification for panels A–F. Tissue sections represent ovaries from the A) long day (LD) control, B) post-transfer (PT) week 0 (week 14 SD), C) PT week 1, D) PT week 2, E) PT week 4, and F) PT week 8 photoperiod groups. PA= preantral follicle; EA= early antral follicle; CL=corpus luteum; TA= terminal atretic follicle.
Figure 6. Immunoreactivity of GRP54 protein in Siberian hamster ovaries during photostimulated recrudescence.
Sections presented are representative of n = 6 hamsters per time-point. Staining is red/ink on purple background. Scale bar (A) depicts 500µm with photographs taken at 4x magnification for panels A–F. Tissue sections represent ovaries from the A) long day (LD) control, B) post-transfer (PT) week 0 (week 14 SD), C) PT week 1, D) PT week 2, E) PT week 4, and F) PT week 8 photoperiod groups. PA= preantral follicle; EA= early antral follicle; CL=corpus luteum; TA= terminal atretic follicle.
Discussion
Results presented here show, for the first time, that KiSS-1/GPR54 mRNA and protein are present in Siberian hamster ovaries. Protein immunostaining was cytosolic, differential, and mostly observed in steroidogenic cells, and, interestingly, both protein and mRNA expression showed differences across the estrous cycle and in response to photoperiod alterations.
Although the expression of KiSS-1 and GPR54 mRNA and protein in the brain of many species (reviews Seminara 2007, Smith et al. 2007, Kaufman et al. 2007) including Siberian (Greives et al. 2007), and Syrian hamsters (Revel et al. 2006) is well established, few studies (Castellano et al. 2006, Gaytan et al. 2007) have examined KiSS-1/GPR54 expression in the ovaries. Our results show cell and estrous stage- specific KiSS-1 staining in hamster ovaries. KiSS-1 immunostaining, most prominently in the theca and granulosa cells, peaked during P and E, and declined sharply during DII (Figure 1, Figure 3). GPR54 immunostaining was also relatively more intense in P and appeared to decline in DII, although the quantification was not significant (p=0.055; Figure 2, Figure 3). The timing of these observed changes in the KiSS/GPR54 system in the current study is consistent with a possible role in ovulation in Siberian hamsters, as has been suggested in rats (Castellano et al. 2006).
With the presence of RFamide structures at the C-terminal, kisspeptins belong to the RF family of amides (Fukusumi et al. 2006). The RFamides are neuropeptides that are distributed primarily in the brain. There is concern about the cross reactivity of commercially available kisspeptin antibodies raised against human peptides to other RF-amide peptides, particularly in brain tissue. While most RFamides have not been examined in ovarian tissue, expression of one purified RFamide, RFRP-1, in rats showed that this neuropeptide was present predominantly in the hypothalamus (2800 fmol/g wet wt), with greatly reduced levels in the thalamus and midbrain; however, was undetected in the ovaries and uterus (Fukusumi et al. 2001, 2006). Although one study (Goodman et al. 2007) showed that the kisspeptin antibody used in this study (human metastin 45–54, Phoenix Pharmaceuticals, Inc.) cross-reacted with some neuropeptides (gonadotropin inhibitory hormone and neuropeptide FF) in sheep brain, manufacturer’s literature indicates low to no cross reactivity towards neuropeptides AF, FF, RFRP-3, and FMRF-NH2 in rat tissue. This antibody does show a high degree of cross reactivity with rat, mouse, and human RFRP-1; however, this is the RFamide found not to be expressed in ovarian tissue (Fukusumi et al. 2001, 2006). In our hands, preabsorption of rabbit antimetastin antibody with kisspeptin peptide showed negligible to no staining in hamster ovaries (Figure 1G), actual staining was accomplished at relatively low concentrations for paraffin immunohistochemistry (1:1500), and staining results were parallel using two additional antibodies from separate manufacturers (data not shown). Together, these data suggest that in hamster ovaries, this kisspeptin antibody shows no cross reactivity. Based on the above information, staining using rabbit antimetastin antibody (Phoenix Pharmaceuticals), observed in the present study represents specific kisspeptin staining in hamster ovaries.
