Abstract
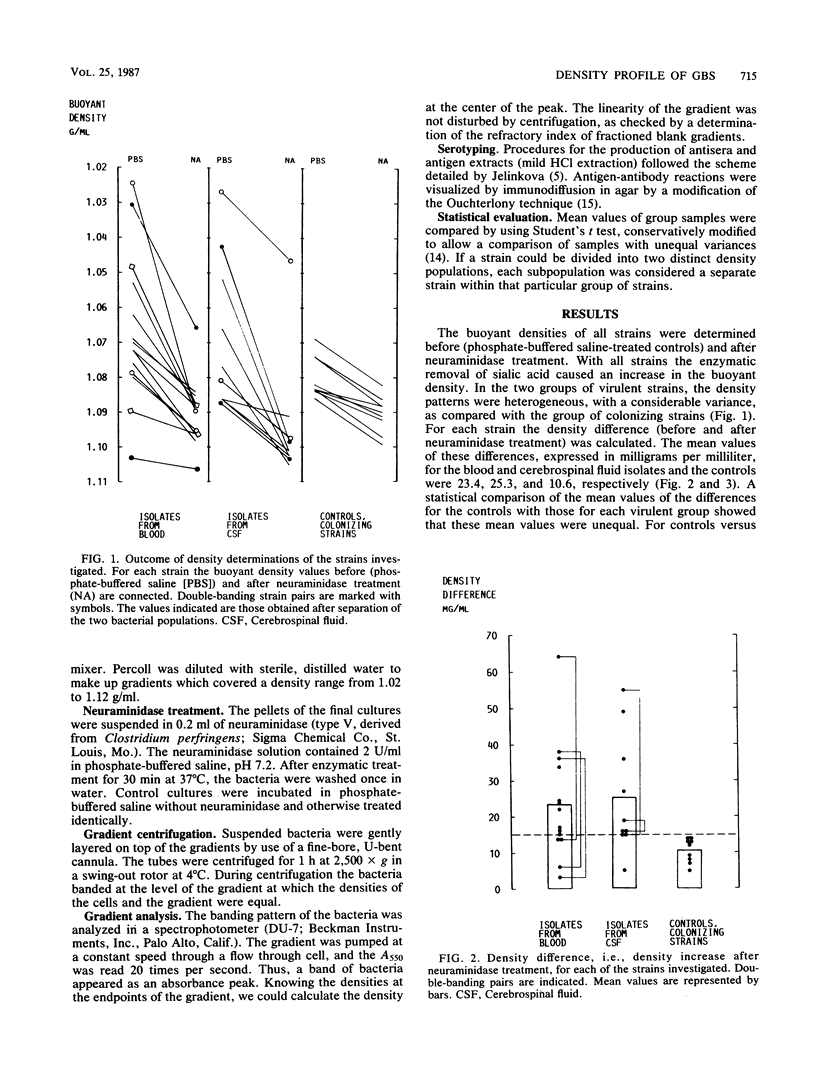
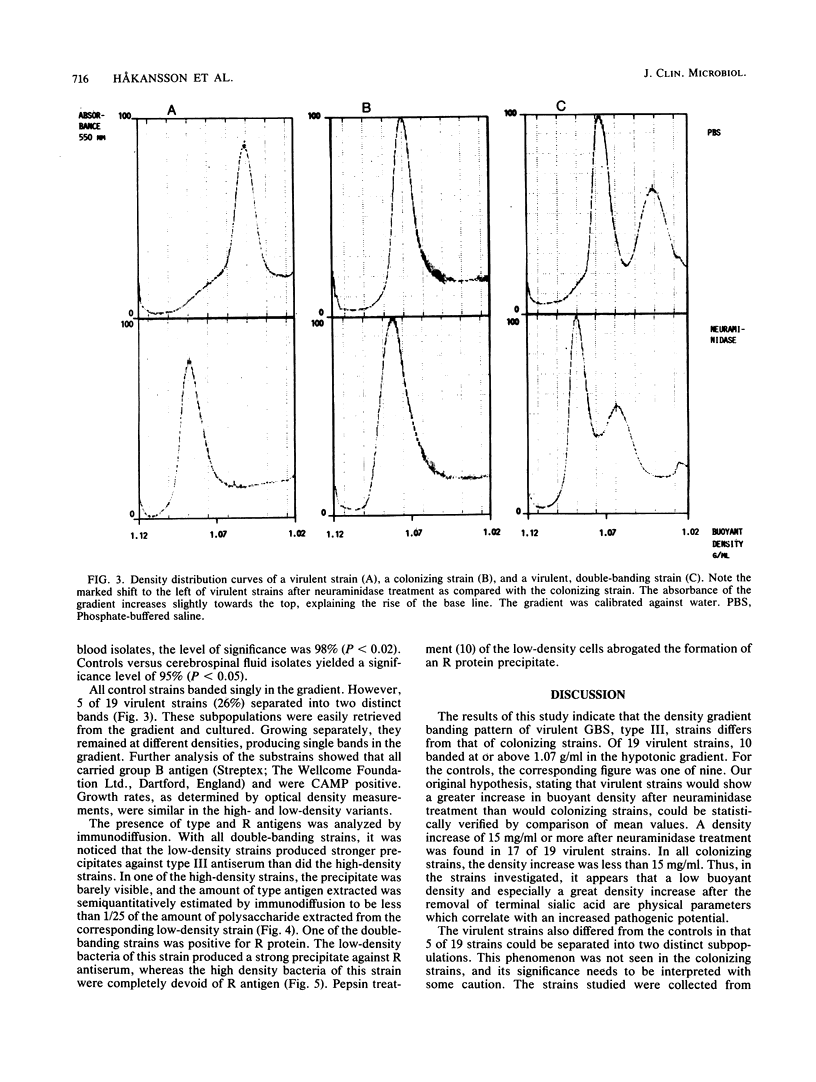
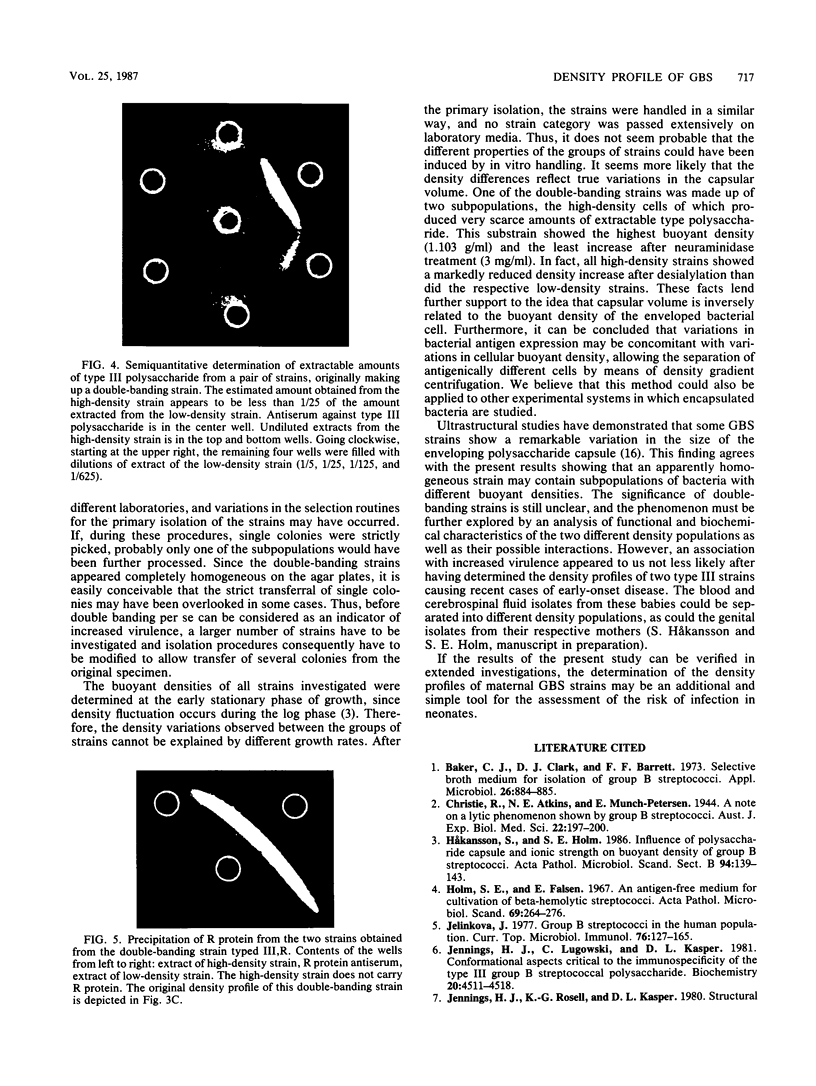
The buoyant densities of virulent and colonizing group B streptococci, type III, were determined by centrifugation of bacteria on a linear, hypotonic density gradient. A total of 28 strains were investigated. Eleven strains were obtained from blood cultures of babies with early-onset disease, and eight strains were isolated from the cerebrospinal fluid of babies with late-onset septicemia and meningitis. Nine colonizing strains were genital isolates from pregnant women subsequently giving birth to healthy children. In each strain the buoyant density was determined before and after neuraminidase treatment. All strains showed an increase in the buoyant density after enzymatic removal of sialic acid, and the density differences before and after desialylation were calculated. The mean values of these differences for blood, cerebrospinal fluid, and colonizing isolates were 23.4, 25.3, and 10.6 mg/ml, respectively. The mean value for the colonizing strains differed significantly from the mean value for each group of virulent strains. All colonizing strains banded singly in the gradient, whereas five of the virulent strains divided into two density populations. Extracts of the low-density cells produced markedly more dense immunoprecipitates with type antiserum than did extracts of the high-density bacteria. One double-banding strain was positive for R protein. After separation of the two density populations, this antigen was detected only in the low-density