Abstract
Dietary supplementation with resveratrol may produce calorie restriction-like effects on metabolic and longevity endpoints in mice. In this study, we sought to determine whether resveratrol treatment elicited other hallmark changes associated with calorie restriction, namely bradycardia and decreased body temperature. We found that during short-term treatment, wild-type mice on a calorie-restricted diet experienced significant decreases in both heart rate and body temperature after only 1 day whereas those receiving resveratrol exhibited no such change after 1 wk. We also used ob/ob mice to study the effects of long-term treatment because previous studies had indicated the therapeutic value of resveratrol against the linked morbidities of obesity and diabetes. After 12 wk, resveratrol treatment had produced no changes in either heart rate or body temperature. Strikingly, and in contrast to previous findings, we found that resveratrol-treated mice had significantly reduced endurance in a treadmill test. Quantitative reverse transcriptase-polymerase chain reaction suggested that a proposed target of resveratrol, Sirt1, was activated in resveratrol-treated ob/ob mice. Thus, we conclude that the bradycardia and hypothermia associated with calorie restriction occur through mechanisms unaffected by the actions of resveratrol and that further studies are needed to examine the differential effects of resveratrol in a leptin-deficient background.—Mayers, J. R., Iliff, B. W., Swoap, S. J. Resveratrol treatment in mice does not elicit the bradycardia and hypothermia associated with calorie restriction.
Keywords: obesity, dieting, body temperature, heart rate
The dietary regimen known as calorie restriction (CR) has long been known as a means to extend life span in rodents (1). In mice, long-term CR results in a host of behavioral, physiological, and metabolic changes, including increased foraging activity; decreased body temperature, heart rate, blood pressure, blood glucose, insulin, fat mass, and body weight; and increased glucose tolerance and insulin sensitivity (2,3,4,5). More recently, work in Saccharomyces cerevisiae and Drosophila melanogaster has demonstrated that Sir2, a NAD+-dependent histone deacetylase, plays a central role in mediating the increase in longevity associated with CR (6,7,8). Both a low NAD/NADH ratio and increased nicotinamide levels, important indicators of cellular metabolic status, inhibit Sir2 (2, 9, 10). A growing body of evidence indicates that the mammalian homologue of Sir2, Sirt1, also plays a significant role in responding to CR. First, expression of Sirt1 is elevated in numerous tissues with CR (11), and transgenic mice that overexpress Sirt1 exhibit a phenotype mirroring some aspects of CR (12). Sirt1 knockout mice, unlike wild types, do not increase their foraging activity in response to a CR diet (13). Other evidence indicates that Sirt1 promotes gluconeogenesis and fatty acid mobilization, two critical components for energy delivery during CR, through its activation of hepatic PGC-1α and by preventing transcription of PPARγ target genes, respectively (14, 15). Finally, Sirt1 has been shown to improve insulin sensitivity—another consequence of a CR diet (2, 16)—by inhibiting the phosphatase activity of PTP-1β (17). It remains to be seen, however, whether Sirt1 plays any role in the regulation of the cardiovascular and body temperature changes associated with CR.
New research has focused on identifying and verifying so-called CR mimetics, or compounds that produce the benefits of CR by targeting the pathways regulating its effects (i.e., Sir2/Sirt1), without requiring a reduction in the number of calories consumed (5, 18). A screen of small-molecule libraries initially identified resveratrol, a polyphenol derived from red grape skins, as an activator of Sir2/Sirt1 in vitro (19,20,21) although subsequent studies have questioned this finding (22, 23). Nevertheless, several studies indicate that resveratrol treatment increases longevity in S. cerevisiae, D. melanogaster, and Caenorhabditis elegans in a Sir2-dependent manner (19, 24, 25); one recent study (26), however, failed to replicate these findings in flies and worms. Resveratrol has shown some potential as a CR mimetic in mice, increasing survival rates in mice fed a high-fat, but not regular, diet (27, 28) and inducing transcriptional programs mirroring aspects of CR (28, 29). It also stimulates other metabolic and physiological changes consistent with CR action, including decreased adiposity, improved endurance, increased metabolic rate, and increased insulin sensitivity and glucose tolerance (12, 27, 30). Other purported Sirt1 activators, structurally distinct from resveratrol, have also shown promise as treatments for type 2 diabetes in mice (31).
Because the adaptive response to CR affects so many different aspects of an organism and the studies of resveratrol to date have focused primarily on the metabolic and longevity endpoints, we sought to determine whether resveratrol treatment had any effect on cardiovascular or behavioral parameters of mice. To do this, we performed 2 studies supplementing a standard diet with 0.1% resveratrol. Using surgically implanted radiotelemeters in free-moving mice, we found that resveratrol treatment did not produce the same decrease in heart rate or body temperature as CR over a 1 wk period in wild-type mice. A second study, in which ob/ob mice received supplemented food for a 12-wk period, found that resveratrol had no lasting effects on heart rate or body temperature. Interestingly, although treated and control animals had similar activity levels within their cages, the ob/ob mice on a resveratrol-supplemented diet exhibited a dramatic decrease in treadmill endurance.
