Abstract
RNA virus replication results in expression of double-stranded RNA (ds-RNA) molecules that trigger innate immune responses through interactions with both intracellular and extracellular receptors. We investigated the contributions of the extracellular and intracellular pathways to innate immunity in murine astrocyte primary cultures using polyinosinic-polycytidylic acid (poly I:C), a synthetic ds-RNA molecule designed to mimic RNA virus infection. Whereas extracellular poly I:C (naked poly I:C) mainly induced the expression of regulated on activation normal T-cell expressed and secreted (RANTES), interleukin-8 (IL-8), and tumor necrosis factor α (TNF-α), intracellular delivery of poly I:C (complexed poly I:C) chiefly induced expression of IFN-β and IL-6. Experiments with astrocytes from Toll-like receptor 3 (TLR-3) knockout mice indicated that naked poly I:C signals via a TLR-3-dependent NF-κB pathway. Complexed poly I:C induced the expression of the intracellular ds-RNA sensor proteins, retinoic acid inducible gene I (RIG-I), and melanoma differentiation-associated gene 5 (MDA-5). However, transfection of astrocytes with dominant negative forms of the helicases implicated MDA-5, but not RIG-I, as the intracellular sensor of poly I:C. Complexed poly I:C-mediated MDA-5 stimulation transmitted “downstream” signals, resulting in activation of the transcription factors NF-κB and IRF-3. Our results illustrate the intricacy of extracellular and intracellular ds-RNA recognition in viral infections of the central nervous system and indicate the importance of MDA-5 helicase as an intracellular ds-RNA sensor in astrocytes. De Miranda, J., Yaddanapudi, K., Hornig, M., Lipkin, W. I. Astrocytes recognize intracellular polyinosinic-polycytidylic acid via MDA-5.
Keywords: ds-RNA, TLR-3, RIG-I, CNS, innate immunity
Immune responses in the central nervous system (CNS) are largely mediated by microglia and astrocytes. Astrocytes integrate information from the microvasculature and neuronal interfaces modulating neuronal excitability, synaptic transmission, and cerebral blood flow. In CNS viral infection, astrocytes induce excitotoxic neuronal apoptosis through a tumor necrosis factor α (TNF-α)-mediated mechanism (1).
The innate immune system recognizes microorganisms via pattern-recognition receptors (PRRs) located in the plasma membrane and cytosol of the cells. PRRs bind to key microbial components known as pathogen-associated molecular patterns (PAMPs), including the double-stranded RNA (ds-RNA) associated with virus replication, thereby engaging host cells in pathogen-specific cell response programs (2). Polyinosinic-polycytidylic acid (poly I:C), a ds-RNA mimic, triggers the innate immune system to secrete the antiviral cytokines IFN-α and IFN-β and proinflammatory cytokines. In the CNS, poly I:C is recognized by resident microglia and astrocytes (3,4,5,6).
The innate immune system has two pathways for the recognition of ds-RNA associated with viral infection. In one pathway, poly I:C present in the extracellular space is internalized through endocytosis, resulting in activation of Toll-like receptor 3 (TLR-3) signaling (7). TLR-3 recognizes poly I:C through its extracellular domain and transduces the signal via the cytoplasmic Toll/Interleukin-1 receptor (TIR) domain. The downstream signaling occurs by recruiting the adaptor protein, TIR domain-containing adaptor inducing IFN-β (TRIF). The signaling cascade culminates in the activation and nuclear migration of NF-κB, resulting in induction of several genes involved in innate and adaptive immunity (8, 9). A second, intracellular pathway detects ds-RNA via the cytosolic sensor proteins melanoma differentiation-associated gene 5 (MDA-5) and retinoic acid inducible gene I (RIG-I). These cytosolic proteins are members of the DexD/H RNA box helicase family with a caspase recruitment domain (CARD) at the N terminus and a helicase domain at the C terminus (10, 11). On binding poly I:C, MDA-5/RIG-I associate with the adaptor protein, IFN-β promoter stimulator 1 (IPS-1; also known as MAVS) (12). IPS-1 then recruits both IKKε/TBK1 and IKKα/β/γ complexes, resulting in activation of the transcription factors interferon regulatory factor 3 (IRF-3), NF-κB, and activating protein 1 (AP-1) (13).
Here, we investigate the contributions of the pathways activated on treatment of murine primary astrocytes with either naked or complexed poly I:C. Our data indicate that naked poly I:C triggers TLR-3-mediated signaling responses, resulting in the release of IFN-β, interleukin (IL)-6, TNF-α, IL-8, and RANTES (regulated on activation normal T-cell expressed and secreted). Complexed poly I:C treatment induces the expression of RIG-I and MDA-5 in murine astrocytes. Furthermore, our data show that recognition of cytosolic poly I:C is mediated through MDA-5, resulting in IRF-3 activation and a more robust release of IFN-β and IL-6.
