Abstract
Methylmalonic acidemia is an autosomal recessive inborn error of metabolism caused by defective activity of methylmalonyl-CoA mutase (MUT) that exhibits multiorgan system pathology. To examine whether mitochondrial dysfunction is a feature of this organic acidemia, a background-modified Mut-knockout mouse model was constructed and used to examine mitochondrial ultrastructure and respiratory chain function in the tissues that manifest pathology in humans. In parallel, the liver from a patient with mut methylmalonic acidemia was studied in a similar fashion. Megamitochondria formed early in life in the hepatocytes of the Mut−/− animals and progressively enlarged. Liver extracts prepared from the mutants at multiple time points displayed respiratory chain dysfunction, with diminished cytochrome c oxidase activity and reduced intracellular glutathione compared to control littermates. Over time, the exocrine pancreas and proximal tubules of the kidney also exhibited megamitochondria, and older mutant mice eventually developed tubulointerstitial renal disease. The patient liver displayed similar morphological and enzymatic findings as observed in the murine tissues. These murine and human studies establish that megamitochondria formation with respiratory chain dysfunction occur in a tissue-specific fashion in methylmalonic acidemia and suggest treatment approaches based on improving mitochondrial function and ameliorating the effects of oxidative stress.—Chandler, R. J., Zerfas, P. M., Shanske, S., Sloan, J., Hoffmann, V., DiMauro, S., Venditti, C. P. Mitochondrial dysfunction in mut methylmalonic acidemia.
Keywords: methylmalonyl-CoA mutase, cytochrome c oxidase, glutathione, oxidant stress, vitamin B12
Hereditary methylmalonic acidemias are a group of inborn errors of metabolism characterized by deficient activity of the mitochondrial matrix enzyme, methylmalonyl-CoA mutase (MUT) (1). These disorders are caused by mutations in the methylmalonyl-CoA mutase apoenzyme or by impaired synthesis of the enzymatic cofactor, 5′deoxyadenosylcobalamin (2). Patients with mutations in the MUT gene typically have severe disease and demonstrate poor outcomes, with early mortality and substantial lifelong morbidity (3,4,5,6,7,8,9). Those affected exhibit multisystemic manifestations, such as “metabolic strokes” of the basal ganglia (10, 11), a propensity to develop pancreatitis (12), progressive renal insufficiency (13), and hepatomegaly, suggestive of underlying liver disease (14, 15). The mechanisms underlying these symptoms are poorly understood, both clinically and pathologically.
Deficient energy metabolism has long been suspected to play a role in methylmalonic acidemia (14, 16). Early reports stressed the effects of widespread methylmalonyl-CoA accumulation in causing symptoms, particularly hypoglycemia (14) and hypothesized that decreased production of succinyl-CoA as a consequence of the enzymatic block might interfere with the function of the Krebs cycle. An inherent bioenergetic defect was also suggested by early clinical observations of unexplained severe lactic acidosis in affected patients (17). However, the only direct evidence for respiratory chain (RC) dysfunction in methylmalonic acidemia has come from the studies of Hayasaka et al. (18), who noted that postmortem liver extracts from a single patient with methylmalonic acidemia and from two patients with propionic acidemia had markedly diminished cytochrome c oxidase (COX) activity compared to control liver samples.
The inhibitory vitamin B12 analog, hydroxy-cobalamin[c-lactam] (HCCL), has been an important tool to understand the effects of perturbed propionyl- and methylmalonyl-CoA metabolism in rat hepatocytes (19,20,21,22,23). After several weeks of continuous subcutaneous infusion with HCCL, rats developed methylmalonic aciduria (19) and decreased activities of complexes I, III, and IV in liver extracts (22). However, other in vitro studies to examine the mitochondrial toxicity of methylmalonic acid (MMA) have not unequivocally proven that chemically induced mitochondrial dysfunction was a pathogenic mechanism in methylmalonic acidemia. Without exception, these studies have relied on exogenous administration of MMA to a variety of normal tissues and extracts, including rat brain (24), mouse muscle (25), and bovine heart (26). While some have reported varying effects on complexes I–IV (24), carefully executed single-chain assays have demonstrated that MMA itself has no direct effect on the RC and suggested that secondary metabolites, such as 2-methylcitrate and malonic acid, cause the metabolic dysfunction seen in this disorder (25). Whether any of these mechanisms operate in vivo is uncertain because neither methylmalonyl-CoA knockout mice nor methylmalonic acidemia patient material were used in these studies.
To gain insight into the pathophysiology of methylmalonic acidemia, and more specifically to examine mitochondrial dysfunction as a putative disease mechanism, we created a modified Mut−/− mouse model (27) by introducing genes from the FVB/N strain into the (C57BL/6×129Sv/Ev) Mut+/− strain and intercrossing the carrier progeny. Mut−/− mice on the (C57BL/6×129Sv/Ev) background uniformly perish within the first days of life. However, a small fraction of the triply mixed [(C57BL/6×129Sv/Ev) × FVB/N] G2 Mut−/− animals survived beyond the neonatal period and were used to examine mitochondrial function. Older mutants were also allowed to age, so that renal pathology (13, 14), not previously observed in animal models of methylmalonic acidemia (27, 28), might manifest. Studies conducted in parallel on a liver specimen from a mut methylmalonic acidemia patient showed morphological and enzymatic changes similar to those seen in the animals, unifying observations between species and highlighting the role of mitochondrial dysfunction in this organic acidemia. Taken together, the murine and human investigations link the energy defect to tissue-specific manifestations in methylmalonic acidemia, support the existence of modifier loci in mice, and suggest new treatment approaches based on improving mitochondrial function and ameliorating the effects of oxidative stress.
MATERIALS AND METHODS
Clinical studies
Patient studies were conducted in compliance with the Helsinki Declaration and were approved by the National Human Genome Research Institute Institutional Review Board as part of U.S. National Institutes of Health (NIH) study 04-HG-0127, Clinical and Basic Investigations of Methylmalonic Acidemia and Related Disorders, after informed consent was obtained.