population. The results indicate that bacterial buoyant density is inversely related to the amount of capsular polysaccharide enveloping the cell and that a determination of the density profile of the bacteria may be used for discriminating strains with an increased pathogenic potential.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. J., Clark D. J., Barrett F. F. Selective broth medium for isolation of group B streptococci. Appl Microbiol. 1973 Dec;26(6):884–885. doi: 10.1128/am.26.6.884-885.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Håkansson S., Holm S. Influence of polysaccaride capsule and ionic strength on buoyant density of group B streptococci. Acta Pathol Microbiol Immunol Scand B. 1986 Jun;94(3):139–143. doi: 10.1111/j.1699-0463.1986.tb03033.x. [DOI] [PubMed] [Google Scholar]
- Jelínkoá J. Group B streptococci in the human population. Curr Top Microbiol Immunol. 1977;76:127–165. [PubMed] [Google Scholar]
- Jennings H. J., Lugowski C., Kasper D. L. Conformational aspects critical to the immunospecificity of the type III group B streptococcal polysaccharide. Biochemistry. 1981 Aug 4;20(16):4511–4518. doi: 10.1021/bi00519a001. [DOI] [PubMed] [Google Scholar]
- Kasper D. L. Bacterial capsule--old dogmas and new tricks. J Infect Dis. 1986 Mar;153(3):407–415. doi: 10.1093/infdis/153.3.407. [DOI] [PubMed] [Google Scholar]
- Klegerman M. E., Boyer K. M., Papierniak C. K., Levine L., Gotoff S. P. Type-specific capsular antigen is associated with virulence in late-onset group B Streptococcal type III disease. Infect Immun. 1984 Apr;44(1):124–129. doi: 10.1128/iai.44.1.124-129.