MATERIALS AND METHODS
Primary astrocyte culture
Postnatal day 1 to 3 C57BL/6J (B6), B6129SF2/J, and B6;129S1-Tlrtm1Flv/J TLR3 knockout mice (Jackson Laboratories; Bar Harbor, ME, USA) were anesthetized and decapitated. Brains were removed directly into ice-cold Hank’s buffered saline solution (HBSS) (14). The cortex was dissected, freed of the meninges, trimmed, and treated with trypsin (0.25%) and DNase (2000U) at 37°C for 10–20 min. Cells were mechanically dissociated, plated in 75 cm2 flasks and incubated in Dulbecco’s modified Eagle medium plus F12 salts (DMEM-F12) supplemented with 10% fetal calf serum and 5 mM glutamine at 37°C for 15–20 days until the mixed glia culture reached confluence. The flasks were agitated in a rotary shaker at 180 rpm. Tissue culture media were removed after 24 h, and the cell monolayer was washed with HBSS solution to remove the contaminant glial cells. The adherent astrocytes were trypsinized and plated in 24-well poly-d-lysine-coated plates at a density of 2 × 105 cells/well. Cells were plated for 24–48 h before poly I:C treatment. The cell culture consisted of 95% or more of astrocytes, as confirmed by glial fibrillary acid protein (GFAP) immunofluorescence (anti-GFAP, 1:50; BD Biosciences, San Jose, CA, USA) and flow cytometric analysis (data not shown).
Poly I:C treatment
Polyinosinic-polycytidylic potassium salt (Sigma-Aldrich, St. Louis, MO, USA) dissolved in PBS was heated to 55°C for 5 min and allowed to cool at room temperature. ds-RNA concentration was measured by UV spectroscopy. For naked poly I:C treatment, the drug was dissolved in culture medium at a concentration of 25 μg/ml. Complexed poly I:C treatment was performed by transfecting 4 μg/ml of ds-RNA coupled to lipofectamine 2000 (2.5 μl/well; Invitrogen, Carlsbad, CA, USA).
RNA extraction and cDNA synthesis
The cell monolayer was washed twice with ice-cold PBS, and RNA was extracted using the RNAeasy kit (Qiagen, Valencia, CA, USA). Following extraction, total RNA was quantified by UV spectroscopy. cDNA was synthesized using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) from 1 μg of RNA in a total 100 μl reaction volume.
Quantitative real-time polymerase chain reaction (PCR)
Intron/exon spanning gene-specific PCR primers and probes labeled at the 5′-end with the fluorescent reporter dye FAM (6-carboxyfluorescein) and at the 3′-end with the quencher dye TAMRA (6-carboxy-tetramethyl-rhodamine) for the mouse homologue of the RIG-I and MDA-5 helicases were purchased from Applied Biosystems. For the determination of the target transcript copy number, PCR products amplified from the primers were subcloned into a pGEM-T Easy vector (Promega Corp., Madison, WI, USA). The plasmid was then linearized, and the concentration was determined by UV spectroscopy. Several 10-fold dilutions of the linearized plasmid were used in the assay to generate standard curves. Samples were normalized by laminin gene expression in the same sample replicates using intron/exon-spanning mouse laminin-specific PCR primers and probe containing the 5′-end reporter dye TET (tetrachloro-6-carboxy-fluorecein) and the 3′-end quencher dye TAMRA (F-CCTCGCAGCGCAGCC, R-ACCATTCTGACGCCTGATCTG P-TGGAGGCAGCGTCACCAAAAAGC). For the quantitative real-time PCR assay, each 25-μl amplification reaction contained 5 μl of the cDNA template, 12.5 μl universal master mix (Applied Biosystems), 1.25 μl of the gene of interest primers and probe mix (Applied Biosystems), 200 nM of the laminin probe, and 300 nM of the laminin primers. The assay was performed on a Model 7700 Sequence Detector System Thermal Cycler (Applied Biosystems) with a profile that consisted of 1 cycle at 50°C for 2 min, 1 cycle at 95°C for 10 min, and 45 cycles at 95°C for 15 s, followed by 60°C for 1 min.
