Abstract
In view of the conventional wisdom in the cardiology literature that apoptosis is extensive early after myocardial ischemia, predicated largely from results with the TUNEL assay known to be nonspecific, this study was performed to delineate its extent with multiple assays and at multiple intervals. Coronary occlusion with and without subsequent revascularization was induced in 10-wk-old C57BL6 mice subjected to 1 or 4 h of transient ligation followed by 24 h of reperfusion, or 24 h persistent ligation. Apoptosis was quantified throughout the left ventricle immunohistochemically by assay of TUNEL, single-stranded DNA (ssDNA), and cleaved caspase 3; electron microscopy (EM); and activity assays of caspase 3 and 8. TUNEL staining was marked, but ssDNA and cleaved caspase 3 staining were significantly less (P<0.001 compared with TUNEL), and apoptosis defined by EM was virtually absent in all groups. Caspase 3 and caspase 8 activities per milligram protein were not significantly different from those in normal hearts. Only rare, potentially apoptotic cells were seen by EM in hearts from any group. Thus, the results with TUNEL were not specific, and the extent of apoptosis was markedly less than that predicated on the results with the TUNEL procedure. Apoptosis is de minimus early after transitory or persistent ischemia, though it is overestimated by TUNEL assays. Thus, antiapoptotic interventions per se are not likely to preserve substantial amounts of myocardium early after ischemic insults.—French, C. J., Spees, J. L., Tarikuz Zaman, A. K. M., Taatjes, D. J., Sobel, B. E. The magnitude and temporal dependence of apoptosis early after myocardial ischemia with or without reperfusion.
Keywords: multiple assays, infarction, TUNEL, caspase 3, ssDNA
The treatment of acute coronary syndromes (ACS) is designed to enhance myocardial perfusion, diminish myocardial oxygen requirements, and preserve myocardium through stabilizing myocardial metabolism (1). Unfortunately, restoration of blood flow to ischemic myocardium may, itself, induce reperfusion injury (2), limiting overall salvage of heart muscle and perhaps contributing to the 10% 30-day mortality and 25% incidence of heart failure that persist (3).
Reperfusion injury has been attributed, in part, to apoptosis, i.e., programmed cell death (4,5,6,7,8,9,10) thought to be extensive early after the onset of ischemia, based largely on results obtained with terminal deoxynucleotidyl transferase [TdT]-mediated dUTP in situ nick-end labeling (TUNEL) assays in studies in experimental animals and patients. Thus, preservation of myocardium has been attempted by interventions designed to attenuate apoptosis (11,12,13). Results with this approach have, unfortunately, been disappointing (11). If apoptosis were extensive early after the onset of myocardial infarction (MI) when jeopardized myocardium could still be salvaged, its amelioration should improve outcomes in patients. If it were not extensive, antiapoptotic strategies would be ineffective.
Studies that have implicated apoptosis as extensive have relied primarily or exclusively on results obtained with TUNEL assays. However, the TUNEL assay is not specific and yields positive results in mitotic, oncotic (cell swelling or necrotic), and apoptotic cell death (14,15,16,17,18,19,20,21,22,23,24,25,26). The gold standard for detection of apoptosis is electron microscopy. However, it is expensive, very labor intensive, and time consuming. Furthermore, fields of view are miniscule. Its use has not detected appreciable amounts of apoptosis in the ischemic heart (23).
This study was performed to determine whether the extent of apoptosis measured with multiple immunohistochemical, biochemical, and electron microscopic procedures (27,28,29) early after myocardial ischemia with or without subsequent reperfusion is sufficient to render antiapoptotic interventions effective in preserving substantial amounts of myocardium.
MATERIALS AND METHODS
All reagents were prepared with distilled water unless otherwise noted.
Induction of MI
Animals were used in conformity with a protocol approved by the University of Vermont Institutional Animal Care and Use Committee and with adherence to the U.S. National Institutes of Health Principles of Animal Care. C57BL6 mice (males, 9 to 10 wk of age) were obtained from Taconic Farms (Albany, NY, USA). The mice were intubated and maintained on a ventilator at 145 strokes/min and 200-μl stroke volume (Harvard Apparatus, Holliston, MA, USA) following induction of anesthesia with 4% isoflurane and oxygen, kept on a heating pad during and for several hours after the surgery, and operated on under aseptic conditions. An 8-mm skin incision was made 2 mm from the left sternal border near the area above the fourth intercostal space. Blunt dissection was used to separate the thoracic muscles, and the chest was opened in the fourth intercostal space with a 5-mm incision between the ribs. Left anterior descending (LAD) ligation (technically, the middle left) with an 8-0 suture was performed as described in detail previously (27). For studies in which reperfusion was induced at selected intervals, we ligated the LAD with a 0.4-inch piece of PE-10 tubing interposed between the vessel wall and the ligature. Its presence facilitated cutting of the suture and hence induction of reperfusion confirmed by visualization of the return of color in the previously pale region and immediate electrocardiographic changes, including resolution of ST segment elevation detected with the use of a base plate (VisualSonics Vevo 770 system). Persistent (24 h) coronary ligation of the LAD was induced in 9- to 10-wk-old mice, and the hearts were harvested 24 h later. To simulate ischemia followed by early reperfusion analogous to percutaneous coronary intervention in patients, we performed the ligations as follows: 4-h transient ligation followed by reperfusion for 24 h and 1 h transient ligation followed by reperfusion for 24 h. Successful reperfusion was confirmed visually (amelioration of pallor and restoration of wall motion) and, in some animals, by injection of 0.5 ml of Evans blue dye [1% in phosphate-buffered saline (PBS), pH 7.4] slowly into the left ventricular cavity through the apex with the use of a 27-gauge needle. The dye suffused reperfused regions that had previously been obviously pale.
Assessment of the extent of infarction
Two-dimensional (2-D) and M-Mode echocardiography were performed immediately before harvest of the hearts 24 h after induction of MI, as described previously (30). Systolic function was analyzed to confirm successful induction of infarction, as described previously (27). A functional assessment with a 13-segment model similar to the American Society of Echocardiography’s 16-segment model was performed, and cardiac output, stroke volume, and fractional shortening were measured to assess systolic function.
