Abstract
Endothelial activation is a central initiating event in atheroma formation. Evidence from our laboratory and others has demonstrated links between activation of early growth response-1 (Egr-1) and atherosclerosis and also has demonstrated that activated protein kinase C (PKC) βII is a critical upstream regulator of Egr-1 in response to vascular stress. We tested the role of PKCβ in regulating key events linked to atherosclerosis and show that the aortas of apoE−/− mice display an age-dependent increase in PKCβII antigen in membranous fractions vs. C57BL/6 animals with a ∼2-fold increase at age 6 wk and a ∼4.5-fold increase at age 24 wk. Consistent with important roles for PKCβ in atherosclerosis, a significant decrease in atherosclerotic lesion area was evident in PKCβ−/−/apoE−/− vs. apoE−/− mice by ∼5-fold, in parallel with significantly reduced vascular transcripts for Egr-1 and matrix metalloproteinase (MMP)-2 antigen and activity vs. apoE−/− mice. Significant reduction in atherosclerosis of ∼2-fold was observed in apoE−/− mice fed ruboxistaurin chow (PKCβ inhibitor) vs. vehicle. In primary murine and human aortic endothelial cells, the PKCβ-JNK mitogen-activated protein kinase pathway importantly contributes to oxLDL-mediated induction of MMP2 expression. Blockade of PKCβ may be beneficial in mitigating endothelial perturbation and atherosclerosis.—Harja, E., Chang, J. S., Lu, Y., Leitges, M., Zou, Y. S., Schmidt, A. M., Yan, S.-F. Mice deficient in PKCβ and apolipoprotein E display decreased atherosclerosis.
Keywords: signal transduction, MMP-2, JNK, Egr-1
Atherosclerosis is a complex disease that begins with endothelial activation, which causes macrophages and other inflammatory cells to adhere to the stimulated endothelium (1,2,3,4). Sustained dysfunction or activation of the endothelium elicited by low-density lipoproteins (LDLs), particularly their oxidatively modified species (oxLDL), is a key step in the initiation of atherosclerosis (2, 4, 5). However, the precise mechanisms by which oxLDL influences the development of atherosclerosis remain incompletely defined.
Our earlier studies demonstrated that activated protein kinase C (PKC), especially the βII isoform, is a critical upstream regulator of early growth response-1 (Egr-1) in the response to acute vascular stresses, such as hypoxia/hypoxemia and ischemia/reperfusion (I/R) (6,7,8). Egr-1, in turn, functions as a master switch controlling the regulation of a diverse array of genes linked to expression of cytokines, chemokines, procoagulant molecules, and cell adherence molecules (9). Evidence from our laboratory and others has accumulated linking activation of Egr-1 to chronic vascular stress, such as atherosclerosis. Pivotal studies in the discovery of the biological impact of Egr-1 in atherosclerosis came from experiments by the laboratories of McCaffrey et al. (10), who showed that transcripts for Egr-1 were up-regulated in human atherosclerotic lesions and in the lesions of mice deficient in the LDL receptor fed a high-fat diet. Moreover, increased Egr-1 expression in the human lesion was associated with an elevation in the expression of several known Egr-1 target genes, such as tumor necrosis factor, intercellular adhesion molecule (ICAM)-1, and macrophage colony-stimulating factor (M-CSF), suggesting that Egr-1 is transcriptionally active in human atheroma (10). We previously reported that Egr-1−/−/apoE−/− mice display less atherosclerosis than apoE−/− control mice at 14 and 24 wk of age (11). In parallel, transcripts for proinflammatory and procoagulant mediators such as JE/MCP-1, interleukin (IL)-1β, vascular cell adhesion molecule (VCAM)-1, ICAM-1, tissue factor (TF) and plasminogen activator inhibitor-1 (PAI-1) were significantly reduced in the aortas of Egr-1−/−/ apoE−/− mice vs. apoE−/− mice (11).
These observations led us to hypothesize that PKCβ might be a central upstream regulator of Egr-1 implicated in atherosclerotic lesion initiation and/or progression in hyperlipidemic stress. Here, we tested these concepts in homozygous PKCβ−/− mice (12) bred into the hypercholesterolemic apoE−/− background. Furthermore, based on the early and prominent up-regulation of Egr-1 antigen in the endothelium in the aortas of apoE−/− mice, we probed the role of this pathway in oxLDL-mediated stress in primary cultures of murine and human aortic endothelial cells (MAECs and HAECs).
MATERIALS AND METHODS
Animal studies
Homozygous apoE−/− mice in the C57BL/6 background were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Homozygous PKCβ−/− mice were backcrossed >10 generations into C57BL/6 in our laboratory, followed by intercrossing with apoE−/− mice. The progeny were used for breeding that generated PKCβ−/−/apoE−/− and PKC-β+/+/apoE−/− littermate offspring. All procedures were carried out with the approval of the Institutional Animal Care and Use Committee of Columbia University. All of the mice were fed normal chow. Genomic DNA was isolated from tail biopsies. Polymerase chain reaction (PCR) analysis was used to identify the deficiency of apoE according to the website of the Jackson Laboratories (www.jax.org). Southern blotting was used to identify the deficiency of PKCβ based on previously published methods (12). In other studies, apoE−/− mice were fed chow containing the PKCβ inhibitor ruboxistaurin (LY-333531; 10 mg/kg daily) or vehicle chow without inhibitor from age 5 to 24 wk (8, 45, 46). Ruboxistaurin and vehicle chow were generously supplied by Dr. Louis Vignati (Eli Lilly, Indianapolis, IN, USA), who provided specific instructions regarding the appropriate dose of ruboxistaurin.
Quantification of atherosclerotic lesion area
After the mice were deprived of food for 4 h and then anesthetized, their blood was withdrawn from the inferior vena cava into heparin, and plasma was stored for analysis. For quantitative PCR studies, aortas were retrieved and rapidly frozen in liquid nitrogen and stored at −80°C before analysis. For histology studies, the aorta and heart were harvested and stored in buffered formalin (10%), and all procedures to prepare histological sections for quantitative analysis of atherosclerosis were performed exactly as described in previous publications (13). Specifically, cryostat sections were prepared after hearts were sequentially embedded in gelatin at concentrations of 5, 10, and 25%. Serial sections, 10 μm in thickness, were cut from the level of the aortic valve leaflets up to ∼480 μm above the leaflets in the aortic sinus; alternate sections were retrieved and placed onto Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA, USA); 4 sections were placed onto each slide for a total of 6 slides. Sections were then stained with Oil Red O and counterstained with hematoxylin/light green (Sigma, St. Louis, MO, USA). Atherosclerotic lesion areas were quantified on 1 section from each slide (beginning at the side where 3 distinct valves first appear) using a Zeiss microscope and Axiovision 4.5 software (Carl Zeiss, Oberkochen, Germany): mean lesion area from slides 2 through 5 is reported. Two of the investigators, masked to the experimental conditions, analyzed the slides and performed the morphometric analysis.
RNA extraction and real-time PCR
Total RNA was extracted from frozen single aorta using TRIzol reagent (Invitrogen, Rockville, MD, USA). The real-time PCR analysis for Egr-1 and 18s rRNA was performed as previously described (11). All reactions were performed in triplicate in ABI Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA). Data are calculated by 2−ΔΔCt method (14) and are presented as fold induction of transcripts for Egr-1 in apoE−/− mice or in PKCβ−/−/apoE−/− mice normalized to 18s rRNA, compared with C57BL/6 mice (arbitrarily defined as 1.0-fold).
