Abstract
Interstitial lung disease encompasses a large group of chronic lung disorders associated with excessive tissue remodeling, scarring, and fibrosis. The evidence of a redox imbalance in lung fibrosis is substantial, and the rationale for testing antioxidants as potential new therapeutics for lung fibrosis is appealing. Current animal models of lung fibrosis have clear involvement of ROS in their pathogenesis. New classes of antioxidant agents divided into catalytic antioxidant mimetics and antioxidant scavengers are being developed. The catalytic antioxidant class is based on endogenous antioxidant enzymes and includes the manganese-containing macrocyclics, porphyrins, salens, and the non–metal-containing nitroxides. The antioxidant scavenging class is based on endogenous antioxidant molecules and includes the vitamin E analogues, thiols, lazaroids, and polyphenolic agents. Numerous studies have shown oxidative stress to be associated with many interstitial lung diseases and that these agents are effective in attenuating fibroproliferative responses in the lung of animals and humans.
LUNG FIBROSIS
Classifications
Human lung fibrosis has been described histopathologically as a group of interstitial pneumonias including usual interstitial pneumonia (UIP), also known as idiopathic lung fibrosis (IPF), desquamative interstitial pneumonia (DIP), respiratory bronchiolitis interstitial lung disease (RB), lymphoid interstitial pneumonia (LIP), cryptogenic organizing pneumonia (OP), diffuse alveolar damage (DAD) or acute interstitial pneumonia (AIP), and nonspecific interstitial pneumonia (NSIP) (108). A large variation is found between the prognosis of the different interstitial pneumonias. NSIP, DIP, RB, and LIP have a 5-year mortality of less than 10% and are usually responsive to corticosteroid treatment. IPF and AIP have poor prognosis with a 5-year mortality >60% and are generally unresponsive to treatment. Some evidence supports a spectrum of inflammatory and fibrotic mechanisms in interstitial pneumonias (IPs), in which the IP forms that are most responsive to corticosteroids are more inflammatory and the unresponsive IP forms are more fibrotic (199).
IPF is one of the most devastating forms of lung fibrosis and is progressive and fatal (173). IPF occurs most often in people older than 50 years, with a higher occurrence in men than in women and an overall incidence of 13 to 20/100,000 individuals (47). IPF is generally nonresponsive to conventional anti-inflammatory and immunomodulatory therapy and currently is in need of new therapeutic approaches (214). IPF patients are thought to respond poorly to antiinflammatory therapies because little inflammation exists at advanced stages of the disease and the fibrosis is driven by a dysregulated repair process with a loss of epithelial cells and accumulation of mesenchymal cells (199).
Pathogenesis
The pathogenesis of lung fibrosis is complex and is thought to involve a number of processes that lead to an altered alveolar environment and to an abnormal repair process with accumulated fibrosis. Several factors, including age, genetic susceptibility, and environmental agents, are known to contribute to lung fibrosis (13, 127, 219, 222). An increased interest exists in mesenchymal cells and, in particular, in the fibroblastic foci that are associated with disease progression (102). These fibroblastic foci are also associated with increased levels of active transforming growth factor beta (TGF-β) in the fibrotic lung. This cytokine is an important mediator of fibroblast differentiation into the myofibroblast phenotype (81). Overex-pression of active TGF-β produces lung fibrosis in animals (180). TGF-β is a major regulator of wound repair and a stimulant of reactive oxygen species (ROS) production in fibroblasts (212). Oxidative stress is often defined as an imbalance between ROS production and antioxidant defenses. Oxidative stress can dysregulate cell signaling (158) and is a potential target for the development of therapeutics to treat lung fibrosis (110).
OXIDATIVE STRESS AND LUNG FIBROSIS
Reactive oxygen species and antioxidant defenses
Part of the altered alveolar environment in lung fibrosis involves oxidative stress that is driven by an imbalance between oxidant production and antioxidant defenses. Reactive oxygen species (ROS) are normal byproducts of cellular metabolism and are continually produced at low levels under basal conditions. Biologically, the ROS superoxide (O2−) is commonly generated from the uncoupling of the cellular electron-transport systems (131). Electron-transport systems that account for a large portion of cellular O2− formation are the mitochondrial electron-transport system (21) and various oxidases including NADPH oxidases (35), cytochrome P450 monoxygenases (33), cyclooxygenases (115), lipoxygenases (115), nitric oxide synthases (114), and xanthine oxidoreductase (130). O2− can also rapidly react with nitric oxide (NO) to form the strong oxidizing and nitrating agent, peroxynitrite (ONOO−). The ROS hydrogen peroxide (H2O2) is generated directly from O2− through a rapid dismutation reaction that can occur either enzymatically with superoxide dismutases (SODs, second-order rate constant of 109 M/sec) or spontaneously (second-order rate constant of 105 M/sec). This means that wherever O2− is generated, formation of H2O2 also occurs. In addition, H2O2 is formed enzymatically as a byproduct of lipid metabolism in peroxisomes (160). H2O2 is stable at biologic pH and easily crosses lipid membranes. H2O2 can participate in hydroxyl radical (HO·) formation in the presence of metals (78). H2O2 readily reacts with thiol functional groups, and this type of reaction is proposed to be a key mechanism by which ROS modulate cell-signaling events (62).