Harvesting of the hearts
Mice were anesthetized with 4% isoflurane and then killed humanely by exsanguination. Their left ventricles were excised, rinsed with PBS, gently cleaned of gross fat, immersed into 3% paraformaldehyde in PBS, and fixed overnight at 4°C. After fixation, the tissue was rinsed several times with PBS, placed in cassettes, dipped in 70% ethanol, and embedded in paraffin. Mouse blood was extracted from the right ventricle at the time of tissue harvest with 75 μl 0.129 M sodium citrate, pH 6.0, as the anticoagulant and centrifuged to provide plasma that was frozen immediately in liquid nitrogen for use of assays later.
TUNEL assays
Paraffin sections of 6 μm each were prepared from the hearts of control mice and those with MI. The sections were incubated for 1 h at 56°C, deparaffinized in xylenes, and rehydrated by an ethanol series. TUNEL assays were performed with the use of ApopTag Plus Fluorescein In Situ Apoptosis Detection Kits, according to the manufacturer’s instructions (Chemicon, Temecula, CA, USA). Photographs were taken with the use of an epifluorescence microscope equipped with an automated x, y, and z stage for deconvolution (Leica DM6000B microscope, Leica CC camera, and Leica Deblur deconvolution software; Leica Microsystems, Wetzlar, Germany). Overall image brightness was adjusted by setting the total RGB channel to the base of the signal distribution in all images (Adobe Photoshop; Adobe Systems, San Jose, CA, USA). The numbers of TUNEL-positive cardiac myocyte nuclei were quantified with the use of Image Pro Plus software (MediaCybernetics, Bethesda, MD, USA). For each heart, 3 tissue sections were quantified spanning 150 μm from the midpapillary region well below the ligation point of the coronary artery. Three separate fields of view were quantified within the zone of infarction from each section, and the numbers of positive cells were averaged. The chosen fields of view represented areas of heaviest TUNEL staining.
Immunohistochemical assay for single-stranded DNA (ssDNA)
Paraffin sections were prepared as described above. After incubation in PBS for 5 min, sections were incubated for 20 min in Saponin (0.2 mg/ml in PBS), then for 20 min at room temperature in 0.02 mg/ml proteinase K, RNA grade solution from Invitrogen (Eugene, OR, USA) in PBS, pH 7.4. The slides were then rinsed in distilled water 3 times and treated with 50% formamide in H2O in a 56°C water bath for 20 min. After washing with cold PBS for 5 min, 3% nonfat dry milk in H2O was added for 15 min, followed by the addition of 100 μl of 10 μg/ml primary antibody (F7–26; Chemicon) working solution (primary concentration of 0.1 mg/ml diluted in 5% fetal bovine serum in PBS). The slides were washed in PBS twice for 5 min, followed by the addition of 100 μl of Alexa 594 conjugated goat anti-mouse IgG secondary antibody (Invitrogen) diluted in 1% nonfat dry milk in PBS–1:400 dilution for 15 min. The slides were then washed with PBS twice for 5 min and mounted in Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA) to preserve fluorescence and for visualization of cell nuclei. Subsequent procedures were the same as those described above for the TUNEL assay.
Immunohistochemical assay for cleaved caspase 3
Paraffin sections were prepared as described above. The slides were rinsed in PBS for 5 min. They were then treated with 3% peroxide in PBS for 30 min, followed by a rinse in PBS for 5 min. The slides were then treated with 100% methanol for 10 min and subsequently washed in PBS for 5 min. The slides were then placed in a solution of 20 μg/ml proteinase K in PBS for 15 min at room temperature; washed in 2 changes of PBS, 5 min each; blocked with 10 ml PBS, 0.5 normal goat serum (191356; MP Biomedicals LLC, Solon, OH, USA), and 40 μl Triton X-100; and incubated in a humid chamber for 1 h. Primary antibody, rabbit anti-cleaved caspase 3 (9661S; Cell Signaling, Danvers, MA, USA) was diluted 1:100 in blocking solution, applied to the slides, and incubated overnight at 4°C. The slides were then rinsed with the PBS 3 times for 5 min each. A dilution of 1:500 was prepared of the secondary antibody (goat anti-rabbit Alexa-488; Invitrogen) in blocking buffer, applied to the slides, and incubated for 1 h at room temperature in the dark. The slides were washed in 2 changes of PBS/1% BSA 5 min each in the dark and mounted with Vectashield with DAPI (Vector Laboratories; H-1200) to preserve fluorescence and to visualize cell nuclei. Photographs were taken as described for the TUNEL assays.
Immunohistochemical assay for thymus (positive controls)
Eight- to 10-wk-old mice were given 250 μl intraperitoneal injections of 4 mg/ml dexamethasone sodium phosphate (American Reagent, Shirley, NY, USA) or an equivalent amount of normal saline. The mice were anesthetized with 4% isoflurane. The thymuses were harvested 10 to 12 h after the dexamethasone or normal saline injections. They were dissected free, rinsed with PBS, gently cleaned of gross fat, and fixed overnight at 4°C in 3% PFA/PBS. After fixation, the tissues were rinsed several times with PBS, placed in cassettes, dipped in 70% ethanol, and embedded in paraffin. They were sectioned and stained for TUNEL, ssDNA, and cleaved caspase 3 as described above.
Caspase 3 and caspase 8 activity assays performed biochemically
The mice were anesthetized with 4% isoflurane and then killed humanely by exsanguination. The left ventricles to be used for biochemical assays were immediately frozen in liquid nitrogen. Caspase 3 activity was measured in mouse left ventricle homogenates with the CaspSELECT Caspase 3 Immunoassay Kit, according to the manufacturer’s instructions (Biovision, Mountain View, CA, USA). A triton lysis buffer (20 mM Tris, pH 7.4; 137 mM NaCl; 25 mM B-glycerolphosphate, pH 7.4; 2 mM PPiNa; 2 mM EDTA, pH 7.4; 1% Triton X-100; 10% glycerol; H2O) was used to ensure lysis of membranes. Caspase 3 activity was measured with a fluorescence plate reader (360/528 nm; HT Synergy, Winooski, VT, USA). Solubilized protein concentrations were determined by the Bradford method (31). Results were expressed relative to values obtained with normal hearts and in absolute terms. Thymuses from mice treated with dexamethasone served as positive controls for apoptosis, and those from mice given saline were used as negative controls for apoptosis. Caspase 8 activity of left ventricular homogenates was measured with the Caspase 8/FLICE Fluorometric Assay Kit (Biovision). Samples were read in a fluorometer with 360-nm excitation and 528-nm emission filter. Results were expressed relative to values obtained with normal hearts and in absolute terms.