Western blot analysis
Astrocytes were washed once with ice-cold PBS and resuspended in the sample buffer (10 mM Tris-HCl, pH 7.5; 10 mM EDTA, 20% v/v glycerol; 1% w/v SDS; 0.005% w/v bromphenol blue; 100 mM dithiothreitol) and size fractionated by 8–10% SDS-PAGE. Proteins were transferred to a polyvinylidene difluoride (PVDF) membrane according to standard protocols. Blots were blocked in a 5% nonfat milk, 0.1% Tween 20, and PBS solution and incubated with anti-IRF-3 (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho-IRF-3 (1:200; Ser-396; Cell Signaling, Danvers, MA, USA), anti-NF-κB2 p100 (1:200; Cell Signaling) or anti-Iκ-Bβ (1:200; eBioscience, San Diego, CA, USA) at 4°C overnight. The membranes were washed 3 times in a PBS-0.1% Tween-20 solution and incubated with peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (1:10,000; Bio-Rad, Hercules, CA, USA) or goat anti-rabbit IgG (1:10,000; Bio-Rad) for 2 h at room temperature. The blots were developed using ECL kit reagents (Amersham Biosciences; Piscataway, NJ, USA). The blots were stripped and reprobed with mouse anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (Ambion, Austin, TX, USA), as a housekeeping gene and for loading control.
Cytokine measurement
Cytokines were measured in tissue culture supernatants using the bead-based Luminex assay. The bead mix was custom designed (Millipore, Billerica, MA, USA), and the assay was performed according to the manufacturer’s protocol. The results were measured on a Luminex 100 system, and the cytokine concentration values were calculated using the LMAT 1.0 software (Luminex Corporation, Austin, TX, USA). IFN-β cytokine analysis was performed by ELISA, according to the manufacturer’s protocol (PBL Biomedical Laboratories, Piscataway, NJ, USA).
Flow cytometric analysis
Astrocytes were untreated or treated with naked poly I:C (25 μg/ml), lipofectamine alone, or complexed poly I:C (4 μg/ml). Cells were harvested after 6 h of culture and analyzed for the expression of intracellular activated caspase-8 by flow cytometry. Cells were washed twice with RPMI 1640 medium supplemented with 0.5% FBS. Cells (106) were stained for caspase expression using the vibrant FAM caspase-8 assay kit (Invitrogen). Cells were analyzed on a LSRII analyzer (BD Biosciences). Data were obtained using FACS DiVa acquisition software (BD Biosciences), and analyzed using FlowJo6.1 software (Tree Star, Ashland, OR, USA) after appropriate gating to exclude dead cells and debris based on forward scatter and side scatter.
Generation and transfection of RIG-I and MDA-5 dominant negative plasmids
Empirical inactivating point mutations at the Walker-type ATP-binding site of RIG-I pUNO and MDA-5 pUNO (Invivogen, San Diego, CA, USA) expressing plasmids were performed using the site mutagenesis kit (Stratagene; La Jolla, CA, USA). A lysine 270 to alanine substitution was introduced in the RIG-I sequence (K270A) using the primer: 5′-GTGCTCCTACAGGTTGTGGAGCAACCTTTGTTTCACTGCTTA-3′. A lysine 335 to alanine substitution was introduced in the MDA-5 sequence (K335A) using the primer: 5′-CTCCCTACAGGGAGTGGAGCAACCAGAGTGGTCGTTTA-3′. Astrocytes were either singly or doubly transfected with 0.75 μg of each of dominant negative plasmids of RIG-I (K270A) or MDA-5 (K335A) for 48 h. Transfection was performed using 2.5 μl of lipofectamine LTX and 3 μl of Plus reagent (Invitrogen). Transfection efficiency was quantitated in astrocytes by flow cytometry. Cells were transfected with plasmid expressing the FLAG-tagged dominant negative mutant form of MDA-5 (MDA-5 K335A). 106 cells were stained for MDA-5 K335A expression using the FITC-conjugated anti-FLAG antibody (Sigma- Aldrich). MDA-5 K335A protein expression was observed in 46.7% of the cells (Supplemental Fig. 1).
Immunofluorescence
Astrocytes were fixed with 4% paraformaldehyde at room temperature for 30 min, permeabilized with 0.1% Triton X-100 for 15 min, and blocked for 2 h in PBS with 10% normal goat serum (Sigma-Aldrich). Cells were incubated overnight at 4°C with anti-IRF-3 (1:50; Santa Cruz Biotechnology) and anti-GFAP (1:50; BD Biosciences) antibodies. Cells were then incubated for 2 h at room temperature with Cy-3 conjugated goat anti-rabbit IgG and Cy-2 conjugated goat anti-mouse IgG (1:200; Jackson Immunoresearch Laboratories; West Grove, PA, USA). Cells were mounted with ProLong Gold antifade reagent with DAPI (Invitrogen). Images were captured with a Zeiss LSM 510 NLO Multiphoton Confocal Microscope and analyzed using Carl Zeiss Confocal Microscope (AIM) Software (Carl Zeiss GmbH; Thornwood, NY, USA).