Because of the peak of ovarian KiSS-1 during proestrus (Figure 1, Figure 3), it is likely that KiSS-1 may have some dependency on LH secretion in the ovary. This association is clear in rats, with administration of GnRH antagonist effectively preventing a preovulatory rise in ovarian KiSS-1 mRNA, and antagonist plus LH replacement (hCG) reinstating KiSS-1 proestrus expression (Castellano et al. 2006). Indeed, in estrogen–primed ovariectomized rats, metastin (kisspeptin) injections into the preoptic area increase plasma LH concentrations, and the LH surge and estrous cyclicity can be effectively blocked using anti-metastin antibody (Kinoshita et al. 2005). Further evidence of systemic involvement of KiSS-1 in estrous cycle regulation includes an increase in LH concentration in rats following preovulatory intercerebral injections of KiSS-1 peptide (Kp-10), and an alteration of sensitivity to Kp-10 during the estrous cycle (Roa et al. 2006). In addition, expression of KiSS-1 in the hypothalamus appears to be central to regulating both positive and negative feedback effects of ovarian steroids on gonadotropin release (Navarro et al. 2004; Smith et al. 2005). Ovariectomy in rats increases hypothalamic KiSS-1 expression and decreases LH levels, but these effects are reversed by estradiol (Navarro et al. 2004). These studies establish a role of hypothalamic KiSS-1 in GnRH release and perhaps in regulating estrus cyclicity. This cyclic nature of hypothalamic KiSS-1 expression is mirrored by the cyclic pattern of KiSS-1/GPR54 expression in rat and Siberian hamster ovaries (Figure 1–Figure 2 and Castellano et al. 2006).
The predominant localization of KiSS-1 and GPR54 to steroidogenic cells in Siberian hamster ovaries along with their differential and temporal expression, suggests that these proteins may play a critical local role in ovarian function. While KiSS-1 was present in thecal and granulosa cells, apparent co-localization with the GPR54 receptor was limited to granulosa cells, and therefore differential function for the KiSS system may exist between these secretory cells. In non-ovarian tissues, KiSS-1/GPR54 expression is often associated with secretory cell types, with a suggested role in hormone secretion. KiSS-1 and GPR54 are expressed in cultured pituitary cells from peripubertal rats (Gutierrez-Pascal et al. 2007) and in bovine and porcine anterior pituitary cells (Suzuki et al. 2008). Addition of kisspeptin (Kp-10) to cultured pituitary cells stimulates an increase in free cytosolic Ca2+, LH, and growth hormone (Gutierrez-Pascal et al. 2007; Kadokawa et al. 2008), and the KiSS-1/GPR54 system plays a major role in pituitary GnRH secretion in the brain (Smith et al. 2007). Additionally, in the pancreas KiSS-1 is expressed only in the endocrine (α and β cells) cells, and exogenous addition of KiSS-1 stimulates insulin secretion from mouse islets (Hauge-Evans et al. 2006). Recently it has been shown that both GnRH and their receptors are locally expressed in rat ovaries and that their expression shows modulation with the estrous cycle (Schurman-Hildesheim et al. 2008). Due of this apparent congruency in function from both central and peripheral systems, in addition to the location of KiSS-1 in secretory cells in hamster ovaries, it is suggested that ovarian KiSS-1 may be associated with steroid release from the ovary, particularly in granulosa cells where both KiSS-1 and GPR54 were localized.