Preparation of tissue for transmission electron microscopy
The ventricles from 14 animals (12 with coronary ligation and 2 sham operated) were divided into the following 3 regions: infarct zone, peri-infarct zone, and normal zone. These areas were cut into small pieces and placed into fixative consisting of 2.5% glutaraldehyde and 1.0% formaldehyde in Millonig’s phosphate buffer overnight at 4°C. Following rinses with buffer, the tissue pieces were postfixed in 1% osmium tetroxide for 60 min at 4°C, rinsed with buffer, dehydrated in a graded series of ethanol, and embedded in Spurr’s epoxy resin. Ultrathin sections were cut with diamond knives, retrieved onto copper mesh grids, contrasted with uranyl acetate and lead citrate, and examined with a JEOL 1210 transmission electron microscope (JEOL USA, Peabody, MA, USA) operating at 60 kV. One grid from each of the 3 regions from 2 animals subjected to each one of the conditions implemented was evaluated. Images were acquired on Kodak 4489 electron microscope film (Eastman Kodak, Rochester, NY, USA), followed by scanning the negatives with a Microtek ScanMaker 8700 (8-bit grayscale, 600 ppi; Microtek, Carson, CA, USA). Contrast of the images was enhanced by performing a levels adjustment (essentially a contrast histogram stretch action) in Adobe Photoshop CS2.
Statistics
Values were compared by means of ANOVA or Student’s t test as appropriate. Variables that demonstrated a lack of normality or of homogeneity of variance were compared with distributions with the use of the Kruskal-Wallis test. Significance was assumed to be present when P ≤ 0.05. Our study was designed to permit us to detect a 4-fold difference between values in normal hearts and those in hearts subjected to MI with a probability of failing to detect a true difference if one were present (β) of 20%.
RESULTS
Consequences of myocardial ischemia and reperfusion
Mortality within 24 h following surgery after either transient or persistent ligation was 10%. Successful reperfusion was confirmed with Evan’s blue dye injections in representative mice following 4 h of transient ligation (Fig. 1). The effect of the coronary ligation was confirmed by echocardiography in representative mice that had sustained 4-h transient ligations followed by 1 day of reperfusion or persistent ligation for 24 h. The average functional scores in the two groups were 16 and 23, respectively, compared with a score in normal hearts of 13.
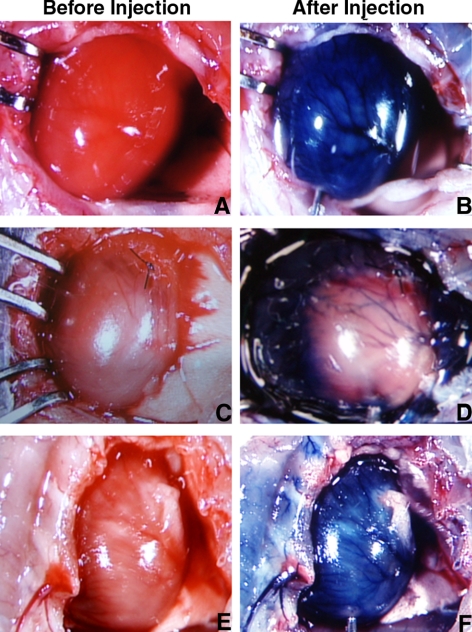
Figure 1.
Confirmation of reperfusion with injection of Evans blue dye in the absence of ligation (A, B), after 4 h of persistent ligation (C, D), and after 4 h of transient ligation followed by reperfusion for 24 h (E, F). In each case, the heart was visualized before (left panels) and after injection of the dye. As can be seen, dye did not appear in nonreperfused regions (D) but did after 24 h of reperfusion (F). White regions in E and F are reflections from fat.
Positive controls for detection of apoptotic cell death
Successful TUNEL, ssDNA, and caspase 3 staining for apoptosis was confirmed with the use of thymus tissue harvested from mice treated with dexamethasone (Fig. 2). The positive thymuses exhibited a significant amount of TUNEL-positive nuclei, whereas the negative controls exhibited only a few positive cells. Thymuses from mice treated with dexamethasone exhibited a substantial number of ssDNA positive nuclei and cleaved caspase 3-positive cells, whereas the negative thymuses exhibited only a few positive cells with each immunostain. These results confirm successful histochemical detection of apoptosis in the thymus with the assays employed.
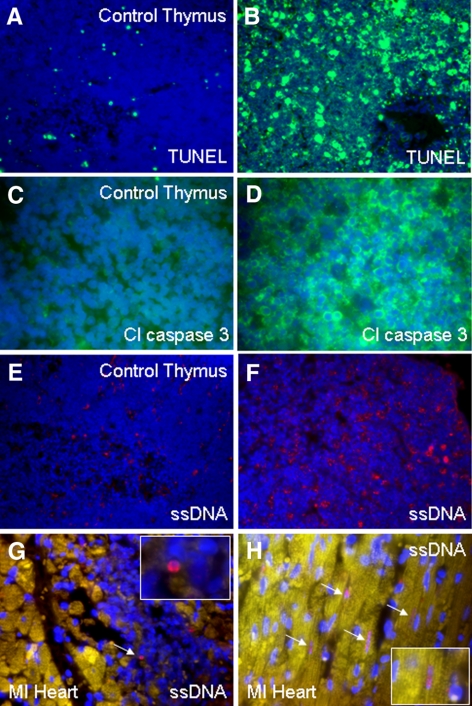
Figure 2.