Statistical analysis
For values that presented with a normal distribution, one- or two-way ANOVA tests were performed (independent variables, dose group, or dose group and genotype, respectively). Nonparametrical approaches were pursued for the analysis of the values in which a non-Gaussian distribution was observed. For the evaluation of differences involving independent variables with ≥3 levels (e.g., controls vs. naked poly I:C vs. complexed poly I:C treatment) the Kruskal-Wallis test was used. For differences involving independent variables with ≤ 2 levels (e.g., control vs. poly I:C), we used the Mann-Whitney U test. All nonparametric tests were 2-tailed. When the Kruskal-Wallis test was significant (nominal α ≤ 0.05 as the threshold of significance), individual Mann-Whitney two-group comparisons were pursued to identify the nature of the significant effect. All statistical analysis was performed using Statview for Windows software (ver. 5.0.1; SAS Institute; Cary, NC, USA).
RESULTS
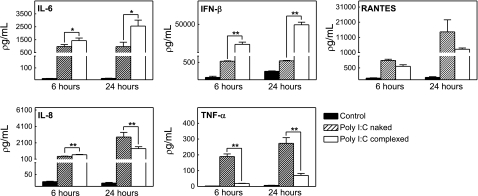
Cytokines and chemokines play important roles in viral infection and innate immune processes. Thus, we examined the influence of naked and complexed poly I:C on cytokine production by cultured murine astrocytes. Naked and complexed poly I:C treatment for 6 and 24 h significantly increased the levels of IFN-β, IL-6, TNF-α, IL-8, and RANTES (Fig. 1). Whereas complexed poly I:C treatment induced elevated levels of IFN-β and IL-6, naked poly I:C treatment had more pronounced effects on TNF-α, IL-8, and RANTES (Fig. 1). Lipofectamine treatment alone (negative control for complexed poly I:C) did not alter cytokine levels at 24 h (data not shown). Taken together, these data implicate the involvement of different signaling cascades following exposure to naked or complexed poly I:C.
Figure 1.
Poly I:C treatment of murine astrocytes results in increased release of IL-6, IFN-β, TNF-α, IL-8, and RANTES. Astrocytes isolated from wild-type C57BL/6J mice were untreated (control) or treated with either naked poly I:C (25 μg/ml) or complexed poly I:C (4 μg/ml) for 6 or 24 h. Cytokines were assayed in cell supernatants. Results indicate mean ± se concentration (pg/ml). *P ≤ 0.05, **P ≤ 0.001; one-way ANOVA with dose group as independent variable.
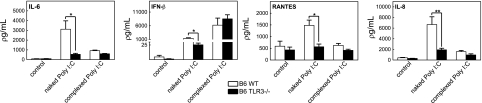
TLR-3-deficient (TLR-3−/−) mice show reduced responses to naked poly I:C (7). To elucidate the role of TLR-3 in poly I:C recognition, we compared the cytokine profiles of astrocytes isolated from wild-type B6129SF2/J and B6;129S1-Tlrtm1Flv/J TLR-3 knockout mice following exposure to naked or complexed poly I:C. Whereas TLR-3−/− astrocytes were deficient in IFN-β, IL-6, IL-8, and RANTES responses after treatment with naked poly I:C, cytokine expression after complexed poly I:C was the same as in wild-type astrocytes (Fig. 2). These results suggest that TLR-3 is involved in the recognition of naked poly I:C and may not play a role in complexed poly I:C signaling.
Figure 2.
Cytokine induction by naked poly I:C but not complexed poly I:C is TLR-3 dependent. Astrocytes isolated from wild-type B6129SF2/J or TLR3 knockout B6;129S1-Tlrtm1Flv/J mice were untreated (control) or treated with either naked poly I:C (25 μg/ml) or complexed poly I:C (4 μg/ml) for 6 h. Cytokines were assayed in cell supernatant. Results indicate mean ± se concentration (pg/ml). *P ≤ 0.05, **P ≤ 0.001; Mann-Whitney.
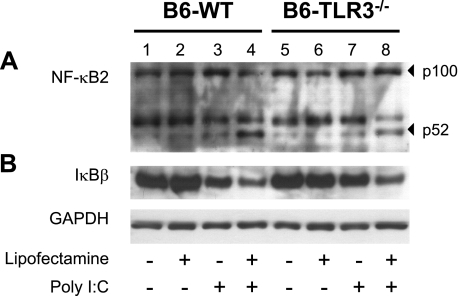
TLR-3 specifically signals for NF-κB activation in response to viral infections (7). In resting cells, NF-κB is present as an inactive complex with its regulatory subunit IκB. On ds-RNA stimulation, the complex becomes a substrate for proteasome degradation, resulting in the degradation of IκB and liberation of the cleaved NF-κB. The active subunit of NF-κB then migrates to the nucleus where it acts as a transcription factor (15). To elucidate the role of TLR-3 in naked and complexed poly I:C-induced NF-κB activation, protein extracts of astrocytes were isolated from wild-type and TLR-3−/− mice and analyzed by Western blot analysis. In wild-type cells, naked poly I:C treatment did not increase the protein levels of the active NF-κB p52 subunit (Fig. 3A, lanes 1, 3). However, naked poly I:C induced significant degradation of the inhibitory protein IκB (Fig. 3B, lanes 1, 3). Complexed poly I:C induced the activation of NF-κB and decreased the intensity of the protein signal corresponding to IκB (Fig. 3A, B; lanes 2, 4). These effects were not observed in TLR-3−/− cells exposed to naked poly I:C (Fig. 3A, lane 7). In contrast, responses were similar in wild-type and TLR-3−/− cells after exposure to complexed poly I:C (Fig. 3A, B; lanes 4, 8).