MATERIALS AND METHODS
Animals and diets
Adult C57Bl/6J mice, ob/ob mice, and their wild-type cohort mates were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Mice were maintained on an AIN-93G standard diet for 2 wk before the start of each experiment. Mice on a calorie-restricted regimen were fed 60% of their 7-day average ad libitum food intake. Resveratrol-treated mice were fed a custom diet (Harlan Teklad, Madison, WI, USA) containing AIN-93G plus 0.1% resveratrol. All chow was stored at −20°C, and fresh food was provided every 2–3 days. Mice were maintained on a 12-h light-dark cycle and at an ambient temperature of 24 to 29°C.
Radiotelemetry
For the implantation of electrocardiographic radiotelemeters (model no. ETAF20; Data Sciences International, St. Paul, MN, USA), mice were anesthetized initially with 5% isoflurane in an oxygen stream and maintained on 1–2% isoflurane. Mice were kept on a heating pad (38°C) throughout implantation of the telemeter in the abdominal cavity and subcutaneous placement of the electrocardiographic leads (32). Mice were kept on a heating pad (38°C) for their first 48 h postoperation.
Data streams at 500 Hz were collected from each animal for a 5-s period every minute over the course of a single day (23 h: 12 dark, 11 light, with the remaining hour reserved for handling and maintenance). Data were collected by using the Dataquest A.R.T. 3.01 acquisition and analysis software (Data Sciences International).
Short-term resveratrol treatment
Six C57Bl/6J male mice (6 months old, ∼28–29 g) were implanted with electrocardiographic telemeters and allowed to recover for 1 wk. The mice were randomized into 3 groups over 3 feeding periods of 7 days each: standard food (control group), resveratrol-containing food (RSV group), and calorie restricted to 60% of ad libitum intake (CR group). Between feeding periods, the mice were fed a standard diet for 4 days. By the end of the experiment, all 6 mice underwent each of the 3 conditions. Food intake and body weight were measured daily. Heart rate data were obtained for 23 h daily and collapsed into light (11 h) and dark cycle (12 h) averages for each animal.
Long-term resveratrol treatment
Procedure
At the start of the experiment, the 8-wk-old male ob/ob mice either remained on the standard diet (ObC; n=5) or were switched to the resveratrol-supplemented diet (ObR; n=6). The male wild-type cohort mates remained on the standard diet (WTC; n=6). Food intake was measured 3×/wk and averaged for the week. Body weight was measured once per week. Two weeks before the initiation of the resveratrol diet, 3 mice in each group were implanted with electrocardiographic telemeters. Data was obtained for 23 h once every 7 days, and the telemeters were turned off in the intervening time to conserve telemeter battery life. Data points from electrocardiographic devices were collapsed into an average for each animal for both the light and dark periods as above.
Indirect calorimetry
Custom-built shoebox cages were fitted with a polycarbonate lid providing a near-airtight seal for continuous determination of oxygen consumption. Air from each cage was sampled for 10 s every 8 min by an O2 analyzer (Ametek S-3A; Applied Electrochemistry, Naperville, IL, USA). Up to 4 cages were run simultaneously, with 1 of the cages left empty to serve as a blank control. Oxygen consumption was measured before resveratrol treatment and after 4 and 11 wk into the treatment. Ambient pressure data was measured electronically (DSI APR-1; Data Sciences International) and collected using the Dataquest A.R.T. 3.01 acquisition and analysis software.
Rotarod
The protocol used herein was adapted from a previous study (27). Mice were tested on the rotarod before resveratrol treatment, and after 3, 6, and 11 wk into the trial. For each time point, the mice were given a habituation trial on the first day, where they were placed on the rotarod (755 Rotarod; IITC Life Sciences, Woodland Hills, CA, USA) at a constant speed of 3 rpm for 45–60 s. The next day, each mouse was given 3 trials during which the rotarod started at 3 rpm and gradually accelerated to 40 rpm over a 5-min period. The maximum trial length was 5 min, and there was a 30-min recovery period between trials. An individual average over the 3 trials was first calculated before finding a total group average.
Treadmill
The protocol used herein was adapted from a previous study (33). Briefly, the mice were tested on the treadmill before resveratrol treatment and after 6 and 11 wk into the study. For each time point, mice were given a habituation trial on the first day, which consisted of 5 min on the treadmill (Mouse Modular Treadmill; Columbus Instruments, Columbus, OH, USA) at 10 m/min for WTC mice or 3 min at 7 m/min for ObC and ObR mice. For the experimental trial, mice were run on the following day. WTC mice ran at 10 m/min for 5 min, and the speed was increased 2 m/min every 2 min thereafter. ObR and ObC mice were started at 7 m/min for 5 min, with the speed increasing 2 m/min every 2 min thereafter. For the final trial during wk 11, the protocol for ObR and ObC mice was modified. Following 3 min at 7 m/min, the speed was increased to 8 m/min for 1 min followed by 9 m/min for 1 min. After this 5-min period, the treadmill was set at 10 m/min, and mice were run until exhaustion. For all trials, the open-ended back of the treadmill was blocked with cardboard. When mice touched the cardboard block, they were prodded with a second piece of cardboard; this served to encourage them to move to the front of the treadmill. For each trial, mice were run until exhausted, with exhaustion being defined as a failure to respond to repeated, firm prodding as well as a reliance on the cardboard support to continue at the set pace. Time to exhaustion and top speed were recorded, and from these numbers total distance was calculated.