Left panels: thymus staining in nondexamethasone-treated mice for TUNEL (green) (A), cleaved caspase 3 (green) (C), and ssDNA (red) (E). Right panels: thymus staining in dexamethasone-treated mice showing significantly more positive staining for TUNEL (B), cleaved caspase 3 (D), and ssDNA (F). Rare positive ssDNA (red) staining is shown in heart from a mouse with MI in a lymphocyte (G) and in four cardiomyocytes (arrows) (H). Nuclei were stained with DAPI (blue). Yellow color is attributable to autofluorescence of tissue. Original views: ×40 (A, B, E, F); ×63 (C, D, G, H).
Immunohistochemical analysis of hearts subjected to persistent ischemia or ischemia for selected intervals followed by reperfusion for 24 h
Positive TUNEL staining was seen in regions of infarction in all groups studied. There was no TUNEL staining in the noninfarct areas or in the peri-infarct areas of any of the hearts 24 h after the onset of persistent ischemia or reperfusion. One hour of transient ligation followed by 24 h of reperfusion led to less TUNEL staining within the zone of infarction (P=0.001), as did 4 h of transient ligation (P=0.009), compared with that seen with 24 h of persistent ligation.
The numbers of positive ssDNA cells were markedly and significantly lower than the numbers of TUNEL-positive cells in contiguous sections (Table 1, P<0.001). We did not detect any positive cleaved caspase 3 cells in the hearts from mice in any of the groups (Table 1). Sections from the infarct zones showed that the vast majority of TUNEL-positive cells did not stain positively for ssDNA or caspase 3 (Fig. 3, Table 1).
TABLE 1.
Results of immunohistochemical analysis of sections of hearts
| Treatment group
|
Animals
|
Total sections
|
Percentage positive cells of total cells (n)
|
||
|---|---|---|---|---|---|
| TUNEL | Cleaved caspase 3 | ssDNA | |||
| 1 h ischemia, 24 h reperfusion | 3 | 9 | 38.6% (n=304)† | 0 (n=100) | 1.0% (n=97) |
| 4 h ischemia, 24 h reperfusion | 3 | 9 | 48.5% (n=362)† | 0 (n=100) | 0.7% (n=91) |
| 24 h ischemia | 3 | 9 | 67.2% (n=294)*,† | 0 (n=100) | 2.8% (n=80) |
P = 0.009 compared with results in the 4 h of ischemia and 24-h reperfusion group and 0.001 compared with those in the 1 h of ischemia and 24-h reperfusion group.
P < 0.001 compared with results for cleaved caspase 3 and for ssDNA.
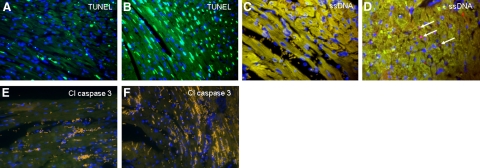
Figure 3.
Sections from a mouse heart undergoing 1 h of transient ligation and 24 h of reperfusion (A, C, E) and from a mouse heart subjected to 24 h of persistent ligation (B, D, F) exhibit positive TUNEL staining (A, B) (green) that is much in excess and is not congruent with positive ssDNA (C, D) (pink) or positive cleaved caspase 3 staining (E, F) (green, none seen). Arrows indicate rare cells positive for ssDNA. Nuclei were stained with DAPI (blue). Yellow color is attributable to autofluorescence of tissue; mustard color is attributable to red blood cells. All panels ×40.
Caspase 3 and caspase 8 biochemical assays
Thymuses from mice treated with dexamethasone showed significantly higher caspase 3 activity than activity in those from normal, untreated mice (Table 2, P=0.0040). There were no differences between caspase 3 activities in the hearts from mice without infarcts and caspase 3 activities in hearts from mice with infarction or ischemia in any group studied. To ascertain whether apoptosis occurred through the receptor-mediated extrinsic caspase 8 apoptotic pathway as opposed to the mitochondrial caspase 3 pathway, we performed caspase 8 biochemical assays on hearts from mice with persistent ligation and those with 4-h transient ligation. Although caspase 8 activity was significantly higher in thymuses from mice treated with dexamethasone compared with controls given saline (P=0.0039), results were the same in hearts with and without infarction (Table 3).
TABLE 2.
Results of caspase 3 biochemical assays
| Treatment group
|
Caspase 3 activity (fluorescence intensity/mg protein)
|
|
|---|---|---|
| Relative value | Absolute value | |
| 4 h ischemia, 24 h reperfusion (n=6 hearts) | 0.77 ± 0.80 | 32.45 ± 29.16 |
| 24 h ischemia (n=6 hearts) | 1.06 ± 0.80 | 35.61 ± 26.72 |
| Controls without ischemia (n=7 hearts) | 1.00 ± 0.89 | 33.50 ± 29.76 |
| Thymuses from mice given dexamethasone (n=6) | 9.04 ± 3.32* | 1071 ± 393* |
| Thymuses from mice given saline (n=6) | 1.0 ± 0.46 | 118.50 ± 54.33 |
Results are means ± sd of ratios of intensity of fluorescence relative to that in normal hearts or to that in thymuses from mice given saline. Absolute values are shown as well.
P = 0.0040 compared with results in thymuses from mice given saline.
TABLE 3.
Results of caspase 8 biochemical assays
| Treatment group
|
Caspase 8 activity (fluorescence intensity/mg protein)
|
|
|---|---|---|
| Relative value | Absolute value | |
| 4 h ischemia, 24 h reperfusion (n=6 hearts) | 0.33 ± 0.60 | 27.64 ± 49.90 |
| 24 h ischemia (n=6 hearts) | 0.82 ± 0.65 | 68.42 ± 53.85 |
| Controls without ischemia (n=7 hearts) | 1.0 ± 0.96 | 83.04 ± 79.65 |
| Thymuses from mice given dexamethasone (n=6) | 6.87 ± 3.05* | 7811 ± 3469* |
| Thymuses from mice given saline (n=6) | 1.0 ± 0.30 | 1134 ± 339 |
Results are means ± sd of ratios of intensity of fluorescence relative to that in normal hearts or thymuses from mice given saline. Absolute values are shown as well.
P = 0.0039 compared with results in thymuses from mice given saline.