Immunofluorescence
Sections from formalin-fixed, paraffin-embedded aortic arch was stained with a rabbit polyclonal anti-mouse Egr-1 antibody (1:50; Cell Signaling Technology Inc., Danvers, MA, USA) or a rabbit polyclonal anti-matrix metalloproteinase (MMP)-2 (1:100; Chemicon International Co., Temecula, CA, USA) antibody and then incubated with a biotinylated goat anti-rabbit immunoglobulin (IgG; 1:200; Vector Laboratories Inc., Burlingame, CA, USA), followed by incubation with Texas Red-avidin D. The sections were blocked with avidin/biotin blocking solution and then incubated with a rat monoclonal anti-mouse platelet endothelial cell adhesion molecule-1 (CD31) antibody (1:250; Fitzgerald Industries International Inc., Concord, MA, USA) or mouse monoclonal anti-α-smooth muscle (SM) actin (Sigma). This was followed by incubation with a biotinylated rabbit anti-rat IgG or goat anti-mouse IgG (1:200; Vector Laboratories Inc.), followed by fluorescein-avidin D. A similar procedure was performed on the adjacent section with a nonimmune IgG, and then nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The signals of individual and merged images for antigen detection were performed using a LaserSharp 2000 scanning confocal microscope with epifluorescent illumination (excitation wavelength 488 nm for fluorescein-avidin D, 568 nm for Texas Red-avidin D; Bio-Rad Laboratories Inc., Richmond, CA, USA).
Biochemical analyses
Levels of total cholesterol and triglyceride were determined in food-deprived mice using chromogenic assays (Thermo Electron, Waltham, MA, USA). Glucose levels were determined from samples of tail vein blood using a glucometer (Abbott Diabetes Care Co., Alameda, CA, USA).
Preparation of oxLDL
Human native LDL (5 mg protein/ml) purchased from Sigma was oxidized in the presence of 10 nmol/L CuCl2 in PBS for 24 h at room temperature (15). Oxidation was terminated by adding EDTA (pH 8.0) to a final concentration of 200 μmol/ml, followed by dialysis against PBS/100 μmol/ml EDTA (pH 8.0) for 24 h at 4°C (16). The degree of oxidation was verified by assessment of thiobarbituric acid reactive substances assay (17). The protein concentration of oxLDL was determined using the Lowry assay.
Cell culture
Three different lines of wild-type (WT) and PKCβ−/− MAECs were established from 3 individual mouse aortas as described previously (18). HAECs were purchased from Lonza (Basel, Switzerland). When cells reached 70 to 80% confluence, they were serum-starved for 24 h and then incubated with oxLDL (5 μg/ml) for the specific times. Cells were preincubated with the inhibitors of PKCβ (LY379196, 30 nM), c-Jun NH2-terminal kinase (JNK) (SP600125 (20 μM), and ERK1/2 (U0126, 5 μM) for 1 h, respectively, followed by coincubation with oxLDL for 1 h or 4 h.
Western blotting
Membrane protein fractions, nuclear extracts, and total proteins were prepared from aorta and cells (6,7,8) and were subjected to Western blot. To detect membrane protein translocation, blots were incubated with the primary rabbit anti-PKCβI, anti-PKCβII, anti-PKCδ, or anti-PKCε IgG (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), To detect phosphorylated JNK or ERK1/2, blots were incubated with the primary anti-phospho-JNK (P-JNK) IgG or anti-phospho-ERK1/2 (P-ERK1/2) IgG and anti-total JNK (T-JNK) or anti-total ERK1/2 (T-ERK1/2) IgG (1:1000; Cell Signaling Technology). To detect Egr-1 antigen in nuclear extracts, blots were incubated with rabbit anti-Egr-1 or Sp1 IgG (1:1000; Santa Cruz Biotechnology). To detect MMP-2 antigen in extracts from a single aorta or in cell supernatant, which were collected and concentrated 100-fold using Amicon Ultra Centrifugal Filter Devices, blots were incubated with rabbit anti-MMP-2 IgG (1:2000; Chemicon). To detect VCAM-1 antigen in total protein from MAECs, blots were incubated with rabbit anti-VCAM-1 IgG (1:1000; Santa Cruz Biotechnology).
Zymography
To detect MMP-2 activity, a single mouse aorta was homogenized, whereas cell culture media were collected and concentrated as above. Electrophoresis was performed on zymogram gelatin gels (Invitrogen). After the suggested developing time, gels were stained with Coomasie Blue (Bio-Rad). Images were obtained with an Alpha-Imager.
Statistical analysis
All data are reported as mean ± sem. Data were analyzed by ANOVA using commercially available software (Statview, version 5.0.1, Berkeley, CA, USA). Probability values < 0.05 were considered statistically significant.
RESULTS
Activation of PKCβII in aorta of apoE−/− mice
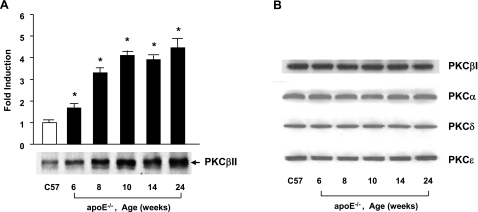
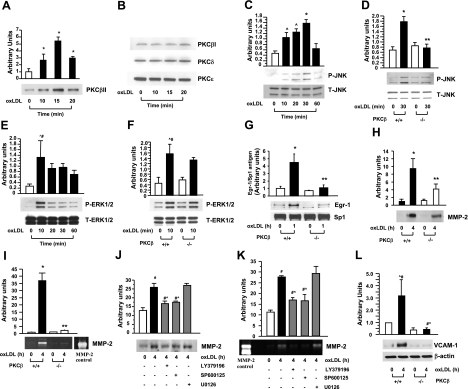
Our previous studies illustrated a time-dependent increase in Egr-1 in the aortas of apoE−/− mice vs. WT C57BL/6 mice and that apoE−/− mice deficient in Egr-1 exhibited decreased atherosclerosis. To dissect the contribution of PKCβ to the regulation of Egr-1 and the pathogenesis of atherosclerosis, we first assessed the extent of activation of the PKCβII isoform in the aortas of apoE−/− mice. Compared with WT C57BL/6, apoE−/− mice displayed an age-dependent increase in translocation of PKCβII from cytosolic to the membrane fraction by Western blotting (Fig. 1A, P<0.0001). Next, we examined the patterns of 4 other PKC isoforms: PKCβI, PKCα, PKCδ, and PKCε. Compared with WT C57BL/6 mice, these 4 distinct PKC isoforms did not reveal any differences in membrane levels at different ages of apoE−/− mice vs. WT C57BL/6 (Fig. 1B). Activation of PKCβII was first evident at age 6 wk (Fig. 1A, P<0.0001), a time at which up-regulation of Egr-1 transcripts, a downstream consequence of PKCβII activity in the vessel wall, was not yet observed (11).
Figure 1.
Detection of PKCs in membrane fractions in aorta of apoE null mice. Membranous fraction was prepared from aorta of apoE−/− and C57 mice and subjected to SDS-PAGE (25 μg of total protein from 3 sets of pooled aortas/lane) and immunoblotting with antibody to PKCβII (A) and PKCβI, PKCα, PKCδ, and PKCε (B). *P < 0.0001 vs. aortas of C57BL6 mice.