In addition to a possible role in ovarian steroid release, the KiSS-1/GPR54 system may be involved in ovulation by its effects on the activity of matrix metalloproteinases. In Siberian hamster ovaries, immunoreactivity of several matrix metalloproteinases (MMPs -1, -2, -9, -13) is substantially higher during P and E but declines sharply during diestrus I and II (Vrooman et al. 2007). This pattern parallels differential expression of KiSS-1/GPR54 immunoreactivity in hamster ovaries during the estrus cycle (Figure 1, Figure 2). Furthermore, kisspeptins down-regulate MMPs-2 and -9 (Yan et al. 2001), and interact with MMP-2 activity in human trophoblasts (Bilban et al. 2004). Since the expression of many MMPs is LH dependent and their stimulation of follicular breakdown is concomitant with progression of ovulation (reviewed by Curry and Osteen 2003), it is likely that the ovarian expression of KiSS-1/GPR54 exerts its effect on the reproductive process, in part, by regulating MMP activity.
Because ovarian function changes substantially during the initiation and cessation of the annual breeding season, local KiSS-1 expression may have additional functions beyond maintenance of the ovarian cycle. Recent studies (Revel et al. 2007; Greives et al. 2007; Smith et al. 2007) have suggested a key role for hypothalamic KiSS-1/GPR54 in seasonal reproduction. Photoperiodic cues are integrated by a photoneuroendocrine system that includes the retina, the suprachiasmatic nucleus of the hypothalamus, and the pineal gland. Biphasic pineal melatonin secretion affects pulsatile secretion of GnRH and thus LH release, and therefore can centrally regulate ovarian function (Bittman et al. 1985). Exposure of male Syrian hamsters to short photoperiods reduces KiSS-1 expression in the hypothalamic arcuate nucleus and this reduction is prevented by pinealectomy, suggesting that melatonin’s regulation of KiSS-1 expression may be a central mechanism for transmission of photoperiodic information (Revel et al. 2006, 2007). The present study corroborates a similar photoperiodic change in KiSS-1 mRNA and protein expression, in hamster ovaries. Immunostaining for both KiSS and GPR54 proteins was at low levels following 14 weeks of SD photoperiod, and exposure to LD increased staining levels (Figure 5, Figure 6). Eight weeks of post transfer LD also induced significant increases in KiSS and GPR54 mRNA expression (Figure 4A, B). This photostimulated increase in KiSS-1 and GPR54 parallels the return of ovarian function, as evidenced by the appearance of preantral, antral follicles, CL, and plasma estradiol concentrations during the first eight weeks of LD stimulation (Salverson et al. 2007). Present results show photoperiod associated changes in both KiSS-1 mRNA and protein although their pattern was generally, but not completely mirrored. While KiSS-1 immunoreactivity was low following SD exposure and increased (qualitatively) at PT weeks 1 and 2, it appeared to decline at PT weeks 4 and 8. The reason for this discrepancy between KiSS protein and mRNA data is not clear; however, it could be due to lower overall KiSS immunoreactivity in the recrudescing ovaries since they were collected during the DII stage (Figure 1, Figure 5). The DII stage was selected to maintain consistency between groups because SD-regressed ovaries remain in a DII-like state while ovarian function is reduced (Beasley et al. 1981). Investigation of the KiSS-1 system during different estrous cycle stages in the newly cycling ovaries during recrudescence would be a valuable next step.
Collectively, our observations for the first time suggest that KiSS-1/GPR54 mRNA and protein are expressed differentially in hamster ovaries during estrous cycle, and their expression is modulated in ovaries undergoing photoperiod-induced recrudescence. While the function of locally-produced KiSS-1/GPR54 in the ovary is currently unidentified, it is likely that this system may be critical during either ovulation or photostimulated return of ovarian function, via its possible effects on steroid secretion, regulation of MMP-mediated ovarian remodeling, or through another, as yet unknown mechanism.