The affected liver tissue used in these experiments was derived from the discarded liver of a 5-yr-old boy with mut methylmalonic acidemia, who underwent a combined renal and hepatic transplant procedure (29). The patient presented with hyperammonemia and metabolic crisis coinciding with extreme MMA elevations on the second day of life. Complementation and [1-14C] propionate incorporation studies in skin fibroblasts indicated a cobalamin nonresponsive, or muto lesion and sequencing of the MUT gene revealed two early nonsense mutations, p.E224X and p.R228X. Control human liver samples (n=5) were obtained from adults who had undergone liver resection for other reasons and then donated their liver tissues to the University of Pittsburgh cell isolation facility (Pittsburgh, PA, USA), established as part of an NIH-funded liver tissue procurement and distribution system.
Murine model
The construction and analysis of the murine methylmalonyl-CoA mutase-deficient animals has been described (27). The null allele produces no Mut mRNA or protein and generates a neonatal lethal phenotype in the homozygote state on the (C57BL/6×129 Sv/Ev) background. (C57BL/6×129 Sv/Ev) Mut+/− carriers were intercrossed for 4 generations, rederived onto C57BL/6, and maintained by random carrier mating for more than 10 generations. The resulting (C57BL/6×129 Sv/Ev) Mut−/− progeny consistently displayed neonatal lethality and have been extensively characterized (27). Triply mixed Mut−/− animals were constructed by crossing (C57BL/6×129 Sv/Ev) Mut+/− mice to the FVB/N strain and then intercrossing the [(C57BL/6×129 Sv/Ev) × FVB/N] F1 Mut+/− offspring to generate [(C57BL/6×129 Sv/Ev) × FVB/N] G2 Mut−/− animals. The National Human Genome Research Institute Animal Care and Use Committee approved the animal experiments. For all murine studies, age- and diet-matched unaffected littermates were sacrificed in parallel with affected mice. Aside from severe growth retardation, the mutants were not in distress or obviously ill when they were euthanized.
Histology and electron microscopy
Samples were fixed in formalin, embedded, and processed for routine histology. For electron microscopy, the tissues were fixed in 2% glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, overnight at 4°C, washed with cacodylate buffer, and then postfixed with 1% OsO4 for 2 h. The tissues were washed again with 0.1 M cacodylate buffer, serially dehydrated in ethanol and propylene oxide, and embedded in Eponate 12 resin (Ted Pella, Redding, CA, USA). Thin sections (∼80 nm) were obtained with the Leica ultracut-UCT ultramicrotome (Leica, Deerfield, IL, USA), placed onto 400-mesh copper grids, and stained with saturated uranyl acetate in 50% methanol and then with lead citrate. The grids were viewed in a Philips 410 electron microscope (FEI, Hillsboro, OR, USA) at 80 kV, and images were recorded on Kodak SO-163 film (Eastman Kodak, Rochester, NY, USA).
Respiratory chain assays
Biochemical analysis of respiratory chain enzyme activities and citrate synthase in liver, muscle, and kidney homogenates was performed as described previously (30).
Metabolite analysis
Blood samples were immediately centrifuged, the plasma removed, diluted in water, and stored at −80°C in screw-top tubes for later analysis. Plasma was analyzed by gas chromatography-mass spectrometry with stable isotopic internal calibration to measure MMA and 2-methylcitrate isomers I and II (31). Plasma amino acid analysis used nihydrin conjugation followed by HPLC detection, and acylcarnitine esters were quantitated using tandem mass spectrometry.
Western blot analysis
Tissue samples were homogenized in a 2-ml Tenbroeck tissue grinder (Wheaton Science Products, Millville, NJ, USA) with T-Per® (Pierce Biotechnology, Rockford, IL, USA) tissue protein extraction buffer in the presence of a Halt™ (Pierce Biotechnology) protease inhibitor cocktail. Aliquots (20–40 μg) of clarified extracts were analyzed by immunoblotting and probed with either antisuperoxide dismutase 2 (Abcam, Cambridge, MA, USA) or with anti-OxPhos Complex III core 2 subunit (Invitrogen, Carlsbad, CA, USA) as a loading control. HRP-conjugated goat anti-rabbit IgG (NA934VS; Amersham Biosciences, Piscataway, NJ, USA) or rabbit anti-goat IgG (sc-2768; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were used as a secondary antibodies and were visualized with chemiluminescent detection (Pierce Biotechnology).
Quantitative real-time polymerase chain reaction (PCR)
To examine Mn-SOD up-regulation, TaqMan Gene Expression Assays (Applied Biosystems Mouse GAPD 4352932E, Mn-SOD Mm00449726_m1 and Citrate Synthase Mm00466043_m1) were analyzed in an Applied Biosystems 7500 Fast Real-Time PCR System following the manufacturers’ instructions (Applied Biosystems, Foster City, CA, USA) (32). All assays were carried out in triplicate and included RNA without added reverse transcriptase as a negative control. Total liver RNA from wild-type mice was used to determine the 100% wild-type mRNA expression. For each cDNA sample, total mRNA not subjected to reverse transcription was used as a negative control for background noise and was subtracted from the resultant CT value of the cDNA sample.
For quantitative PCR (qPCR) to determine mitochondrial DNA (mtDNA) copy number, we used the TaqMan assay, probes for mtDNA and nuclear DNA, and the 7000 IBI PRISM detection system, all from Applied Biosystems (33, 34). All assays were carried out in triplicate, and DNA quantitation was based on a standard curve obtained using between 1–750 ng of template.
Glutathione measurements
Murine liver samples were snap-frozen prior to the study, and 50- to 100-mg aliquots were homogenized in 10 vol of 50 mM phosphate buffer, pH 7.4. The homogenates were spun at 2000 g for 10 min to eliminate debris, and the supernatant was transferred to a clean tube. This homogenate was used to measure the concentration of reduced glutathione (GSH) and oxidized glutathione (GSSG) by an enzymatic recycling method using the GSH Assay kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturers’ instructions. The protein concentration of each homogenate was determined using the Bradford method.
Statistical analysis
Kaplan-Meier survival statistics were used to compare the mutant groups with different genetic backgrounds. The primary null hypothesis was that the two survival curves were identical and was tested with a log rank statistic using SAS software version 9 (SAS Institute, Cary, NC, USA). The end point for the survival analysis was defined as survival to day 21 of life. A paired Student’s t test was used to assess the significance of the differences between the plasma MMA, amino acid, carnitine, and individual acylcarnitine concentrations and respiratory enzyme chain activities between control and mutant mice. Values of P < 0.05 were considered significant.