Up-regulation of Egr-1 transcripts and antigen in apoE−/− mice: effect of PKCβ deletion
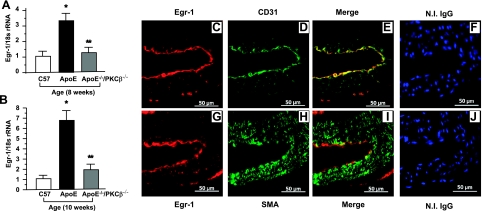
To determine whether PKCβ modulated the expression of Egr-1 in the vessel wall of apoE−/− mice, we performed real-time PCR on RNA prepared from aortas retrieved from apoE−/− mice vs. apoE−/− mice devoid of PKCβ (PKCβ−/−/apoE−/−) mice and WT C57BL6 mice at age 8 and 10 wk (Fig. 2A, B). Transcripts for Egr-1 were significantly lower in the aortas of PKCβ−/−/apoE−/− mice vs. apoE−/− mice (P<0.0001, Fig. 2A, B).
Figure 2.
Evaluation of induction of Egr-1 transcripts and antigen in apoE−/− and PKCβ−/−/apoE−/− mice. A, B) Real time PCR analysis of RNA extracted from aorta retrieved from C57BL/6, apoE−/− and PKCβ−/−/apoE−/− male mice at age 8 wk (A) and 10 wk (B). Data are calculated by 2−ΔΔCt method and are presented as the fold induction of mRNA for Egr-1 normalized to 18s rRNA and relative to control C57BL/6. *P < 0.0001 vs. aortas of C57BL6 mice. **P < 0.0001 vs. aortas of apoE−/− mice. C–E) Immunofluorescence double-stained on the sections of aorta from apoE−/− male mice at age 8 wk with anti-Egr-1 IgG (red; C) and anti-CD31IgG (green; D); merged image is also shown (E). F) No immunofluorescence stained with nonimmune IgG was shown, but nuclei were stained with DAPI (blue). G–I) Immunofluorescence double-stained on the sections of aorta from apoE−/− male mice at age 8 wk with anti-Egr-1 IgG (red; G) and anti-α-smooth muscle actin (green; H); merged image is also shown (I). J) No immunofluorescence stained with nonimmune IgG was shown, but nuclei were stained with DAPI (blue). Scale bars = 50 μm.
To determine the cell types expressing Egr-1 in early atherogenesis in the vasculature of apoE−/− mice, we performed immunohistochemistry. At age 8 wk (Fig. 2C–F), aorta of apoE−/− mice displayed immunoreactivity for Egr-1 (Fig. 2C) in ECs. The Egr-1-expressing ECs were detected by colocalization using anti-Egr-1 and anti-CD31 IgG (Fig. 2C–E). In contrast, Egr-1 antigen was undetectable in SM cells in aorta of apoE−/− mice at age of 8 wk (Fig. 2G–J).
Effects of genetic deletion or blockade of PKCβ on atherosclerosis in apoE−/− mice
Based on the prominent role of PKCβ in regulation of Egr-1 in apoE−/− mice, it was logical to assess the impact of the PKCβ axis in atherosclerosis. Thus, we bred PKCβ−/− mice into apoE−/− background and studied the impact of genetic deletion of PKCβ in the apoE background on the course of atherosclerosis.
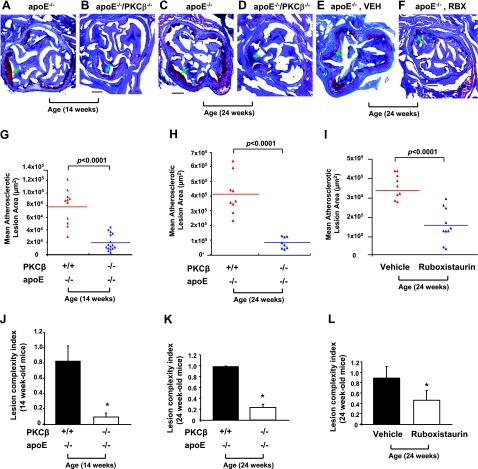
At 14 wk of age, a ∼4-fold lower atherosclerotic lesion area at the aortic root was observed in PKCβ−/−/apoE−/− mice (19,256.4±3340.4 μm2, n=16) vs. littermate apoE−/− expressing PKCβ (77,653.5±8493.2 μm2, n=11); P < 0.0001 (Fig. 3A, B, G). PKCβ−/−/apoE+/+ animals displayed no atherosclerotic lesions (not shown). The effects of PKCβ on atherosclerosis were sustained beyond the earliest stages of lesion formation. Specifically, at 24 wk of age, similar results were observed. The differences between double-null and single-null apoE−/− mice, however, were even more striking: ∼0.5-fold lower mean atherosclerotic lesion area was observed in PKCβ−/−/apoE−/− mice (83,718.6±13,607.7 μm2, n=8) vs. littermate apoE−/− animals (412,458±44,468.9 μm2, n=9); P < 0.0001 (Fig. 3C, D, H).
Figure 3.
Assessment of atherosclerotic lesion area and complexity in apoE−/− and PKCβ−/−/apoE−/− mice. A–F) Histological analysis. Typical histological sections from the aortic sinus are shown from apoE−/− and apoE−/−/PKCβ−/− male mice at age 14 wk (A, B) and 24 wk (C, D), and from apoE−/− male mice fed vehicle chow (VEH; E) or Ruboxistaurin (RBX; F) between ages 5 and 24 wk. Arrows indicate areas of atherosclerotic lesion as determined by staining with oil red O. Scale bars = 200 μm. G–I) Quantification of atherosclerotic lesion areas. Mean atherosclerotic lesion areas (μm2) were determined in apoE−/− (n=11 and n=9) and PKCβ−/−/apoE−/− male mice (n=16 and n=8) at age 14 wk (G) and 24 wk (H) and in apoE−/− male mice fed VEH (n=10) or RBX (n=9) at age 24 wk (I). J–L) Lesion complexity index was calculated in the above apoE−/−, PKCβ−/−/apoE−/− male mice and apoE−/− male mice fed VEH or RBX. *P < 0.0001 vs. aortas of apoE−/− mice or apoE−/− mice fed VEH.
To further probe roles for the PKCβ pathway, we tested an inhibitor of this enzyme. ApoE−/− male mice were fed normal chow containing the PKCβ inhibitor, ruboxistaurin (LY333531) or vehicle chow from ages 5 to 24 wk. At 24 wk of age, an ∼2-fold lower atherosclerotic lesion area at the aortic root was observed in apoE−/− male mice fed ruboxistaurin (153,210.7±23,781.6 μm2, n=10) vs. apoE−/− male mice fed vehicle chow (338,800.9±20,833.7 μm2, n=9); P < 0.0001 (Fig. 3E, F, I).
In parallel with decreased atherosclerotic lesion area, the lesion complexity index was determined by the ratio of complex lesions to the total number of lesions studied. Complex lesions were defined as those characterized by fibrous caps, cholesterol clefts, or lesion necrosis (13). The complexity index was significantly lower in PKCβ−/−/apoE−/− mice vs. littermate apoE−/− mice at age of 14 wk (0.100±0.067 vs. 0.833±0.105; P < 0.0001; Fig. 3J) and 24 wk (0.24±0.06 vs. 0.98±0.02; P < 0.0001; Fig. 3K). Further, the complexity index was significantly lower in apoE−/− mice fed ruboxistaurin vs. vehicle (0.47±0.19 vs. 0.9±0.22; P < 0.0001; Fig. 3L).