Figure 3.
Poly I:C induces NF-κB activation in murine astrocytes. Astrocytes isolated from wild-type B6129SF2/J and TLR3 knockout B6;129S1-Tlrtm1Flv/J mice were untreated (control) or treated with either naked poly I:C (25 μg/ml), lipofectamine alone, or complexed poly I:C (4 μg/ml) for 6 h. A) Protein extracts from astrocyte cultures were subjected to immunoblot analysis using anti-NF-κB2 antibody. Inactive (p100) and active (p52) forms of the protein are indicated. B) Immunoblot analysis of astrocyte cell extracts using anti-IκBβ antibody. Anti-GAPDH was used as loading control.
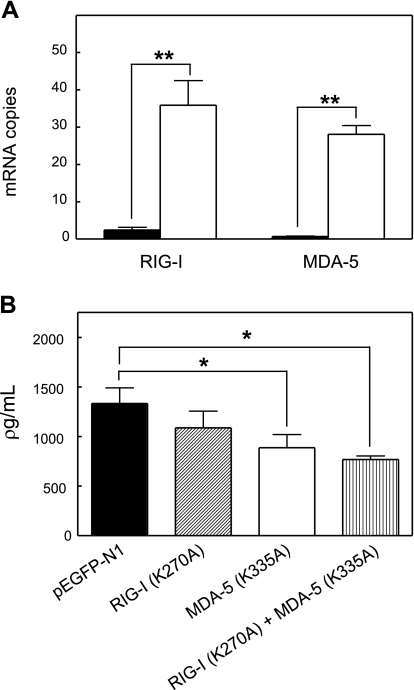
The cytoplasmic proteins RIG-I and MDA-5 have been identified as intracellular sensors of ds-RNA (11). However, expression of RIG-I and MDA-5 has not been previously reported in murine astrocytes. Quantitative real-time PCR demonstrated that complexed poly I:C induced a 21-fold and 25-fold increase in RIG-I and MDA-5 mRNA levels, respectively (Fig. 4A). To further address the function of RIG-I and MDA-5 in complexed poly I:C signaling, we examined their activities in astrocytes transfected with constructs that directed expression of proteins, wherein inactivating point mutations were introduced at ATP-binding sites: RIG-I (K270A) and MDA-5 (K335A) (16). We also used cells doubly transfected with constructs to enable simultaneous expression of MDA-5 mutant protein and RIG-I mutant protein. Whereas levels of IL-6 were significantly reduced in cells transfected with mutant MDA-5 or with both mutant MDA-5 and mutant RIG-I, no reduction was seen in cells transfected with mutant RIG-I (Fig. 4B). These results are compatible with MDA-5 serving as an essential regulator of complexed poly I:C signaling in astrocytes.
Figure 4.
MDA-5 but not RIG-I is the intracellular sensor of complexed poly I:C in astrocytes. Astrocytes isolated from wild-type C57BL/6J mice were untreated (control, solid bar) or treated with complexed poly I:C (4 μg/ml, white bar) for 6 h. A) Endogenous mRNA levels of RIG-I and MDA-5 were measured by quantitative real-time PCR. Results are expressed as the mean numbers of mRNA copies of RIG-I and MDA-5 relative to the value obtained for laminin mRNA; **P ≤ 0.001; Mann-Whitney U test. B) Wild-type C57BL/6J astrocytes expressing dominant-negative forms of RIG-I (K270A), MDA-5 (K335A), RIG-I (K270A), and MDA-5 (K335A) or GFP control protein were treated with complexed poly I:C (4 μg/ml) for 6 h. Levels of IL-6 protein were measured in culture supernatants. Results indicate mean ± se concentration (pg/ml). *P ≤ 0.05; one-way ANOVA with dose group as independent variable.