Transmission electron microscopy
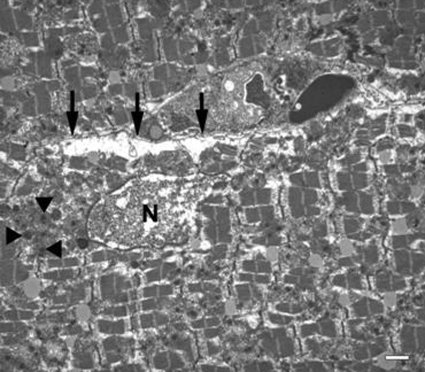
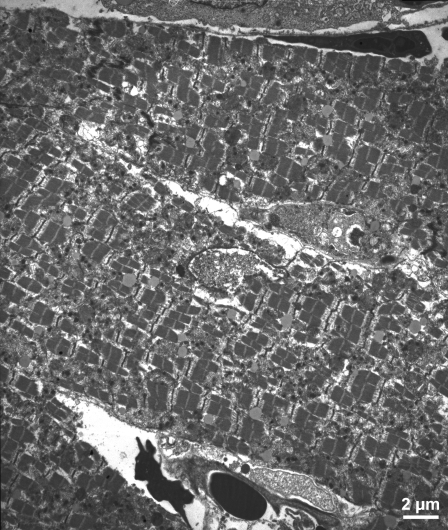
Using the morphological criteria provided by Hayakawa et al. 32) and Abbate et al. (33), only very rare cardiomyocytes could be identified as potentially apoptotic in sections from 14 animals (12 with coronary artery ligation and 2 unoperated controls). A good example is shown in the peri-infarct zone from an animal subjected to 4 h of coronary ligation, followed by 24 h of reperfusion (Fig. 4). Some cardiomyocytes displayed a degree of nuclear condensation and atypical nuclear shape, but they were observed in sections from control unoperated animals as well (Fig. 5). The slightly swollen mitochondria may be a response to fixation. The interstitial cells evident in Fig. 5 display a condensed nuclear pattern. However, this image was taken from a sham-operated animal, and no further morphological evidence for apoptosis was apparent in these cells. Accordingly, the nuclear chromatin pattern is not a sufficient criterion establishing apoptosis; indeed, many endothelial cell nuclei in sham-operated animals, also showed similar chromatin condensation. The major morphological features of cardiomyocytes in infarct zones were indicative of necrosis as first described by electron microscopy 50 years ago (34, 35): disintegrated nucleus, cytoplasmic swelling, membrane and myofibrillar disruptions, and electron-dense flocculent mitochondrial inclusions (Figs. 6 and 7).
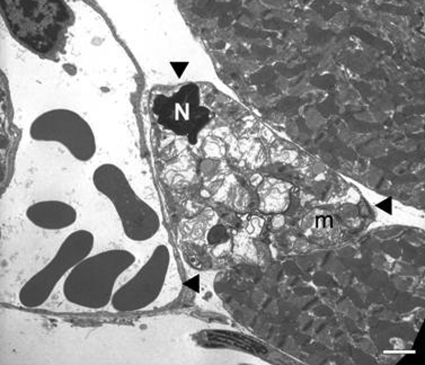
Figure 4.
Transmission electron micrograph from the peri-infarct zone in the heart from an animal subjected to 4 h of transient ligation and 24 h of reperfusion. Cardiomyocyte in the middle (arrowheads) displays morphology potentially consistent with apoptosis in this cell type, including a shrunken nucleus (N) with condensed chromatin and abnormal swollen mitochondria (m) with “wrinkled” bodies. Scale bar = 2 μm.
Figure 5.
Transmission electron micrograph from the ventricle of a control animal not subjected to surgery. Nucleus (arrowheads) in the center of the field displays marginated chromatin and atypical nuclear morphology. This is likely due to the plane of sectioning rather than apoptosis, since the rest of the cell evinces normal ultrastructural characteristics. Scale bar = 1 μm.
Figure 6.
Transmission electron micrograph from the infarct zone in the heart from an animal subjected to 4 h transient coronary ligation and 24 h of reperfusion. Note the classic morphological features of necrosis rather than apoptosis: disrupted nucleus (N) with loss of nuclear content, electron-dense floccular deposits in mitochondria (arrowheads), and ruptured plasma membrane (arrows). Scale bar = 1 μm.
Figure 7.
Another example of a transmission electron micrograph from an infarct zone from a mouse with 4-h transient coronary ligation and 24 h of reperfusion exhibiting a typical necrotic reaction. Scale bar = 2 μm.
DISCUSSION
It has been hoped and conjectured, largely on the basis of results with TUNEL assays, that amelioration of apoptosis will preserve substantial amounts of jeopardized myocardium early after ischemia. One such approach, namely, use of the presumably antiapoptotic agent erythropoietin elicited favorable effects on ventricular function in mice subjected to MI (36). Three clinical trials of this agent are under way (37).
By contrast, previous studies by our group have shown a surprising paucity of apoptosis in the heart following MI. In one study in plasminogen activator inhibitor (PAI-1) knockout mice, we detected very low levels (38). In addition, we observed a lack of correlation between results with TUNEL and more specific methods. The nonspecificity of the TUNEL assay has been well established (14,15,16,17,18,19,20,21,22,23,24,25,26). TUNEL will yield positive staining of cells undergoing oncotic (ischemic) death (characterized by cell swelling), apoptotic cell death, mitotic cells (28), cells in the process of gene transcription (22), cells undergoing DNA repair (39), and cells subjected to a microtomy-sectioning procedure (15).
Transmission electron microscopy is often deemed to be the “gold standard” for the morphological identification of apoptotic cells (40). Typical ultrastructural characteristics of apoptotic cells have been described in a variety of cell types, and include rounding up of the cell, nuclear fragmentation into a “half-moon” appearance, cell shrinkage, plasma membrane blebbing, and little to no modification of intracellular organelles (41). However, cardiomyocytes in vivo do not appear to display these canonical morphological features of apoptosis. Both Hayakawa et al. (32) and Abbate et al. (33) described characteristic features of apoptotic cardiomyocytes as condensed nuclear chromatin in a “doughnut-like” pattern, myofibrillar degeneration or derangement, and cytoplasmic lipid-like vesicles. Although both groups report the presence of “wrinkled” bodies in the mitochondria of apoptotic cardiomyocytes, Hayakawa et al. describe mitochondrial condensation, whereas Abbate et al. describe mitochondrial swelling. Regardless, the unequivocal identification of apoptotic cardiomyocytes by transmission electron microscopy is limited by subjectivity.