There were no significant differences in levels of cholesterol or triglyceride in PKCβ−/−/apoE−/− animals vs. apoE−/− mice at age 14 and 24 wk or in apoE−/− mice fed ruboxistaurin vs. vehicle chow (Table 1).
TABLE 1.
Levels of plasma cholesterol and tryglyceride
| Age (wk) | Plasma | Cholesterol
|
Tryglyceride
|
||||
|---|---|---|---|---|---|---|---|
| n | mg/dl | P | n | mg/dl | P | ||
| 14 | apoE−/− | 11 | 203.01 ± 21.04 | 0.63 | 8 | 114.16 ± 5.27 | 0.82 |
| apoE−/−/PKCβ−/− | 13 | 188.92 ± 18.78 | 8 | 102.71 ± 3.71 | |||
| 24 | apoE−/− | 8 | 297.00 ± 14.32 | 0.74 | 7 | 126.82 ± 3.77 | 0.32 |
| apoE−/−/PKCβ−/− | 7 | 288.24 ± 21.56 | 7 | 118.00 ± 1.15 | |||
| 24 | apoE−/− and VEH | 9 | 322.80 ± 17.70 | 0.65 | 5 | 86.40 ± 9.90 | 0.27 |
| apoE−/− and RBX | 8 | 312.70 ± 12.60 | 5 | 102.10 ± 8.60 | |||
Induction of MMP-2 in apoE−/− mice: effect of PKCβ deletion
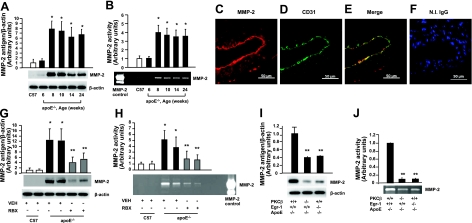
We next sought to delineate the impact of PKCβ on gene expression in the apoE−/− mouse aorta. Extracellular matrix metalloproteinases (MMPs) degrade the matrix and participate in multiple aspects of atherogenesis and then lesion progression, and previous studies (19) suggested that Egr-1 regulated MMP2 gelatinase activity. To probe the potential role of PKCβ in regulation of MMP2, we prepared protein extracts from aortas of apoE−/− mice and tested levels of MMP2 antigen and activity by Western blotting and zymography assay, respectively. MMP-2 antigen and activity in the aorta of apoE−/− mice was increased in an age-dependent manner compared with WT C57BL6 mice (P<0.0001; Fig. 4A, B). ECs were the primary MMP2-expressing cells in the aorta of apoE−/− mice. At age 8 wk (Fig. 4C–F), aortas of apoE−/− mice displayed immunoreactivity for MMP2 (Fig. 4C) in ECs based on colocalization studies using anti-MMP2 and anti-CD31 IgG (Fig. 4C–E).
Figure 4.
Determination of antigen and activity of MMP-2 in apoE−/− mice and PKCβ−/−/apoE−/− mice. A, B) Antigen level or activity of MMP-2 from aortic tissue in apoE−/− male mice was determined by Western blot (A) or zymography (B) as indicated in time course of ages. *P < 0.0001 vs. aortas of C57 mice. C–F) Immunofluorescence double-stained on the aorta from apoE−/− male mice at age 8 wk with anti-MMP-2 IgG (red; C) and anti-CD31 IgG (green; D); merged image is also shown (E). F) No immunofluorescence stained with nonimmune IgG was shown but nuclei were stained with DAPI (blue). Scale bars = 50 μm. G, H) Antigen level or activity of MMP-2 from aortic tissue in apoE−/− male mice fed VEH or RBX at age 8 wk was determined by Western blot (G) or zymography (H). **P < 0.0001 vs. aortas of apoE−/− mice fed VEH. I, J) Antigen level or activity of MMP-2 from aortic tissue in apoE−/−, apoE−/−/PKCβ−/− and apoE−/−/Egr-1−/− male mice at age 8 wk was determined by Western blot (I) or zymography (J). **P < 0.0001 vs. aortas of apoE−/− mice.
Next, we assessed the role of PKCβ. Levels of MMP2 antigen and activity of MMP2 were suppressed in the aorta of apoE−/− mice fed a PKCβ inhibitor, ruboxistaurin (P<0.0001; Fig. 4G, H).
We predicted that PKCβ-mediated reduction in vascular inflammation and atherosclerosis was, at least in part, reflective of PKCβ’s effects on regulation of Egr-1, so we addressed these concepts directly in mice devoid of Egr-1 in the apoE−/− background. The levels of MMP2 antigen and activity of MMP2 were suppressed in aortas of PKCβ−/−/apoE−/− or Egr-1−/−/apoE−/− mice (P<0.0001; Fig. 4I, J, respectively).
PKCβII regulates secretion of MMP-2 in MAECs via activation of mitogen-activated protein (MAP) kinases and Egr-1 pathway in response to oxLDL
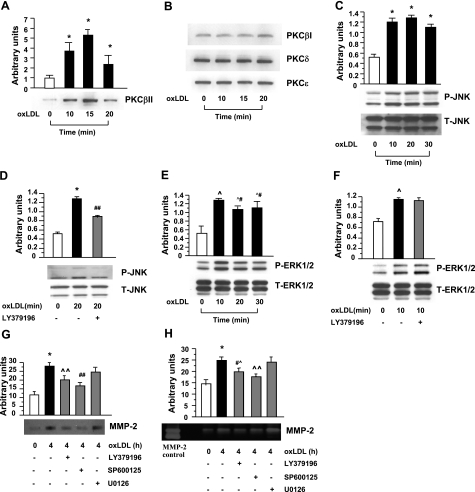
Based on our studies revealing that at very early stages of atherogenesis in apoE−/− mouse aorta, ECs were the principal cell types expressing both Egr-1 and MMP-2, we prepared primary cultures of aortic endothelial cells from PKCβ+/+ and PKCβ−/− mice. First, PKCβ+/+ MAECs were incubated with oxLDL (5 μg/ml). Immunoblots of membrane fractions prepared from PKCβ+/+ MAECs exposed to oxLDL for 10, 15, and 30 min revealed that PKCβII membrane translocation was significantly increased in a time-dependent manner, beginning at 10 min (P<0.0001; Fig. 5A), with peak translocation of PKCβII from cytosol to membrane observed at 15 min (P<0.0001; Fig. 5A). Three other PKC isoforms—PKCβI, PKCδ, and PKCε (Fig. 5B)—revealed no changes in membrane translocation over this duration of incubation with oxLDL.
Figure 5.