RNA isolation and quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
At the time of sacrifice, brown adipose tissue (BAT) was removed and flash frozen in liquid nitrogen for RNA analysis. Thawed BAT was homogenized in 4 M guanidine thiocyanate and extracted in phenol/chloroform; then the RNA was precipitated with isopropanol. The RNA was resuspended in diethyl pyrocarbonate-treated water. Following DNase treatment, quantitative real-time RT-PCR was carried out in a 2-step process. The iScript cDNA synthesis kit (Bio-Rad, Hercules, CA, USA) was used for cDNA synthesis (1 μg) and quantitative real-time PCR was performed on an iQ5 Real-Time PCR Detection System (Bio-Rad) with the following protocol: 95°C, 15 min; 40 cycles (95°C, 30 s; 55°C, 30 s; 72°C, 30 s) followed by a melt-curve analysis to confirm PCR products. Alternatively, total RNA was isolated from liver and heart tissues (also collected at the time of sacrifice) using Trizol reagent (Invitrogen, Carlsbad, CA, USA). Reverse transcription was carried out with superscript first-strand cDNA synthesis system (Applied Biosystems Inc., Foster City, CA, USA) using 1 μg of RNA. Quantitative real-time RT-PCR was performed on a real-time PCR thermal cycler (Applied Biosystems Inc.) The PCR program was 2 min 30 s at 95°C for enzyme activation, 40–50 cycles of 15 s at 95°C, 30 s at 58°C, and 1 min at 72°C for extension. Melting-curve analysis was performed to confirm the real-time PCR products. All quantitations were normalized to the β-actin (BAT) or 18S rRNA (heart and liver) levels as indicated. Primer sequences used are provided in Table 1.
TABLE 1.
Primer sequences for quantitative RT-PCR
| Gene | Primer name | Sequence |
|---|---|---|
| PGC-1α | Forward | TGAAGAGCGCCGTGTGATT |
| Reverse | TTCTGTCCGCGTTGTGTCA | |
| UCP-1 | Forward | TGGAGGTGTGGCAGTGTTCA |
| Reverse | GCTCTGGGCTTGCATTCTG | |
| IL-6 | Forward | ACAACCACGGCCTTCCCTACTT |
| Reverse | CACGATTTCCCAGAGAACATGTG | |
| iNOS | Forward | CCAAGCCCTCACCTACTTCC |
| Reverse | CTCTGAGGGCTGACACAAGG | |
| G6Pase | Forward | CGACTCGCTATCTCCAAGTGA |
| Reverse | GTTGAACCAGTCTCCGACCA | |
| PEPCK | Forward | CTGCATAACGGTCTGGACTTC |
| Reverse | CAGCAACTGCCCGTACTCC | |
| 18S | Forward | AGTCCCTGCCCTTTGTACACA |
| Reverse | CGATCCGAGGGCCTCACTA | |
| β-actin | Forward | ACGGCCAGGTCATCACTATTG |
| Reverse | CAAGAAGGAAGGCTGGAAAAGA |
Statistics
All results are reported as means ± se. In the short-term study, a repeated-measures ANOVA was used with treatment as a repeated measure, followed by a least significant difference (LSD) test for statistical significance. Anatomical and physiological parameters in the long-term study were analyzed with 2-factor repeated-measures ANOVA with group as a between-subjects factor and treatment as a repeated measure, followed by an LSD test, unless otherwise noted. Estimates of power were calculated based on the design of the experiments and the sample sizes used. The power estimates ranged from 0.925 for body temperature in the long-term resveratrol treatment to >0.99 for most of the remaining measurements, including all of the measurements in the short-term treatment study. The 0.05 level of confidence was accepted for statistical significance.
RESULTS
Short-term resveratrol treatment has no effect on heart rate or body temperature in wild-type mice
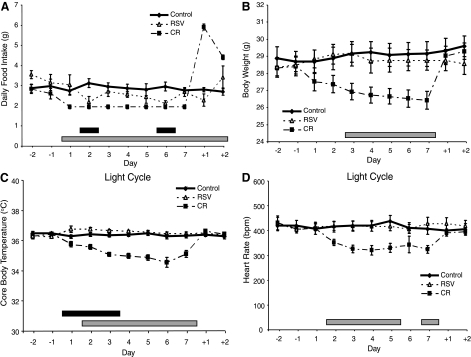
The heart rate, body temperature, and activity levels of wild-type mice were analyzed over a 1 wk period of CR treatment (CR group), resveratrol treatment (RSV group), and ad libitum feeding of standard chow (control group). The diet supplemented with 0.1% resveratrol resulted in an average dose of 87 mg resveratrol · kg bw−1 · day−1. The food intake of mice during resveratrol treatment was significantly lower than intake during control treatment on days 2 and 6 but not significantly different on any other day (Fig. 1A). During CR, mice consumed 60% of control values. During the recovery period after 1 wk of CR, food intake increased significantly (Fig. 1A). The body weights of mice decreased over the 1 wk CR feeding period, but body weights did not change during resveratrol and control periods (Fig. 1B).
