Abstract
PHYTOCHROME INTERACTING FACTOR 3-LIKE5 (PIL5) is a basic helix-loop-helix transcription factor that inhibits seed germination by regulating the expression of gibberellin (GA)- and abscisic acid (ABA)-related genes either directly or indirectly. It is not yet known, however, whether PIL5 regulates seed germination solely through GA and ABA. Here, we used Chromatin immunoprecipitation-chip (ChIP-chip) analysis to identify 748 novel PIL5 binding sites in the Arabidopsis thaliana genome. Consistent with the molecular function of PIL5 as a transcription regulator, most of the identified binding sites are located in gene promoter regions. Binding site analysis shows that PIL5 binds to its target sites mainly through the G-box motif in vivo. Microarray analysis reveals that phytochromes regulate a large number of genes mainly through PIL5 during seed germination. Comparison between the ChIP-chip and microarray data indicates that PIL5 regulates 166 genes by directly binding to their promoters. Many of the identified genes encode transcription regulators involved in hormone signaling, while some encode enzymes involved in cell wall modification. Interestingly, PIL5 directly regulates many transcription regulators of hormone signaling and indirectly regulates many genes involved in hormone metabolism. Taken together, our data indicate that PIL5 inhibits seed germination not just through GA and ABA, but also by coordinating hormone signals and modulating cell wall properties in imbibed seeds.
INTRODUCTION
The timing of germination is critical for the survival of plants. A seed monitors various environmental factors, such as temperature, light, and water, and integrates these external conditions into plant hormonal signals inside the seeds that trigger germination at an optimal time. For many seeds, two plant hormones, namely, gibberellins (GAs), which break seed dormancy, and abscisic acid (ABA), which establishes and maintains seed dormancy, play important roles for seed germination. After dormancy is broken, ABA inhibits seed germination, whereas GA promotes seed germination (Finch-Savage and Leubner-Metzger, 2006). In addition to ABA and GA, other hormones, including ethylene, brassinosteroids (BRs), auxins, and cytokinins, have also been reported to promote (ethylene and BR) or inhibit (auxins and cytokinins) seed germination (Kucera et al., 2005). Various environmental conditions are thought to be integrated into plant hormonal signaling for the regulation of seed germination, but the precise modes of action are not yet fully understood.
Phytochrome-mediated light signaling provides a good model system for investigating how environmental conditions are integrated into plant hormonal signaling pathways in seeds. The pioneering work by Borthwick et al. (1952) showed that phytochromes are the major photoreceptors that promote seed germination in various plant species (Borthwick et al., 1952; Shinomura et al., 1994). Phytochromes undergo a reversible photoisomerization between the inactive Pr form and the active Pfr form in response to far-red and red light, respectively (Rockwell et al., 2006). The inactive Pr form is localized in the cytosol, whereas the red light–activated Pfr form is translocated to the nucleus (Yamaguchi et al., 1999; Nagy et al., 2000). In the nucleus, phytochromes activate various light responses, including seed germination, by modulating the activities of various phytochrome-interacting proteins (Bae and Choi, 2008).
In Arabidopsis thaliana, five phytochromes (PHYA to PHYE) regulate shared but distinct light responses (Mathews, 2006). PHYA is the primary phytochrome promoting seed germination in response to the very low fluence response and the far-red high irradiance response, whereas PHYB is the primary phytochrome promoting seed germination in response to the red light low fluence response. In Arabidopsis, PHYA is expressed only after prolonged imbibition, whereupon it exerts its effects (Shinomura et al., 1994, 1996). In addition to PHYA and PHYB, PHYE also promotes seed germination in response to low fluence response and far-red high irradiance in imbibed seeds (Hennig et al., 2002).
Phytochromes promote seed germination partly through GA. Light induces the expression of two GA anabolic genes, the GA 3-oxidase genes (GA3ox1 and GA3ox2), which encode enzymes that catalyze the final step in GA biosynthesis (GA9 and GA20 to GA4 and GA1, respectively). In addition, light represses a GA catabolic gene, GA 2-oxidase 2 (GA2ox2), which encodes an enzyme that converts active GAs (GA4 and GA1) to inactive catabolites (GA54 and GA8) (Yamaguchi et al., 1998; Yamauchi et al., 2007). This reciprocal regulation of GA metabolic genes results in a high level of bioactive GA in the light (Oh et al., 2006). This promotes degradation of the DELLA proteins, which are nuclear-localized GA negative signaling components that inhibit seed germination in conjunction with SCFSLY1 and GIBBERELLIN INSENSITIVE DWARF1 (GID1) (Itoh et al., 2008). Consistent with the expression patterns of these GA metabolic genes, mutations in ga3ox1 and ga3ox2 cause low germination frequencies in response to light, whereas mutation of ga2ox2 causes a high germination frequency even under far-red irradiation. In addition, light also enhances GA signaling, as shown by the increased GA responsiveness of the ga1 mutant in the light (Oh et al., 2007). This increased GA responsiveness is partly due to the transcriptional repression of GA INSENSITIVE (GAI) and REPRESSOR OF GA (RGA), two of the five DELLA genes (GAI, RGA, RGA-LIKE 1 [RGL1], RGL2, and RGL3) in Arabidopsis. Consistent with the repression of GAI and RGA by light, the gai-t6 rga double loss-of-function mutant is hypersensitive to red light for seed germination. The further loss of RGL2, another major DELLA protein that inhibits seed germination, abolishes the light requirement completely even in the ga requiring1 (ga1) mutant background, as shown by the light-independent germination of the gai-t6 rga rgl2 ga1 quadruple mutant (Cao et al., 2005). Taken together, these previous findings indicate that phytochromes promote seed germination by lowering the level of DELLA proteins through transcriptional repression of two DELLA genes and by increasing the level of bioactive GA, which activates the degradation of DELLA proteins.
Phytochromes also promote seed germination partly through ABA. In contrast with GA, light reduces the ABA level in seeds (Seo et al., 2006), primarily by repressing ABA anabolic genes (ABA DEFICIENT1 [ABA1], NINE-CIS-EPOXYCAROTENOID DIOXYGENASE6 [NCED6], and NCED9) and activating an ABA catabolic gene (CYP707A2) (Oh et al., 2007). The decreased level of ABA cannot inhibit seed germination because ABA positive signaling components, such as ABA INSENSITIVE3 (ABI3), ABI4, and ABI5, are not fully activated. Consistent with the expression patterns of ABA metabolic genes, mutations in ABA biosynthetic genes cause hypersensitivity to red light, whereas mutation of the ABA catabolic gene causes hyposensitivity to red light (Seo et al., 2006). It is not clearly understood how light regulates ABA signaling genes. However, microarray data indicate that the level of ABI3 mRNA is increased in phyB mutant seeds, suggesting that light represses ABA signaling not just through changes in ABA metabolism, but also by repressing an ABA positive signaling gene (Nakabayashi et al., 2005).
Phytochromes regulate hormone metabolism and signaling in seeds partly through PIL5 (also known as PHYTOCHROME INTERACTING FACTOR1 [PIF1] and BASIC HELIX-LOOP-HELIX PROTEIN 015) (Oh et al., 2007), which is a phytochrome-interacting basic helix-loop-helix (bHLH) transcription factor that inhibits seed germination (Huq et al., 2004; Oh et al., 2004). In the dark, PIL5 activates the expression of ABA anabolic genes (ABA1, NCED6, and NCED9) and a GA catabolic gene (GA2ox2) but represses an ABA catabolic gene (CYP707A2) and two GA anabolic genes (GA3ox1 and GA3ox2), resulting in seeds having a low GA level and a high ABA level (Oh et al., 2006, 2007). In addition, PIL5 also activates the expression of GAI and RGA, causing reduced GA responsiveness. Due to the increased ABA level, decreased GA level, and increased DELLA protein level, seeds do not germinate in the dark. In the light, phytochromes interact with PIL5 and activate the degradation of PIL5 protein. The degradation of PIL5 protein by light reverses the action of PIL5, causing a decreased ABA level, increased GA level, and decreased DELLA protein level. In response to these changes, seeds germinate.
Chromatin immunoprecipitation (ChIP) assays have shown that PIL5 directly binds to the promoter regions of GAI and RGA through a G-box motif (CACGTG), whereas it does not bind to the promoter regions of GA and ABA metabolic genes (Oh et al., 2007). Other unknown factors have been suggested to mediate the transcriptional regulation of these metabolic genes downstream of PIL5. The recent identification of SOMNUS (SOM) as a PIL5 direct target gene that regulates ABA and GA metabolic genes further supports the hypothesis that PIL5 target genes play important roles in seed germination (Kim et al., 2008). To identify PIL5 direct target genes at the genome level, we herein performed a ChIP assay coupled with genome tiling microarray (ChIP-chip). This analysis identified 748 PIL5 binding sites, most of which contain G-box elements (CACGTG). Comparison between the ChIP-chip and microarray data indicates that PIL5 regulates 166 of the genes by directly binding to their promoters in imbibed seeds. These 166 direct target genes include previously identified PIL5 direct target genes (RGA and SOM). In addition, the target genes include those encoding various hormone-related transcription regulators, such as ABI3, ABI5, AUXIN RESPONSE FACTOR18 (ARF18), BES1-INTERACTING MYC-LIKE PROTEIN1 (BIM1), and JASMONATE-ZIM-DOMAIN PROTEIN1 (JAZ1), and genes encoding cell wall–localized enzymes, such as expansins and xyloglucan endoglycosyltransferases. These results suggest that PIL5 regulates seed germination not just by regulating ABA and GA signaling, but also by coordinating hormone signaling and modulating cell wall properties in imbibed seeds.
RESULTS
Determination of PIL5 Binding Sites by ChIP-Chip Analysis
Previous studies showed that PIL5 regulates the expression of various GA metabolic genes (GA3ox1, GA3ox2, and GA2ox2), GA signaling genes (GAI and RGA), and ABA metabolic genes (ABA1, NCED6, and CYP707A2) to inhibit seed germination in the dark (Oh et al., 2006, 2007). Among them, PIL5 directly binds to the promoter regions of GAI and RGA, but not to those of any of the metabolic genes (Oh et al., 2007), suggesting that the identification of other PIL5 direct target genes might be useful for helping to delineate light signaling during seed germination.
We performed ChIP-chip analysis to identify PIL5 direct target genes at the genome level using PIL5-OX3 that expresses functional myc-tagged PIL5 protein (Oh et al., 2006). For the assay, ChIP was performed using imbibed seeds, and the precipitated DNA fragments were amplified, labeled, and hybridized to Arabidopsis whole-genome tiling array chips. The Arabidopsis whole-genome tiling array chips used in this report bore a total of 1,148,028 probes (50-mers) located every 90 bp throughout the Arabidopsis genome. A total of three biological replicates were performed for the ChIP-chip analysis.
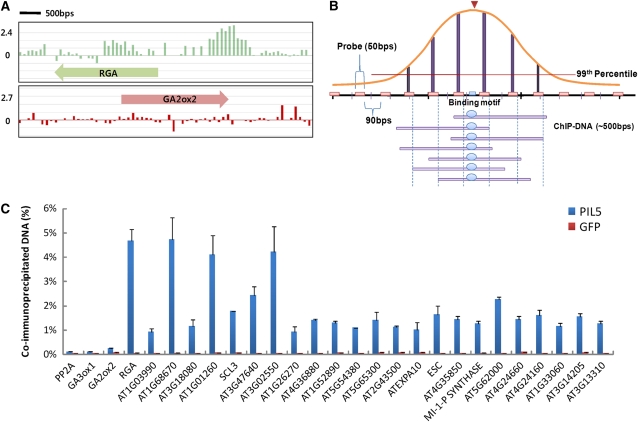
A graphic presentation of a ChIP-chip data set comprising the regions around the RGA and GA2ox2 genes is shown in Figure 1A. Probes in the promoter of RGA show high signal intensities, with a peak signal intensity around the G-box element, whereas probes in the promoter of GA2ox2 show low signal intensities. These results indicate that PIL5 binding sites can be distinguished from nonbinding sites by their clustered high signal intensities.