In the present study, in addition to using electron microscopy, we used highly specific methods that have been verified in previous studies by our group and others (28, 29, 38). In our PAI-1 knockout mouse study, we confirmed the specificity of ssDNA and cleaved caspase 3 immunohistochemical assays (38). The specificity of the assays in the present study is supported by the positive control results with thymus from dexamethasone-treated mice (42, 43).
The intervals of ischemia and reperfusion that we employed were chosen to correspond roughly to the time course of ischemia and reperfusion in patients undergoing early revascularization for treatment of acute MI. Although rats studied by others exhibited apoptosis in the heart several weeks after induction of persistent coronary ligation (44), its magnitude was modest, and the timing of data acquisition was quite dissimilar from that which we employed.
The results of the present study indicate that apoptosis occurs in the heart after MI but to a much more modest extent than that inferred from results with TUNEL assays. They demonstrate also that early reperfusion injury is not associated with marked apoptosis in the heart.
The amounts of apoptosis detected by ssDNA and cleaved caspase 3 immunohistochemistry indicated that a maximum of 2.8% of cells in the infarct zone were apoptotic, and that only 1% of cells in the infarct zone after reperfusion were apoptotic. As judged only from cleaved caspase 3 immunohistochemistry, there was no detectable apoptosis. We confirmed the limited extent of apoptosis in the heart with the use of electron microscopy; results were concordant with the immunohistochemical results. Only rare, morphological features suggestive of apoptotic cells were seen by electron microscopy in hearts from any group.
Apoptosis can occur through two major pathways in cells, a receptor-mediated (extrinsic pathway) and a mitochondrial (intrinsic) pathway (45). Caspase 8 is involved in the extrinsic pathway, whereas caspase 3 is a downstream effector caspase in the intrinsic pathway. To exclude failure to detect apoptosis in the heart if it were to be mediated via the extrinsic pathway, we assessed caspase 8 activity biochemically. Similarly, as observed with our caspase 3 biochemical assay results, there was no significant increase in caspase 8 activity in any of the hearts with infarction compared with that in normal hearts. The relative values seen in the positive control tissue (thymuses from mice treated with dexamethasone) were 6- to 9-fold greater than values seen in thymuses from saline-treated controls. Thus, if there were marked amounts of apoptosis present in the hearts with MI, the statistical power (β=0.20) should have been sufficient for detection of them with the biochemical assays employed.
Although the variances in the results from the caspase 3 and caspase 8 assays were large in percentage terms, the values in hearts with MI were very low in absolute terms compared with those in positive thymus controls and compared with values in normal hearts. Furthermore, the point estimate values for hearts subjected to ischemia were generally lower or about the same as those in normal hearts. Thus, the absolute magnitude of apoptosis reflected by the biochemical data is not sufficient to suggest that antiapoptotic measures would salvage substantial amounts of myocardium early after the onset of myocardial ischemia.
Previous studies have implicated apoptosis as a contributor to chronic heart disease (46,47,48). Although smoldering apoptosis seen in chronic cardiac conditions may accumulate or be deleterious at a low but persistent level in aggregate over time and impair cardiac function, the minute amount of apoptotic cell death we observed early after acute MI is too modest to significantly alter function. Thus, even if antiapoptotic agents prove to be beneficial to patients with chronic cardiac conditions, as judged from our results, it is very unlikely that antiapoptotic agents will preserve substantial amounts of heart muscle in patients with acute MI.
Acknowledgments
This work was supported in part by U.S. National Institutes of Health grant NIH RO1 HL085210 (J.L.S.). We appreciate the expert technical assistance of Ms. Patricia Baumann, Ms. Keara McElroy-Yaggy, Ms. Dagnija Neimane, and Ms. Marilyn Wadsworth and preparation of the manuscript by Ms. Lori Dales. We appreciate also helpful discussions during the course of our study with Drs. Ralph Budd and David Schneider.
References
- Sobel B E, Dauerman H L. The rationale underlying pharmacoinvasive therapy. Dauerman H L, Sobel BE, editors. New York: Taylor & Francis; Pharmacoinvasive Therapy in Acute Myocardial Infarction. 2005:3–31. [Google Scholar]
- Yellon D M, Hausenloy D J. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- Keeley E C, Boura J A, Grines C L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- Xiang G, Schuster M D, Seki T, Witkowski P, Eshghi S, Itescu S. Downregulated expression of plasminogen activator inhibitor-1 augments myocardial neovascularization and reduces cardiomyocyte apoptosis after acute myocardial infarction. J Am Coll Cardiol. 2005;46:536–541. doi: 10.1016/j.jacc.2005.04.047. [DOI] [PubMed] [Google Scholar]