RESULTS
Background modification
An intercross between F1 [(C57BL/6×129Sv/Ev) × FVB/N] Mut+/− carriers yielded Mut+/+, Mut+/−, and Mut−/− mice in a 1:2:1 ratio. A small fraction of the affected G2 [(C57BL/6×129Sv/Ev) × FVB/N] Mut−/− mice survived postweaning. Table 1 summarizes the Mut−/− survival of 7 litters from the parental (C57BL/6×129Sv/Ev) Mut−/− mice compared to 19 litters from the triply mixed G2 [(C57BL/6×129Sv/Ev) × FVB/N] animals. A Kaplan Meier statistic (Table 1) comparing the survival of the Mut−/− animals between the two strains was highly significant (P<0.0001). An extended survival curve from 46 G2 [(C57BL/6×129Sv/Ev) × FVB/N] Mut−/− animals derived from 40 litters is depicted in Supplemental Fig. 1. Knockout G2 [(C57BL/6×129Sv/Ev) × FVB/N] Mut−/− animals that survived showed early and significant growth retardation (P<0.01 at all time points) that persisted throughout life, achieving on average only 66, 67, 69, and 51% of age-matched littermate weight on day of life 6, 14, 18, and 230, respectively (Supplemental Table 1). Surviving mutants had elevations of MMA in the plasma (517±83 μM; n=4) vs. controls (6.9±0.4 μM; n=12). Full urine organic acid profiles were not obtained because urine could not be reliably collected without contaminating stool, but spot measurements in one ill Mut−/− animal showed a concentration greater than 80 mM, comparable to what has been documented in neonatal affected animals (27). Examination of other plasma metabolites (Supplemental Table 2) in the older mutant animals compared to age and diet-matched controls showed decreased free carnitine, lower valine, isoleucine, and ornithine, and elevated propionylcarnitine and alanine. Neither methylmalonyl-CoA mutase mRNA nor immunoreactive enzyme was detected in the surviving mutants (Supplemental Fig. 1). Tissues were obtained from affected and control littermates at various time points for electron micrographic, histological, and enzymatic studies.
TABLE 1.
Summary of survival statistics of Mut−/− mice on C57BL/6×129Sv/Ev compared to [(C57BL/6×129 Sv/Ev) × FVB/N] background
| Variable | (C57BL/6× 129Sv/Ev) Mut−/− | G2[(BL6×129)× FVB/N] Mut−/− |
|---|---|---|
| Litters | 7 | 19 |
| Animals | 73 | 210 |
| Mut−/− survivors at DOL 21 (number born) | 0 (17) | 16 (46) |
| Significance vs background | N.A. | P < 0.0001 |
P value denotes significance of difference in survival between the two Mut−/− groups, calculated by a Kaplan Meier statistic. DOL, day of life; N.A., not applicable.
Light and electron microscopic studies in the Mut−/− mice
Hepatic histology
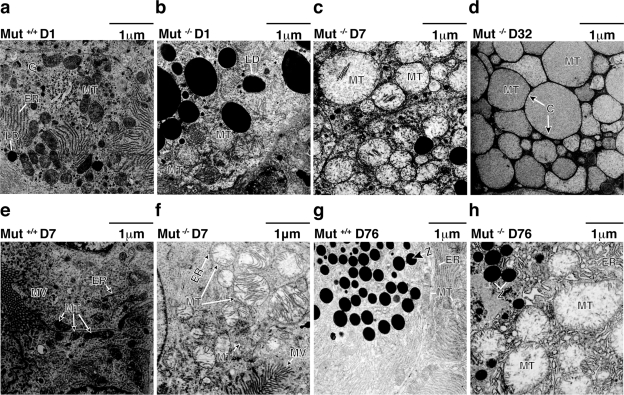
Routine histological evaluation of the early neonatal lethal animals showed hepatic lipidosis, which has also been reported in another mouse model of Mut methylmalonic acidemia (28). However, eosinophilic vesicles in the cytoplasm of hepatocytes from older mice were observed and were not entirely consistent in appearance with lipid accumulation (see Supplemental Fig. 3a), which normally washes out during fixation. Therefore, we resorted to electron microscopy to further investigate these findings. Electron micrographs from wild-type mice were studied in parallel with age-matched Mut−/− mutant littermates. As shown in Fig. 1a, a 1-day-old control animal had normally shaped mitochondria, glycogen, small lipid droplets, and abundant rough endoplasmic reticulum. However, the age-matched Mut−/− littermate (Fig. 1b) displayed a large increase in the number and size of the lipid droplets and slightly enlarged mitochondria, some of which retained intact ultrastructural features. Oil-red O staining confirmed hepatic lipidosis in the neonatal mutants (data not presented). As time progressed, the hepatic mitochondria in the mutant mice enlarged and started showing dysmorphic cristae, intramitochondrial lamellar inclusion body formation, and a less electron-dense mitochondrial matrix (Fig. 1c, d). Because of their increased size, these mitochondria are called megamitochondria (35). Megamitochondria were apparent in the livers of all Mut−/− knockout mice so studied but absent in the livers from the controls. By day 32 of life (Fig. 1d), the hepatic Mut−/− mitochondria were markedly enlarged and had shortened cristae, barely visible at the borders of the mitochondria (labeled C in Fig. 1d). In addition, the mitochondria were massively enlarged and occupied most of the cytoplasm, obscuring other prominent organelles, such as rough endoplasmic reticulum.
Figure 1.
a–d) Electron microscopy of liver samples from a control animal (a) and Mut−/− knockout mice sacrificed on day 1 (b), day 7 (c), or day 32 (d). Many cellular elements can be seen in control mouse liver (a), including golgi network, endoplasmic reticulum, lipid droplets, and mitochondria. On day 1, mutant liver shows enlarged mitochondria and significantly increased lipid droplets (b). Changes progress, and by day 7 (c), mitochondria are much larger and pale, with diminished cristae. Lipid is present but is much less prominent than on day 1. By day 32 (d), mitochondria still retain a characteristic double membrane but are enormous, and cristae are barely visible at the periphery. e, f) Electron microscopy of kidney samples from a Mut+/+ control animal (e) and Mut−/− knockout littermate sacrificed on day 7 (f). Both pictures are from proximal tubule. Microvilli near brush border are present. Mitochondria in mutant are enlarged and have disorganized cristae. g, h) Electron microscopy of pancreas samples from a Mut+/+ control animal (g) and Mut−/− knockout littermate sacrificed on day 76 (h). Both pictures are from exocrine pancreas. Mitochondria in mutant are enlarged and display a pale matrix. Number of zymogen granules is less in the mutant than in the control, and endoplasmic reticulum is disorganized. C, cristae; ER, endoplasmic reticulum; LD, lipid droplets; MT, mitochondria; MV, microvilli; Z, zymogen. All views ×11,000. Scale bars =1 μm.