The impact of ROS may be especially important in the lung because of its large surface area and its exposure to higher oxygen levels than other tissues. The lung counters this with a formable array of antioxidant defense systems, starting with high levels of antioxidants in the epithelial lining fluid (ELF). Glutathione (GSH) is a major water-soluble antioxidant thiol in the lung ELF (27), and its levels are lower in subjects with IPF (26). In addition to GSH, the lung ELF contains other antioxidants such as ascorbate, urate, albumin, mucins, and metal-binding proteins, which are all effective at limiting oxidative damage. The lung also has a number of antioxidant enzyme systems including SODs, catalase, glutathione peroxidases (GPxs), thioredoxin, glutaredoxin, and peroxiredoxins (155). Overexpression of many of these antioxidant enzyme systems is protective against lung fibrosis (74, 99, 150). Many of these antioxidant systems are upregulated during lung fibrosis via the Nrf2 redox-sensitive transcription factor (95), that when deficient, enhances lung fibrotic responses (36). It is likely that inadequate antioxidant adaptive responses play a key role in lung fibrosis.
Oxidative stress and disruption of cell signaling
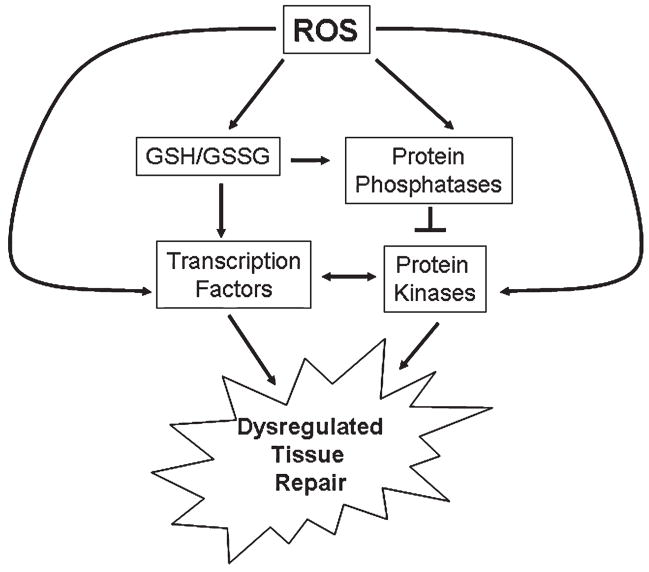
Several phosphatases contain sensitive thiol residues that are inhibited on oxidation (31). The inactivation of phosphatases is often associated with a perceived activation of their respective kinase(s), many of which play prominent roles in inflammatory responses (154). The phosphorylation state of a protein is a steady-state level set by the relative rates of kinases and phosphatases. As steady-state levels of oxidants increase, an increase occurs in the inactivation of phosphatases and a corresponding increase in the levels and duration of phosphorylated proteins (73, 189). An increase in steady-state ROS results in altering the cellular glutathione (GSH)/glutathione disulfide (GSSG) redox couple, which can in turn alter the relative oxidation status of protein thiols. In addition, H2O2 can alter cell signaling directly by reacting with specific thiols on transcription factors, such as reducing factor-1, activator protein-1, and inhibiting factor-κB (89, 103). Many of these types of mechanisms may contribute to the often observed unregulated repair responses associated with lung fibrosis (Fig. 1).
FIG. 1. Role of ROS in the dysregulation of tissue repair.
Several phosphatases contain sensitive thiol residues that are inhibited on oxidation. As steady-state levels of oxidants increase, an increase in the inactivation of phosphatases and a corresponding increase in the levels and duration of phosphorylated proteins occur. An increase in steady-state ROS results in altering the cellular glutathione (GSH)/glutathione disulfide (GSSG) redox couple, which can in turn alter the relative oxidation status of protein thiols. In addition, ROS can alter cell signaling directly by reacting with specific thiols on transcription factors. Many of these types of mechanisms are thought to contribute to the often observed unregulated tissue-repair responses associated lung fibrosis.