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nealon T. J., Mattingly S. J. Kinetic and chemical analyses of the biologic significance of lipoteichoic acids in mediating adherence of serotype III group B streptococci. Infect Immun. 1985 Oct;50(1):107–115. doi: 10.1128/iai.50.1.107-115.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos J. I., Shigeoka A. O., Hill H. R. Strain differences in virulence of group B streptococci. Pediatr Res. 1982 May;16(5):347–350. doi: 10.1203/00006450-198205000-00005. [DOI] [PubMed] [Google Scholar]
- Shigeoka A. O., Rote N. S., Santos J. I., Hill H. R. Assessment of the virulence factors of group B streptococci: correlation with sialic acid content. J Infect Dis. 1983 May;147(5):857–863. doi: 10.1093/infdis/147.5.857. [DOI] [PubMed] [Google Scholar]
- WADSWORTH C. COMPARATIVE TESTING OF A NEW PHOTOGRAPHIC MATERIAL FOR RAPID REGISTRATION OF IMMUNOPRECIPITATES. Int Arch Allergy Appl Immunol. 1963;23:103–114. doi: 10.1159/000229408. [DOI] [PubMed] [Google Scholar]
- Wagner M., Wagner B., Kubín V. R. Immunoelectron microscopic study of the location of group-specific and type-specific polysaccharide antigens on isolated walls of group B streptococci. J Gen Microbiol. 1980 Oct;120(2):369–376. doi: 10.1099/00221287-120-2-369. [DOI] [PubMed] [Google Scholar]
- Yeung M. K., Mattingly S. J. Biosynthetic capacity for type-specific antigen synthesis determines the virulence of serotype III strains of group B streptococci. Infect Immun. 1984 May;44(2):217–221. doi: 10.1128/iai.44.2.217-221.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]