Materials and Methods
Animals
Adult (> 60 days of age) female Siberian hamsters were obtained from the breeding colony of Dr. Katherine Wynne-Edwards, Queen’s University (Kingston, Ontario, Canada) and tissues were used for multiple experiments in our laboratory. Hamsters were housed individually in polypropylene cages (28 × 7.5 × 13 cm) at 20 ± 2°C, receiving food and water ad libitum. All experiments were conducted in our AAALAC-approved facilities, in accordance with California State University, Long Beach and NRC guidelines for use of laboratory animals. After a two-week period of acclimatization hamsters were subjected to the following treatments:
Estrous cycle experiment
Hamsters were kept in LD (16 hours of light: 8 hours of dark) photoperiod for 3 weeks. To maintain estrous cycling, four male hamsters were housed in the same room as the female hamsters. Estrous cycles were synchronized by placing soiled male bedding into individual female cages prior to planned tissue collection (Dodge et al., 2002). Vaginal cytology was used to stage ovaries (n=6/group) at proestrus (P), estrus (E), diestrus I (DI) and diestrus II (DII) as described previously (Moffatt-Blue et al. 2006). Ovaries were fixed in formalin, paraffin embedded, and sectioned (6 µm).
Recrudescence experiment
Hamsters were exposed to either LD (n=18) or SD (8L: 16D; n=50) for 14 weeks. Subsequently, SD exposed hamsters were transferred to LD for 0, 1, 2, 4 or 8 weeks (n= 10/group) and, for consistency, were euthanized while in the DII phase for reproductive tissue collection as described before (Moffatt-Blue et al. 2006; Salverson et al. 2008). Controls were maintained in LD and reproductive tissue collected on weeks 0, 2, and 8 (n=6/group). One ovary was fixed in formalin, embedded, sectioned (6µm) and used for immunohistochemistry. The contralateral ovary was frozen in liquid nitrogen for RNA extraction. Portions of this tissue set have been used for different experiments (Salverson et al. 2007).
Immunohistochemical detection of KiSS-1 and GPR54
Ovary sections (6µ) were deparaffinized in xylene, rehydrated through a graded series of ethanol, washed in phosphate buffer solution (PBS), and heated under pressure in Citra Antigen Unmasking solution (Vector Laboratories, Burlingame, CA) for 10 min, cooled, and washed in PBS. Sections were then placed in a 3% hydrogen peroxide/methanol solution, blocked with normal goat serum/Tween-20 (0.1%) for 40 min, washed in PBS, and then incubated with either rabbit antimetastin (45–54)-NH2 (human) antibody (1:1500) or rabbit anti GPR54 (mouse) antibody (1:200) (Phoenix Pharmaceuticals, CA) overnight at 4°C in humidified chambers. Biotinylated goat anti-rabbit immunoglobulin G (Vectastain Elite ABC kit, Vector Laboratories) and NovaRed Substrate (Vector Laboratories, Burlingame, CA) were used as a secondary antibody and colorimetric substrate according to manufacturer’s protocol. Sections were counterstained with hematoxylin, dehydrated, and mounted. Negative controls were processed without primary antibody. To verify the specificity of the primary antibodies in hamsters, primary antibodies were pre-absorbed with 10 µg of either GPR54 (375–396) or KiSS-1 (kp-10) amide peptides overnight at 4°C, centrifuged (15,000 rpm, 30 min), and the supernatant was used in place of primary antibodies and processed exactly as above. In addition, two other anti-KiSS-1 antibodies: rabbit anti-kisspeptin -13 (4–13) human (Pennisula Laboratories, LLC, San Carlos, CA) and rabbit anti-kisspeptin 10 (Chemicon International, Inc) were also tested at a 1:500 dilution. Rabbit anti-kisspeptin antibody kp-13 (Peninsula Laboratories) is specific for kisspeptin staining in AVPV and ARC (Grieves et al. 2007). The KiSS-1 staining pattern in the ovaries with antibodies from both Peninsula Laboratories and Chemicon (Kp-13 and Kp-10) were similar to the rabbit anti-metastin (45–54) antibody used in the present study (data not shown). We selected the latter antibody to detect kisspeptin because staining intensity matched the other antibodies but was specific at a higher dilution (1:1500), and because the manufacturer’s data suggests low cross-reactivity with several RF-amides (see discussion). Furthermore, preabsorption of this antibody with specific KiSS or GPR54 peptides, showed little or no staining.