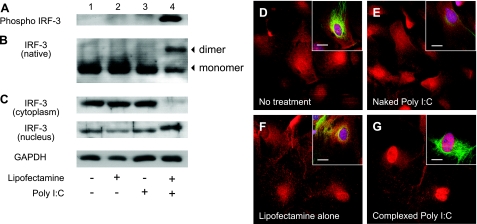
RIG-I and MDA-5 transduce signals that lead to type 1 IFN production by triggering the phosphorylation, dimerization, and nuclear translocation of active IRF-3 (12). To assess the role of MDA-5 in naked and complexed poly I:C-induced IRF-3 activation, protein extracts of astrocytes isolated from wild-type mice were analyzed by Western blot analysis. Complexed poly I:C treatment induced phosphorylation of IRF-3, as detected by an antibody specific for phospho-IRF-3 (Ser-396) (Fig. 5A, lane 4). Constitutive formation of IRF-3 dimers was augmented by complexed poly I:C (Fig. 5B, lane 4). Western blot analysis of cytoplasmic and nuclear cell fractions demonstrated that complexed poly I:C induced the nuclear translocation of IRF-3 (Fig. 5C, lane 4). IRF-3 phosphorylation, dimerization, and nuclear translocation were not observed with control, naked poly I:C or lipofectamine alone (Fig. 5A–C, lanes 1–3). Immunofluorescence analysis confirmed that IRF-3 staining was restricted to the nuclear compartment of astrocytes treated with complexed poly I:C (Fig. 5G). IRF-3 was present in the cytoplasm, and, to a lower extent, in the nucleus of control, naked, or lipofectamine treated astrocytes (Fig. 5D–F).
Figure 5.
Treatment of astrocytes with complexed poly I:C but not naked poly I:C induces IRF-3 activation. Astrocytes isolated from wild-type C57BL/6J mice were untreated (control) or treated with either naked poly I:C (25 μg/ml), lipofectamine alone, or complexed poly I:C (4 μg/ml) for 6 h. A) Astrocyte cell extracts were subjected to immunoblot analysis using anti-phospho IRF-3 antibody. B) Anti-IRF-3 immunoblot of astrocyte cell extracts separated by native PAGE. Protein monomer and dimer forms are indicated. C) Protein lysates from cytoplasmic (top) and nuclear cell fractions (bottom) were subjected to immunoblot analysis using anti-IRF-3 antibody. Corresponding immunoblot signals for GAPDH are shown (loading control). D–G) Immunofluorescence analysis of IRF-3 protein in untreated astrocytes (D) or in astrocytes treated with naked poly I:C (E), lipofectamine alone (F), or complexed poly I:C (G) for 6 h. Insets: triple labeling of cells with anti-IRF-3 (red), anti-GFAP (green), and nuclear counterstain (blue). Scale bars = 20 μm.
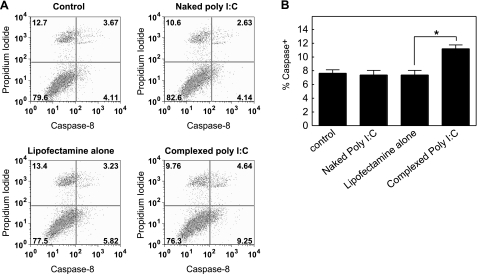
MDA-5/RIG-I signaling induces a caspase-8/10 mediated NF-κB activation in a TLR-3-independent manner (17). To test whether complexed poly I:C-induced NF-κB activation occurred through caspase-8/10 signaling, expression levels of activated caspase-8 were measured in astrocytes by flow cytometry. Complexed poly I:C treatment resulted in a 1.5-fold increase in the percentage of cells positive for activated caspase-8 (percentage of caspase-8+ astrocytes exposed to complexed poly I:C, 11.2±0.5%; lipofectamine-treated cells, 7.3±0.6%, Fig. 6). No significant induction of activated caspase-8 was observed in untreated cells or cells treated with naked poly I:C (Fig. 6).
Figure 6.
Complexed poly I:C treatment of astrocytes induces caspase-8 activation. Astrocytes isolated from wild-type C57BL/6J mice were untreated (control) or treated with either naked poly I:C (25 μg/ml), lipofectamine alone, or complexed poly I:C (4 μg/ml) for 6 h. After staining with caspase-8-FLICA and propidium iodide (PI; marker for apoptosis or necrosis), cells were analyzed by flow cytometry. A) Dot plots of caspase-8 and PI fluorescence in untreated, lipofectamine-treated, or poly I:C-treated astrocytes. Numbers in quadrants represent percentages of each subpopulation. B) Percentages of caspase-8+ cells in untreated, lipofectamine-treated, or poly I:C-treated astrocytes. Values are means ± se. *P ≤ 0.05; Mann-Whitney U test.