The ultrastructural studies led to the conclusion that the swollen hepatocytic cytosol seen on light microscopy was mostly due to the enlargement of mitochondria and not the result of lipid accumulation, which does occur at earlier time points but becomes less prominent as the animals age. Cardiac and skeletal muscle from the Mut−/− mice examined by EM at early and late time points showed no mitochondrial abnormalities (data not presented), indicating that morphological and—possibly—functional changes were tissue-specific and not global in nature. This led us to examine other tissues with pathological findings seen in patients with methylmalonic acidemia, specifically the kidney and pancreas.
Renal histology
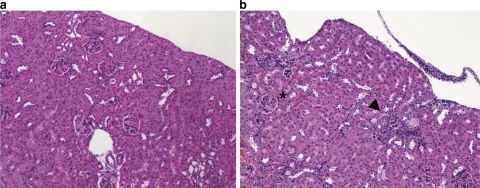
Electron micrographs from the kidneys of all 6 wild-type mice, examined at the same time points as the animals above, showed normal histology and, in particular, morphologically intact mitochondria (Fig. 1e). As we had observed in the mutant hepatocytes, megamitochondria with underdeveloped cristae and peculiar lamellations were also present in the proximal tubular cells of the kidney, beginning on day 7 in the Mut−/− mice (Fig. 1f) and were not observed in younger mice. Mitochondrial changes in the kidney progressed over time, but only the proximal tubular cells were affected at any time in the mutants, indicating that the process affected specific cells within the complex cytoarchitecture of the kidney. The oldest mutant survivors lived slightly longer than 1 yr (n=3) and displayed severe and widespread tubulointerstitial changes (Fig. 2b), similar to the tubulointerstital nephritic changes seen in patients with methylmalonic acidemia patients and renal insufficiency (13, 14).
Figure 2.
Histology (hematoxylin and eosin stain) of kidney from a Mut+/− control animal (a) and a [(C57BL/6×129 Sv/Ev) × FVB/N] Mut−/− knockout littermate (b) sacrificed at 13 mo of age. In mutant (b), tubulointerstitial inflammation can be appreciated near arrow, and kidney outline is distorted. Some glomeruli (indicated by asterisk) are normal in mutant.
Pancreas histology
Because individuals with methylmalonic acidemia can develop pancreatitis (12), we performed electron microscopy on the pancreas from Mut−/− and wild-type mice. Mitochondrial changes were not apparent in the pancreas of the Mut−/− mice until later in life. The pancreas from a 76-day-old wild-type animal is shown in Fig. 1g. However, a mutant littermate of the same age (Fig. 1h) showed megamitochondria and greatly reduced numbers of zymogen granules in the exocrine pancreas. In addition, insulin granules also appeared to be decreased in the small number of beta cells visualized in the mutants (data not presented). These alterations were not seen in the age-matched wild-type littermate (Fig. 1g). Finally, similar studies on liver, renal, and pancreatic tissue samples from Mut+/− animals (not presented) had a normal ultrastructural appearance as observed in wild-type mice.
Mitochondrial studies
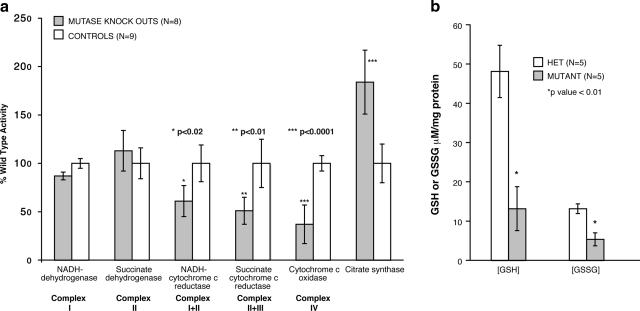
To determine whether the morphological changes seen in the mitochondria correlated with mitochondrial function, RC enzyme activities were measured in liver, kidney, and skeletal muscle from mutants and from age- and diet-matched control littermates. Hepatic extracts from mutants (n=8) and controls (n=9) from four different litters were studied individually and then used for comparisons. For each respiratory chain measurement in the two groups, we calculated average and sd; then we assessed P values using a Student’s t test (2 sides, 2 tails) to examine the differences between mutant and controls. Consistent with the ultrastructural studies demonstrating enlarged mitochondria and suggesting increased mitochondrial mass, the mutant hepatic samples all showed significant increases of citrate synthase activities (P<0.0001) compared to control mice (Fig. 3a). The activities of the other complexes of the respiratory chain were variably affected. The linked assays of complex I + III and complex II + III activities were significantly reduced in the mutants (P<0.02). However, the most pronounced change affected the activity of COX (complex IV), which was decreased more than 50% in the Mut−/− mice (P<0.0001). A smaller number of mutant and matched control skeletal muscle (Mut−/−=3, controls=3) and whole kidney (Mut−/−=3, controls=3) extracts were studied in the same fashion and did not show any differences between the groups (data not presented). Mut−/− mice exhibited abnormal mitochondria localized to the proximal tubules of the kidney, which could not be isolated for RC assay and could account for our inability to detect changes in RC activity. Finally, we found comparable abundance of mtDNA copy numbers in a subset of liver samples, thus excluding the possibility that the decreased RC enzyme activities in the Mut−/− mice could be due to an mtDNA depletion syndrome.
Figure 3.
a) Respiratory chain activity in murine liver extracts. Eight mutants from 4 different litters ranging from 10 to 30 days were studied with 9 age- and diet-matched littermates. Error bars surround the 95% confidence interval. Specific enzyme activities were measured as described in text and normalized to controls. Mut−/− knockout mice show diminished activities of complex III and complex IV and increased citrate synthase. *P < 0.02; **P < 0.01; ***P < 0.0001. b) GSH and GSSG concentrations in liver extracts in mutant compared to heterozygote controls. *P < 0.01.