Figure 1.
Identification of PIL5 Binding Sites by ChIP-Chip Analysis of the Entire Arabidopsis Genome.
(A) Graphic representation of ChIP-chip data from around the RGA and GA2ox2 genes. Vertical bars (green and red) indicate the log ratio of precipitated DNA signal to input DNA signal. Thick arrows represent the transcribed regions of the RGA and GA2ox2 genes. Transcription starts at the tail of the arrow.
(B) Schematic diagram of the criteria used to determine PIL5 binding sites. If six consecutive probes had signals higher than the top 1%, the peak made by those signals was designated as a PIL5 binding site. A black horizontal bar (DNA) with pink boxes (probes) is indicated in the middle. Vertical bars represent signal ratios. Sonicated DNA fragments (∼500 bp; purple bars) that were cross-linked to PIL5 (blue circle) are indicated at the bottom. A red inverted triangle indicates a peak made by six consecutive signals, and its corresponding DNA region is indicated by a blue box on the DNA (black bar).
(C) Recapitulation of ChIP-chip data by manual ChIP. Twenty-three PIL5 binding sites were selected from the ChIP-chip data and tested for PIL5 binding using manual ChIP. PIL5-myc overexpressing (PIL5) and GFP-myc overexpressing (GFP) transgenic seeds were used for the ChIP assay. Negative controls (PP2A, GA3ox1, and GA2ox2) and a positive control (RGA) were included. Error bars indicate sd of triplicate experiments.
We adopted a Tamalpais peak-calling algorithm to analyze our ChIP-chip data for identification of PIL5 binding sites (Bieda et al., 2006). Briefly, if a minimum of six consecutive probes had signal intensities in the top 1% of all probe signal intensities, then the peak made by those probes was identified as a putative PIL5 binding peak (P value < 0.00001), and the genomic region corresponding to the peaks was called a putative PIL5 binding site (Figure 1B). Among the putative PIL5 binding sites, only those identified in at least two of the three biological replicates were considered final binding sites. This approach is thought to be a reliable method for determining binding sites from an extensive ChIP-chip data set (Bieda et al., 2006; Kim et al., 2007). Using these criteria, we identified a total of 748 PIL5 binding sites (see Supplemental Data Set 1 online).
To examine whether the employed criteria correctly identified true binding sites, we selected 23 of the putative PIL5 binding sites and determined whether the corresponding regions could also be enriched by a conventional ChIP assay. We included a positive ChIP assay control (the RGA promoter) and three negative controls (the PP2A, GA3ox1, and GA2ox2 promoters). Consistent with previous reports (Oh et al., 2007), our ChIP assay of GFP-OX seeds expressing myc-tagged green fluorescent protein (GFP) was indiscriminately enriched for relatively low levels of all promoters (Figure 1C). By contrast, the ChIP assay using the PIL5-OX3 seeds expressing myc-tagged PIL5 was highly enriched for the RGA promoter but not for the three negative control promoters. The same ChIP assay was highly enriched for all 23 of the putative PIL5 binding sites that we tested for in this analysis (Figure 1C). Among the sites, three regions were enriched to levels as high as that of the RGA promoter. The others were enriched to a lesser degree than the RGA promoter, but at levels that were still much higher than those of the negative control promoters. Taken together, these results suggest that the ChIP-chip assay and the employed criteria correctly identified PIL5 binding sites.
Distribution of PIL5 Binding Sites and Their Assignment to Genes
Figure 2A shows the proportion of probe numbers and PIL5 binding sites for each chromosome. Chromosome 1 contains slightly more PIL5 binding sites (26%) compared with other chromosomes (18 to 20%). However, the PIL5 binding sites are distributed more or less evenly across the five chromosomes (Figure 2A). In each chromosome, the binding sites are mainly located in gene-rich regions and are relatively rare in the centromeres and their surrounding regions (Figure 2B). Taken together, these data show that the PIL5 binding sites are distributed more or less evenly across all chromosomal regions except for the gene-poor centromeres and their surrounding regions.
Figure 2.
PIL5 Binding Sites Are Concentrated at the Proximal Regions of Promoters.
(A) The percentage of PIL5 binding sites for each Arabidopsis chromosome, adjusted to the number of probes in each chromosome.
(B) Distribution of PIL5 binding sites in the five Arabidopsis chromosomes. Bars represent the positions of the PIL5 binding sites on each chromosome. An orange circle indicates the location of the centromere.
(C) Distribution of PIL5 binding sites in the Arabidopsis genome.
(D) Distribution of PIL5 binding sites in the promoter regions (−3000 to +500 bp).
The locations of the PIL5 binding sites were determined in relation to nearby transcription start sites, and the results revealed that most of the binding sites (71%) are located in promoter regions (from −3000 to +500 bp of the start site) (Figure 2C). In the promoter regions, the locations of the PIL5 binding sites are further skewed toward regions immediately upstream of the transcription start sites, with the peak region being at −200 to −400 bp. Only a small portion of binding sites are present at 0 to +500 bp (6.7%) (Figure 2D). This distribution pattern is consistent with the molecular function of PIL5 as a transcription factor. The remaining 29% of the PIL5 binding sites are located either within intergenic regions (14%, upstream of −3000 bp) or within genic regions (15%, +501 to the 3′ untranslated region). Of the binding sites in genic regions, 46% are located in exons, 41% are located in introns, and 13% are located in 3′ untranslated region.
We next assigned the identified binding sites to their neighboring genes. Briefly, if a binding peak was located in the promoter region (−3000 to +500 bp) of a gene, we assigned the binding site to that gene. If a binding site was located within the promoters of two adjacent genes, we assigned the binding site to both genes. Binding sites located in intergenic or genic regions were not assigned to any gene. Using these criteria, we assigned a total of 748 binding sites to 750 genes, which are hereafter referred to as PIL5 direct target genes (see Supplemental Data Set 2 online). The PIL5 direct target genes include three previously identified PIL5 direct target genes, GAI, RGA, and SOM, suggesting that our analysis correctly identified the known target genes.
Analysis of PIL5 Binding Motifs
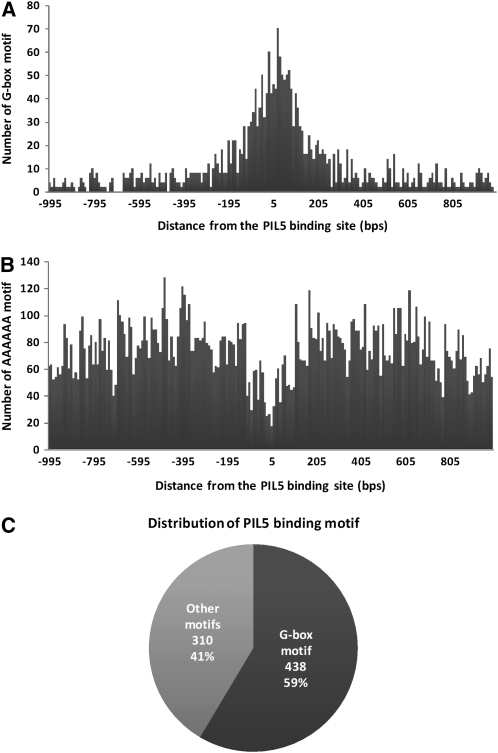
PIL5 binding motifs are likely to be present within +250 to −250 bp of the PIL5 binding sites. Consistent with a previous study showing that the G-box motif is a PIL5 binding motif in vivo (Oh et al., 2007), many of the identified PIL5 binding sites have G-box motifs in their surrounding regions. To determine the distribution pattern of PIL5 binding motifs, we plotted the frequency of G-box motifs in relation to the predicted PIL5 binding sites. As shown in Figure 3A, across the identified regions the frequency of a G-box motif increases near the predicted PIL5 binding sites and decreases to a background level beyond approximately +250 and −250 bp from the PIL5 binding site. By contrast, the frequency of a negative control hexameric sequence (AAAAAA) does not increase near the predicted PIL5 binding sites (Figure 3B). These results indicate that PIL5 binding motifs are mainly present in the 500-bp fragments within +250 to −250 bp of each predicted PIL5 binding site. Hereafter, these regions will be referred to as PIL5 binding regions.
Figure 3.
PIL5 Binds to the G-Box Motif in Vivo.
(A) Distribution of G-box motifs around the PIL5 binding sites (−1000 to +1000 bp).
(B) Distribution of AAAAAA motifs around the PIL5 binding sites (−1000 to +1000 bp).
(C) Percentage of PIL5 binding sites containing at least one G-box motif.
We then used the ab initio motif-finding programs, AlignACE (Roth et al., 1998) and MDscan (Liu et al., 2002), to discover PIL5 binding motifs in the PIL5 binding regions. Statistically overrepresented motifs were searched for in the 748 PIL5 binding regions. Both programs identified the G-box motif and failed to discover any other novel motif. The G-box motif is 15 times more frequent in the PIL5 binding regions compared with the whole genome. Consistent with this, a large number (438) of the identified PIL5 binding regions contain one or more G-box motifs (Figure 3C). However, the remaining 310 PIL5 binding regions do not contain any G-box motif, suggesting that PIL5 binds to these regions through other motifs.
Determination of PIL5-Regulated Genes in Imbibed Seeds by Microarray Analysis
We next sought to determine which of the PIL5 direct target genes are transcriptionally regulated by PIL5 in imbibed seeds. We performed microarray analysis using imbibed seeds of Columbia-0 (Col-0) and the pil5 mutant. RNA samples were extracted from imbibed seeds following irradiation with either a far-red light pulse alone [Col-0(D) and pil5(D)] or a far-red light pulse immediately followed by a red light pulse [Col-0(R) and pil5(R)]. Three independent biological replicates were used for the microarray analysis. Genes having low signal intensity (<64) were excluded from the analysis to reduce the inconsistency associated with low-intensity signals. Differentially expressed genes (DEGs) were identified using the Limma package with the false discovery rate (FDR) set to 5% and a 1.5-fold change.
The microarray analysis shows that PIL5-mediated phytochrome signaling alters the expression of ∼10% of the whole genome in imbibed seeds. Red light alters the expression of 2031 genes in the Col-0 seeds (Figure 4A); 1006 and 1025 of these genes are activated and repressed, respectively, indicating that red light activates and represses genes to a similar degree in imbibed seeds. By contrast, none of these 2031 genes are differentially expressed in the pil5 mutant seeds (Figure 4B), indicating that these genes are all regulated by PIL5 (either directly or indirectly) in imbibed seeds.
Figure 4.
PIL5 Mediates Red Light Signaling in Imbibed Seeds.
(A) Venn diagram showing an overlap between the DEG sets of Col-0(D)/Col-0(R) and Col-0(D)/pil5(D). DEGs were determined based on fold change (>1.5), P value (<0.01), and FDR (<0.05).
(B) Venn diagram showing no overlap between the DEG sets of Col-0(D)/Col-0(R) and pil5(D)/pil5(R).
(C) Hierarchical cluster display of expression ratios in Col-0(D)/Col-0(R), Col-0(D)/pil5(D), and pil5(D)/pil5(R). The DEGs in at least one sample pair were included in this cluster. Expression ratios are given as log values.