Detection of PKCs in membrane fractions, phosphorylated MAP kinases, Egr-1 protein, and secretion of MMP-2 and expression of VCAM-1 in MAECs on stimulation with oxLDL. A, B) Membranous fraction from PKCβ+/+ MAECs after incubation with or without oxLDL (5 μg/ml) for the indicated times, subjected to SDS-PAGE (25 μg/lane) and immunoblotting with antibody to PKCβII (A), PKCβI, PKCδ, and PKCε (B). C–G) Total protein or nuclear protein was prepared from PKCβ+/+ and PKCβ−/− MAECs after incubation with or without oxLDL (5 μg/ml) for the indicated times, subjected to SDS-PAGE (5–10 μg/lane) and immunoblotting with antibody to P-JNK and T-JNK (C, D), P-ERK1/2 and T-ERK1/2 (E, F), Egr-1 and SP1 (G). H, I) Antigen level or activity of MMP-2 in supernatant of PKCβ+/+ and PKCβ−/− MAECs after incubation with or without oxLDL (5 μg/ml) was detected by immunoblotting with antibody to MMP-2 (H) or zymography (I). J, K) After PKCβ+/+ MAECs were preincubated with or without inhibitors of PKCβ (LY379196, 30 nM), JNK (SP600125, 20 μM) and ERK1/2 (U0126, 5 μM) for 1 h before coincubation with oxLDL (5 μg/ml) for 4 h, antigen level or activity of MMP-2 in supernatants was detected by Western blot (J) or zymography (K). Antigen level of VCAM-1 in total protein of PKCβ+/+ and PKCβ−/− MAECs after incubation with or without oxLDL (5 μg/ml) was detected by immunoblotting with antibody to VCAM-1 (L). *P < 0.0001, #P < 0.001, ^P < 0.01, ^#P < 0.05 vs. unstimulated PKCβ+/+ MAECs. **P < 0.0001, #^P < 0.05 vs. stimulated PKCβ+/+ MAECs.
Stimulated by these findings, we tested the potential impact of oxLDL on activation of signaling mechanisms, such as JNK MAP kinase, which might mediate PKCβ-dependent cellular activation in ECs. Immunoblots of extracts from PKCβ+/+ MAECs exposed to oxLDL for 10, 20, 30, and 60 min displayed time-dependent phosphorylation of JNK evident by 10 min, with maximal activation by 30 min (P<0.0001; Fig. 5C). In contrast, PKCβ−/− MAECs exposed to oxLDL displayed a significantly diminished phosphorylation of JNK (P<0.0001; Fig. 5D). In addition, we evaluated the effect of ablation of PKCβ in oxLDL-stimulated MAECs on distinct signaling molecules implicated in cell stress, such as ERK1/2 and p38. Immunoblots of extracts from PKCβ+/+ MAECs exposed to oxLDL for 10, 20, 30, and 60 min displayed a ∼5-fold increase in a peak intensity of phosphorylated ERK1/2 at 10 min (P<0.05; Fig. 5E). In parallel, the level of phosphorylation of ERK1/2 in PKCβ−/− MAECs exposed to 10 min of incubation with oxLDL was enhanced compared with PKCβ−/− MAECs not exposed to oxLDL (Fig. 5F). These data demonstrated that PKCβ modulated phosphorylation of JNK but not ERK1/2 in oxLDL stimulated MAECs. In PKCβ+/+ ECs, phosphorylation of p38 in response to oxLDL was not observed (data not shown).
To determine if PKCβ-JNK signaling pathway was linked to oxLDL-mediated regulation of Egr-1, in parallel with expression and activity of MMPs, such as MMP-2, both PKCβ+/+ and PKCβ−/− MAECs were incubated with oxLDL (5 μg/ml). Egr-1 antigen was increased by ∼9-fold at 1 h of incubation with oxLDL compared with untreated PKCβ+/+ MAECs (P<0.0001; Fig. 5G). However, Egr-1 antigen in PKCβ−/− MAECs was significantly lower at 1 h of incubation with oxLDL compared with PKCβ+/+ MAECs (P<0.0001; Fig. 5G). Consistent with these observations, MMP-2 antigen in PKCβ+/+ MAECs was strikingly increased at 4 h of incubation with oxLDL compared with untreated PKCβ+/+ MAECs (P<0.0001; Fig. 5H). In contrast, MMP-2 antigen in PKCβ−/− MAECs was significantly attenuated at 4 h of incubation with oxLDL compared with PKCβ+/+ MAECs (P<0.0001; Fig. 5H). In parallel, significantly lower activity of MMP-2 in PKCβ−/− MAECs at 4 h of incubation with oxLDL was observed (P<0.0001; Fig. 5I).
To trace the signaling pathways by which oxLDL up-regulated MMP2 in MAECs, PKCβ+/+ MAECs were pretreated with inhibitors of PKCβ (LY379196, 30 nM), JNK (SP600125, 20 μM), or ERK1/2 (U0126, 5 μM) for 1 h, followed by stimulation with oxLDL. Compared with cells treated with oxLDL alone, pretreatment with the inhibitor of PKCβ or JNK followed by coincubation with oxLDL significantly reduced expression of MMP-2 antigen (P<0.05; Fig. 5J) and the activity of MMP-2 (P<0.05; Fig. 5K), but pretreatment with the ERK1/ 2 inhibitor followed by coincubation with oxLDL did not suppress oxLDL-induced expression of MMP-2 antigen and activity of MMP-2 (Fig. 5J, K).
In view of the strong endothelial expression of Egr-1 in apoE−/− aorta, we also tested the effects of PKCβ signaling on a distinct marker of endothelial activation, VCAM-1, in MAECs. Compared with control MAECs, incubation with oxLDL resulted in a significant increase in VCAM-1 antigen in PKCβ+/+ MAECs (P<0.001; Fig. 5L). In PKCβ−/− MAECs, however, no increase in VCAM-1 antigen was noted compared with untreated PKCβ−/− MAECs (Fig. 5L).
Activation of PKCβII mediates secretion of MMP-2 in HAECs via activation of MAP kinase pathway in response to oxLDL
To extend the implications of these findings to human atherosclerosis and endothelial activation, we tested the role of PKCβ in human ECs. Immunoblots of membrane fractions prepared from HAECs exposed to oxLDL displayed time-dependent induction of PKCβII membrane translocation evident by 10 min (4-fold, P<0.0001; Fig. 6A), with maximum increase by 15 min (∼5-fold, P < 0.0001; Fig. 6A). However, 3 other PKC isoforms—PKCβI, PKCδ, and PKCε (Fig. 6B)—revealed no changes in membrane translocation over this duration of incubation with oxLDL.
Figure 6.
Detection of PKCs in membrane fractions, phosphorylated MAP kinase, and secretion of MMP-2 in HAECs on stimulation with oxLDL. A, B) Membranous fraction was prepared from HAECs after incubation with or without oxLDL (5 μg/ml) for the indicated times, subjected to SDS-PAGE (25 μg/lane) and immunoblotting with antibody to PKCβII (A), PKCβI, PKCδ, and PKCε (B). C–F) Total protein was prepared from HAECs after preincubation with or without PKCβ inhibitor, LY379196 (30 nM) for 1 h, followed by coincubation with or without oxLDL (5 μg/ml) for the indicated times, SDS-PAGE (5–10 μg/lane), and immunoblotting with antibody to P-JNK and T-JNK (C, D), P-ERK1/2, and T-ERK1/2 (E, F). G, H) After HAECs were preincubated with or without inhibitors of PKCβ (LY379196, 30 nM), JNK (SP600125, 20 μM), and ERK1/2 (U0126, 5 μM) for 1 h followed by coincubation with oxLDL (5 μg/ml) for 4 h, antigen level or activity of MMP-2 in supernatants was detected by Western blot (G) or zymography (H). *P < 0.0001, ^P < 0.01, ^#P < 0.05 vs. unstimulated HAECs. ##P < 0.001, ^P < 0.01, #^P < 0.05 vs. stimulated HAECs.