Oxidative stress
The severe morphological changes and RC enzyme deficiencies in the mutant animals prompted us to examine hepatic glutathione stores, especially since symptomatic glutathione deficiency has been described in patients with methylmalonic acidemia (36). The concentrations of GSH and GSSG were measured in liver extracts from age- and diet-matched mutants (n=5) and heterozygous littermates (n=5) from two different litters and normalized to total protein content. RC assays of these liver samples had shown increased citrate synthase and markedly impaired COX activities in the mutants, as described above. As seen in Fig. 3b, the mutant liver extracts contain significantly lower concentrations of both GSH and GSSG (P<0.01 for each) than controls, indicating that glutathione is indeed depleted in the liver and suggesting that oxidant injury might contribute to the pathophysiology of the liver involvement in the mice.
We then examined the mitochondrial enzyme Mn-SOD by Western blot analysis and qPCR to determine whether this oxidant stress pathway might be activated in response to diminished hepatic glutathione stores. Western blot analysis using murine anti-MnSOD antibodies yielded results suggestive of increased MnSOD in some older animals (see Supplemental Fig. 3b). However, the results of qPCR experiments with mutant and control liver RNAs to examine Mn-SOD up-regulation were inconclusive (data not presented).
Methylmalonic acidemia patient studies
We obtained liver samples near the time of organ removal from a 5-yr-old mut methylmalonic acidemia patient, who underwent elective combined liver-kidney transplantation and used them for ultrastructural and enzymatic studies. The patient was clinically stable at the time of surgery. Previous investigations had demonstrated that liver extracts from this patient were completely devoid of immunoreactive methylmalonyl-CoA mutase protein (29), consistent with the genotype data showing two early nonsense mutations (pE224X and pR228X) in the MUT gene. A set of 5 unrelated control liver samples obtained from anonymous adult liver donors was processed and studied in an identical fashion and used to generate a range of control values for respiratory chain enzymes.
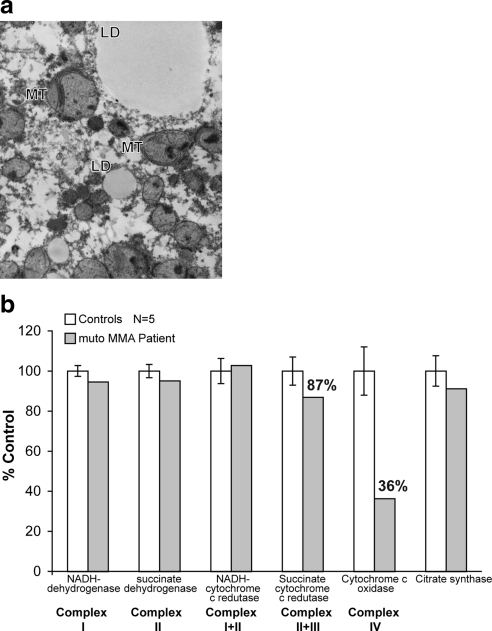
Routine histology of the mut methylmalonic acidemia liver revealed nonspecific microvesicular and macrovesicular steatosis (data not presented). However, electron microscopy demonstrated widespread mitochondrial changes (Fig. 4a). All hepatocytes contained megamitochondria, many of which had inclusions with abnormal membranous lamellation, very similar to what was observed in the murine Mut−/− tissues. Electron microscopic studies from 3 unrelated control liver samples showed neither mitochondrial nor any other morphological change seen in the mut patient liver. Hepatic extracts from the patient and liver donor samples (n=5) were prepared and used for respiratory chain activity assays: control ranges were established from the donor samples and used for comparative purposes. As shown in Fig. 4b, the most pronounced change was a greater than 40% decrease in the activity of COX (complex IV) in the mut methylmalonic acidemia liver compared to controls processed in an identical fashion. The linked complex II + III activity was slightly reduced, but the other complexes were within the control ranges. Thus, the pattern in the patient liver is that of COX deficiency. In the mut human liver—as in the Mut −/− murine liver—assessment of mtDNA copy number showed no differences between patient and controls, again excluding mtDNA depletion as causative of the biochemical phenotype.
Figure 4.
a) Electron microscopy of a mut methylmalonic acidemia patient liver (×10,000). Lipid droplets (LD) and mitochondria (MT) are enlarged, have a pale matrix, and some have lamellar inclusions. b) Respiratory chain activity in a mut methylmalonic acidemia patient liver extract compared to controls (n=5). Specific enzyme activities were measured as described in text and normalized to controls. The mut methylmalonic acidemia patient liver shows diminished activities of complex IV and possibly complex II + III compared to controls.
DISCUSSION
We present several lines of experimental evidence that demonstrate mitochondrial dysfunction is a pathological characteristic of methylmalonic acidemia. The generation of triply mixed [(C57BL/6×129Sv/Ev) × FVB/N] Mut −/− mice with increased survival compared to the (C57BL/6×129 Sv/Ev) parental strain allowed us to generate knockout animals that could survive past weaning, and rarely, beyond (Supplemental Fig. 1). Although the biochemical basis of this effect is still unknown, it is certainly not related to the expression of the Mut enzyme from a different locus, since the surviving mutant mice produce neither Mut RNA nor protein and display massively elevated levels of circulating metabolites. Strain effects, specifically those able to modify a neonatal lethal murine mitochondrial phenotype, have been previously described (37) and generally are well recognized in mouse genetic studies (38). Of particular relevance to this report is that mitochondrial transcription factor A (Tfam)-knockout mice had significantly increased neonatal survival after genes from the FVB strain were introduced (37). The increased survival of both Mut and Tfam mutant mice appears to depend on background effects and suggests that the introduction of genes from this strain might extend survival of other mouse models of metabolic disorders. In the future, genetic analysis, mapping, and subsequent positional cloning of Mut−/− survival modifiers might yield important insight into the pathophysiology of methylmalonic acidemia and other organic acidemias.