Evidence of oxidative stress in the pathogenesis of lung fibrosis
When ROS production and antioxidant defenses are mismatched, an increase in ROS steady-state levels leads to an increase in the oxidation of cellular macromolecules. ROS are difficult to measure directly and often are assessed by measuring oxidative footprints in fluids and tissues, such as markers of protein, lipid, and DNA oxidation. Subjects with IPF have increased levels of oxidized proteins in their ELF that correlate with the percentage of neutrophils in the ELF (15, 121, 166). IPF subjects also have lower antioxidant capacity in their ELF than do healthy subjects (157). IPF subjects have higher levels of exhaled ethane (a marker of lipid peroxidation) than do normal subjects, and these levels are inversely correlated with PaO2 (100). Breath condensate has been investigated as a noninvasive method to assess lung oxidative stress, and IPF subjects have higher levels of H2O2 and isoprostanes than do control subjects (151). Evidence also exists of increased nitrosative stress in IPF subjects (196). These data support the concept of increased oxidant formation with decreased antioxidant capacity in IPF, which is the hallmark of oxidative stress and the rationale for antioxidant therapy (Table 1).
Table 1.
Evidence of Oxidative Stress in IPF
| Blood | Sputum | BALF | BAL cells |
|---|---|---|---|
| ↓ GSH | ↓ GSH | ↓ GSH | ↑ O2− |
| ↑ GSSG | ↑ PMN | ↑ GSSG | |
| ↑ O2− | ↑ IL-8 | ↑ PMN | |
| ↑ MPO | |||
| ↑ Nitrite/nitrate | |||
| ↑ Oxidized proteins |
ANTIOXIDANTS
Antioxidants have been defined many different ways, and in a very broad sense, they are agents that decrease steady-state ROS levels and protect cellular macromolecules from oxidative modification. The mechanisms by which this is achieved are many, and some are even paradoxic. For instance, some agents can produce a mild oxidative stress that results in a cellular adaptive response that increases endogenous antioxidant defenses. Some agents may inhibit cellular sources of ROS. A classic antioxidant is an agent that can rapidly react with ROS, producing less-reactive species. Catalytic antioxidants are not consumed in the reaction and are regenerated. Regardless of the mechanisms, antioxidants decrease oxidative stress and restore redox balance in biologic systems.
Catalytic antioxidant mimetics
Endogenous antioxidant enzymes are examples of catalytic antioxidants and have been used as models for the development of catalytic antioxidant mimetics. The three most prominent classes are the SOD, catalase, and GPx mimics. Most of these compounds contain a ligated transitional metal or selenium. They are generally broad-spectrum antioxidants that can scavenge O2−, H2O2, ONOO−, and a variety of lipid peroxides. The SOD and catalase mimic class include macrocyclics, metallo-porphyrins, salens, and nitroxides. The GPx class includes selenium- and tellurium-based compounds.
The potencies of these catalytic antioxidants are often compared by using their rate constants obtained under tightly defined simple chemical systems, which may or may not be relevant in more complex biologic systems. Another important note is that many of these agents can obtain electrons from cellular sources (54). These properties have two important consequences that affect the in vivo rate constant for the reaction with ROS and can also result in the inhibition of ROS production. The finding that many of these diverse compounds are effective in similar oxidative stress models confirms the basic concept that small, efficient, catalytic antioxidants show promise in the treatment of ROS-mediated conditions associated with injury and tissue dysfunction.
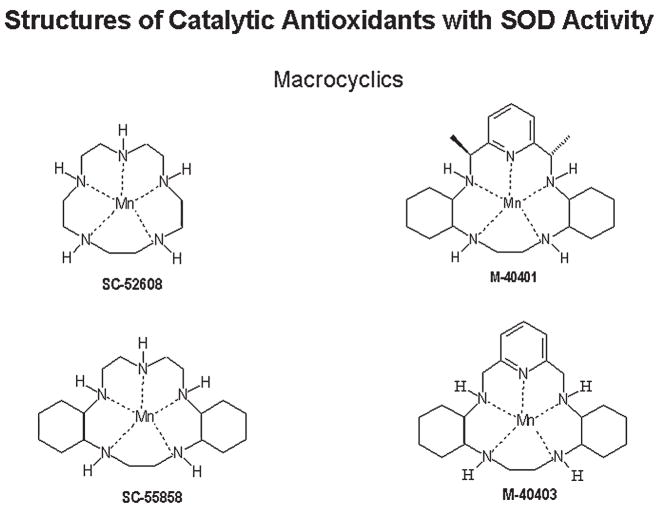
The pentaazamacrocyclic ligand-based mimetics [M40403 is currently being developed by ActivBiotics (http://www.activbiotics.com)] are unique in that they are relatively specific O2− scavengers, because the manganese atom (Mn) is held by five coordination points in the macrocyclic structure and is available only for one-electron transfers (Fig. 2). Mn(II) macrocylics function in the dismutation reaction with O2− by alternate oxidation and reduction, changing its valence between Mn(II) and Mn(III) (11). This unique aspect of these compounds gives them selectivity toward O2− under highly defined conditions. However, in biologic systems, it is unclear whether it is only with O2− that these compounds interact. A number of endogenous compounds also can partake in one-electron reactions beside O2−; these include flavins and ubiquinones. The macrocyclics are effective in many of the same oxidative paradigms in which nonselective catalytic antioxidant mimetics have been used. The different classes of catalytic antioxidant mimetics have not been directly compared in experimental models; therefore, the conditions under which one class holds an advantage over the others are currently not known.