Consistent with the major role of PIL5, both pil5(D) and Col-0(R) express similar sets of genes. When we compared the DEGs among samples, we found that the DEG set of Col-0(D)/pil5(D) overlaps with that of Col-0(D)/Col-0(R) (1156 out of 1680; 69%) (Figure 4A) and shows a high expression correlation (r = 0.893) (Figure 4C). Taken together, these results show that the gene expression pattern of pil5(D) is similar to that of Col-0(R), indicating that imbibed pil5 mutant seeds are similar to imbibed, red light–treated wild-type seeds. Since phytochromes promote the degradation of PIL5 in imbibed seeds, these results further suggest that phytochromes regulate gene expression mainly by decreasing the level of PIL5.
Determination of PIL5-Regulated Direct Target Genes by Comparing PIL5-Regulated Genes and PIL5 Direct Target Genes
We defined PIL5-regulated direct target genes as the PIL5 direct target genes that show PIL5-mediated expression changes during seed germination. To determine the PIL5-regulated direct target genes, we compared the PIL5-regulated genes identified from our microarray analysis with the PIL5 direct target genes identified from our ChIP-chip assay. Due to the limited number of probes contained within the Affymetrix microarray, only 655 out of the 750 PIL5 direct target genes had available expression data; thus, only these 655 genes were used in the comparison. For the PIL5-regulated genes, we used the union DEG set of Col-0(D)/Col-0(R) and Col-0(D)/pil5(D) (2555 genes; see Supplemental Data Set 3 online).
The comparison identified 166 genes as PIL5-regulated direct target genes, indicating that PIL5 binds to the promoters of these 166 genes and regulates their expression either positively or negatively in imbibed seeds (Table 1). Consistent with previous reports (Oh et al., 2007; Kim et al., 2008), the PIL5-regulated direct target genes include RGA and SOM, but not the ABA and GA metabolic genes. GAI is not included in the list because of its relatively high FDR value (0.16). Among the 166 PIL5-regulated direct target genes, 105 and 61 of them are positively and negatively regulated by PIL5, respectively (Figure 5A). This is in contrast with the similar numbers of positively and negatively regulated genes contained within the overall set of PIL5-regulated genes (1267 and 1288 genes, respectively). These results indicate that PIL5 activates twice as many of its direct target genes than it represses in imbibed seeds.
Table 1.
List of PIL5-Regulated Direct Target Genes
| Gene | FC | Gene | FC | Gene | FC | Gene | FC |
|---|---|---|---|---|---|---|---|
| AT3G16150 | −9.00 | AT1G25550 | −1.68 | AT2G44920 | 1.84 | AT3G03150 | 2.77 |
| PGP4 | −6.50 | JA Z1 | −1.67 | AT4G24110 | 1.85 | ATHSD5 | 2.83 |
| CP1 | −5.47 | AT3G21540 | −1.66 | AT2G45910 | 1.89 | AT4G27460 | 2.84 |
| EXP8 | −4.53 | DSO | −1.65 | AT4G 19700 | 1.92 | AT1G08570 | 2.85 |
| CRF3 | −4.52 | CAX2 | −1.64 | AT5G49710 | 1.92 | AT1G 16730 | 2.85 |
| EXP10 | −4.27 | KUP3 | −1.64 | CYP78A7 | 1.95 | AT1G62290 | 2.89 |
| RSW 10 | −3.73 | AT3G24050 | −1.64 | AHA4 | 1.98 | AT1G03790 | 2.93 |
| HAT1 | −3.49 | AT3G17830 | −1.63 | AT3G47640 | 1.99 | AT5G61590 | 2.95 |
| AT5G65300 | −3.49 | AT5G60670 | −1.63 | AT1G78700 | 1.99 | CAT3 | 2.98 |
| AT5G51550 | −3.48 | AT5G03370 | −1.62 | RGA | 2.04 | AT5G01950 | 3.16 |
| AT1G74940 | −3.40 | VPS24.1 | −1.62 | ARF18 | 2.04 | AT3G60690 | 3.16 |
| THE1 | −3.31 | AT1G32920 | −1.61 | AT4G37550 | 2.05 | HSFA7B | 3.31 |
| CRF2 | −3.18 | AT5G10750 | −1.60 | AT5G24930 | 2.07 | AT1G30110 | 3.34 |
| IAA16 | −3.18 | IQ D22 | −1.60 | RAD23-3 | 2.07 | AT3G52500 | 3.35 |
| AT5G05600 | −2.90 | AT3G21090 | −1.55 | AT5G08139 | 2.07 | GID1A | 3.45 |
| LBD41 | −2.79 | AT1G79150 | −1.55 | AT1G22060 | 2.09 | AT3G25870 | 3.48 |
| AT2G27500 | −2.78 | AT1G60190 | −1.52 | AT5G63530 | 2.11 | AT1G68670 | 3.50 |
| XTR2 | −2.75 | AT1G03850 | −1.52 | ABI5 | 2.17 | BIM2 | 3.52 |
| AT1G 19450 | −2.73 | AT1G80530 | −1.50 | N IT1 | 2.18 | AT1G01260 | 3.56 |
| AGP15 | −2.71 | AT2G45620 | 1.53 | PHYA | 2.20 | AT1G27990 | 3.67 |
| RALFL34 | −2.66 | AT5G23850 | 1.54 | GBF3 | 2.24 | AT4G33800 | 3.83 |
| CRF1 | −2.55 | AT3G19590 | 1.54 | AT5G02580 | 2.24 | AT2G38465 | 3.84 |
| AT2G16660 | −2.52 | AT5G65380 | 1.54 | AT5G01720 | 2.25 | AT3G56350 | 3.88 |
| AT-GTL2 | −2.48 | ABI3 | 1.55 | MRP5 | 2.26 | AT1G49500 | 4.11 |
| HEC1 | −2.42 | SIR | 1.55 | AT1G01650 | 2.26 | DRM 1 | 4.19 |
| SKS4 | −2.30 | M I-1-P SYNTHASE | 1.58 | ACR3 | 2.27 | AT1G75490 | 4.22 |
| AT1G80440 | −2.29 | AT1G03590 | 1.59 | PHYB | 2.30 | AT3G57020 | 4.38 |
| ATSIP2 | −2.26 | AT5G35660 | 1.60 | AT5G66730 | 2.32 | AT1G03610 | 4.51 |
| UGT84A2 | −2.22 | AT2G45820 | 1.61 | LBD40 | 2.33 | AHA11 | 4.59 |
| AT2G43500 | −2.21 | AT3G47650 | 1.63 | AT1G16840 | 2.35 | AT5G58650 | 4.61 |
| AT3G62630 | −2.20 | GRF8 | 1.66 | AT1G14910 | 2.41 | AT3G12920 | 4.63 |
| AT3G54000 | −2.14 | AT2G28420 | 1.69 | ANAC002 | 2.43 | AT3G18080 | 4.96 |
| AT1G31240 | −2.11 | AT1G69840 | 1.69 | AT5G07020 | 2.49 | AT5G66590 | 5.11 |
| AT2G42770 | −1.96 | AT1G70790 | 1.69 | AT5G06980 | 2.51 | AT4G18650 | 5.17 |
| AT1G26270 | −1.92 | AT2G45590 | 1.69 | AT1G07430 | 2.56 | AT5G07010 | 5.35 |
| AtGRF5 | −1.90 | AT4G31390 | 1.70 | ATBAG7 | 2.63 | AT4G28240 | 6.34 |
| ANAC019 | −1.90 | AT3G14360 | 1.73 | AT5G10740 | 2.63 | ALDH2C4 | 8.18 |
| AT5G42250 | −1.88 | AT2G46710 | 1.76 | AT1G67310 | 2.73 | AT1G17830 | 8.43 |
| AHUS5 | −1.85 | AT5G28300 | 1.77 | AT5G64260 | 2.74 | AT1G16850 | 8.64 |
| M FP1 | −1.74 | AT1G03600 | 1.79 | AT1G56220 | 2.74 | FHL | 29.78 |
| AT5G65900 | −1.69 | AT1G19110 | 1.81 | AT2G31980 | 2.75 | PIL2 | 34.53 |
| AT2G21520 | −1.69 | AT1G75810 | 1.82 |
Positive values indicate upregulated genes by PIL5. FC, fold change.
Figure 5.
Identification of PIL5-Regulated Direct Target Genes by Comparison between the Microarray and ChIP-Chip Data.
(A) Venn diagram showing the overlap between the PIL5-regulated genes and the PIL5 direct target genes. Genes in the overlap (166 genes) are defined as PIL5-regulated direct target genes, indicating that PIL5 binds to their promoters and regulates their expression.
(B) PIL5 direct target genes whose expression is not altered in the pil5 mutant can be regulated by PIL5 in PIL5-OX seeds. Expression levels were normalized to that of PP2A. Bars indicate sd across three PCR reactions.
(C) GO analysis of PIL5-regulated direct target genes. The size of each circle is proportional to the number of genes annotated to that node. The yellow color of the circles represents enriched categories based on the FDR-corrected P value ranging from 0.05 (yellow) or below (darker yellow).
The number of PIL5-regulated direct target genes is quite small compared with the total number of PIL5-regulated genes (166 out of 2555 genes; 6.5%) (Figure 5A), suggesting that PIL5 indirectly regulates the majority of its target genes. This may occur through transcriptional cascades initiated by transcription factors encoded by the PIL5-regulated direct target genes. Consistent with this idea, genes encoding transcription factors are enriched (34 out of 166; 21%) among the PIL5-regulated direct target genes (Table 1), suggesting that these transcription factors could regulate the expression of many genes downstream of PIL5. In addition, PIL5 could indirectly regulate the expression of genes through hormonal and biochemical metabolism.
Only 25% of the total PIL5 direct target genes are PIL5 regulated (166 out of 655 genes) (Figure 5A), indicating that three-fourths of the PIL5 direct target genes are not differentially regulated by PIL5 in imbibed seeds. A previous ChIP-chip analysis of ELONGATED HYPOCOTYL5 (HY5) also showed that only 19% of HY5 direct target genes are differentially regulated by HY5 in seedlings (Lee et al., 2007), suggesting that both PIL5 and HY5 regulate only a subset of their direct target genes. The 75% of PIL5 direct target genes not regulated by PIL5 under the tested conditions could be regulated by PIL5 during other developmental stages or under other environmental conditions. To investigate this possibility, we chose 21 of the nonregulated PIL5 direct target genes and determined whether they are differentially expressed when PIL5 is ectopically expressed. Consistent with our microarray data, none of these 21 genes are differentially expressed in pil5 mutant seeds compared with Col-0 seeds (see Supplemental Data Set 3 online), but the expression of 10 out of the 21 genes is significantly increased in PIL5-OX1 seeds (Figure 5B). These results suggest that approximately half of the nonregulated PIL5 direct target genes may be regulated by ectopically expressed PIL5. The remaining half of the nonregulated PIL5 direct target genes are not differentially expressed in the pil5 mutant or in PIL5-overexpressing seeds.