Investigations into mitochondrial changes began with the histological observations of eosinophilic inclusions in the hepatocytes in older Mut−/− mice (Supplemental Fig. 3a), which ultrastructural studies later determined to be megamitochondria (35). The affected animals, other than displaying growth retardation, were otherwise not overtly intoxicated, suggesting that the observed enzymatic and microscopic changes were not secondary to acute illness. Electron microscopic analysis was extended to other tissues in the Mut−/− mice, especially to those organs that are affected in patients, and established that megamitochondria were present in the proximal tubular cells of the kidney and in the pancreas but not in other high-energy tissues such as the heart or skeletal muscle. The changes in the tissues appeared to be morphologically progressive and identifying markers that correlate with these changes will be the subject of future investigations. Attempts to remove and study the mouse lentiform nuclei, structures that correspond to the basal ganglia in humans, were unsuccessful. Other than a single necropsy of an older mutant that revealed an increased BUN compared to reference values (data not presented), the very small number of mutants (n=3) that survived long term prevented more detailed studies on renal function but illustrates the potential that murine models will be useful to study methylmalonic acidemia-related kidney disease. When generated, transgenic and/or partial deficiency Mut mice might be ideally suited to further examine renal pathophysiology, especially if such models were inducible. The fact that mitochondrial changes similar to those seen in various murine tissues were seen in the liver of a patient with mut methylmalonic acidemia harvested during a state of relative health, but not in controls, further indicates that mitochondrial morphological and RC abnormalities are characteristic of certain cell types in methylmalonic acidemia.
To examine whether the ultrastructural morphological changes had functional correlates, we measured the main components of the respiratory chain. In these analyses, mutant and matched controls were studied, and the results were pooled because of the small numbers of mutant animals that could be obtained from each litter. Compared to controls, the murine liver extracts (Fig. 3a) showed severely decreased NADH-cytochrome c reductase (complex I+III), succinate cytochrome c reductase (complex II+III), and COX (complex IV) activities contrasting with increased activity of citrate synthase, a commonly used marker of mitochondrial “mass”. This biochemical pattern resembles that seen in hepatic mitochondria from hydroxycobalamin[c-lactam]-treated rats (22), chemical models of methylmalonic acidemia, where the activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) were markedly reduced after 5 wk of continuous infusion with the inhibitor. In addition, electron microscopy of the liver from such a treated rat revealed megamitochondria (23). Despite the different mechanisms used to produce methylmalonic acidemia in these rodent models (chemical inhibitor vs. genetic), the RC impairment is consistent and supports the hypothesis that the morphologically abnormal mitochondria seen in the Mut−/− hepatocytes harbor a functional oxidative phosphorylation defect, which will need to be further examined with respiratory measurements of O2 consumption and ATP production. The ultrastructural and enzymatic studies on the mut patient liver paralleled the murine findings, further proving that mitochondrial abnormalities and RC dysfunction are cardinal aspects of methylmalonic acidemia in humans as well as in rodents.
To further characterize the metabolic consequences of the mitochondrial dysfunction seen in methymalonic acidemia, the concentrations of reduced and oxidized glutathione were measured in liver extracts from mutant and control mice. The mutants had depressed GSH and GSSG levels in liver extracts. This is consistent with the symptomatic glutathionine deficiency previously observed in mut methymalonic acidemia (36) and with the documentation of low mitochondrial GSH levels in other well-characterized models of oxidant injury, such as the murine model of manganese superoxide dismutase deficiency (39). Increases in SOD2 (MnSOD) protein, another marker of mitochondrial oxidative stress, were also noted in some of the Mut−/− mice and support the previous observations that this enzyme is increased in cell lines from patients with methylmalonic acidemia (40). Because the RC accounts for most of the reactive oxygen species (ROS) generated in normal mitochondria (41), the functional impairment that we observed in the liver of both mice and humans is likely responsible for an increase in ROS. Oxidative stress is also known to cause a loss of mitochondria membrane potential (42) and can result in severe structural changes of the mitochondria (43), similar to what we observed in both mice and humans with methylmalonic acidemia. The precise mechanisms that might engender increased oxidant stress will be the subject of future study but reactive acryloyl-CoA species derived from propionyl-CoA, long hypothesized to form in methylmalonic and propionic acidemia (44), are obvious candidate molecules to consider in the pathogenesis of mitochondrial dysfunction and GSH depletion. We postulate that abnormal accretion of one or more of such reactive metabolites in the mitochondrial matrix results from the enzymatic block at the methylmalonyl-CoA mutase step and cause the RC dysfunction. The permeability and chemical properties of these compounds to the inner and outer mitochondrial membranes are not understood. If the transport of these metabolites out of the matrix does not match the rate of production, damage and eventual effects on mitochondrial morphology and function might ensue as a result of concentration dependent toxicity. That other intermediary pathways, such as ureagenesis, carnitine metabolism, gluconeogenesis, and Krebs cycle function, are perturbed is consistent with both patient observations and the finding of decreased ornithine, free carnitine, and increased alanine (Supplemental Table 2) in the mutant mice.
We have demonstrated that the liver, and more specifically the hepatocyte, is a major target of mitochondrial pathology in methylmalonic acidemia. This observation might explain the drastic improvement in metabolic stability observed in the patients who successfully undergo elective liver transplantation (45, 46), despite the fact that they demonstrate significant methylmalonic acidemia (27, 45, 47, 48). Liver-targeted gene delivery experiments and hepatocyte transplantation studies in the Mut murine model should be useful to assess the physiological consequences of organ-specific correction on this whole-body metabolic defect and to examine whether transduced Mut−/− hepatocytes or wild-type cells display in vivo growth advantages in a mutant background.
Supplementary Material
Acknowledgments
Patient participation and physician referral were critical to these studies. Cherry Yang (NIH) assisted with animal husbandry. Dr. Steven Strom and Kenneth Dorko (University of Pittsburgh School of Medicine, Pittsburgh, PA, USA) provided control human liver samples. R.J.C., J.S., and C.P.V. were supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, NIH. S.S. and S.D. were supported by grant HD32062 from the National Institute of Child Health and Development, NIH, and the Marriott Mitochondrial Disorder Clinical Research Fund.