Next, to test the potential impact of oxLDL on activation of JNK in HAECs. immunoblots of extracts from HAECs exposed to oxLDL for 10, 20, and 30 min displayed significantly increased phosphorylation of JNK compared with untreated HAECs (P<0.0001; Fig. 6C). Phosphorylation of JNK was significantly suppressed in HAECs pretreated with the inhibitor of PKCβ, LY379196 (30 nM) before coincubation with oxLDL compared with HAECs incubated with oxLDL alone (P<0.001; Fig. 6D). In addition, we tested the effect of PKCβ inhibitor in oxLDL stimulated HAECs on signaling molecules studied in MAECs, including ERK1/2 and p38. Immunoblots of extracts from HAECs exposed to oxLDL for 10, 20, and 30 min displayed significantly increased phosphorylated ERK1/2 (P<0.01 and P<0.05; Fig. 6E), but no elevation of phosphorylation of p38 was observed (data not shown). In parallel, the level of phosphorylation of ERK1/2 in HAECs exposed to 10 min incubation with oxLDL was enhanced in the presence of PKCβ inhibitor to the same degree as in those cells in the absence of PKCβ inhibitor (Fig. 6F). These data demonstrated that PKCβ modulated phosphorylation of JNK but not ERK1/2 and p38 in oxLDL-stimulated HAECs.
Finally, we sought to determine whether the PKCβ-JNK signaling pathway was linked to oxLDL-mediated expression and activity of MMP-2 in HAECs. MMP-2 antigen in HAECs was significantly increased at 4 h of incubation with oxLDL compared with untreated HAECs (P<0.0001; Fig. 6G), and this was significantly attenuated when HAECs were pretreated with the inhibitor of PKCβ or JNK before 4 h of coincubation with oxLDL compared with HAECs incubated with oxLDL alone (P<0.01 or P<0.001; Fig. 6G). Increased activity of MMP-2 in HAECs at 4 h of incubation with oxLDL (P<0.0001; Fig. 6H) was significantly lower in HAECs pretreated with the inhibitor of PKCβ or JNK before oxLDL (P<0.05 or P<0.01; Fig. 6H). Inhibitors of ERK1/2, however, had no effect (Fig. 6G, H).
DISCUSSION
The present study indicates important roles for PKCβ in vascular activation triggered by nondiabetic hypercholesterolemia in the atherosclerosis-prone apoE−/− mouse model. PKC is a family of multifunctional isoenzymes that play a central role in signal transduction and intracellular crosstalk by phosphorylating an array of substrates at serine/threonine residues (20). Activation of PKCβ, indicated by translocation from the cytosol to the plasma membrane, is known to trigger expression of Egr-1 and a range of proinflammatory and procoagulant molecules in the settings of acute vascular stresses, such as hypoxia (6, 7) or I/R (8). Consistent with these observations, our present data demonstrated that an age-dependent increase in PKCβII antigen was evident in membranous vs. cytosolic extracts from aortas of apoE−/− mice vs. WT C57BL/6 animals that precedes increased expression of Egr-1. These findings suggested that activation of PKCβII in a hyperlipidemic environment may potentially reflect a response to chronic vascular stress stimulated by accumulation of modified lipoproteins in the vessel wall. The prominent expression of Egr-1 in the ECs in the aortas of mice deficient in apoE suggested key roles for this pathway in early events linked to atherosclerosis.
In our published studies, we examined the role of PKCβ in the response to hypoxia or I/R and in modulating expression of Egr-1 (6,7,8) by using PKCβ−/− mice (12, 21, 22). These mice, deficient in both PKCβI/II (because both isoforms result from the same gene), are viable and fertile yet display immunodeficiency. In the present study, we bred PKCβ−/− mice into apoE−/− background. A striking decrease in atherosclerotic lesion area and complexity was evident at age 14 and 24 wk in PKCβ−/−/apoE−/− vs. apoE−/− mice, in parallel with significantly reduced vascular transcripts for Egr-1 at age of 8 and 10 wk, compared with mice solely deficient in apoE. No changes in cholesterol or triglycerides, however, were observed in either group of mice.
PKCβII is preferentially activated in ECs but also in SM cells (23, 24). Links between PKCβ activation and endothelial dysfunction in diabetes have been elucidated, such as impaired NO-mediated vasodilatation, increased release of endothelin-1, increased expression of inducible NOS and ICAM-1, and enhanced monocyte adhesion to the vessel wall (25,26,27,28,29,30). Because LDL, particularly its oxidatively modified species, is a major cause of injury to the endothelium and underlying SM (31,32,33) and modified lipoproteins are enriched in atherosclerosis before the activation of PKCβ, our findings in vitro, in both MAECs and HAECs, provided the first evidence that activation of PKCβ contributes to oxLDL-mediated induction of MMP-2 expression and activity via endothelial JNK signaling pathway, which may represent crucial endothelial processes involved in an early event in atheroma formation.
The activation and functions of JNK have been intensively studied in response to a variety of stressors such as UV irradiation (34), inflammatory cytokines (35), heat shock and mechanical stress (36,37,38), angioplasty (39), and ischemia (8, 40). Of note, increased phosphrylation of JNK has been observed in atherosclerotic lesions of cholesterol-fed rabbits (41) and in human atherosclerotic lesions (42). Our present data reinforce these concepts and illustrate that JNK signaling may modulate endothelial properties, and not merely be associated with them, because oxLDL stimulated increased phosphorylation of JNK in both WT MAECs and HAECs, in a manner suppressed by genetic deletion or pharmacological blockade of PKCβ. Furthermore, the demonstration that regulation of MMP-2 is modulated by PKCβ or JNK antagonism supports etiological roles for these pathways in ECs stress.
Pharmacological inhibition of PKCβ provides an essential and supportive means to suppress the effects of PKCβ in vivo. A selective inhibitor of PKCβ, ruboxistaurin (LY333531), was applied in the present study. Amelioration of atherosclerosis in apoE−/− mice fed ruboxistaurin chow was observed compared with the mice fed vehicle chow as control. Previous studies using this compound have suggested that in diabetes, significant impact on vascular function may be achieved, as assessed in animal models by improved retinal blood flow, reduced vascular endothelial growth factor-induced retinal permeability and neovascularization, excess production of extracellular matrix proteins in the glomerulus of db/db mice in parallel with reduced albumin excretion and mesangial expansion, and neural dysfunction (43,44,45,46,47,48). Beckman et al. (49) demonstrated that administration of ruboxistaurin to healthy human subjects exposed to hyperglycemic conditions prevented the reduction in endothelium-dependent vasodilation. Because determination of endothelial-dependent vasodilation may provide a window into the status of macrovascular activation and dysfunction, these findings highlight functional relevance for PKCβ in macrovascular disease.
Taken together, these data provide mechanistic support for the link between PKCβ-JNK signaling-mediated up-regulation of Egr-1 and MMP-2 and the pathogenesis of atherosclerosis, suggesting important implications for blockade of this pathway in atherosclerosis–even in the absence of diabetes.
Acknowledgments
We gratefully acknowledge grant support provided by the LeDucq Foundation, the Surgical Research Fund of Columbia University, and National Heart, Lung, and Blood Institute grants (RO1 HL073325) and (HL60901). We are grateful to L. Woods for her expert assistance in preparation of the manuscript.