- Freude B, Masters T N, Robicsek F, Fokin A, Kostin S, Zimmermann R, Ullmann C, Lorenz-Meyer S, Schaper J. Apoptosis is initiated by myocardial ischemia and executed during reperfusion. J Mol Cell Cardiol. 2000;32:197–208. doi: 10.1006/jmcc.1999.1066. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Cheng W, Reiss K, Clark W A, Sonnenblick E H, Krajewski S, Reed J C, Olivetti G, Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- Kumar D, Jugdutt B I. Apoptosis and oxidants in the heart. J Lab Clin Med. 2003;142:288–297. doi: 10.1016/S0022-2143(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Saraste A, Pulkki K, Kallajoki M, Henriksen K, Parvinen M, Voipio-Pulkki L M. Apoptosis in human acute myocardial infarction. Circulation. 1997;95:320–323. doi: 10.1161/01.cir.95.2.320. [DOI] [PubMed] [Google Scholar]
- Veinot J P, Gattinger D A, Fliss H. Early apoptosis in human myocardial infarcts. Hum Pathol. 1997;28:485–492. doi: 10.1016/s0046-8177(97)90039-3. [DOI] [PubMed] [Google Scholar]
- Zhao Z Q, Nakamura M, Wang N P, Wilcox J N, Shearer S, Ronson R S, Guyton R A, Vinten-Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc Res. 2000;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- Lipsic E, van der Meer P, Voors A A, Westenbrink B D, van den Heuvel A F, de Boer H C, van Zonneveld A J, Schoemaker R G, van Gilst W H, Zijlstra F, van Veldhuisen D J. A single bolus of a long-acting erythropoietin analogue darbepoetin alpha in patients with acute myocardial infarction: a randomized feasibility and safety study. Cardiovasc Drugs Ther. 2006;20:135–141. doi: 10.1007/s10557-006-7680-5. [DOI] [PubMed] [Google Scholar]
- Zhao Z Q, Nakamura M, Wang N P, Velez D A, Hewan-Lowe K O, Guyton R A, Vinten-Johansen J. Dynamic progression of contractile and endothelial dysfunction and infarct extension in the late phase of reperfusion. J Surg Res. 2000;94:133–144. doi: 10.1006/jsre.2000.6029. [DOI] [PubMed] [Google Scholar]
- Doukas J, Wrasidlo W, Noronha G, Dneprovskaia E, Fine R, Weis S, Hood J, Demaria A, Soll R, Cheresh D. Phosphoinositide 3-kinase gamma/delta inhibition limits infarct size after myocardial ischemia/reperfusion injury. Proc Natl Acad Sci U S A. 2006;103:19866–19871. doi: 10.1073/pnas.0606956103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura G, Fujiwara H. Morphological aspects of apoptosis in heart diseases. J Cell Mol Med. 2006;10:56–75. doi: 10.1111/j.1582-4934.2006.tb00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloop G D, Roa J C, Delgado A G, Balart J T, Hines M O, 3rd, Hill J M. Histologic sectioning produces TUNEL reactivity. A potential cause of false-positive staining. Arch Pathol Lab Med. 1999;123:529–532. doi: 10.5858/1999-123-0529-HSPTR. [DOI] [PubMed] [Google Scholar]
- Walker J A, Quirke P. Viewing apoptosis through a ‘TUNEL.’. J Pathol. 2001;195:275–276. doi: 10.1002/path.979. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Hitomi M, van der Wee K, Rothenberg F, Fisher S A, Zucker R, Svoboda K K, Goldsmith E C, Heiskanen K M, Nieminen A L. The pros and cons of apoptosis assays for use in the study of cells, tissues, and organs. Microsc Microanal. 2002;8:375–391. doi: 10.1017/S1431927602010346. [DOI] [PubMed] [Google Scholar]
- Collins R J, Harmon B V, Gobe G C, Kerr J F. Internucleosomal DNA cleavage should not be the sole criterion for identifying apoptosis. Int J Radiat Biol. 1992;61:451–453. doi: 10.1080/09553009214551201. [DOI] [PubMed] [Google Scholar]
- Dong Z, Saikumar P, Weinberg J M, Venkatachalam M A. Internucleosomal DNA cleavage triggered by plasma membrane damage during necrotic cell death. Involvement of serine but not cysteine proteases. Am J Pathol. 1997;151:1205–1213. [PMC free article] [PubMed] [Google Scholar]
- Kang P M, Izumo S. Apoptosis in heart failure: is there light at the end of the tunnel (TUNEL)? J Card Fail. 2000;6:43–46. doi: 10.1016/s1071-9164(00)80005-6. [DOI] [PubMed] [Google Scholar]
- Kockx M M, Muhring J, Bortier H, De Meyer G R, Jacob W. Biotin- or digoxigenin-conjugated nucleotides bind to matrix vesicles in atherosclerotic plaques. Am J Pathol. 1996;148:1771–1777. [PMC free article] [PubMed] [Google Scholar]
- Kockx M M, Muhring J, Knaapen M W, de Meyer G R. RNA synthesis and splicing interferes with DNA in situ end labeling techniques used to detect apoptosis. Am J Pathol. 1998;152:885–888. [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Takemura G, Ohno A, Misao J, Hayakawa Y, Minatoguchi S, Fujiwara T, Fujiwara H. “Apoptotic” myocytes in infarct area in rabbit hearts may be oncotic myocytes with DNA fragmentation: analysis by immunogold electron microscopy combined with in situ nick end-labeling. Circulation. 1998;98:1422–1430. doi: 10.1161/01.cir.98.14.1422. [DOI] [PubMed] [Google Scholar]
- Sakahira H, Enari M, Ohsawa Y, Uchiyama Y, Nagata S. Apoptotic nuclear morphological change without DNA fragmentation. Curr Biol. 1999;9:543–546. doi: 10.1016/s0960-9822(99)80240-1. [DOI] [PubMed] [Google Scholar]
- Charriaut-Marlangue C, Ben-Ari Y. A cautionary note on the use of the TUNEL stain to determine apoptosis. Neuroreport. 1995;7:61–64. [PubMed] [Google Scholar]
- Buja L M, Entman M L. Modes of myocardial cell injury and cell death in ischemic heart disease. Circulation. 1998;98:1355–1357. doi: 10.1161/01.cir.98.14.1355. [DOI] [PubMed] [Google Scholar]
- Zaman A K, Fujii S, Schneider D J, Taatjes D J, Lijnen H R, Sobel B E. Deleterious effects of lack of cardiac PAI-1 after coronary occlusion in mice and their pathophysiologic determinants. Histochem Cell Biol. 2007;128:135–145. doi: 10.1007/s00418-007-0300-z. [DOI] [PubMed] [Google Scholar]