Figure 1.
Physiological effects of resveratrol on wild-type mice are different from effects of CR. Food consumption (A), body weight (B), light-cycle core temperature (C), and light-cycle heart rate (D) of C57Bl mice during ad libitum feeding (control), restriction to 60% of normal caloric intake (CR), and consumption of standard diet supplemented with 0.1% resveratrol (RSV). Days −2 and −1, days preceding treatment; days 1–7, treatment days; days +1 and +2, days following treatment. Black bar: P < 0.05, RSV vs. control; gray bar: P < 0.05, CR vs. control. Data shown as means ± se.
Core body temperature was lower during CR than during control feeding over the last 6 days of the 1 wk feeding period, as shown previously (2, 4, 34, 35). Body temperature was higher for resveratrol than for control treatment on the first 3 days of the feeding period, with no other differences on any of the other days (Fig. 1C). Heart rates during CR were significantly lower than during control feeding for the last 6 days of the 1 wk period, as shown previously (2, 36, 37). Heart rates during resveratrol feeding were not significantly different from control (Fig. 1D). Activity levels did not differ among the 3 treatments (data not shown).
Long-term resveratrol treatment has no effect on heart rate, body temperature, or metabolic rate in ob/ob mice
For the longer-term experiment, ob/ob mice were used as experimental subjects because recent data has highlighted the potential of resveratrol as a treatment for obesity and type 2 diabetes (27, 30), and ob/ob mice have been used for decades to study these linked morbidities (38, 39).
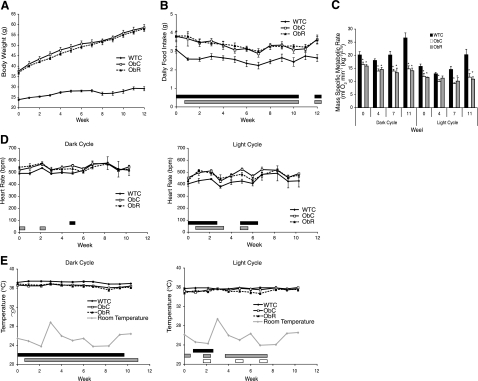
The metabolic, cardiovascular, and behavioral effects of resveratrol were monitored in a cohort of ob/ob mice (ObC and ObR groups) and age-matched WTC mice over the course of a 12 wk study. The dosage of 0.1% resveratrol in the food resulted in an average of 101 mg resveratrol · kg bw−1 · day−1 at the start of the study and 60 mg resveratrol · kg bw−1 · day−1 at its conclusion. The decrease in dosage was the result of weight gain in the ob/ob mice throughout the study without an increase in food consumed. Resveratrol had no effect on body weight in the ob/ob mice (Fig. 2A). No difference in food intake was observed between the ObC and ObR groups, whereas both groups consumed significantly more food than the WTC group throughout the study (Fig. 2B).
Figure 2.
Resveratrol has little effect on various anatomical and physiological measurements. A) Body weight of WTC, ObC, and ObR mice. Both ObC and ObR mice weighed significantly more than WTC mice during the entire 12-wk study; P < 0.05. B) Daily food intake averaged per week. C) Dark- and light-cycle mass-specific metabolic rate [ml O2·min−1·(kg−1)0.75]. *P < 0.05 vs. WTC. D) Dark- and light-cycle heart rate in beats per minute. Black bar; P < 0.05, ObC vs. WTC; gray bar: P < 0.05, ObR vs. WTC. E) Dark- and light-cycle core body temperature (°C). Black and gray bars: same as D; white bar: P < 0.05, ObC vs. ObR. Data shown as means ± se.
Resveratrol had no effect on the mass specific metabolic rate (MSMR) of ob/ob mice during either the dark or light cycles (Fig. 2C), and both groups had a lower MSMR than WTC mice. Heart rate was monitored throughout the duration of the study in ObC, ObR, and WTC groups using electrocardiographic telemetry devices. The average dark- and light-cycle heart rates were unchanged by resveratrol at all time points. ObR and ObC mice generally had elevated heart rates (albeit small increases) in the light cycle, at least during the first half of the study (Fig. 2D). As reported previously (39, 40), the dark-cycle core body temperature (Tb) of both groups of ob/ob mice was generally lower compared with WTC mice throughout the study (Fig. 2E). Resveratrol treatment, however, had no effect on core body temperature (Fig. 2E). During the light cycle, the core body temperature of WTC mice was not consistently higher than that of either ob/ob group (Fig. 2E). Resveratrol did lower core body temperature slightly and transiently during wk 2, 5, and 7 (Fig. 2E).