References
- Fenton W A, Gravel R A, Rosenblatt D S. Disorders of propionate and methylmalonate metabolism. Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Kinzler K W, Vogelstein B, editors. New York: McGraw-Hill; The Metabolic and Molecular Bases of Inherited Disease. 2001:2165–2192. [Google Scholar]
- Fenton W A, Rosenblatt D S. Inherited disorders of folate and cobalamin transport and metabolism. Scriver C R, Beaudet A L, Sly W S, Valle D, Childs B, Kinzler K W, Vogelstein B, editors. New York: McGraw-Hill; The Metabolic and Molecular Bases of Inherited Disease. 2001:3897–3933. [Google Scholar]
- Matsui S M, Mahoney M J, Rosenberg L E. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308:857–861. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- Baumgarter E R, Viardot C. Long-term follow-up of 77 patients with isolated methylmalonic acidaemia. J Inherit Metab Dis. 1995;18:138–142. doi: 10.1007/BF00711749. [DOI] [PubMed] [Google Scholar]
- Van der Meer S B, Poggi F, Spada M, Bonnefont J P, Ogier H, Hubert P, Depondt E, Rapoport D, Rabier D, Charpentier C, Parvy P, Kamoun P, Saudubray J M. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr. 1994;125:903–908. doi: 10.1016/s0022-3476(05)82005-0. [DOI] [PubMed] [Google Scholar]
- Nicolaides P, Leonard J, Surtees R. Neurological outcome of methylmalonic acidaemia. Arch Dis Child. 1998;78:508–512. doi: 10.1136/adc.78.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Baulny H O, Benoist J F, Rigal O, Touati G, Rabier D, Saudubray J M. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28:415–423. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- Horster F, Baumgartner M R, Viardot C, Suormala T, Burgard P, Fowler B, Hoffmann G F, Garbade S F, Kolker S, Baumgartner E R. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB) Pediatric Res. 2007;62:225–230. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- Dionisi-Vici C, Deodato F, Roschinger W, Rhead W, Wilcken B. ‘Classical’ organic acidurias, propionic aciduria, methylmalonic aciduria and isovaleric aciduria: long-term outcome and effects of expanded newborn screening using tandem mass spectrometry. J Inherit Metab Dis. 2006;29:383–389. doi: 10.1007/s10545-006-0278-z. [DOI] [PubMed] [Google Scholar]
- Korf B, Wallman J K, Levy H L. Bilateral lucency of the globus pallidus complicating methylmalonic acidemia. Ann Neurol. 1986;20:364–366. doi: 10.1002/ana.410200317. [DOI] [PubMed] [Google Scholar]
- Heidenreich R, Natowicz M, Hainline B E, Berman P, Kelley R I, Hillman R E, Berry G T. Acute extrapyramidal syndrome in methylmalonic acidemia: “metabolic stroke” involving the globus pallidus. J Pediatr. 1988;113:1022–1027. doi: 10.1016/s0022-3476(88)80574-2. [DOI] [PubMed] [Google Scholar]
- Kahler S G, Sherwood W G, Woolf D, Lawless S T, Zaritsky A, Bonham J, Taylor C J, Clarke J T, Durie P, Leonard J V. Pancreatitis in patients with organic acidemias. J Pediatr. 1994;124:239–243. doi: 10.1016/s0022-3476(94)70311-6. [DOI] [PubMed] [Google Scholar]
- Walter J H, Michalski A, Wilson W M, Leonard J V, Barratt T M, Dillon M J. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr. 1989;148:344–348. doi: 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- Oberholzer V G, Levin B, Burgess E A, Young W F. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch Dis Child. 1967;42:492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow G, 3rd, Barness L A, Auerbach V H, DiGeorge A M, Ando T, Nyhan W L. Observations on the coexistence of methylmalonic acidemia and glycinemia. J Pediatr. 1969;74:680–690. doi: 10.1016/s0022-3476(69)80130-7. [DOI] [PubMed] [Google Scholar]
- Stokke O, Eldjarn L, Norum K R, Steen-Johnsen J, Halovorsen S. Methylmalonic acidemia: A newborn error of metabolism which may cause fatal acidosis in the neonatal period. Scand J Clin Lab Invest. 1967;20:313–328. [Google Scholar]
- Lindblad B, Lindblad B S, Olin P, Svanberg B, Zetterstrom R. Methylmalonic acidemia. A disorder associated with acidosis, hyperglycinemia, and hyperlactatemia. Acta Paediatr Scand. 1968;57:417–424. doi: 10.1111/j.1651-2227.1968.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Hayasaka K, Metoki K, Satoh T, Narisawa K, Tada K, Kawakami T, Matsuo N, Aoki T. Comparison of cytosolic and mitochondrial enzyme alterations in the livers of propionic or methylmalonic acidemia: a reduction of cytochrome oxidase activity. Tohoku J Exp Med. 1982;137:329–334. doi: 10.1620/tjem.137.329. [DOI] [PubMed] [Google Scholar]
- Stabler S P, Brass E P, Marcell P D, Allen R H. Inhibition of cobalamin-dependent enzymes by cobalamin analogues in rats. J Clin Invest. 1991;87:1422–1430. doi: 10.1172/JCI115148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl S, Ray D B, Stabler S P, Allen R H, Brass E P. Increased hepatic mitochondrial capacity in rats with hydroxy-cobalamin[c-lactam]-induced methylmalonic aciduria. J Clin Invest. 1990;86:2054–2061. doi: 10.1172/JCI114942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brass E P, Allen R H, Ruff L J, Stabler S P. Effect of hydroxycobalamin[c-lactam] on propionate and carnitine metabolism in the rat. Biochem J. 1990;266:809–815. [PMC free article] [PubMed] [Google Scholar]
- Krahenbuhl S, Chang M, Brass E P, Hoppel C L. Decreased activities of ubiquinol:ferricytochrome c oxidoreductase (complex III) and ferrocytochrome c:oxygen oxidoreductase (complex IV) in liver mitochondria from rats with hydroxycobalamin[c-lactam]-induced methylmalonic aciduria. J Biol Chem. 1991;266:20998–21003. [PubMed] [Google Scholar]
- Tandler B, Krahenbuhl S, Brass E P. Unusual mitochondria in the hepatocytes of rats treated with a vitamin B12 analogue. Anat Rec. 1991;231:1–6. doi: 10.1002/ar.1092310102. [DOI] [PubMed] [Google Scholar]