- Ito Y, Shibata M A, Kusakabe K, Otsuki Y. Method of specific detection of apoptosis using formamide-induced DNA denaturation assay. J Histochem Cytochem. 2006;54:683–692. doi: 10.1369/jhc.5A6799.2006. [DOI] [PubMed] [Google Scholar]
- Frankfurt O S, Robb J A, Sugarbaker E V, Villa L. Monoclonal antibody to single-stranded DNA is a specific and sensitive cellular marker of apoptosis. Exp Cell Res. 1996;226:387–397. doi: 10.1006/excr.1996.0240. [DOI] [PubMed] [Google Scholar]
- Sobel B E, Schneider D J, Lee Y H, Pratley R E. Insulin resistance increases PAI-1 in the heart. Biochem Biophys Res Commun. 2006;346:102–107. doi: 10.1016/j.bbrc.2006.05.078. [DOI] [PubMed] [Google Scholar]
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Hayakawa K, Takemura G, Koda M, Kawase Y, Maruyama R, Li Y, Minatoguchi S, Fujiwara T, Fujiwara H. Sensitivity to apoptosis signal, clearance rate, and ultrastructure of fas ligand-induced apoptosis in in vivo adult cardiac cells. Circulation. 2002;105:3039–3045. doi: 10.1161/01.cir.0000018651.89208.69. [DOI] [PubMed] [Google Scholar]
- Abbate A, De Falco M, Morales C, Gelpi R J, Prisco M, De Luca A, Palleiro J, Fedele V, Feroce F, Baldi F, Vetrovec G W, Baldi A. Electron microscopy characterization of cardiomyocyte apoptosis in ischemic heart disease. Int J Cardiol. 2007;114:118–120. doi: 10.1016/j.ijcard.2005.11.025. [DOI] [PubMed] [Google Scholar]
- Bryant R E, Thomas W A, O'Neal R M. An electron microscopic study of myocardial ischemia in the rat. Circ Res. 1958;6:699–709. doi: 10.1161/01.res.6.6.699. [DOI] [PubMed] [Google Scholar]
- Caulfield J, Klionsky B. Myocardial ischemia and early infarction: an electron microscopic study. Am J Pathol. 1959;35:489–523. [PMC free article] [PubMed] [Google Scholar]
- Moon C, Krawczyk M, Ahn D, Ahmet I, Paik D, Lakatta E G, Talan M I. Erythropoietin reduces myocardial infarction and left ventricular functional decline after coronary artery ligation in rats. Proc Natl Acad Sci U S A. 2003;100:11612–11617. doi: 10.1073/pnas.1930406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K N, Binbrek A S, Sobel B E. Heart disease and erythropoietin. Future Cardiol. 2008;4:57–64. doi: 10.2217/14796678.4.1.57. [DOI] [PubMed] [Google Scholar]
- Taatjes D J, Wadsworth M P, Zaman A K, Schneider D J, Sobel B E. A novel dual staining method for identification of apoptotic cells reveals a modest apoptotic response in infarcted mouse myocardium. Histochem Cell Biol. 2007;128:275–283. doi: 10.1007/s00418-007-0323-5. [DOI] [PubMed] [Google Scholar]
- Kanoh M, Takemura G, Misao J, Hayakawa Y, Aoyama T, Nishigaki K, Noda T, Fujiwara T, Fukuda K, Minatoguchi S, Fujiwara H. Significance of myocytes with positive DNA in situ nick end-labeling (TUNEL) in hearts with dilated cardiomyopathy: not apoptosis but DNA repair. Circulation. 1999;99:2757–2764. doi: 10.1161/01.cir.99.21.2757. [DOI] [PubMed] [Google Scholar]
- Taatjes D J, Sobel B E, Budd R C. Morphological and cytochemical determination of cell death by apoptosis. Histochem Cell Biol. 2008;129:33–43. doi: 10.1007/s00418-007-0356-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluzzi L, Maiuri M C, Vitale I, Zischka H, Castedo M, Zitvogel L, Kroemer G. Cell death modalities: classification and pathophysiological implications. Cell Death Differ. 2007;14:1237–1243. doi: 10.1038/sj.cdd.4402148. [DOI] [PubMed] [Google Scholar]
- Sun X M, Dinsdale D, Snowden R T, Cohen G M, Skilleter D N. Characterization of apoptosis in thymocytes isolated from dexamethasone-treated rats. Biochem Pharmacol. 1992;44:2131–2137. doi: 10.1016/0006-2952(92)90339-k. [DOI] [PubMed] [Google Scholar]
- Zavitsanou K, Nguyen V, Greguric I, Chapman J, Ballantyne P, Katsifis A. Detection of apoptotic cell death in the thymus of dexamethasone treated rats using [123I]annexin V and in situ oligonucleotide ligation. J Mol Histol. 2007;38:313–319. doi: 10.1007/s10735-007-9104-7. [DOI] [PubMed] [Google Scholar]
- Abbate A, Morales C, De Falco M, Fedele V, Biondi Zoccai G G, Santini D, Palleiro J, Vasaturo F, Scarpa S, Liuzzo G, Severino A, Baldi F, Crea F, Biasucci L M, Vetrovec G W, Gelpi R J, Baldi A. Ischemia and apoptosis in an animal model of permanent infarct-related artery occlusion. Int J Cardiol. 2007;121:109–111. doi: 10.1016/j.ijcard.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Crow M T, Mani K, Nam Y J, Kitsis R N. The mitochondrial death pathway and cardiac myocyte apoptosis. Circ Res. 2004;95:957–970. doi: 10.1161/01.RES.0000148632.35500.d9. [DOI] [PubMed] [Google Scholar]
- Hikoso S, Ikeda Y, Yamaguchi O, Takeda T, Higuchi Y, Hirotani S, Kashiwase K, Yamada M, Asahi M, Matsumura Y, Nishida K, Matsuzaki M, Hori M, Otsu K. Progression of heart failure was suppressed by inhibition of apoptosis signal-regulating kinase 1 via transcoronary gene transfer. J Am Coll Cardiol. 2007;50:453–462. doi: 10.1016/j.jacc.2007.03.053. [DOI] [PubMed] [Google Scholar]
- Narula J, Haider N, Virmani R, DiSalvo T G, Kolodgie F D, Hajjar R J, Schmidt U, Semigran M J, Dec G W, Khaw B A. Apoptosis in myocytes in end-stage heart failure. N Engl J Med. 1996;335:1182–1189. doi: 10.1056/NEJM199610173351603. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Abbi R, Quaini F, Kajstura J, Cheng W, Nitahara J A, Quaini E, Di Loreto C, Beltrami C A, Krajewski S, Reed J C, Anversa P. Apoptosis in the failing human heart. N Engl J Med. 1997;336:1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]