Gene Ontology Analysis of PIL5-Regulated Direct Target Genes
Gene Ontology (GO) analysis using BiNGO (Maere et al., 2005) shows that nuclear-localized proteins are enriched among the proteins encoded by the PIL5-regulated target genes compared with the entire Arabidopsis proteome (Figure 5C). In regard to molecular function, proteins with transcription regulator, transcription factor, and DNA binding activity are highly enriched among those encoded by PIL5-regulated direct target genes. Thirty-four of the 166 PIL5-regulated direct target genes encode transcriptional regulators (20%); this is significantly higher than the proportion of such genes in the whole Arabidopsis genome (5.9%) (Riechmann et al., 2000). Consistent with this finding, transcription is the most enriched biological process among the PIL5-regulated direct target genes. These results support the notion that PIL5 regulates the expression of PIL5-regulated genes at the upper hierarchy of the transcriptional cascades in imbibed seeds. In addition, proteins localized in the cell wall are also enriched among the PIL5-regulated direct target genes, suggesting that PIL5 also modulates cell wall properties by directly regulating genes involved in cell wall modification.
Functional Classification of PIL5-Regulated Direct Target Genes: Hormone Metabolic and Signaling Genes
The enriched transcription regulators include various hormone-related transcription regulators (Table 2), such as ABA signaling genes (ABI3 and ABI5), auxin signaling genes (INDOLE ACETIC ACID-INDUCED PROTEIN16 [IAA16] and ARF18), a BR signaling gene (BIM2), cytokinin signaling genes (CYTOKININ RESPONSE FACTOR1 [CRF1], CRF2, and CRF3), a GA signaling gene (RGA), and a JA signaling gene (JAZ1). These results indicate that PIL5 regulates various hormone signals by directly binding to the promoters of these genes that regulate the transcription of other genes and regulating their expression in imbibed seeds. The inclusion of various hormone genes in the set of PIL5-regulated genes further suggests that PIL5 regulates not just one or two hormone signals, but rather coordinates various hormonal signals during seed germination. To analyze the role of PIL5 in the various hormone signaling pathways, we discuss (below) these hormone-related PIL5 direct target genes together with their indirectly regulated hormone metabolic genes.
Table 2.
List of PIL5-Regulated Hormone-Related Genes
| Hormone | Class | AGI | Name | FC | Direct | Hormone | Class | AGI | Name | FC | Direct |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ABA | Signaling | AT4G26080 | ABI1 | −1.94 | X | Cytokinin | Signaling | AT1G03430 | AHP5 | 1.91 | X |
| ABA | Signaling | AT3G24650 | ABI3 | 1.55 | O | Cytokinin | Signaling | AT4G11140 | CRF1 | −2.55 | O |
| ABA | Signaling | AT2G40220 | ABI4 | −3.10 | X | Cytokinin | Signaling | AT4G23750 | CRF2 | −3.18 | O |
| ABA | Signaling | AT2G36270 | ABI5 | 2.17 | O | Cytokinin | Signaling | AT5G53290 | CRF3 | −4.52 | O |
| ABA | Metabolic | AT5G67030 | ABA1 | 2.56 | X | Ethylene | Signaling | AT3G04580 | EIN4 | −1.53 | X |
| ABA | Metabolic | AT2G29090 | CYP707A2 | −2.36 | X | Ethylene | Signaling | AT3G23150 | ETR2 | −1.72 | X |
| ABA | Signaling | AT5G66880 | SNRK2.3 | −1.91 | X | Ethylene | Signaling | AT3G20770 | EIN3 | 1.67 | X |
| ABA | Signaling | AT1G52920 | GCR2 | 2.00 | X | GA | Signaling | AT3G05120 | GID1A | 3.45 | O |
| ABA | Signaling | AT1G72770 | HAB1 | 1.86 | X | GA | Signaling | AT5G27320 | GID1C | 2.16 | X |
| Auxin | Signaling | AT4G28640 | IA A11 | 2.26 | X | GA | Signaling | AT2G01570 | RGA | 2.04 | O |
| Auxin | Signaling | AT3G04730 | IAA16 | −3.18 | O | GA | Signaling | AT1G66350 | RGL1 | −2.84 | X |
| Auxin | Signaling | AT3G61830 | ARF18 | 2.04 | O | GA | Metabolic | AT5G25900 | GA3 | 1.74 | X |
| Auxin | Signaling | AT5G42190 | ASK2 | 1.66 | X | GA | Metabolic | AT5G51810 | GA20OX2 | 3.92 | X |
| Auxin | Metabolic | AT1G08980 | AM I1 | 3.27 | X | GA | Metabolic | AT5G07200 | GA20OX3 | 4.32 | X |
| Auxin | Metabolic | AT3G44310 | N IT1 | 2.18 | O | GA | Metabolic | AT1G 15550 | GA3OX1 | −7.12 | X |
| Auxin | Metabolic | AT3G44320 | N IT3 | 2.74 | X | GA | Metabolic | AT1G80340 | GA3OX2 | −14.51 | X |
| Auxin | Signaling | AT2G35635 | RUB2 | −1.63 | X | GA | Metabolic | AT1G30040 | GA2OX2 | 9.32 | X |
| Auxin | Metabolic | AT5G20960 | AAO 1 | 1.62 | X | JA | Signaling | AT1G 19180 | JAZ1 | −1.67 | O |
| Auxin | Metabolic | AT2G20610 | SUR1 | −2.64 | X | ||||||
| Auxin | Metabolic | AT4G37390 | YDK1 | 3.33 | X | ||||||
| Auxin | Metabolic | AT5G54510 | DFL1 | −3.41 | X | ||||||
| BR | Signaling | AT5G42750 | BKI1 | −2.13 | X | ||||||
| BR | Signaling | AT4G18710 | BIN2 | 1.78 | X | ||||||
| BR | Signaling | AT5G08130 | BIM 1 | 1.95 | X | ||||||
| BR | Signaling | AT1G69010 | BIM2 | 3.52 | O | ||||||
| BR | Metabolic | AT3G50660 | DW F4 | −3.32 | X |
FC, fold change; O, PIL5 direct target gene; X, not PIL5 direct target gene.
GA Metabolic and Signaling Genes
PIL5 indirectly regulates various GA metabolic and signaling genes (Table 2). Among the GA metabolic genes, PIL5 negatively regulates two GA anabolic genes (GA3ox1 and GA3ox2) and positively regulates one GA catabolic gene (GA2ox2). In addition, the PIL5-regulated gene set includes three GA anabolic genes (GA3 [encoding ent-kaurene oxidase] and two GA 20-oxidase genes [GA20ox2 and GA20ox3]). In contrast with the two negatively regulated GA 3-oxidases, the three GA anabolic genes are positively regulated by PIL5, suggesting that the level of bioactive GA is determined not by the simple dichotomous transcriptional regulation of anabolic and catabolic genes in imbibed seeds, but rather by the summed action of various positively and negatively regulated GA metabolic genes.
Among the GA signaling genes, two GA receptor genes (GID1A and GID1C) are positively regulated, and the DELLA genes, RGA and RGL1, are positively and negatively regulated, respectively, by PIL5. PIL5 regulates GID1A and RGA1 directly, while it regulates GID1C and RGL1 indirectly. The positive regulation of RGA by PIL5 is consistent with previous reports demonstrating that PIL5 inhibits GA signaling (Oh et al., 2007). However, the positive regulation of two GA receptor genes and the negative regulation of RGL1 are contrary to the proposed negative role of PIL5 in GA signaling (Oh et al., 2007). Since many hormone-related genes are under feedback regulation, opposite expressions of these genes might be caused by feedback regulations. Taken together, the results suggest that, similar to the case of GA metabolism, the role of PIL5 in GA signaling is the summed result of both positively and negatively regulated GA signaling components.
ABA Metabolic and Signaling Genes
PIL5 also regulates both ABA metabolic and signaling genes either directly or indirectly (Table 2). Among the ABA metabolic genes, PIL5 activates the expression of the ABA anabolic gene ABA1 but represses the expression of the ABA catabolic gene CYP707A2. However, our PIL5-regulated gene set does not include two other ABA anabolic genes (NCED6 and NCED9) that were previously shown to be positively regulated by PIL5 (Oh et al., 2007), suggesting that the criteria we used for identifying DEGs did not encapsulate all PIL5-regulated genes. In addition to ABA metabolic genes, our PIL5-mediated gene set includes a number of ABA signaling genes (ABI1, ABI2, ABI3, ABI5, G PROTEIN COUPLED RECEPTOR2 [GCR2], HOMOLOGY TO ABI1 [HAB1], and SNF-RELATED PROTEIN KINASE2.3 [SnRK2.3]). PIL5 activates the expression of HAB1, GCR2, ABI3, and ABI5, while it represses the expression of ABI1, ABI4, and SnRK2.3. Among these ABA-related genes, PIL5 directly regulates ABI3 and ABI5, two central positive ABA signaling transcription factors, but indirectly regulates the other components. Similar to the case of the GA signaling genes, however, PIL5 does not activate or repress ABA signaling components simply based on their positive or negative roles in ABA signaling, further suggesting that the role of PIL5 in ABA signaling is the summed activity of numerous positively and negatively regulated signaling components. As for some GA-related genes, opposite directions of gene expression could be caused by feedback regulations.
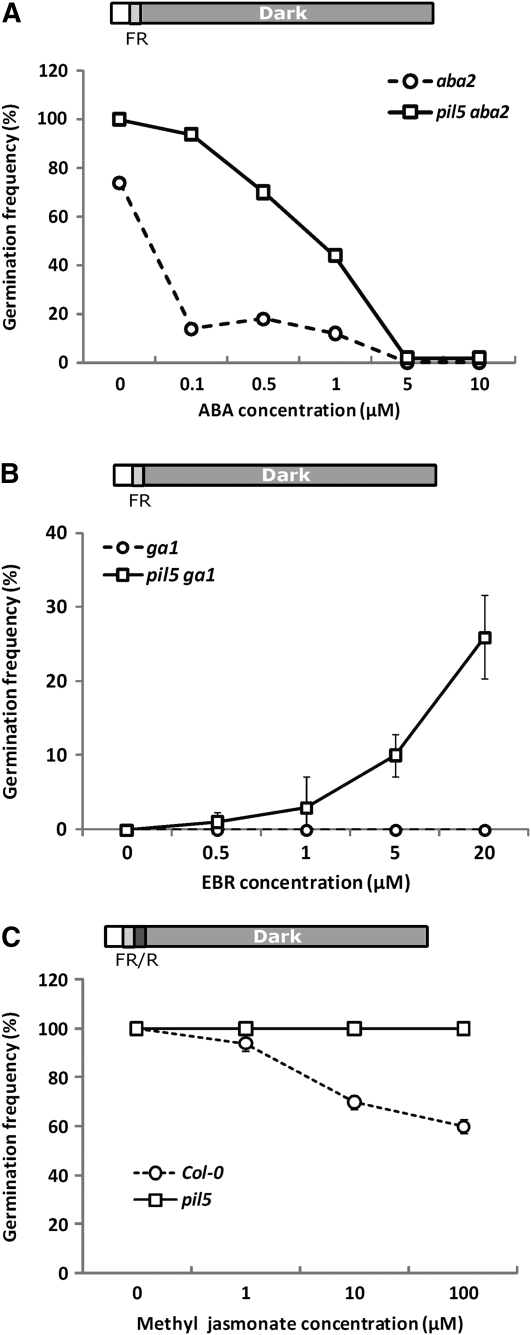
To further investigate the role of PIL5 in ABA signaling, we determined the germination frequency of aba2 and pil5 aba2 seeds in the presence of various concentrations of ABA. As shown in Figure 6A, both aba2 and pil5 aba2 seeds germinate after far-red light irradiation, but their germination frequencies decrease as the ABA concentration increases. Notably, the aba2 seeds respond to exogenous ABA more strongly than the pil5 aba2 seeds, indicating that PIL5 enhances the ABA responsiveness of imbibed seeds. This enhanced responsiveness to ABA is likely due to the summed activity of both positively and negatively regulated ABA-related genes (e.g., the PIL5-mediated repression of CYP707A2 and activation of ABI3 and ABI5).