References
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990’s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Glass K, Witztum J L. Atherosclerosis: the road ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- Erl W, Weber P C, Weber C. Monocytic cell adhesion to endothelial cells stimulated by oxidized low-density lipoprotein is mediated by distinct endothelial ligands. Atherosclerosis. 1998;136:297–303. doi: 10.1016/s0021-9150(97)00223-2. [DOI] [PubMed] [Google Scholar]
- Steinberg D. Low density lipoprotein oxidation and its pathobiological significance. J Biol Chem. 1997;272:20963–20966. doi: 10.1074/jbc.272.34.20963. [DOI] [PubMed] [Google Scholar]
- Yan S F, Lu J, Zou Y S, Soh-Won J, Cohen D M, Buttrick P M, Cooper D R, Steinberg S F, Mackman N, Pinsky D J, Stern D M. Hypoxia-associated induction of early growth response-1 gene expression. J Biol Chem. 1999;274:15030–15040. doi: 10.1074/jbc.274.21.15030. [DOI] [PubMed] [Google Scholar]
- Yan S F, Lu J, Zou Y S, Kisiel W, Mackman N, Leitges M, Steinberg S, Pinsky D, Stern D. Protein kinase C-beta and oxygen deprivation. A novel Egr-1 dependent pathway for fibrin deposition in hypoxemic vasculature. J Biol Chem. 2000;275:11921–11928. doi: 10.1074/jbc.275.16.11921. [DOI] [PubMed] [Google Scholar]
- Fujita T, Asai T, Andrassy M, Stern D M, Pinsky D J, Zou Y S, Okada M, Naka Y, Schmidt A M, Yan S F. PKCβ regulates ischemia/reperfusion injury in the lung. J Clin Invest. 2004;113:1615–1623. doi: 10.1172/JCI19225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S F, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky D J, Stern D M. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–1361. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- McCaffrey T A, Fu C, Du B, Eksinar S, Kent K C, Bush H, Kreiger K, Rosengart T, Cybulsky M I, Silverman E S, Collins T. High level expression of Egr-1 and Egr-1 inducible genes in mouse and human atherosclerosis. J Clin Invest. 2000;105:653–662. doi: 10.1172/JCI8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harja E, Bucciarelli L G, Lu Y, Stern D M, Zou Y S, Schmidt A M, Yan S F. Early growth response-1 promotes atherogenosis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ Res. 2004;94:333–339. doi: 10.1161/01.RES.0000112405.61577.95. [DOI] [PubMed] [Google Scholar]
- Leitges M, Schmedt C, Guinamard R, Davoust J, Schaal S, Stabel S, Tarakhosky A. Immunodeficiency in protein kinase Cβ deficient mice. Science. 1996;273:788–791. doi: 10.1126/science.273.5276.788. [DOI] [PubMed] [Google Scholar]
- Park L, Raman K G, Lee K J, Lu Y, Ferran L J, Jr, Chow W S, Stern D, Schmidt A M. Suppression of accelarated diabetic atherosclerosis by the soluble receptor for advanced glycation endproducts. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- Livak K J, Schmittgen T D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Hein T W, Liao J C, Kuo L. oxLDL specifically impairs endothelium-dependent, NO-mediated dialation of coronary arterioles. Am J Physiol Heart Circ Physiol. 2000;278:H175–H183. doi: 10.1152/ajpheart.2000.278.1.H175. [DOI] [PubMed] [Google Scholar]
- Fischer B, von Knethen A, Brune B. Dualism of oxidized lipoproteins in provoking and attenuating the oxidative burst in macrophages: role of peroxisome proliferator-activated receptor-γ. J Immuno. 2002;168:2828–2834. doi: 10.4049/jimmunol.168.6.2828. [DOI] [PubMed] [Google Scholar]
- Chait A. Methods for assessing lipid and lipoprotein oxidation. Curr Opin Lipidol. 1992;3:389–394. [Google Scholar]
- Kobayashi M, Inoue K, Warabi E, Minutesami T, Kodama T. A simple method of isolating mouse aortic endothelial cells. J Atheroscler Thromb. 2004;12:138–142. doi: 10.5551/jat.12.138. [DOI] [PubMed] [Google Scholar]
- Seguin C A, Pilliar R M, Madri J A, Kandel R A. TNF-α induces MMP2 gelatinase activity and MT1-MMP expression in an in vitro model of nucleus pulposus tissue degeneration. Spine. 2008;33:356–365. doi: 10.1097/BRS.0b013e3181642a5e. [DOI] [PubMed] [Google Scholar]
- Idris I, Gray S, Donnelly R. Protein kinase C activation: isozyme-specific effects on metabolism and cardiovascular complications in diabetes. Diabetologia. 2001;44:659–673. doi: 10.1007/s001250051675. [DOI] [PubMed] [Google Scholar]
- Nechushtan H, Leitges M, Cohen C, Kay G, Razin E. Inhibition of degranulation and IL-6 production in mast cells derived from mice deficient in PKCβ. Blood. 2000;95:1752–1757. [PubMed] [Google Scholar]
- Standaert M L, Bandyopadhyay G, Galloway L, Soto J, Ono Y, Kikkawa U, Farese R V, Leitges M. Effects of knockout of the PKCβ gene on glucose transport and glucose homeostasis. Endocrinology. 1999;140:4470–4477. doi: 10.1210/endo.140.10.7073. [DOI] [PubMed] [Google Scholar]
- Braiman L, Sheffi-Freidman L, Bak A, Tennenbaum T, Sampson S. Tyrosine phosphorylation of specific protein kinase C isoenzymes participates in insulin stimulation of glucose transport in primary cultures of rat skeletal muscle. Diabetes. 1999;48:1922–1929. doi: 10.2337/diabetes.48.10.1922. [DOI] [PubMed] [Google Scholar]
- Bandyopadhyay G, Standaert M L, Galloway L, Moscat J, Farese R V. Evidence for involvement of protein kinase C zeta and noninvolvement of diacylglycerol sensitive PKCs in insulin-stimulated glucose transport in L6 myotubes. Endocrinology. 1997;138:4721–4731. doi: 10.1210/endo.138.11.5473. [DOI] [PubMed] [Google Scholar]
- Park J Y, Takahara N, Gabriele A. Induction of endothelin-1 expression by glucose: an effect of PKC activation. Diabetes. 2000;49:1239–1248. doi: 10.2337/diabetes.49.7.1239. [DOI] [PubMed] [Google Scholar]
- Takagi C, Bursell S E, Lin Y W, Takagi H, Duh E, Jiang Z, Clermont A C, King G L. Regulation of retinal hemodynamics in diabetic rats by increased expression and action of ET-1. Invest Ophthalmol Vis Sci. 1996;37:2504–2518. [PubMed] [Google Scholar]
- Sharma K, Danoff T, DePierro A, Ziyadeh F N. Enhanced expression of inducible nitric oxide synthase in murine macrophages and glomerular mesangial cells by elevated glucose levels: possible mediation by PKC. Biochem Biophys Res Commun. 1995;207:80–88. doi: 10.1006/bbrc.1995.1156. [DOI] [PubMed] [Google Scholar]