Resveratrol treatment negatively affects endurance in ob/ob mice
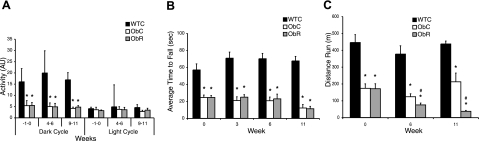
General cage activity, obtained from the telemeters, was lower in the dark cycle within both groups of ob/ob mice compared with wild-type mice (Fig. 3A). In the light cycle, no difference in general cage activity was detected among any of the groups (Fig. 3A). Resveratrol treatment did not affect activity in either the dark or light cycle. As a measure of agility, the mice were subjected to a rotarod test on a gradually accelerating rotarod before resveratrol treatment, and after 3, 6, and 11 wk into the trial. Both ob/ob groups performed significantly inferior to the WTC mice (Fig. 3B). In addition, the average time to fall for the WTC group remained stable over the course of the study—there was no significant change in average fall time between wk 0 and 11. Performance on the rotarod in both ObC and ObR groups significantly diminished over the 12 wk of the study (paired t test, P<0.05 for both the ObC and ObR groups). This finding is likely the result of the weight gain in these animals (Fig. 2A).
Figure 3.
Decreased motor functioning with resveratrol treatment. A) Dark- and light-cycle activity levels. B) Average time to fall in rotarod test. C) Time to exhaustion on treadmill endurance test. Data shown as means ± se. *P < 0.05 vs. WTC; #P < 0.05 vs. ObC.
The mice were also subjected to a treadmill run test before the initiation of treatment, and at wk 6 and 11 during the study (Fig. 3C). As with the rotarod test, the endurance of the wild-type mice remained unchanged from wk 0 to 11, indicating that the frequency of testing did not have a training effect on these mice. The distance run for this group was significantly farther than for either of the ob/ob groups at each time point (Fig. 3C). At wk 6, ObR mice ran a significantly shorter distance (∼50 m less) than ObC mice. The running protocol for the ob/ob animals was modified in wk 11 such that the animals were run at a moderate speed (see Materials and Methods). With this new protocol, ObC mice were able to run even farther than during wk 6 (Fig. 3C). The distance run by the resveratrol-treated animals, however, decreased. The actual difference in the distance run by the groups was dramatic, with the ObC group running more than 5× as far as the ObR group (Fig. 3C).
Resveratrol alters expression profiles in brown adipose, heart, and liver tissues
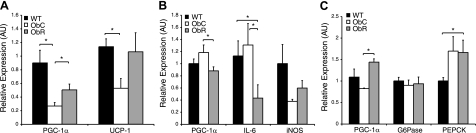
Expression levels of several genes were assessed in BAT and in heart and liver tissues via quantitative real-time PCR (Fig. 4). Levels of UCP-1 in the BAT (Fig. 4A) of ObC mice were significantly lower than those in WTC mice (<50%), in agreement with previously published data (41). Resveratrol treatment normalized BAT UCP-1 compared with WTC mice. Also consistent with previous reports (42), PGC-1α levels were significantly depressed in the BAT of ObC mice relative to wild types (∼30% of WTC). As with UCP-1, resveratrol treatment led to the up-regulation of PGC-1α transcripts.
Figure 4.
Resveratrol alters expression profiles in brown adipose, heart, and liver tissues. A) Relative expression of PGC-1α and UCP-1 levels in BAT. Data are normalized to β-actin levels and all expression levels displayed are relative to WTC. B) Relative expression of PGC-1α, IL-6, and iNOS levels in the heart. C) Relative expression of PGC-1α, G6Pase, and PEPCK levels in the liver. Data in B and C are normalized to 18S. Data shown as means ± se. *P < 0.05 as indicated.
In the cardiac tissue of ObR mice, levels of PGC-1α and interleukin (IL) -6 (a marker of inflammation) were significantly reduced compared with ObC (Fig. 4B). The former result matches previous findings in wild-type animals (29), and the latter extends from data indicating that resveratrol treatment can reduce IL-6 levels in the aortas of older or obese mice (28). It has also been established that resveratrol treatment can increase expression of iNOS in the hearts of wild-type mice, providing protection of ischemic injury (43). Here, we extend these findings to ob/ob mice with a trend (P=0.066) toward an elevation of iNOS in the hearts of mice treated with resveratrol.
As in BAT, resveratrol treatment led to increased expression of PGC-1α in the livers of ObR mice (Fig. 4C). Because resveratrol has been shown to activate AMP-activated protein kinase (AMPK) in the livers of wild-type mice (43, 44), we also checked the expression of gluconeogenic genes, G6Pase and PEPCK, targets known to be repressed by activated AMPK (45). Surprisingly, we saw no effect on the expression levels of these genes vs. ObC.
DISCUSSION
Despite recently published data indicating that a 3-decades-long increase in childhood obesity rates may have reached a plateau (46), obesity and its associated adverse health consequences—high cholesterol, asthma, hypertension, heart disease, and type 2 diabetes—remain a chief concern as one of the major causes of morbidity and mortality in the United States (47, 48). As of 2005–2006, more than one-third of all U.S. adults were obese (49).