Figure 6.
PIL5 Regulates ABA, BR, and JA Hormone Signaling to Inhibit Seed Germination.
(A) Germination frequency of aba2 and pil5 aba2 seeds on various concentrations of ABA. Top diagram depicts the light condition used for the germination assay.
(B) Germination frequency of ga1 and pil5 ga1 seeds on various concentrations of EBR.
(C) Germination frequency of Col-0 and pil5 seeds on various concentrations of MeJA. Error bars indicate sd of triplicate experiments.
BR Metabolic and Signaling Genes
PIL5 binds to the promoter of BES1-INTERACTING MYC-LIKE PROTEIN2 (BIM2), a positive regulator of BR signaling, thereby increasing its expression. In addition, PIL5 indirectly increases the expression of another positive regulator, BIM1, suggesting that PIL5 enhances BR signaling by promoting the expression of two positive transcription factors involved in BR signaling. Since BR signaling is known to promote seed germination, the positive regulation of BIM1 and BIM2 is contradictory to the inhibitory role of PIL5 in seed germination. However, the expression patterns of other BR-related genes suggest that PIL5 represses BR signaling. Notably, PIL5 indirectly represses the expression of the BR anabolic gene DWARF4 (DWF4), which encodes an enzyme known to catalyze a rate-limiting step of BR biosynthesis in Arabidopsis (Kim et al., 2006). Furthermore, PIL5 represses the expression of BKI, which encodes the BR receptor complex, while activating the expression of BIN2, which encodes a protein kinase that represses BR signaling. The mixed expression patterns of the various BR metabolic and signaling genes suggest that the effect of PIL5 on BR signaling is also determined by the sum of these positively and negatively regulated genes. Similar to GA- and ABA-related genes, opposite expression of some of the BR signaling genes could be caused by feedback regulation.
To investigate the net effect of PIL5 on BR signaling during seed germination, we measured the germination frequency of ga1 and pil5 ga1 seeds in the presence of various concentrations of 24-epibrassinolide (EBR) following the application of a far-red pulse. Due to the absence of GA synthesis, both ga1 and pil5 ga1 mutant seeds fail to germinate in the absence of exogenous EBR. The addition of exogenous EBR promotes the germination of pil5 ga1 seeds but not ga1 seeds (Figure 6B). Since PHYB destabilizes PIL5 protein in seeds, these results are qualitatively similar to previous data showing that EBR promotes the germination of ga1 seeds under light conditions (Steber and McCourt, 2001). Taken together, these results indicate that seeds become more sensitive to EBR if PIL5 is removed by either light or mutation, further suggesting that PIL5 coordinates the expression of BR metabolic and signaling genes to inhibit seed germination.
JA Signaling Genes
PIL5 directly binds to the promoter of JAZ1, a negative regulator of JA signaling (Thines et al., 2007), thereby repressing its expression. The functional role of JA signaling in seed germination is not clearly defined. To examine the role of JA signaling during seed germination, we determined the germination frequency of Col-0 and pil5 seeds in the presence of various concentrations of methyl jasmonate (MeJA). We found that MeJA inhibits the germination of Col-0 seeds, but not that of pil5 seeds (Figure 6C). This result indicates that JA signaling inhibits seed germination, but the pil5 mutant is less sensitive to JA signaling for the inhibition of seed germination. The direct repression of JAZ1 by PIL5 suggests that PIL5 inhibits seed germination at least in part by activating JA signaling in imbibed seeds.
Auxin Metabolic and Signaling Genes
Our analysis further shows that PIL5 directly or indirectly regulates auxin metabolism and auxin signaling in imbibed seeds (Table 2). PIL5 increases the expression of four auxin anabolic genes (ALDEHYDE OXIDASE1 [AAO1], AMIDASE1, NITRILASE1 (NIT1), and NIT3), while it decreases the expression of a gene encoding an IAA-conjugating enzyme (DWARF IN LIGHT1 [DFL1]). It also negatively regulates the SUPERROOT 1 (SUR1) gene, whose mutant causes auxin overexpression in Arabidopsis. These antagonistic regulations of the auxin metabolic genes suggest that PIL5 increases auxin levels in imbibed seeds. In addition to auxin metabolic genes, PIL5 regulates the expression of auxin signaling genes. It activates the expression of the ARF18 gene, which encodes a positive signaling transcription factor, and represses the expression of the IAA16 gene, which encodes a negative signaling component. Among these genes, PIL5 directly binds to the promoters of one auxin anabolic gene (NIT1) and two auxin signaling genes (IAA16 and ARF18), suggesting that PIL5 directly regulates both auxin signaling and auxin metabolism. Since auxin is known to inhibit seed germination (Liu et al., 2007), the data further suggest that PIL5 inhibits seed germination by activating auxin signaling in imbibed seeds.
Cytokinin and Ethylene Signaling Genes
PIL5 directly represses the expression of the CRF genes (CRF1, CRF2, and CRF3), but indirectly activates the expression of the ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN5 (AHP5) gene (Table 2). The CRFs, which are AP2 domain transcription factors, positively regulate a subset of cytokinin responses (Rashotte et al., 2006). Similarly, AHP5 positively regulates cytokinin signaling. The functional significance of these signaling components in seed germination is not clearly understood. The functional role of cytokinin itself has not yet been fully elucidated. Previous studies have shown that cytokinin promotes germination by decreasing sensitivity to ABA in lettuce (Lactuca sativa) seeds (Khan, 1968; Fountain and Bewley, 1976). By contrast, the triple mutant of Arabidopsis cytokinin receptors (ahk2, ahk3, and ahk4) shows increased germination, suggesting that cytokinin inhibits seed germination. The opposing regulation of two positive signaling genes by PIL5 suggests that PIL5 affects cytokinin signaling in imbibed seeds, but additional work is necessary to clarify the functional significance of this interaction during seed germination.
PIL5 does not directly bind to the promoters of any ethylene-related genes, but it does indirectly repress the expression of two ethylene receptor genes (ETR2 and ETHYELENE INSENSITIVE4 [EIN4]) and activate the expression of an ethylene positive signaling component (EIN3) (Table 2). Since ethylene promotes seed germination by repressing its receptor activity, the expression patterns of these genes are contradictory to the inhibitory role of PIL5 in seed germination (Kucera et al., 2005). However, our findings are consistent with a previous report showing that a dominant gain-of-function ethylene receptor mutant (etr1-2) shows increased GA levels but does not germinate well (Chiwocha et al., 2005). As is the case for cytokinin signaling, the expression patterns of the ethylene signaling genes suggest that PIL5 affects ethylene signaling in imbibed seeds, but the role of this regulation remain to be clarified.
Functional Classification of PIL5-Regulated Direct Target Genes: Genes Encoding Cell Wall–Related Enzymes
Germination is likely to be accompanied by drastic modification of cell wall properties, as embryonic cells elongate out through the endothelial layer and testa. The PIL5-regulated direct target genes identified herein include a few that encode cell wall–modifying enzymes. PIL5 binds to the promoters of two expansin genes (EXP8 and EXP10) and one xyloglucan endotransglycosylase homolog (XTH28), thereby directly repressing the expression of these genes (Table 3). In addition, PIL5 also indirectly represses the expression of four other EXP genes and six other XTH genes. Among the EXP and XTH genes, however, only XTH11 is activated by PIL5. Since these two classes of enzymes are involved in cell wall loosening, our results suggest that PIL5 regulates cell wall properties by repressing the expression of these cell wall–loosening enzymes either directly or indirectly.
Table 3.
List of PIL5-Regulated Cell Wall–Modifying Enzyme Genes
| AGI | Name | FC | Direct |
|---|---|---|---|
| AT1G69530 | EXP1 | −3.01 | X |
| AT2G37640 | EXP3 | −4.62 | X |
| AT2G40610 | EXP8 | −4.53 | O |
| AT1G26770 | EXP10 | −4.27 | O |
| AT2G03090 | EXP15 | −2.89 | X |
| AT2G 18660 | EXLB3 | −2.25 | X |
| AT2G06850 | XTH4/EXGT-A1 | −8.95 | X |
| AT5G 13870 | XTH5/EXGT-A4 | −4.53 | X |
| AT4G03210 | XTH9 | −7.41 | X |
| AT3G48580 | XTH11 | 2.23 | X |
| AT3G23730 | XTH16 | −4.26 | X |
| AT5G57560 | XTH22/TCH4 | −4.64 | X |
| AT1G 14720 | XTH28/XTR2 | −2.75 | O |
FC, fold change; O, PIL5 direct target gene; X, not PIL5 direct target gene.
DISCUSSION
We previously showed that PIL5, a phytochrome-interacting bHLH transcription factor, inhibits seed germination partly through ABA and GA signaling (Oh et al., 2007). However, it was not known whether PIL5 regulates seed germination through just these two hormonal signaling pathways. We herein report the use of ChIP-chip and microarray analysis to identify 166 PIL5-regulated direct target genes in imbibed seeds. These PIL5-regulated direct target genes are enriched in transcription factors (Table 1), suggesting that PIL5 regulates the expression of various genes at the upper hierarchy of the transcriptional cascades in imbibed seeds. Notably, PIL5 directly regulates many hormone-related transcription regulators, including ABA- (ABI3 and ABI5), auxin- (IAA16 and ARF18), BR- (BIM2), cytokinin- (CRF1, CRF2, and CRF3), GA- (RGA), and JA- (JAZ1) related transcriptional regulators (Table 2). Various hormone metabolic genes and other signaling genes are also regulated by PIL5 both directly and indirectly. In addition to transcription regulators, the PIL5-regulated direct target genes are enriched with genes encoding several cell wall–modifying enzymes, suggesting that PIL5 directly regulates the properties of the cell wall. Taken together, these findings suggest that PIL5 inhibits seed germination not simply via ABA and GA hormone signaling pathways in imbibed seeds, but rather by coordinating various hormone signaling pathways and modifying cell wall properties (Figure 7).
Figure 7.
PIL5 Regulates Seed Germination by Coordinating Various Hormonal Signaling Pathways and Modulating Cell Wall Properties.
PIL5 directly regulates various hormone signaling genes, including those involved in ABA (ABI3 and ABI5), auxin (IAA16 and ARF18), BR (BIM2), cytokinin (CRF1, CRF2, and CRF3), GA (GAI, RGA, and GID1A), and JA (JAZ1) signaling. Various hormone metabolism genes are also regulated indirectly by PIL5. In addition to altering the hormone-related genes, PIL5 inhibits the expression of genes encoding cell wall–modifying enzymes either directly (EXP8, EXP10, and XTH28) or indirectly (four EXP genes and six XTH genes). Taken together, our results indicate that PIL5 inhibits seed germination by coordinating germination-promoting and -inhibiting hormone signals and by modulating cell wall properties. When phytochromes (PHY) are activated, they promote the degradation of PIL5, leading to seed germination. We did not include other seed germination-related PIL5 direct target and regulated genes in the diagram. The notations used in the figure are as follows. Different colored rounded boxes indicate the hormone anabolic genes (light red), hormone catabolic (light blue), and hormone signaling genes (light green). Genes that are upregulated by PIL5 are represented by red letters, whereas those that are downregulated are represented by blue letters. PIL5 direct target genes are enclosed in yellow rectangles. Asterisks indicate that the role of cytokinin signaling in seed germination is still controversial.