FIG. 2.
Chemical structures of macrocyclic catalytic antioxidant mimetics with SOD activity.
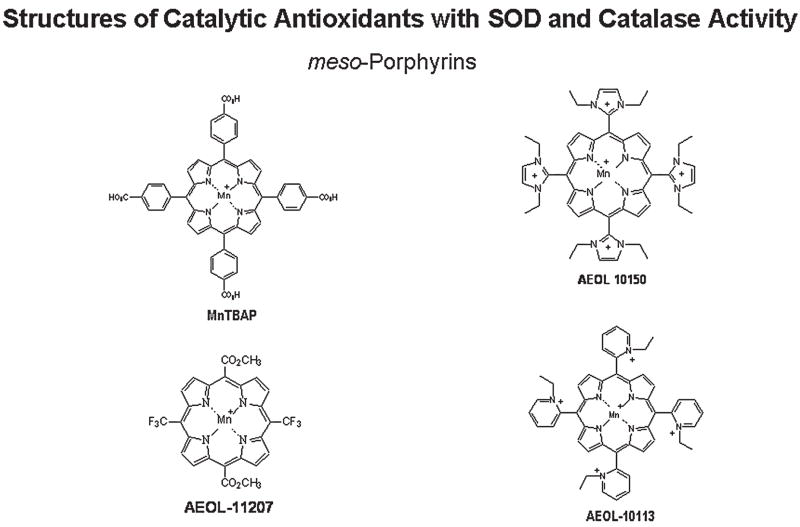
Metalloporphyrins [AEOL series is currently being developed by Aeolus Pharmaceuticals (http://www.aeoluspharma.com)] are structurally different from endogenous protoporphyrins and are classified as synthetic meso-substituted porphyrins (Fig. 3). Metalloporphyrins have been shown to possess at least four distinct antioxidant properties, which include scavenging O2− (149), H2O2 (53), ONOO− (191), and lipid peroxides (51). Most metalloporphyrins contain either an Fe or Mn that is coordinated by four nitrogen axial ligands. The catalase-like activity of metalloporphyrins is thought to be due to their extensive conjugated ring system that can undergo reversible one-electron transfer in addition to the one-electron transfer on the metal center (70). This mechanism is similar to that proposed for the heme prosthetic groups of endogenous catalase and peroxidases. The two classes of metalloporphyrins include one group in which the SOD activities track with their catalase activities, and another group that has very little SOD activity and high catalase activity. An example of a manganese porphyrin with both high SOD and catalase-like activities is AEOL 10150 (98), whereas an example of a compound with low SOD activity and high catalase activity is AEOL 11207 (122). The compounds with high catalase-like activity still only possess a fraction of the native catalase enzyme activity under chemically defined conditions, yet they can protect cells from H2O2-mediated toxicity (53). This may not be a fair comparison because catalase is hard to saturate with H2O2 and has a relative high km for H2O2. Under biologically relevant steady-state levels of H2O2, the metalloporphyrins are more comparable to catalase (32). Metalloporphyrins have been shown to be effective in ameliorating oxidative stress, inflammation, and injury in a large number of animal models of human disease (50). Metalloporphyrins have plasma half-lives that range from 4 to 48 hours. Most metalloporphyrins are not extensively metabolized by the body and are largely excreted unchanged in the urine. A previous limitation of the metalloporphyrin class of compounds has been poor oral bioavailability, but several compounds in the AEOL 112 series have good oral bioavailability and longer plasma half-lives that should make them better candidates for treating chronic diseases (122).
FIG. 3.
Chemical structures of metalloporphyrin catalytic antioxidant mimetics with SOD and catalase activities.