CR is associated with a host of physiological changes that are linked to a longer, healthier life: decreased body weight and adiposity; decreased blood glucose, insulin, triglyceride and cholesterol levels; and increased insulin sensitivity (5). Because this condition represents the converse of the obese phenotype, much effort has been expended to identify the pathways responsible for regulating the CR phenotype and to find compounds capable of manipulating those pathways for clinical benefit. Within the past decade, a histone deacetylase, Sir2 (and its mammalian homologue Sirt1), and resveratrol, a compound found in foods such as red grape skins, have emerged as potential candidates for these roles (6, 8, 19). Indeed, recent work in mice indicates that resveratrol can mimic many of the transcriptional, metabolic, and physiological effects of CR (27,28,29,30). With this study, we wished to expand on these findings to determine whether resveratrol could mimic the cardiovascular and behavioral characteristics associated with CR as well. Using a radiotelemetry system that allows for continuous monitoring of physiological parameters in free-moving mice, we provide novel evidence that the previously reported CR-mimicking actions of resveratrol do not extend to heart rate and body temperature regulation over either short- or long-term periods of administration. In addition, a treadmill test revealed that resveratrol negatively affected the endurance of ob/ob mice over a 12-wk course of treatment.
Substantial bradycardia is a hallmark of CR (2, 3, 50). In our short-term experiment, we found that mice experienced significant bradycardia within 1 day of CR (Fig. 1D). Resveratrol treatment had no effect, however, on heart rate, either within wild-type mice over a short-term (Fig. 1D) or within ob/ob mice over a longer term (Fig. 2D). Because the bradycardia associated with CR is associated with elevated parasympathetic influence over the heart, diminished sympathetic influence over the heart, and a lowered intrinsic heart rate (51), we conclude that resveratrol treatment does not significantly affect the autonomic nervous system influence over the heart nor the intrinsic beating rate. Similarly, wild-type mice on a short-term CR diet experienced a significant and sustained decrease in body temperature, yet in both studies, resveratrol treatment (Figs. 1C and 2E) produced only transient alterations in body temperature that were inconsistent with the CR phenotype (2, 3). Hence, it appears that resveratrol also fails to mimic the core body temperature effects of CR.
Given previous reports describing the beneficial effect of resveratrol on endurance in mice fed a high-fat diet (30), we were surprised to find that the ObR mice performed more than 5-fold worse than ObC mice in a treadmill endurance test (Fig. 3C). Our findings are consistent, however, with another report demonstrating that Sirt1-deficient mice have increased treadmill endurance (13), in that active Sirt1, potentially activated in our experiments with resveratrol, may hinder stamina. Thus, the beneficial effects on motor functioning observed in a diet-induced model of obesity may not be universal to all models of obesity.
It is possible that the lack of an effect by resveratrol on heart rate or body temperature indicates a nonoptimal dosage of resveratrol. The dosage level for this study, however, was selected as a midpoint between 2 previous studies—4-fold greater than Baur et al. (27) and 4-fold less than Lagouge et al. (30). The dosage used here is also well within the range of other published studies (43). In addition, gene expression data in several tissues of ob/ob mice (Fig. 4) are mostly consistent with previously reported in vivo effects of resveratrol.
Our data hint that resveratrol may enhance Sirt1 activity in vivo. Sirt1 is known to deacetylate and activate PGC-1α (15). The expression of both PGC-1α and UCP-1 is depressed in the BAT of ObC mice compared with WTC mice (41, 42), and resveratrol treatment is known to increase PGC-1α expression in skeletal muscle (30). In addition, UCP-1 is induced on activation of PGC-1α in BAT (52); we also find that UCP-1 levels return to wild-type levels in ob/ob mice treated with resveratrol. Interestingly, PGC-1α levels in the heart are elevated with obesity (Fig. 4B) (53), a condition which reseveratrol treatment can reverse (29). We also examined the transcriptional profiles of several other genes in ob/ob mice indicative of the antioxidant and AMPK-activating properties of resveratrol. From our data, it appears that the antioxidant effects of resveratrol (28, 43) do indeed extend to the ob/ob model of obesity, whereas the AMP kinase activating activity does not. This latter finding could result from the leptin-deficient background of our mice; leptin is an established activator of AMPK in skeletal muscle (54), although little is known of its effects in the liver. In addition, the lack of AMPK activation may explain the reduced endurance in the ObR mice. Regardless, the altered transcriptional profiles of 3 distinct tissues suggest that resveratrol did indeed have an effect on these ob/ob mice.
To summarize, because of the alarming prevalence of obesity and obesity-related conditions, a significant amount of work has gone into studying the therapeutic potential of resveratrol because of its ability to mimic the effects of CR in yeast and lower animals (19, 24). Although evidence exists that resveratrol can improve the metabolic predicament of mice fed a high-fat diet (27, 30), resveratrol had no major effect on most of the physiological parameters considered in this study, affecting only the treadmill endurance of ob/ob mice. Therefore, two conclusions become apparent: it is likely that the changes in heart rate and body temperature that occur during CR are not mediated by resveratrol targets and that a further investigation of the effects of resveratrol in a leptin-deficient background is needed.