DISCUSSION
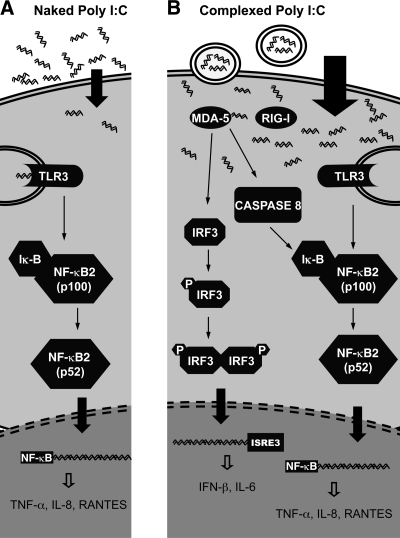
Astrocytes respond to viral infections with the induction of type I interferons, cytokines, and chemokines (6). We examined the mechanisms responsible for the activation of astrocytes by extracellular and intracellular ds-RNA associated with viral infection using naked or complexed poly I:C. Our findings corroborate recent data indicating that TLR-3 is important for responses of astrocytes to naked poly I:C (3). TLR-3, expressed in astrocytes, has been identified as the molecule that participates in the innate immune response to West Nile virus by facilitating its entry into the CNS (18). Our experiments with TLR-3−/− astrocytes demonstrated that TLR-3 stimulation by naked poly I:C resulted in NF-κB activation, which, in turn, induced the production of proinflammatory cytokines such as IL-8, RANTES (Fig. 7A).
Figure 7.
Activation of different poly I:C-mediated signaling pathways depends on the mode of delivery of poly I:C to the cells. A) When delivered to the extracellular milieu (naked poly I:C), ds-RNA is recognized by TLR-3 receptors present on the endosomes and plasma membranes of astrocytes. Binding of poly I:C to TLR-3 results in NF-κB cleavage and activation. Cleaved 52-kDa product of NF-κB translocates to the nucleus and induces the expression of RANTES, IL-8, and TNF-α. B) Intracellular delivery of poly I:C (complexed poly I:C) engages the intracellular helicase MDA-5. Complexed poly I:C-dependent MDA-5 signaling results in IRF-3 phosphorylation. Phospho-IRF-3 forms homodimers and migrates to the cell nuclei. Activated IRF-3 binds to the ISRE elements in the cell nuclei and stimulates the synthesis of IFN-β and IL-6. Binding of complexed poly I:C to MDA-5 also triggers caspase-8 cleavage and activation. Cleaved caspase-8 induces TLR-3-independent NF-κB activation observed after complexed poly I:C treatment of astrocytes.
TLR-3-mediated signaling mechanisms leading to the production of IFN-β and IL-6 involve the activation of the transcription factors IRF-3 and IRF-7 (19). Although IRF-3 and IRF-7 function as direct transducers of TLR-3 signaling, we did not observe significant IRF-3 activation after naked poly I:C treatment. We speculate, therefore, that TLR-3-dependent IFN-β and IL-6 production in naked poly I:C-stimulated astrocytes may involve an IRF-7 signaling pathway.
The cytoplasmic helicases RIG-I and MDA-5 recognize viral nucleic acids and are essential for the production of type I interferons in the context of a viral infection (20). In vivo studies have shown that intracellular recognition of poly I:C is mediated by MDA-5 (10), but little is known about its role during viral infections of the CNS. We found that complexed poly I:C treatment of astrocytes induced the expression of both RIG-I and MDA-5 proteins; however, only MDA-5 participated in intracellular poly I:C recognition. Moreover, MDA-5-mediated signaling is a prerequisite for IRF-3 activation. IRF-3 can form homodimers or heterodimers with IRF-7; these dimers may be responsible for the induction of IL-6 and IFN-β production observed with complexed poly I:C treatment. Alternatively, induction of IFN-β observed with complexed poly I:C may be mediated through the interaction of transcription factors IRF-3, IRF-7, and NF-κB in the nuclear compartment. Assembly of these transcription factors on the IFN-β gene promoter (enhanceosome complex) is known to result in a more robust and prolonged induction of IFN-β gene (13).
Gene-targeting studies in mice have shown that ds-RNA-dependent protein kinase (PKR), an intracellular ds-RNA sensor, participates in poly I:C recognition in astrocytes (3). Activation of PKR by ds-RNA results in PKR autophosphorylation and the downstream activation of eukaryotic initiation factor 2α-subunit (eIF-2α). In our model, poly I:C did not alter the phosphorylation status of PKR and eIF-2α at 6 h post-treatment (data not shown). Although these results suggest that PKR may not contribute to early cytokine expression after ds-RNA exposure, they do not exclude a role in late and prolonged cytokine induction.
Our results with TLR-3−/− astrocytes demonstrate that complexed poly I:C induced a TLR-3-independent NF-κB activation. Therefore, the observed NF-κB activation could be mediated through alternate signaling mechanisms. Earlier reports demonstrated that recognition of ds-RNA by MDA-5 results in cleavage of caspase-8 and -10 and that the activated, cleaved caspases interact with downstream effectors to activate NF-κB (17). Complexed poly I:C up-regulated activated caspase-8 in astrocytes, suggesting its involvement in complexed poly I:C-mediated NF-κB activation (Fig. 7B).