Staining intensity and extent was quantified in the estrous cycle experiment using a scale of 0 (no staining) to 3 (high staining) by investigators blind to experimental conditions. Three sections per animal and 3–6 animals per group were used to assess staining extent. Results are presented as the mean relative degree (0–3) of KiSS-1 or GPR54 immunostaining per group as analyzed by a Kruskal-Wallis test followed by a Dunn's Multiple Comparison post hoc test (Prism 4 statistical software 240 package; GraphPad Software, Inc., San Diego, CA). Staining intensity in the recrudescing ovary sections was low and therefore difficult to quantify.
KiSS-1 and GPR54 mRNA RT-PCR
Total RNA from frozen ovaries was isolated using the RNeasy Micro Kit (QIAGEN, Valencia, CA) according to their protocol. One µg of total RNA was used for cDNA synthesis using ImProm Reverse Transcription System (Promega, Madison, WI). The PCR reaction mixture consisted of 1.5 µl of cDNA, 1 µl each of specific primers (10 µM) and 12.5 µl of GoTaq Green Promega PCR reagents (Promega, Madison, WI) in a total volume of 25 µl. Primer sequences and PCR conditions are listed in Table 1. We validated mRNA expression of KiSS-1/GPR54 mRNA using total RNA from the arcuate nucleus of Siberian hamsters as a positive control. Results are presented as the ratio of KiSS-1 or GPR54 to reference gene β-actin mRNA expression. Band density was determined by using NIH Image software as directed.
Table 1.
| Sequence | PCR conditions | |
|---|---|---|
| KiSS-1 | F- TCGTTAATGCCTGGGAAAAG | 96° (5m, 1 cycle) |
| R- CGAAGGAGTTCCAGTTGTAG | 96° (30s) 55°(1m) 72°(1m), | |
| 219 bp | 40 cycles, 72° (7m) | |
| GPR54 | F- GGCTGGTTCCTCTGTTCTTC | 95° (5m, 1 cycle) |
| R- TGGCGATGTAGAAGTTGGTC | 95° (30s) 54°(1m) 72°(1m), | |
| 166 bp | 36 cycles, 72° (7m) | |
| β-actin | F- GAAATCGTGCGTGACATC | 94° (1m, 1 cycle) |
| R- GCTTGCGATCCACATCT | 94° (30s) 55°(1m, 32 cycles) | |
| 500 bp | 72°(1m), 72° (7m) | |
Statistical analysis
One-way ANOVAs followed by a Neuman-Keuls post hoc test (Prism 4 statistical software 240 package; GraphPad Software, Inc., San Diego, CA) were used to analyze PCR data. A p value of < 0.05 was considered statistically significant.
Acknowledgments
Special thanks to Greer McMichael and Lisa Vrooman (embedding and sectioning), Trevor Salverson and Jonathan Sury (RNA extraction), Carling McMichael, and Melissa Bagnell (tissue harvesting) for their invaluable support for this project. This research was supported by NIH grant S06 GM063119-05 (KAY).