Acknowledgments
This work was supported by U.S. National Institutes of Health grant R15 HL081101-01 to S.J.S.
References
- McCay C M, Crowell M F, Maynard L A. The effect of retarded growth upon the length of life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- Overton J M, Williams T D. Behavioral and physiologic responses to caloric restriction in mice. Physiol Behav. 2004;81:749–754. doi: 10.1016/j.physbeh.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Wolf G. Calorie restriction increases life span: a molecular mechanism. Nutr Rev. 2006;64:89–92. doi: 10.1301/nr.2006.feb.89-92. [DOI] [PubMed] [Google Scholar]
- Chen D, Guarente L. SIR2: a potential target for calorie restriction mimetics. Trends Mol Med. 2007;13:64–71. doi: 10.1016/j.molmed.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Lin S J, Defossez P A, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–2128. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Chen W P, Chi T C, Chuang L M, Su M J. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–277. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand S L. Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci U S A. 2004;101:15998–16003. doi: 10.1073/pnas.0404184101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R M, Bitterman K J, Wood J G, Medvedik O, Sinclair D A. Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature. 2003;423:181–185. doi: 10.1038/nature01578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S J, Kaeberlein M, Andalis A A, Sturtz L A, Defossez P A, Culotta V C, Fink G R, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Cohen H Y, Miller C, Bitterman K J, Wall N R, Hekking B, Kessler B, Howitz K T, Gorospe M, de Cabo R, Sinclair D A. Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 2004;305:390–392. doi: 10.1126/science.1099196. [DOI] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta M C, van Veen E, Czopik A, Steele A D, Crowe H, Marmor S, Luo J, Gu W, Guarente L. SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 2007;6:759–767. doi: 10.1111/j.1474-9726.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- Chen D, Steele A D, Lindquist S, Guarente L. Increase in activity during calorie restriction requires Sirt1. Science. 2005;310:1641. doi: 10.1126/science.1118357. [DOI] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R, Leid M, McBurney M W, Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers J T, Lerin C, Haas W, Gygi S P, Spiegelman B M, Puigserver P. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- Lane M A, Ingram D K, Roth G S. Calorie restriction in nonhuman primates: effects on diabetes and cardiovascular disease risk. Toxicol Sci. 1999;52:41–48. doi: 10.1093/toxsci/52.2.41. [DOI] [PubMed] [Google Scholar]
- Sun C, Zhang F, Ge X, Yan T, Chen X, Shi X, Zhai Q. SIRT1 improves insulin sensitivity under insulin-resistant conditions by repressing PTP1B. Cell Metab. 2007;6:307–319. doi: 10.1016/j.cmet.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Ingram D K, Zhu M, Mamczarz J, Zou S, Lane M A, Roth G S, deCabo R. Calorie restriction mimetics: an emerging research field. Aging Cell. 2006;5:97–108. doi: 10.1111/j.1474-9726.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Howitz K T, Bitterman K J, Cohen H Y, Lamming D W, Lavu S, Wood J G, Zipkin R E, Chung P, Kisielewski A, Zhang L L, Scherer B, Sinclair D A. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Pervaiz S. Resveratrol: from grapevines to mammalian biology. FASEB J. 2003;17:1975–1985. doi: 10.1096/fj.03-0168rev. [DOI] [PubMed] [Google Scholar]
- Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–1526. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman E A, Caldwell S D, Napper A, Curtis R, DiStefano P S, Fields S, Bedalov A, Kennedy B K. Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 2005;280:17038–17045. doi: 10.1074/jbc.M500655200. [DOI] [PubMed] [Google Scholar]
- Borra M T, Smith B C, Denu J M. Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 2005;280:17187–17195. doi: 10.1074/jbc.M501250200. [DOI] [PubMed] [Google Scholar]
- Wood J G, Rogina B, Lavu S, Howitz K, Helfand S L, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430:686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Viswanathan M, Kim S K, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Bass T M, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128:546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Baur J A, Pearson K J, Price N L, Jamieson H A, Lerin C, Kalra A, Prabhu V V, Allard J S, Lopez-Lluch G, Lewis K, Pistell P J, Poosala S, Becker K G, Boss O, Gwinn D, Wang M, Ramaswamy S, Fishbein K W, Spencer R G, Lakatta E G, Le Couteur D, Shaw R J, Navas P, Puigserver P, Ingram D K, de Cabo R, Sinclair D A. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson K J, Baur J A, Lewis K N, Peshkin L, Price N L, Labinskyy N, Swindell W R, Kamara D, Minor R K, Perez E, Jamieson H A, Zhang Y, Dunn S R, Sharma K, Pleshko N, Woollett L A, Csiszar A, Ikeno Y, Le Couteur D, Elliott P J, Becker K G, Navas P, Ingram D K, Wolf N S, Ungvari Z, Sinclair D A, de Cabo R. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8:157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barger J L, Kayo T, Vann J M, Arias E B, Wang J, Hacker T A, Wang Y, Raederstorff D, Morrow J D, Leeuwenburgh C, Allison D B, Saupe K W, Cartee G D, Weindruch R, Prolla T A. A low dose of dietary resveratrol partially mimics caloric restriction and retards aging parameters in mice. PLoS ONE. 2008;3:e2264. doi: 10.1371/journal.pone.0002264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, Geny B, Laakso M, Puigserver P, Auwerx J. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell. 2006;127:1109–1122. doi: 10.1016/j.cell.2006.11.013. [DOI] [PubMed] [Google Scholar]