- Pettenuzzo L F, Ferreira Gda C, Schmidt A L, Dutra-Filho C S, Wyse A T, Wajner M. Differential inhibitory effects of methylmalonic acid on respiratory chain complex activities in rat tissues. Int J Dev Neurosci. 2006;24:45–52. doi: 10.1016/j.ijdevneu.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Kolker S, Schwab M, Horster F, Sauer S, Hinz A, Wolf N I, Mayatepek E, Hoffmann G F, Smeitink J A, Okun J G. Methylmalonic acid, a biochemical hallmark of methylmalonic acidurias but no inhibitor of mitochondrial respiratory chain. J Biol Chem. 2003;278:47388–47393. doi: 10.1074/jbc.M308861200. [DOI] [PubMed] [Google Scholar]
- Okun J G, Horster F, Farkas L M, Feyh P, Hinz A, Sauer S, Hoffmann G F, Unsicker K, Mayatepek E, Kolker S. Neurodegeneration in methylmalonic aciduria involves inhibition of complex II and the tricarboxylic acid cycle, and synergistically acting excitotoxicity. J Biol Chem. 2002;277:14674–14680. doi: 10.1074/jbc.M200997200. [DOI] [PubMed] [Google Scholar]
- Chandler R J, Sloan J, Fu H, Tsai M, Stabler S, Allen R, Kaestner K H, Kazazian H H, Venditti C P. Metabolic phenotype of methylmalonic acidemia in mice and humans: the role of skeletal muscle. BMC Med Genet. 2007;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters H, Nefedov M, Sarsero J, Pitt J, Fowler K J, Gazeas S, Kahler S G, Ioannou P A. A knock-out mouse model for methylmalonic aciduria resulting in neonatal lethality. J Biol Chem. 2003;278:52909–52913. doi: 10.1074/jbc.M310533200. [DOI] [PubMed] [Google Scholar]
- Chandler R J, Tsai M S, Dorko K, Sloan J, Korson M, Freeman R, Strom S, Venditti C P. Adenoviral-mediated correction of methylmalonyl-CoA mutase deficiency in murine fibroblasts and human hepatocytes. BMC Med Genet. 2007;8:24. doi: 10.1186/1471-2350-8-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S, Servidei S, Zeviani M, DiRocco M, DeVivo D C, DiDonato S, Uziel G, Berry K, Hoganson G, Johnsen S D, Johnson P C. Cytochrome c oxidase deficiency in Leigh syndrome. Ann Neurol. 1987;22:498–506. doi: 10.1002/ana.410220409. [DOI] [PubMed] [Google Scholar]
- Allen R H, Stabler S P, Savage D G, Lindenbaum J. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42:978–988. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- Chandler R J, Venditti C P. Adenovirus-mediated gene delivery rescues a neonatal lethal murine model of mut(0) methylmalonic acidemia. Hum Gene Ther. 2008;19:53–60. doi: 10.1089/hum.2007.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naini A, Shanske S. Detection of mutations in mtDNA. Methods Cell Biol. 2007;80:437–463. doi: 10.1016/S0091-679X(06)80022-1. [DOI] [PubMed] [Google Scholar]
- Spinazzola A, Zeviani M. Disorders of nuclear-mitochondrial intergenomic communication. Biosci Rep. 2007;27:39–51. doi: 10.1007/s10540-007-9036-1. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T. Megamitochondria formation—physiology and pathology. J Cell Mol Med. 2002;6:497–538. doi: 10.1111/j.1582-4934.2002.tb00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treacy E, Arbour L, Chessex P, Graham G, Kasprzak L, Casey K, Bell L, Mamer O, Scriver C R. Glutathione deficiency as a complication of methylmalonic acidemia: response to high doses of ascorbate. J Pediatr. 1996;129:445–448. doi: 10.1016/s0022-3476(96)70080-x. [DOI] [PubMed] [Google Scholar]
- Li H, Wang J, Wilhelmsson H, Hansson A, Thoren P, Duffy J, Rustin P, Larsson N G. Genetic modification of survival in tissue-specific knockout mice with mitochondrial cardiomyopathy. Proc Natl Acad Sci U S A. 2000;97:3467–3472. doi: 10.1073/pnas.97.7.3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau J H. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- Williams M D, Van Remmen H, Conrad C C, Huang T T, Epstein C J, Richardson A. Increased oxidative damage is correlated to altered mitochondrial function in heterozygous manganese superoxide dismutase knockout mice. J Biol Chem. 1998;273:28510–28515. doi: 10.1074/jbc.273.43.28510. [DOI] [PubMed] [Google Scholar]
- Richard E, Alvarez-Barrientos A, Perez B, Desviat L R, Ugarte M. Methylmalonic acidaemia leads to increased production of reactive oxygen species and induction of apoptosis through the mitochondrial/caspase pathway. J Pathol. 2007;213:453–461. doi: 10.1002/path.2248. [DOI] [PubMed] [Google Scholar]
- Chance B, Sies H, Boveris A. Hydroperoxide metabolism in mammalian organs. Physiol Rev. 1979;59:527–605. doi: 10.1152/physrev.1979.59.3.527. [DOI] [PubMed] [Google Scholar]
- Kokoszka J E, Coskun P, Esposito L A, Wallace D C. Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci U S A. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebovitz R M, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk M M. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci U S A. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ando T, Rasmussen K, Nyhan W L, Hull D. 3-hydroxypropionate: significance of -oxidation of propionate in patients with propionic acidemia and methylmalonic acidemia. Proc Natl Acad Sci U S A. 1972;69:2807–2811. doi: 10.1073/pnas.69.10.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van 't Hoff W G, Dixon M, Taylor J, Mistry P, Rolles K, Rees L, Leonard J V. Combined liver-kidney transplantation in methylmalonic acidemia. J Pediatr. 1998;132:1043–1044. doi: 10.1016/s0022-3476(98)70407-x. [DOI] [PubMed] [Google Scholar]
- Leonard J V, Walter J H, McKiernan P J. The management of organic acidaemias: the role of transplantation. J Inherit Metab Dis. 2001;24:309–311. doi: 10.1023/a:1010395724012. [DOI] [PubMed] [Google Scholar]
- Nagarajan S, Enns G M, Millan M T, Winter S, Sarwal M M. Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Dis. 2005;28:517–524. doi: 10.1007/s10545-005-0517-8. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Ficicioglu C, Mazur A T, Palmieri M J, Berry G T. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Molec Genet Metab. 2006;88:322–326. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.