PIL5 Binds to Various Gene Promoters Largely through the G-Box Element in Vivo
Our ChIP-chip analysis identified a total of 748 PIL5 binding sites in the Arabidopsis genome. Consistent with the molecular function of PIL5 as a transcription factor, most of the identified PIL5 binding sites (71%) are located in the promoter regions (−3000 to +500 bp) of the annotated genes (Figure 2C). Within the promoter regions, the frequency of PIL5 binding sites is higher in the proximal promoter regions than in the distal regions (Figure 2D). Similar preferential bindings to promoter regions were also reported for other Arabidopsis transcription factors, such as HY5 and TGACG MOTIF BINDING FACTOR2 (TGA2) (Thibaud-Nissen et al., 2006; Lee et al., 2007). This is in contrast with some human transcription factors, such as Sp1, cMYC, and p53, which bind to promoter regions less preferentially (Cawley et al., 2004). The remaining 29% of the identified PIL5 binding sites are located in either intergenic regions (14%) or genic regions (15%) (Figure 2C). Some of these binding sites may serve as enhancers for long-distance regulation of transcription, but future work will be required to clarify the exact roles of these binding sites.
A previous study showed that PIL5 binds to the G-box motif (CACGTG) in the promoter regions of the GAI and RGA genes in vivo (Oh et al., 2007). Consistent with these previous reports, our genome-wide binding motif analysis showed that the G-box motif is highly overrepresented in the PIL5 binding sites, indicating that the G-box motif is indeed the major PIL5 binding motif in vivo. However, the G-box motif is not present in all of the identified PIL5 binding sites; 59% of the PIL5 binding regions contain at least one G-box motif, while the remaining 41% do not (Figure 3C). This may indicate that PIL5 interacts with these other binding regions through less conserved non-G-box sequence motifs, either as a PIL5 homodimer or as a heterodimer with other bHLH proteins. Alternatively, since the ChIP procedure involves cross-linking, PIL5 may be attached to these regions through various protein–protein interactions rather than by direct DNA binding.
Although PIL5 binds to some G-box motifs in vivo, not all G-box motifs act as binding sites for PIL5 in vivo. Our ChIP-chip analysis shows that only a fraction of G-box motifs serve as PIL5 binding sites; the majority of G-box motifs, such as the motif present in the promoter region of GA3ox1 (Oh et al., 2007), do not serve as PIL5 binding sites. This observation suggests that a G-box motif alone is not sufficient for PIL5 binding in vivo. Other factors, such as flanking sequences, neighboring sequence motifs, DNA methylation status, and/or the nucleosome density around the G-box motif, might play important roles in determining in vivo binding. We failed to detect any obvious flanking or neighboring sequence motifs. When we compared the identified PIL5 binding sites to the low nucleosome density (LND) regions, only 33.82% (253/748) of the PIL5 binding sites overlapped with LND regions (Zhang et al., 2007), suggesting that the LND regions are not strictly consistent with all of the in vivo PIL5 binding G-box motifs. Similarly, DNA methylation status does not explain all of the in vivo binding sites. However, the LND regions and DNA methylation status were determined in seedlings rather than in imbibed seeds, so we cannot say for certain that these regions do not play a role in determining the in vivo binding sites in imbibed seeds. Alternatively, many of these factors, rather than a single dominant factor, might act together to determine in vivo PIL5 binding site selection.
PIL5 Mainly Mediates Phytochrome Signaling in Imbibed Seeds
Our microarray analysis showed that red light regulates the expression of a large number (2031) of genes in imbibed wild-type seeds (Figure 4A). Previous reports showed that red light regulates 10 to ∼30% of all Arabidopsis genes in seedlings (Ma et al., 2001), suggesting that red light alters the expression of similarly large numbers of genes in imbibed seeds and seedlings. To determine whether red light regulates similar sets of genes in seeds and seedlings, we analyzed a previously reported seedling microarray data set (the AtGenExpress light treatment data set; GSE5617) using the criteria we adopted in our analysis and compared the DEG sets. In seedlings, red light alters the expression of 1154 genes (see Supplemental Data Set 4 online). When we compared the DEG sets between seeds and seedlings, we found that the two sets do not overlap much (see Supplemental Figure 1 online). Noticeably, photosynthesis-related genes are represented much more highly among seedling DEGs than among seed DEGs, whereas hormone-related genes are less abundant among seedling DEGs than among seed DEGs. Overall, of the analyzed genes, only 198 are differentially expressed in both seeds and seedlings, and the correlation coefficient of the DEGs is only 0.165. Taken together, these results suggest that red light regulates the expression of large but distinct gene sets in imbibed seeds and seedlings.
The microarray data further show that red light signaling is mainly mediated by PIL5 in imbibed seeds. A large number of genes (2031 genes) are differentially expressed following red light exposure of imbibed wild-type seeds (Figure 4A). By contrast, no genes are differentially expressed in response to red light in the pil5 mutant seeds, indicating that PIL5 is necessary for the differential expression of genes in imbibed seeds in response to red light. The DEGs of Col-0(D)/pil5(D) largely overlap with the DEGs of Col-0(D)/Col-0(R) (Figure 4C), with a correlation coefficient of expression of 0.893, supporting the major role of PIL5. These expression data are consistent with previous reports showing that PIL5 is the major mediator of phytochrome signaling for seed germination (Oh et al., 2006, 2007). Since phytochromes activate the degradation of PIL5 in seeds, our results further suggest that phytochromes alter the expression of a large set of genes in imbibed seeds mainly by destabilizing PIL5.
It is surprising that phytochrome signaling is mediated almost solely by PIL5, which is only one of numerous phytochrome-interacting proteins. Many of the genes encoding these phytochrome-interacting proteins are expressed in seeds, indicating that this effect is not due to a lack of their expression at the seed stage (see Supplemental Figure 2 online). Some of these phytochrome-interacting proteins may not directly regulate gene expression downstream of phytochromes, but instead may play roles in regulating either the nuclear localization of phytochromes (type I phytochrome-interacting proteins) or the output activity of phytochromes (type II phytochrome-interacting proteins) (Bae and Choi, 2008). However, some of the genes, such as those encoding phytochrome-interacting proteins whose activities are regulated by phytochromes (type III phytochrome-interacting proteins), are also expressed in seeds. The representative type III interacting proteins include PIL5/PIF1, PIL2, PIF3, PIF4, PIL6/PIF5, and PIF7 (collectively known as PIFs/PILs). Among these, PIL2 and PIF3 are expressed at comparable levels to PIL5 in dry seeds. In imbibed seeds, both PIL2 and PIF3 are expressed at a lower level than PIL5 (see Supplemental Figure 2 online). Thus, if these proteins are functional in seeds, their interacting genes should still be light responsive through PIL2 and PIF3 in the pil5 mutant. The absence of any light-responsive genes in the pil5 mutant seeds, however, indicates that the other type III phytochrome-interacting proteins play only negligible roles in mediating light signals in seeds.
A few different possibilities may account for the apparent lack of roles for the other type III phytochrome-interacting proteins in seeds. First, PIL5 might function solely by heterodimerizing with other type III interacting proteins. If the PIL5-PIF3 heterodimer is the sole functional form, we would expect light signaling to be disrupted in the pil5 single mutant even in the presence of PIF3. A previous analysis, however, indicated that the pif3 mutant behaves more like the wild type than like the pil5 mutant in terms of seed germination, arguing against this possibility (Oh et al., 2004). Second, PIL5 and other PIFs/PILs might be modified differently in seeds, or seeds might contain cofactors that might specifically activate PIL5 by forming a complex. Third, other PIFs/PILs might not be present due to either translation blockade or selective degradation. If the other PIFs/PILs proteins are absent from seeds, then phytochrome signaling will be mediated mainly by PIL5. Further investigation is needed to determine precisely why PIL5 is the major mediator of phytochrome signaling in imbibed seeds.
PIL5 Directly Regulates Large Numbers of Transcription Factor Genes
PIL5-regulated direct target genes are enriched for transcription factors and related proteins. We identified 166 PIL5-regulated direct target genes by comparing the ChIP-chip and microarray data. These PIL5-regulated direct target genes are enriched for proteins localized in the nucleus and cell wall, as shown by our GO analysis. The same analysis also indicated that the process of transcription is enriched in the PIL5-regulated direct target genes. Consistent with this, 34 PIL5-regulated direct target genes encode transcription factors or related proteins (Table 1). These results suggest that PIL5 directly regulates a small number of genes in imbibed seeds, but indirectly regulates many other genes partly through transcriptional cascades. The enrichment of transcription factors has also been noted among HY5 direct target genes (Lee et al., 2007). HY5, which is a bZIP transcription factor that promotes seedling photomorphogenesis, is stabilized in the light and regulates large numbers of genes (Osterlund et al., 2000). A similar ChIP-chip analysis coupled with microarray analysis showed that HY5 directly regulates 219 genes out of 1144 HY5-regulated genes. Among the direct target genes, 37 are transcription factors, suggesting that HY5 also indirectly regulates many genes partly through transcriptional cascades (Lee et al., 2007).
It is not clear, however, whether all transcription factors directly regulate many other transcription factors. Different transcription factors may directly regulate other transcription factors to different degrees, depending on their roles in the regulatory hierarchy. Alternatively, all transcription factors may directly regulate a similar number of other transcription factors, thus forming extensive networks. PIL5 and HY5 play key roles in light-dependent seed germination and seedling photomorphogenesis, respectively. Thus, it is not surprising to find that the two transcription factors directly regulate many other transcription factors, thereby initiating transcriptional cascades. Unlike PIL5 and HY5, however, the Arabidopsis transcription factor TGA2 enriches mainly kinase genes, whereas human p53 enriches mainly cell adhesion mobility genes. TGA2 and p53 play key roles in systemic acquired resistance and tumorigenesis, respectively, suggesting that not all transcription factors, even those playing key roles, enrich other transcription factor genes (Thibaud-Nissen et al., 2006; Wei et al., 2006). In the future, it will be useful to analyze the direct target genes of more transcription factors to assess what properties of PIL5 and HY5 allow them to directly regulate the genes encoding many other transcription factors.
PIL5 Inhibits Seed Germination by Coordinating Various Hormonal Signaling Pathways
Geminating seeds need to coordinate a variety of hormonal signaling pathways to orchestrate the various physiological and developmental processes. The process of germination involves the mobilization of stored nutrients, the elongation of embryonic root cells, and the rupture of both the endosperm and the testa. In addition, seeds must reactivate biochemical and cellular processes to revive specialized cellular activities and cell proliferation. Since these processes must occur throughout all cells (Finch-Savage and Leubner-Metzger, 2006), seeds might use various hormone signals to coordinate the necessary physiological and developmental processes during seed germination. Thus, germination is likely to be regulated not just by one or two hormones, but rather by the coordinated actions of numerous hormones.
PIL5 directly regulates not only GA signaling transcription regulators but also other hormone signaling transcription regulators. We showed previously that PIL5 directly regulates GA signaling genes such as GAI and RGA. In this study, we showed that PIL5 also directly regulates ABA signaling transcription regulators (ABI3 and ABI5), auxin signaling transcription regulators (IAA16 and ARF18), cytokinin signaling transcription regulators (CRFs), a BR signaling transcription regulator (BIM2), and a JA signaling transcription regulator (JAZ1) (Table 2). Some of the signaling transcription regulators (GAI, RGA, ABI3, and ABI5) inhibit seed germination, whereas several others have not yet been studied for their roles in seed germination. However, hormones such as auxin, cytokinin, BR, ethylene, and JA are known to regulate seed germination, suggesting that their transcription regulators are likely to affect hormonal signaling during seed germination.