- Milne J C, Lambert P D, Schenk S, Carney D P, Smith J J, Gagne D J, Jin L, Boss O, Perni R B, Vu C B, Bemis J E, Xie R, Disch J S, Ng P Y, Nunes J J, Lynch A V, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair D A, Olefsky J M, Jirousek M R, Elliott P J, Westphal C H. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden G L, Drucker D J, Weinshenker D, Swoap S J. Oxyntomodulin increases intrinsic heart rate in mice independent of the glucagon-like peptide-1 receptor. Am J Physiol. 2007;292:R962–R970. doi: 10.1152/ajpregu.00405.2006. [DOI] [PubMed] [Google Scholar]
- Arany Z, Lebrasseur N, Morris C, Smith E, Yang W, Ma Y, Chin S, Spiegelman B M. The transcriptional coactivator PGC-1beta drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 2007;5:35–46. doi: 10.1016/j.cmet.2006.12.003. [DOI] [PubMed] [Google Scholar]
- Lane M A, Baer D J, Rumpler W V, Weindruch R, Ingram D K, Tilmont E M, Cutler R G, Roth G S. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A. 1996;93:4159–4164. doi: 10.1073/pnas.93.9.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikke B A, Yerg J E, 3rd, Battaglia M E, Nagy T R, Allison D B, Johnson T E. Strain variation in the response of body temperature to dietary restriction. Mech Ageing Dev. 2003;124:663–678. doi: 10.1016/s0047-6374(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Mager D E, Wan R, Brown M, Cheng A, Wareski P, Abernethy D R, Mattson M P. Caloric restriction and intermittent fasting alter spectral measures of heart rate and blood pressure variability in rats. FASEB J. 2006;20:631–637. doi: 10.1096/fj.05-5263com. [DOI] [PubMed] [Google Scholar]
- Swoap S J, Weinshenker D, Palmiter R D, Garber G. Dbh(-/-) mice are hypotensive, have altered circadian rhythms, and have abnormal responses to dieting and stress. Am J Physiol. 2004;286:R108–R113. doi: 10.1152/ajpregu.00405.2003. [DOI] [PubMed] [Google Scholar]
- Coleman D L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978;14:141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Friedman J M, Halaas J L. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Stanley S, Wynne K, McGowan B, Bloom S. Hormonal regulation of food intake. Physiol Rev. 2005;85:1131–1158. doi: 10.1152/physrev.00015.2004. [DOI] [PubMed] [Google Scholar]
- Commins S P, Watson P M, Padgett M A, Dudley A, Argyropoulos G, Gettys T W. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- Kakuma T, Wang Z W, Pan W, Unger R H, Zhou Y T. Role of leptin in peroxisome proliferator-activated receptor gamma coactivator-1 expression. Endocrinology. 2000;141:4576–4582. doi: 10.1210/endo.141.12.7804. [DOI] [PubMed] [Google Scholar]
- Baur J A, Sinclair D A. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Rabinovitch P S. Medicine: grapes versus gluttony. Nature. 2006;444:280–281. doi: 10.1038/nature05308. [DOI] [PubMed] [Google Scholar]
- Lochhead P A, Salt I P, Walker K S, Hardie D G, Sutherland C. 5-aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the 2 key gluconeogenic genes PEPCK and glucose-6-phosphatase. Diabetes. 2000;49:896–903. doi: 10.2337/diabetes.49.6.896. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Flegal K M. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299:2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, Curtin L R, McDowell M A, Tabak C J, Flegal K M. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Mokdad A H, Ford E S, Bowman B A, Dietz W H, Vinicor F, Bales V S, Marks J S. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–79. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- Ogden C L, Carroll M D, McDowell M A, Flegal K M. Hyattsville, MD, USA: NCHS Data Brief 1. National Center for Health Statistics; Obesity among adults in the United States–no change since 2003–2004. 2007 [PubMed] [Google Scholar]
- Varady K A, Hellerstein M K. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- Swoap S J, Li C, Wess J, Parsons A D, Williams T D, Overton J M. Vagal tone dominates autonomic control of mouse heart rate at thermoneutrality. Am J Physiol. 2008;294:H1581–H1588. doi: 10.1152/ajpheart.01000.2007. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Wu Z, Park C W, Graves R, Wright M, Spiegelman B M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- Pagano C, Calcagno A, Granzotto M, Calabrese F, Thiene G, Federspil G, Vettor R. Heart lipid accumulation in obese non-diabetic rats: effect of weight loss. Nutr Metab Cardiovasc Dis. 2008;18:189–197. doi: 10.1016/j.numecd.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim Y B, Peroni O D, Fryer L G, Muller C, Carling D, Kahn B B. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]