We conclude that differences in poly I:C treatment lead to the recruitment of different, partially redundant intracellular pathways, engaging astrocytes in two distinct cytokine profiles. Participation of MDA-5 in the recognition of poly I:C suggests a crucial role for this helicase in the innate immune response to viral ds-RNA in the CNS and further highlights the importance of astrocytes in innate immune pathogen recognition.
Acknowledgments
The authors thank Vishal Kapoor for technical assistance and Robert Serge for help with statistical analysis. This work was supported by a grant from the U.S. National Institutes of Health (Northeast Biodefense Center U54-AI057158-Lipkin).
References
- Volterra A, Meldolesi J. Astrocytes, from brain glue to communication elements: the revolution continues. Nat Rev Neurosci. 2005;6:626–640. doi: 10.1038/nrn1722. [DOI] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Carpentier P A, Williams B R, Miller S D. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia. 2007;55:239–252. doi: 10.1002/glia.20450. [DOI] [PubMed] [Google Scholar]
- Town T, Jeng D, Alexopoulou L, Tan J, Flavell R A. Microglia recognize double-stranded RNA via TLR3. J Immunol. 2006;176:3804–3812. doi: 10.4049/jimmunol.176.6.3804. [DOI] [PubMed] [Google Scholar]
- Scumpia P O, Kelly K M, Reeves W H, Stevens B R. Double-stranded RNA signals antiviral and inflammatory programs and dysfunctional glutamate transport in TLR3-expressing astrocytes. Glia. 2005;52:153–162. doi: 10.1002/glia.20234. [DOI] [PubMed] [Google Scholar]
- Park C, Lee S, Cho I H, Lee H K, Kim D, Choi S Y, Oh S B, Park K, Kim J S, Lee S J. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–256. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt A C, Medzhitov R, Flavell R A. Recognition of double-stranded RNA and activation of NF-κB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii K J, Yamaguchi O, Otsu K, Tsujimura T, Koh C S, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Takeuchi O, Akira S. MDA5/RIG-I and virus recognition. Curr Opin Immunol. 2008;20:17–22. doi: 10.1016/j.coi.2008.01.002. [DOI] [PubMed] [Google Scholar]
- Kawai T, Takahashi K, Sato S, Coban C, Kumar H, Kato H, Ishii K J, Takeuchi O, Akira S. IPS-1, an adaptor triggering RIG-I- and Mda5-mediated type I interferon induction. Nat Immunol. 2005;6:981–988. doi: 10.1038/ni1243. [DOI] [PubMed] [Google Scholar]
- Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6:644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- Giulian D, Li J, Bartel S, Broker J, Li X, Kirkpatrick J B. Cell surface morphology identifies microglia as a distinct class of mononuclear phagocyte. J Neurosci. 1995;15:7712–7726. doi: 10.1523/JNEUROSCI.15-11-07712.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederberger E, Geisslinger G. The IKK-NF-κB pathway: a source for novel molecular drug targets in pain therapy? FASEB J. 2008;22:3432–3442. doi: 10.1096/fj.08-109355. [DOI] [PubMed] [Google Scholar]
- Yoneyama M, Kikuchi M, Natsukawa T, Shinobu N, Imaizumi T, Miyagishi M, Taira K, Akira S, Fujita T. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Kawai T, Kumar H, Sato S, Yonehara S, Akira S. Roles of caspase-8 and caspase-10 in innate immune responses to double-stranded RNA. J Immunol. 2006;176:4520–4524. doi: 10.4049/jimmunol.176.8.4520. [DOI] [PubMed] [Google Scholar]
- Wang T, Town T, Alexopoulou L, Anderson J F, Fikrig E, Flavell R A. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366–1373. doi: 10.1038/nm1140. [DOI] [PubMed] [Google Scholar]
- Doyle S, Vaidya S, O'Connell R, Dadgostar H, Dempsey P, Wu T, Rao G, Sun R, Haberland M, Modlin R, Cheng G. IRF3 mediates a TLR3/TLR4-specific antiviral gene program. Immunity. 2002;17:251–263. doi: 10.1016/s1074-7613(02)00390-4. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Mikamo-Satoh E, Hirai R, Kawai T, Matsushita K, Hiiragi A, Dermody T S, Fujita T, Akira S. Length-dependent recognition of double-stranded ribonucleic acids by retinoic acid-inducible gene-I and melanoma differentiation-associated gene 5. J Exp Med. 2008;205:1601–1610. doi: 10.1084/jem.20080091. [DOI] [PMC free article] [PubMed] [Google Scholar]