The role of PIL5 in hormonal signaling is not limited to the direct regulation of signaling transcription regulators. We previously showed that PIL5 indirectly regulates both GA and ABA metabolic genes (Oh et al., 2007). In this study, we show that PIL5 indirectly regulates not just GA and ABA metabolism, but also the metabolism of other hormones, including auxin, BR, and ethylene (Table 2). Prominently, PIL5 activates the expression of four auxin anabolic genes and represses DWF4, a BR anabolic gene encoding an enzyme that catalyzes the rate-limiting step of BR biosynthesis. Since auxin inhibits seed germination and BR promotes seed germination, the expression patterns of these metabolic genes are consistent with the inhibitory role of PIL5. Taken together, our data suggest that PIL5 inhibits seed germination not simply by regulating GA and ABA metabolism and signaling, but rather by coordinating various hormonal metabolic and signaling pathways in imbibed seeds. Future work will be required to fully understand the detailed role of each hormone in this process.
Type I Errors in the Analysis
As for many large-scale experiments, our analysis is likely to include both type I errors (the exclusion of true target genes in our final target gene list) and type II errors (the inclusion of false target genes in the final list). Several of the adopted procedures might have introduced type I errors. First, type I errors can be introduced during microarray analysis. The presence of this type I error in our data is evident by the exclusion of GAI from the final list of PIL5-regulated direct target genes. We previously showed that PIL5 directly regulates GAI, as revealed by manual ChIP analysis and quantitative RT-PCR analysis (Oh et al., 2007). In our current large-scale analysis, GAI was not found in the set of PIL5-regulated direct target genes because it was not included in the 2555 PIL5-regulated genes identified by the microarray analysis, although it was included in the 750 PIL5 direct target genes identified by the ChIP-chip analysis (see Supplemental Data Set 2 online). GAI was excluded from the PIL5-regulated gene set because it had a high FDR value, due to signal variation across the microarray chips. Therefore, it is likely that other true PIL5-regulated direct target genes were also excluded from our final gene list. Second, type I errors can be introduced by the ChIP-chip analysis itself. We determined the PIL5 binding sites using relatively strong criteria for ChIP-chip analysis; this will likely have excluded weak binding sites. Third, type I errors could have been introduced during the process of assigning PIL5 binding sites to the corresponding genes. If a PIL5 binding site was located in the promoter region of a gene (−3000 to +500 bp), we assigned it to the specific gene. However, we did not assign sites that were located outside the conventional promoter regions. Using these criteria, we assigned 69% of the putative PIL5 binding sites to their corresponding genes but did not assign 31% of them to any genes. Since enhancer elements can regulate genes from a great distance, even on the interchromosomal level, it is likely that at least some of the unassigned PIL5 binding sites correspond to genes. Finally, type I errors could have been introduced due to the microarray chip we used. The used ATH1 chip contains only 23,288 out of a total of 33,282 Arabidopsis genes, meaning that we did not have expression level data for 9994 genes. Because of this, we compared only 655 out of 750 PIL5 direct target genes with microarray data. Some of the 95 PIL5 direct target genes left out of the analysis are likely to be regulated by PIL5 in imbibed seeds. One example of this is the PIL5 binding site located in the promoter region of microRNA MIR159a (see Supplemental Data Set 1 online). MIR159a mediates the cleavage of two myb transcription factor mRNAs, MYB33 and MYB101, which act as positive regulators of ABA signaling during seed germination (Reyes and Chua, 2007). MIR159a is not represented in the ATH1 chip; thus, we determined the level of MIR159a expression using quantitative RT-PCR. As shown in Supplemental Figure 3 online, the level of pri-miR159a is indeed decreased in the pil5 mutant, indicating that PIL5 directly regulates the expression of pri-miR159a. Thus, some true PIL5-regulated direct target genes were excluded due to the lack of expression data. Because our data include some type I errors, the gene list identified in this study should not be considered a comprehensive list, but rather a partial list of PIL5 target genes.
METHODS
Plant Materials and Growth Conditions
Arabidopsis thaliana plants were grown in a growth room with a 16-h light/8-h dark cycle at 22 to 24°C for general growth and seed harvesting. For germination assays, seeds were dried at 22°C in white paper bags for at least 1 month. The pil5-1 mutant (Salk_072677), ga1 mutant (Salk_109115), and aba2-1 were obtained from the Salk Institute. All other plants used in this study, including PIL5-OX3 that expresses myc-tagged PIL5 (see Supplemental Figure 4 online), were of the Col-0 ecotype background.
Germination Assay
Germination assays were performed as previously described (Oh et al., 2006). Fifty seeds were sowed on various concentrations of hormone medium and irradiated by far-red light (3.2 μmol m−2 s−1) for 5 min or red light (13 μmol m−2 s−1) for 10 s. The seeds were then incubated in the dark for 5 d (BR and JA) or 8 d (ABA), and germination frequency was measured. Seeds with protruded radicles were counted as germinated seeds.
ChIP-Chip Assay
For the ChIP-chip assay, PIL-OX3 seeds were far-red light irradiated, incubated in the dark for 6 h, and then cross-linked in 1% formaldehyde solution under a vacuum for 1 h. The seeds were then ground to powder in liquid nitrogen, and chromatin complexes were isolated and sonicated as previously described (Oh et al., 2007). The sonicated chromatin complexes were precipitated with a monoclonal anti-myc antibody. The cross-linking was then reversed, and precipitated DNA was purified using the QIAquick PCR purification kit (Qiagen) and the relative amount of DNA was determined by real-time PCR using specific primers and SYBR Green as fluorescence dye (see Supplemental Table 1 online).
To amplify the input DNA and precipitated DNA, the annealed linker consisting of two oligomers (5′-GCGGTGACCCGGGAGATCTGAATTC-3′ and 5′-GAATTCAGATC-3′) was ligated to the end of blunted DNA. The linked DNA was amplified by two round of PCR using the primer 5′-GCGGTGACCCGGGAGATCTGAATTC-3′. Then, 500 ng of amplified DNA was labeled by CY5 or CY3, and the CY5- or CY3-labeled probes were hybridized with the Arabidopsis whole-genome tiling array chip (NimbleGen) as previously described (Thibaud-Nissen et al., 2006).
ChIP-Chip Peak Detection
Signal intensity data were extracted from the scanned images of each array using NimbleScan, NimbleGen's data extraction software. The log2 ratio of the Cy5-labeled PIL5 ChIP amplicons to Cy3-labeled total DNA amplicons was calculated and scaled using the bi-weight mean algorithm, implemented in NimbleScan. To remove the effect of outlier probes, Gaussian weighted smoothing was applied with a window size of five probes. Then, putative PIL5 binding sites were identified using the Tamalpais peak-calling algorithm (Bieda et al., 2006). However, a slight adjustment was made due to the difference in probe spacing between ENCODE arrays (38 bp) and the Arabidopsis whole-genome tiling array (90 bp). Briefly, the peaks with a minimum of six consecutive probes in the top 1% of all probes on the array were identified as putative PIL5 binding sites (P value < 0.00001). Among putative PIL5 binding sites, only those peaks that were identified in at least two of three biological replicates were considered as final binding sites.
Microarray Analysis
For microarray analysis, Col-0 and pil5 seeds were irradiated with far-red light or red light and incubated in the dark for 12 h, and total RNA was extracted using the Spectrum Plant Total RNA kit (Sigma-Aldrich) according to the manufacturer's protocol. For biological replicates, seeds were plated and sampled independently.
Double-strand cDNA was synthesized from 10 μg of total RNA using a SuperScript Choice cDNA synthesis kit (Invitrogen) with an oligo(dT) primer containing a T7 polymerase promoter recognition site at the 3′ end. Biotin-labeled cRNA was synthesized by T7 RNA polymerase using the double-strand cDNA as a template (Bioarray High Yield RNA transcript labeling kit; Enzo Diagnostics) and purified using an RNeasy RNA purification kit (QiagenA). The labeled cRNA was fragmented and hybridized to a GeneChip microarray (ATH1 GenomeArray; Affymetrix) for 16 h at 42°C. After hybridization, the arrays were washed and stained with biotinylated antistreptavidin antibody (Vector Laboratories) and a phycoerythrin-streptavidin conjugate (Molecular Probes) according to the manufacturer's protocol. Signals were scanned using a confocal microscope scanner (Gene Array Scanner; Hewlett-Packard) at 570 nm. Signal values for individual genes were obtained using statistical algorithms on Microarray Suite software (version 5.0; Affymetrix).
The GeneChip Arabidopsis ATH1 Genome Array, which contains >22,500 probe sets representing ∼24,000 genes, was used. Microarray data analysis was performed with use of a linear statistical model and robust test statistics as described in the Limma package (Smyth, 2005) in the Bioconductor R project (http://www.bioconductor.org/). First, signal intensities were normalized using the robust multiple-array average method (Irizarry et al., 2003). Second, a linear model was fitted to the log2 expression data for each probe using lmFit and estimated coefficients, and standard errors were computed using contrasts.fit. Third, an empirical Bayes method, ebayes, was used to calculate the moderated t-statistics and rank genes in order of evidence for differential expression. The P values were adjusted for multiple testing by the Benjamini and Hochberg's method to control the FDR (Benjamini and Hochberg, 1995). For GO analysis with BiNGO, we used the Benjamini and Hochberg FDR correction.
Gene Expression Analysis
For quantitative RT-PCR, total RNA was extracted using the Spectrum Plant Total RNA kit (Sigma-Aldrich) according to the manufacturer's protocol. The total RNA was used to synthesize cDNA, the fragments of interest were amplified by real-time PCR using specific primers and SYBR Green as fluorescence dye (see Supplemental Table 1 online), and the resulting expression levels were normalized versus that of PP2A (Han and Kim, 2006).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL databases under the following accession numbers: PIL5 (AT2G20180), GAI (AT1G14920), RGA (AT2G01570), ABI3 (AT3G24650), ABI5 (AT2G36270), IAA16 (AT3G04730), ARF18 (AT3G61830), CRF1 (AT4G11140), CRF2 (AT4G23750), CRF3 (AT5G53290), BIM2 (AT1G69010), JAZ1 (AT1G19180), EXP8 (AT2G40610), EXP10 (AT1G26770), and XTH28 (AT1G14720).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Venn Diagram Showing the Overlap between Differentially Expressed Genes of Col-0(D)/Col-0(R) in Seeds and Seedlings.
Supplemental Figure 2. Relative Expression Levels of Phytochrome Interacting Factor Genes in the Dry Seeds and Imbibed Seeds.
Supplemental Figure 3. pri-miR15a Expression Is Decreased in the pil5 Mutant.
Supplemental Figure 4. Relative Expression Levels of PIL5 in PIL5-OX3.
Supplemental Table 1. Primer List for Gene Expression Analysis and ChIP PCR.
Supplemental Data Set 1. List of PIL5 Binding Sites.
Supplemental Data Set 2. List of PIL5 Direct Target Genes.
Supplemental Data Set 3. List of PIL5-Regulated Genes.
Supplemental Data Set 4. List of Red Light–Regulated Genes in Seedlings.
Supplementary Material
Acknowledgments
We thank Taku Demura and Sachiko Oyama (RIKEN Plant Science Center) for technical assistance on microarray analysis. We also thank the Korea Institute of Science and Technology Information Supercomputing Center for its support. This work was supported in part by grants from the Korea Science Engineering Foundation (Grants R0A-2007-000-20024-0, PF06302-03, and M10601000088 to G.C.) and the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (20570049) to S.Y. D.L. was supported by the Korea Systems Biology Program and a grant (No. 2006-01508).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantcell.org) is: Giltsu Choi (gchoi@kaist.edu).