References
- Beasley LJ, Johnston PG, Zucker I. Photoperiodic regulation of reproduction in postpartum Peromyscus leucopus. Biol Reprod. 1981;24:962–966. doi: 10.1095/biolreprod24.5.962. [DOI] [PubMed] [Google Scholar]
- Bilban M, Ghaffari-Tabrizi N, Hintermann E, Bauer S, Molzer S, Zoratii C, Malli R, Sharabi A, Hiden U, Graier W, Knofler M, Andreae F, Wagner O, Quaranta V, Desoye G. Kisspeptin-10, a KiSS-1/metastin-derived decapeptide, is a physiological invasion inhibitor of primary human trophoblasts. J Cell Sci. 2004;117:1319–1328. doi: 10.1242/jcs.00971. [DOI] [PubMed] [Google Scholar]
- Bittman EL, Kaynard AH, Olster DH, Robinson JE, Yellon SM, Karsch FJ. Pineal melatonin mediates photoperiodic control pulsatile lutenizing hormone secretion in the ewe. Neuroendocrinology. 1985;40:409–418. doi: 10.1159/000124106. [DOI] [PubMed] [Google Scholar]
- Castellano JM, Gayton M, Vigo J, Navarro VM, Bellido C, Aguilar DE, Sanchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M. Expression of KiSS-1 in rat ovary: Putative local regulator of ovulation. Endocrinology. 2006;147:4852–4862. doi: 10.1210/en.2006-0117. [DOI] [PubMed] [Google Scholar]
- Colledge WH. GPR54 and Kisspeptins. Results Probl Cell Differ. 2008 Jan 12; doi: 10.1007/400_2007_050. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Curry TE, Osteen KG. The matrix metalloproteinase system: Changes, Regulation, and impact throughout the ovaraian and uterine reproductive cycle. Endocrine Rev. 2003;24:428–465. doi: 10.1210/er.2002-0005. [DOI] [PubMed] [Google Scholar]
- d'Anglemont de Tassigny X, Fagg LA, Dixon JP, Day K, Leitch HG, Hendrick AG, Zahn D, Franceschini I, Caraty A, Carlton MB, Aparicio SA, Colledge WH. Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proc Natl Acad Sci USA. 2007;104:10714–10719. doi: 10.1073/pnas.0704114104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodge JC, Kristal MB, Badura LL. Male-induced estrus synchronization in the female Siberian hamster (Phodopus sungorus sungorus) Physiological Behavior. 2002;7:227–231. doi: 10.1016/s0031-9384(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Dungan HM, Clifton DK, Steiner RA. Minireview: Kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology. 2006;147:1154–1158. doi: 10.1210/en.2005-1282. [DOI] [PubMed] [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ. Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotropin-releasing hormone/lutenizing hormone surge in ewe suggests stimulatory role for kisspeptins in oestrogen-positive feedback. J Neuroendocrinol. 2006;18:806–809. doi: 10.1111/j.1365-2826.2006.01485.x. [DOI] [PubMed] [Google Scholar]
- Fukusumi S, Fujii R, Hinuma S. Recent advances in mammalian Rfamide peptides: the discovery and functional analyses of PrRP, RFRPs and QRFP. Peptides. 2006;27:1073–1086. doi: 10.1016/j.peptides.2005.06.031. [DOI] [PubMed] [Google Scholar]
- Fukusumi S, Habata Y, Yoshida H, Iijiima N, Kawamata Y, Hoysoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Charecterstics and distribution of endogenous RFamide -related peptide-1. Biochem Biophys Acta. 2001;1540:221–232. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- Gaytan M, Castellano JM, Roa J, Sanchez-Criado JE, Tena-Sempere M, Gaytan F. Expression of KiSS-1 in rat oviduct: possible involvement in prevention of ectopic implantation? Cell Tissue Res. 2007;329:571–579. doi: 10.1007/s00441-007-0426-2. [DOI] [PubMed] [Google Scholar]
- Goodman RL, Lehman MN, Smith JT, Coolen LM, De Oliveira CVR, Jafarzadehshirazi MR, Periera A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- Gottsch ML, Cunningham MJ, Smith JT, Pospa SM, Acohido BV, Crowley WF, Seminara S, Clifton DK, Steiner RA. A role of kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145:4073–4077. doi: 10.1210/en.2004-0431. [DOI] [PubMed] [Google Scholar]