- Kreuzer J, Denger S, Schmidts A, Jahn L, Merten M, von Hodenberg E. Fibrinogen promotes monocyte adhesion via a PKC dependent mechanism. J Mol Med. 1996;74:161–165. doi: 10.1007/BF01575449. [DOI] [PubMed] [Google Scholar]
- Kalra V K, Shen Y, Sultana C, Rattan V. Hypoxia induces PECsAM-1 phophorylation and transendothelial migration of monocytes. Am J Physiol. 1996;271:H2025–H2034. doi: 10.1152/ajpheart.1996.271.5.H2025. [DOI] [PubMed] [Google Scholar]
- Park C W, Kim J H, Lee J W, Kim Y S, Ahn H J, Shin Y S, Choi E J, Chang Y S, Bang B K, Lee J W. High glucose induced intercellular adhesion molecule-1 expression through an osmotic effect in rat mesangial cells is PKC-NF-κΒ-dependent. Diabetologia. 2000;43:1544–1553. doi: 10.1007/s001250051567. [DOI] [PubMed] [Google Scholar]
- Navab M, Berliner J A, Watson A D, Hama S Y, Territo M C, Lusis A J, Shih D M, Van Lenten B J, Frank J S, Demer L L, Edwards P A, Fogelman A M. The Yin and Yang of oxidation in the development of the fatty streak: a review based on the 1994 George Lyman Duff memorial lecture. Atheroscler Thromb Vasc Biol. 1996;16:831–842. doi: 10.1161/01.atv.16.7.831. [DOI] [PubMed] [Google Scholar]
- Morel D W, Hessler J R, Chisholm G M. Low density lipoprotein cytotoxicity induced by free radical peroxidation of lipid. J Lipid Res. 1983;24:1070–1076. [PubMed] [Google Scholar]
- Griendling K K, Alexander R W. Oxidative stress and cardivascular disease. Circulation. 1997;96:3264–3265. [PubMed] [Google Scholar]
- Adler V, Fuchs S Y, Kim J, Kraft A, King M P, Pelling J, Ronai Z. Jun-NH2-terminal kinase activation mediated by UV-induced DNA lesions in melanoma and fibroblast cells. Cell Growth Differ. 1995;6:1437–1446. [PubMed] [Google Scholar]
- Modur V, Zimmerman G A, Prescott S M, McIntyre T M. Endothelial cell inflammatory responses to tumor necrosis factor α Ceramide-dependent and -independent mitogen-activated protein. J Biol Chem. 1996;271:13094–13102. doi: 10.1074/jbc.271.22.13094. [DOI] [PubMed] [Google Scholar]
- Hu Y, Metzler B, Xu Q. Discordant activation of stress-activated protein kinases or c-Jun NH2-terminal protein kinases in tissues of heat-stressed mice. J Biol Chem. 1997;272:9113–9119. doi: 10.1074/jbc.272.14.9113. [DOI] [PubMed] [Google Scholar]
- Reusch H P, Chan G, Ives H E, Nemenoff R A. Activation of JNK/SAPK and ERK by mechanical strain in vascular smooth muscle cells depends on extracellular matrix composition. Biochem Biophys Res Commun. 1997;237:239–244. doi: 10.1006/bbrc.1997.7121. [DOI] [PubMed] [Google Scholar]
- Hamada K, Takuwa N, Yokoyama K, Takuwa Y. Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of purinoceptors. J Biol Chem. 1998;273:6334–6340. doi: 10.1074/jbc.273.11.6334. [DOI] [PubMed] [Google Scholar]
- Hu Y, Cheng L, Hochleitner B W, Xu Q. Activation of mitogen activated protein kinases (ERK/JNK) and AP-1 transcription factor in rat carotid arterties after balloon injury. Atheroscler Thromb Vasc Biol. 1997;17:2808–2816. doi: 10.1161/01.atv.17.11.2808. [DOI] [PubMed] [Google Scholar]
- Yue TL, Ma XL, Gu JL, Ruffolo RR, Jr, Feuerstein GZ. Carvedilol inhibits activation of stress-activated protein kinase and reduces reperfusion injury in perfused rabbit ear. Eur J Pharmacol. 1980;345:61–65. doi: 10.1016/s0014-2999(98)00053-3. [DOI] [PubMed] [Google Scholar]
- Metzler B, Hu Y, Dietrich H, Xu Q. Increased expression and activation of stress-activated protein kinases/ c-Jun NH2-terminal protein kinases in atherosclerotic lesions coincide with p53. Am J Pathol. 2000;156:1875–1886. doi: 10.1016/S0002-9440(10)65061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio H, Matsui K, Tsuji H, Tamura A, Suzuki K. Immunochemical study of the phosphorylated and activated form of c-Jun NH2-terminal kinase in human aorta. Histochem J. 2001;33:167–171. doi: 10.1023/a:1017952310800. [DOI] [PubMed] [Google Scholar]
- Aiello L P, Bursell S E, Clermont A, Duh E, Ishii H, Takagi C, Mori F, Ciulla T A, Ways K, Jirousek M, Smith L E, King G L. Vascular endothelial growth factor induced retinal permeability is mediated by PKC in vivo and suppressed by an orally effective beta-isoform selective inhibitor. Diabetes. 1997;46:147–1480. doi: 10.2337/diab.46.9.1473. [DOI] [PubMed] [Google Scholar]
- Ishii H, Jirousek M R, Koya D, Takagi C, Xia P, Clermont A, Bursell S E, Ken T S, Ballas L M, Heath W F, Stramm L E, Feener E P, King G L. Amelioration of vascular dysfunctions in diabetic rats by an oral PKC beta inhibitor. Science. 1996;272:728–731. doi: 10.1126/science.272.5262.728. [DOI] [PubMed] [Google Scholar]
- Bursell S E, Takagi C, Clermont A C, Takagi H, Mori F, Ishii H, King G L. Specific retinal DAG and PKCβ isoform modulation mimics abnormal retinal hemodynamics in diabetic rats. Invest Ophthalmol Vis Sci. 1997;38:2711–2720. [PubMed] [Google Scholar]
- Danis R P, Bingaman D P, Jirousek M, Yang Y. Inhibition of intraocular neovacularization caused by retinal ischemia in pigs by PKCβ inhibition with LY333531. Invest Ophthalmol Vis Sci. 1998;39:171–179. [PubMed] [Google Scholar]
- Koya D, Haneda M, Nakagawa H, Isshiki K, Sata H, Maeda S, Sugimoto T, Yasuda H, Kashiwagi A, Ways DK, King GL, Kikkawa, King R GL. Amelioration of acclerated diabetic mesangial expansion by treatment with a PKCβ inhibitor in diabetic db/db mice, a rodent model of type 2 diabetes. FASEB J. 2000;14:439–447. doi: 10.1096/fasebj.14.3.439. [DOI] [PubMed] [Google Scholar]
- Nakamura J, Kato K, Hamada Y, Nakayama M, Chaya S, Nakashima E, Naruse K, Kasuya Y, Mizubayashi R, Miwa K, Yasuda Y, Kamiya H, Ienaga K, Sakakibara F, Koh N, Hotta N. A protein kinase C-beta selective inhibitor ameliorates neural dysfunction in stz-diabetic rats. Diabetes. 1999;48:2090–2095. doi: 10.2337/diabetes.48.10.2090. [DOI] [PubMed] [Google Scholar]
- Beckman J A, Goldfine A B, Gordon M B, Garrett L A, Creager M A. Inhibition of PKCβ prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans. Circ Res. 2002;90:5–7. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]