Online version contains Web-only data.
References
- Bae, G., and Choi, G. (2008). Decoding of light signals by plant phytochromes and their interacting proteins. Annu. Rev. Plant Biol. 59 281–311. [DOI] [PubMed] [Google Scholar]
- Benjamini, Y., and Hochberg, Y. (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. Roy. Statist. Soc. Ser. B. Methodological 57 289–300. [Google Scholar]
- Bieda, M., Xu, X., Singer, M.A., Green, R., and Farnham, P.J. (2006). Unbiased location analysis of E2F1-binding sites suggests a widespread role for E2F1 in the human genome. Genome Res. 16 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick, H.A., Hendricks, S.B., Parker, M.W., Toole, E.H., and Toole, V.K. (1952). A reversible photoreaction controlling seed germination. Proc. Natl. Acad. Sci. USA 38 662–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, D., Hussain, A., Cheng, H., and Peng, J. (2005). Loss of function of four DELLA genes leads to light- and gibberellin-independent seed germination in Arabidopsis. Planta 223 105–113. [DOI] [PubMed] [Google Scholar]
- Cawley, S., et al. (2004). Unbiased mapping of transcription factor binding sites along human chromosomes 21 and 22 points to widespread regulation of noncoding RNAs. Cell 116 499–509. [DOI] [PubMed] [Google Scholar]
- Chiwocha, S.D., Cutler, A.J., Abrams, S.R., Ambrose, S.J., Yang, J., Ross, A.R., and Kermode, A.R. (2005). The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J. 42 35–48. [DOI] [PubMed] [Google Scholar]
- Finch-Savage, W.E., and Leubner-Metzger, G. (2006). Seed dormancy and the control of germination. New Phytol. 171 501–523. [DOI] [PubMed] [Google Scholar]
- Fountain, D.W., and Bewley, J.D. (1976). Lettuce seed germination: Modulation of pregermination protein synthesis by gibberellic acid, abscisic acid, and cytokinin. Plant Physiol. 58 530–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, S., and Kim, D. (2006). AtRTPrimer: Database for Arabidopsis genome-wide homogeneous and specific RT-PCR primer-pairs. BMC Bioinformatics 7 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig, L., Stoddart, W.M., Dieterle, M., Whitelam, G.C., and Schafer, E. (2002). Phytochrome E controls light-induced germination of Arabidopsis. Plant Physiol. 128 194–200. [PMC free article] [PubMed] [Google Scholar]
- Huq, E., Al-Sady, B., Hudson, M., Kim, C., Apel, K., and Quail, P.H. (2004). Phytochrome-interacting factor 1 is a critical bHLH regulator of chlorophyll biosynthesis. Science 305 1937–1941. [DOI] [PubMed] [Google Scholar]
- Irizarry, R.A., Hobbs, B., Collin, F., Beazer-Barclay, Y.D., Antonellis, K.J., Scherf, U., and Speed, T.P. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4 249–264. [DOI] [PubMed] [Google Scholar]
- Itoh, H., Ueguchi-Tanaka, M., and Matsuoka, M. (2008). Molecular biology of gibberellins signaling in higher plants. Int Rev Cell Mol Biol. 268 191–221. [DOI] [PubMed] [Google Scholar]
- Khan, A.A. (1968). Inhibition of gibberellic acid-induced germination by abscisic acid and reversal by cytokinins. Plant Physiol. 43 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, D.H., Yamaguchi, S., Lim, S., Oh, E., Park, J., Hanada, A., Kamiya, Y., and Choi, G. (2008). SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 20 1260–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, H.B., Kwon, M., Ryu, H., Fujioka, S., Takatsuto, S., Yoshida, S., An, C.S., Lee, I., Hwang, I., and Choe, S. (2006). The regulation of DWARF4 expression is likely a critical mechanism in maintaining the homeostasis of bioactive brassinosteroids in Arabidopsis. Plant Physiol. 140 548–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, T.H., Abdullaev, Z.K., Smith, A.D., Ching, K.A., Loukinov, D.I., Green, R.D., Zhang, M.Q., Lobanenkov, V.V., and Ren, B. (2007). Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell 128 1231–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera, B., Cohn, M.A., and Leubner-Metzger, G. (2005). Plant hormone interactions during seed dormancy release and germination. Seed Sci. Res. 15 281–307. [Google Scholar]
- Lee, J., He, K., Stolc, V., Lee, H., Figueroa, P., Gao, Y., Tongprasit, W., Zhao, H., Lee, I., and Deng, X.W. (2007). Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 19 731–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P.P., Montgomery, T.A., Fahlgren, N., Kasschau, K.D., Nonogaki, H., and Carrington, J.C. (2007). Repression of AUXIN RESPONSE FACTOR10 by microRNA160 is critical for seed germination and post-germination stages. Plant J. 52 133–146. [DOI] [PubMed] [Google Scholar]
- Liu, X.S., Brutlag, D.L., and Liu, J.S. (2002). An algorithm for finding protein-DNA binding sites with applications to chromatin-immunoprecipitation microarray experiments. Nat. Biotechnol. 20 835–839. [DOI] [PubMed] [Google Scholar]
- Ma, L., Li, J., Qu, L., Hager, J., Chen, Z., Zhao, H., and Deng, X.W. (2001). Light control of Arabidopsis development entails coordinated regulation of genome expression and cellular pathways. Plant Cell 13 2589–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maere, S., Heymans, K., and Kuiper, M. (2005). BiNGO: A Cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics 21 3448–3449. [DOI] [PubMed] [Google Scholar]
- Mathews, S. (2006). Phytochrome-mediated development in land plants: Red light sensing evolves to meet the challenges of changing light environments. Mol. Ecol. 15 3483–3503. [DOI] [PubMed] [Google Scholar]
- Nagy, F., Kircher, S., and Schafer, E. (2000). Nucleo-cytoplasmic partitioning of the plant photoreceptors phytochromes. Semin. Cell Dev. Biol. 11 505–510. [DOI] [PubMed] [Google Scholar]
- Nakabayashi, K., Okamoto, M., Koshiba, T., Kamiya, Y., and Nambara, E. (2005). Genome-wide profiling of stored mRNA in Arabidopsis thaliana seed germination: Epigenetic and genetic regulation of transcription in seed. Plant J. 41 697–709. [DOI] [PubMed] [Google Scholar]
- Oh, E., Kim, J., Park, E., Kim, J.I., Kang, C., and Choi, G. (2004). PIL5, a phytochrome-interacting basic helix-loop-helix protein, is a key negative regulator of seed germination in Arabidopsis thaliana. Plant Cell 16 3045–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, E., Yamaguchi, S., Hu, J., Yusuke, J., Jung, B., Paik, I., Lee, H.S., Sun, T.P., Kamiya, Y., and Choi, G. (2007). PIL5, a phytochrome-interacting bHLH protein, regulates gibberellin responsiveness by binding directly to the GAI and RGA promoters in Arabidopsis seeds. Plant Cell 19 1192–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh, E., Yamaguchi, S., Kamiya, Y., Bae, G., Chung, W.I., and Choi, G. (2006). Light activates the degradation of PIL5 protein to promote seed germination through gibberellin in Arabidopsis. Plant J. 47 124–139. [DOI] [PubMed] [Google Scholar]
- Osterlund, M.T., Hardtke, C.S., Wei, N., and Deng, X.W. (2000). Targeted destabilization of HY5 during light-regulated development of Arabidopsis. Nature 405 462–466. [DOI] [PubMed] [Google Scholar]
- Rashotte, A.M., Mason, M.G., Hutchison, C.E., Ferreira, F.J., Schaller, G.E., and Kieber, J.J. (2006). A subset of Arabidopsis AP2 transcription factors mediates cytokinin responses in concert with a two-component pathway. Proc. Natl. Acad. Sci. USA 103 11081–11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes, J.L., and Chua, N.H. (2007). ABA induction of miR159 controls transcript levels of two MYB factors during Arabidopsis seed germination. Plant J. 49 592–606. [DOI] [PubMed] [Google Scholar]
- Riechmann, J.L., et al. (2000). Arabidopsis transcription factors: genome-wide comparative analysis among eukaryotes. Science 290 2105–2110. [DOI] [PubMed] [Google Scholar]
- Rockwell, N.C., Su, Y.S., and Lagarias, J.C. (2006). Phytochrome structure and signaling mechanisms. Annu. Rev. Plant Biol. 57 837–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, F.P., Hughes, J.D., Estep, P.W., and Church, G.M. (1998). Finding DNA regulatory motifs within unaligned noncoding sequences clustered by whole-genome mRNA quantitation. Nat. Biotechnol. 16 939–945. [DOI] [PubMed] [Google Scholar]
- Seo, M., et al. (2006). Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 48 354–366. [DOI] [PubMed] [Google Scholar]
- Shinomura, T., Nagatani, A., Chory, J., and Furuya, M. (1994). The induction of seed germination in Arabidopsis thaliana is regulated principally by phytochrome B and secondarily by phytochrome A. Plant Physiol. 104 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura, T., Nagatani, A., Hanzawa, H., Kubota, M., Watanabe, M., and Furuya, M. (1996). Action spectra for phytochrome A- and B-specific photoinduction of seed germination in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 93 8129–8133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, G.K. (2005). Limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor, R. Gentlemen, V. Carey, S. Dudoit, R. Irizarry, and W. Huber, eds (New York: Springer), 397–420.
- Steber, C.M., and McCourt, P. (2001). A role for brassinosteroids in germination in Arabidopsis. Plant Physiol. 125 763–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibaud-Nissen, F., Wu, H., Richmond, T., Redman, J.C., Johnson, C., Green, R., Arias, J., and Town, C.D. (2006). Development of Arabidopsis whole-genome microarrays and their application to the discovery of binding sites for the TGA2 transcription factor in salicylic acid-treated plants. Plant J. 47 152–162. [DOI] [PubMed] [Google Scholar]
- Thines, B., Katsir, L., Melotto, M., Niu, Y., Mandaokar, A., Liu, G., Nomura, K., He, S.Y., Howe, G.A., and Browse, J. (2007). JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448 661–665. [DOI] [PubMed] [Google Scholar]
- Wei, C.L., et al. (2006). A global map of p53 transcription-factor binding sites in the human genome. Cell 124 207–219. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, R., Nakamura, M., Mochizuki, N., Kay, S.A., and Nagatani, A. (1999). Light-dependent translocation of a phytochrome B-GFP fusion protein to the nucleus in transgenic Arabidopsis. J. Cell Biol. 145 437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi, S., Smith, M.W., Brown, R.G., Kamiya, Y., and Sun, T. (1998). Phytochrome regulation and differential expression of gibberellin 3beta-hydroxylase genes in germinating Arabidopsis seeds. Plant Cell 10 2115–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi, Y., Takeda-Kamiya, N., Hanada, A., Ogawa, M., Kuwahara, A., Seo, M., Kamiya, Y., and Yamaguchi, S. (2007). Contribution of gibberellin deactivation by AtGA2ox2 to the suppression of germination of dark-imbibed Arabidopsis thaliana seeds. Plant Cell Physiol. 48 555–561. [DOI] [PubMed] [Google Scholar]
- Zhang, X., Clarenz, O., Cokus, S., Bernatavichute, Y.V., Pellegrini, M., Goodrich, J., and Jacobsen, S.E. (2007). Whole-genome analysis of histone H3 lysine 27 trimethylation in Arabidopsis. PLoS Biol. 5 e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.