- Greives TJ, Mason AO, Scotti ML, Levine M, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptins: implications for seasonal reproduction. Endocrinology. 2007;148:1158–1166. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Pascal E, Martiznez-Fuentes AJ, Pinilla L, Malagon NM, Castano JP. Direct pituitary effects of kisspeptin: activation of gonadotrophs and somatographs and stimulation of lutenizing hormone and growth hormone secretion. J Neuroendocrinol. 2007;19:521–530. doi: 10.1111/j.1365-2826.2007.01558.x. [DOI] [PubMed] [Google Scholar]
- Hauge-Evan AC, Richardson CC, Milne HM, Christie MR, Persaud SJ, Jones PM. A role of kisspeptin in islet function. Diabetologia. 2006;49:2131–2135. doi: 10.1007/s00125-006-0343-z. [DOI] [PubMed] [Google Scholar]
- Kadokawa H, Suzuki S, Hashizume T. Kisspeptin-10 stimulates the secretion of growth hormone and prolactin directly from cultured bovine anterior pituitary cells. Anim Reprod Sci. 2008;105:404–408. doi: 10.1016/j.anireprosci.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signalling in the neuroendocrine regulation of reproduction. Trends in Neurosci. 2007;30:504–511. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, Yamada S, Inoue K, Ohtaki T, Matsumoto H, Maeda K-I. Involvment of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146:4431–4436. doi: 10.1210/en.2005-0195. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Planpain C, Schiffmann SN, Vassart G, Parmentier M. The metastsis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor. J Biol Chem. 2001;276:34631–34363. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Lee JH, Miele ME, Hicks DJ, Phillips KK, Trent JM, Weissman BE, Welsh DR. A novel human malignant melanoma metastsis-supressor gene. J Natl Cancer Inst. 1996;88:1731–1737. doi: 10.1093/jnci/88.23.1731. [DOI] [PubMed] [Google Scholar]
- Maeda K, Adaci S, Inoue K, Ohkura S, Tsukamura H. Metastin/Kisspeptin and control of estrus cycle. Rev Endocr Metab Diord. 2007;8:21–29. doi: 10.1007/s11154-007-9032-6. [DOI] [PubMed] [Google Scholar]
- Malpaux B, Migaud M, Tricoire H, Chemineau P. Biology of mammalian photoperiodism and the critical role of pineal gland and melatonin. J Biol Rhythms. 2001;16:336–347. doi: 10.1177/074873001129002051. [DOI] [PubMed] [Google Scholar]
- Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Horm Behav. 2007;52:492–498. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins are novel potent vasoconstrictors in humans, with a discrete localization of their receptor GPR54, to atherosclerosis prone vessels. Endocrinology. 2006;148:140–147. doi: 10.1210/en.2006-0818. [DOI] [PubMed] [Google Scholar]
- Mead EJ, Maguire JJ, Kuc RE, Davenport AP. Kisspeptins: a multifunctional peptide system with a role in reproduction, cancer and the cardiovascular system. British J Pharmacol. 2007;151:1143–1153. doi: 10.1038/sj.bjp.0707295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MBL, Colledge WH, Caraty A. Kisspeptins directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proc Natl Aca Sci. 2005;102:1761–1766. doi: 10.1073/pnas.0409330102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt-Blue C, Sury JJ, Young KA. Short photoperiod-induced ovarian regression is mediated by apotosis in Siberian hamsters (Phodopus sungorus) Reproduction. 2006;131:771–782. doi: 10.1530/rep.1.00870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Castellano JM, Fernandez-Fernandez R, Barreiro ML, Roa J, Sanchez-Criado JE, Aguilar E, Dieguez C, Pinilla L, Tena-Sempere M. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor GPR54 in rat hypothalamus and potent LH releasing activity KiSS-1 peptide. Endocrinology. 2004;145:4565–4574. doi: 10.1210/en.2004-0413. [DOI] [PubMed] [Google Scholar]
- Ohtaki T, Shintani Y, Honda S, Matsumoto H, Ishibashi Y, Watanabe T, Asada M, Yamada T, Suenaga M, Fujino C, Usuki S, Kurokawa T, Onda H, Nishimura O, Fujino M. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G protein-coupled receptor. Nature. 2001;411:613–617. doi: 10.1038/35079135. [DOI] [PubMed] [Google Scholar]
- Pevet P. The role of pineal gland in the photoperiodic control of reproduction in different hamster species. Reprod Nutr Dev. 1988;28:443–458. doi: 10.1051/rnd:19880310. [DOI] [PubMed] [Google Scholar]
- Revel FG, Ansel L, Klosen P, Saboureau, Pevet P, Mikklsen JD, Simonneaux V. Kisspeptin: A key link to seasonal breeding. Rev Endocr Metab Disord. 2007;8:57–65. doi: 10.1007/s11154-007-9031-7. [DOI] [PubMed] [Google Scholar]
- Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Curr Biol. 2006;16:1730–1735. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Roa J, Tena-Sempere M. KiSS-1 system and reproduction:comparative aspects and roles in the control of female gonadotropic axis in mammals. Gen Comp Endocrinol. 2007;153:132–140. doi: 10.1016/j.ygcen.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Salverson TJ, McMichael GE, Sury JJ, Shahed A, Young KA. Differential expression of matrix mealloproteinases during stimulated ovarian recrudescence in Siberian hamsters (Phodopus sungorus) Gen Comp Endocrinol. 2008;155:749–761. doi: 10.1016/j.ygcen.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriman-Hildesheim TD, Gershon E, Litichever N, Galiani D, Ben-Aroya N, Dekel N, Koch Y. Local production of the gonadotropic hormones in the rat ovary. Mol Cell Endocrinol. 2008;282:32–38. doi: 10.1016/j.mce.2007.11.014. [DOI] [PubMed] [Google Scholar]
- Seminara SB. Kisspeptins in reproduction. Semin Reprod Med. 2007;25:337–343. doi: 10.1055/s-2007-984739. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr, Apraicio SA, Colledge WH. The GPR54 gene as a regulator or puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahab M, Mastonardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Aca Sci USA. 2005;102:2129–2134. doi: 10.1073/pnas.0409822102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonneaux V, Ribelayga C. Generation of the melatonin endocrine message in mammals: a review of the complex regulation of melatonin synthesis by norepinephrine, peptides and other pineal transmitters. Pharmacol Rev. 2003;55:325–395. doi: 10.1124/pr.55.2.2. [DOI] [PubMed] [Google Scholar]
- Smith JT. Kisspeptin signaling in the brain: Steroid regulation in rodent and ewe. Brain Res Rev. 2007 doi: 10.1016/j.brainresrev.2007.04.002. [Epub April 19 ahead of print] [DOI] [PubMed] [Google Scholar]
- Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–1157. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA. Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/en.2005-0488. [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA. Kiss1 neurons in the forebrain as central processor for generating the preovulatory lutenizing hormone surge. J Neurosci. 2006;26:6687–6694. doi: 10.1523/JNEUROSCI.1618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle JH, von Gall C, Schomerus C, Korf HW. Regulation of melatonin synthesis in mammal pineal: Of rodents and ungulates and melatonin: creating a uniform code for darkness by different signaling mechanisms. J Biol Rhythms. 2001;16:312–325. doi: 10.1177/074873001129002033. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Kadokawa H, Hashizume T. Direct kisspeptin-10 stimulation on lutenizing hormone secretion from bovine and porcine anterior pituitary cells. Anim Reprod Sci. 2008;103:360–365. doi: 10.1016/j.anireprosci.2007.05.016. [DOI] [PubMed] [Google Scholar]
- Vrooman L, Moffatt-Blue C, Young KA. Differential MMP and TIMP expression during the estrous cycle and photoperiod-induced ovarian regression in Siberian hamsters (Phodopus sungorus) Biology of Reproduction Scientific Program for the 2007 Annual Meeting of the Society for the Study of Reproduction. :59. Abstract 621. [Google Scholar]
- Yan C, Wang H, Boyd DD. KiSS-1 represses 92-kDa typr IV collagenase expression by down-regulating NF-kappa B binding to the promoter as a consequence of Ikappa-balpha-induced block of p65/p50 nuclear translocation. J Biol Chem. 2001;276:1164–1172. doi: 10.1074/jbc.M008681200. [DOI] [PubMed] [Google Scholar]