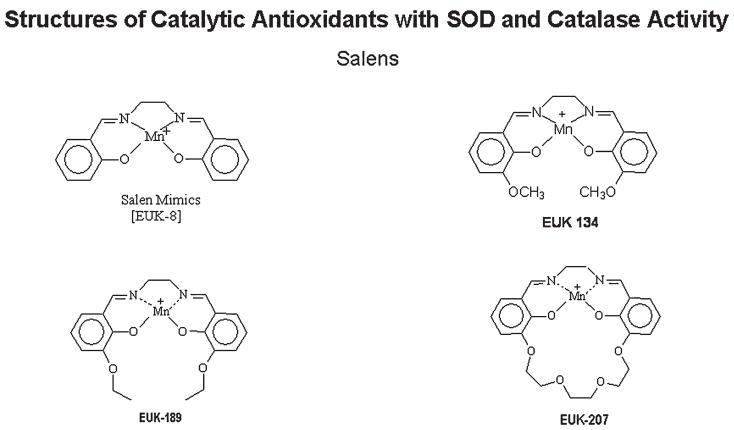
The salen class of catalytic antioxidant mimetics (EUK series) is currently being developed by Proteome Systems (http://www.proteomesystems.com) (Fig. 4). Generically, salens are aromatic, substituted ethylenediamine metal complexes. The Mn(III)-containing salen complexes have both O2− and H2O2 dismutation activities (63). However, like the metalloporphyrins, these compounds are not selective and can react with O2− and other peroxides, including ONOO− (178). The Mn moiety of the salen is coordinated by four axial ligands. One of the unique features of these compounds is that the metal center is coordinated to oxygen and nitrogen atoms, which is in contrast to the porphyrins, in which the metal is coordinated to nitrogen atoms. The coordination of Mn by four axial ligands results in the formation of several possible valance states that give these compounds their broad ROS-scavenging capabilities. The rates at which reported salens scavenge H2O2 are similar to those reported for metalloporphyrins, but are many orders less than those documented for catalase under similarly defined conditions (63). Salens have also been shown to protect cells against oxidative stress and are protective in a large number of animal models of human diseases (50). One of the current limitations of the salens is the stability of the parent compounds in biologic matrix, which makes it difficult to determine tissue levels and half-lives.
FIG. 4.
Chemical structures of salen catalytic antioxidant mimetics with SOD and catalase activities.
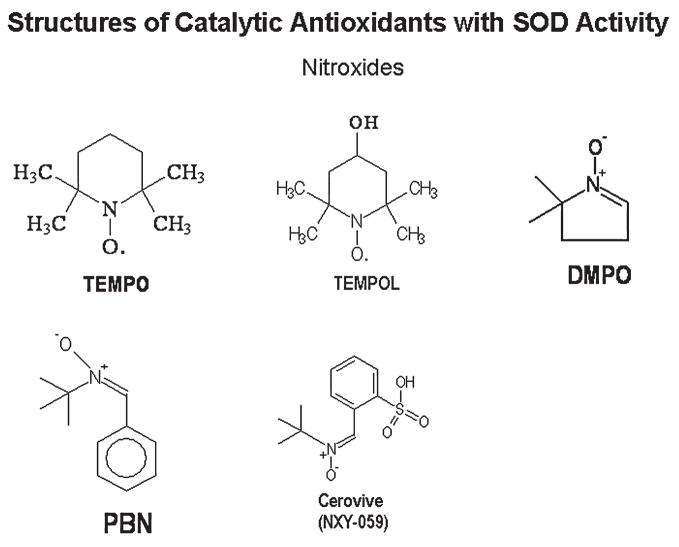
A number of compounds initially developed as free radical spin traps have been shown to have antioxidant properties in cell and animal systems (136). These compounds react with free radicals and form more-stable free radical products. The most frequently used compounds are the nitroxides and include α-phenyl-tert-butylnitrone (PBN) and 2,2,6,6-tetramethylpiperidine N-oxyl (TEMPO) (Fig. 5). These compounds have also been described as non–metal-containing SOD mimics (5). The rate of reaction with O2− is relatively low and thus requires large amounts (often millimolar levels) of these compounds to be present in the system to be effective (190). Fortunately, these compounds are well tolerated in animals and can achieve high tissue levels (136). These agents have a number of properties other than the reaction with ROS that could also explain some of their protective properties in models of oxidative stress. Many of these compounds can be metabolized to release NO and can inhibit enzymes that are endogenous sources of ROS (34, 229).
FIG. 5.
Chemical structures of nitroxide catalytic antioxidant mimetics with SOD activity.
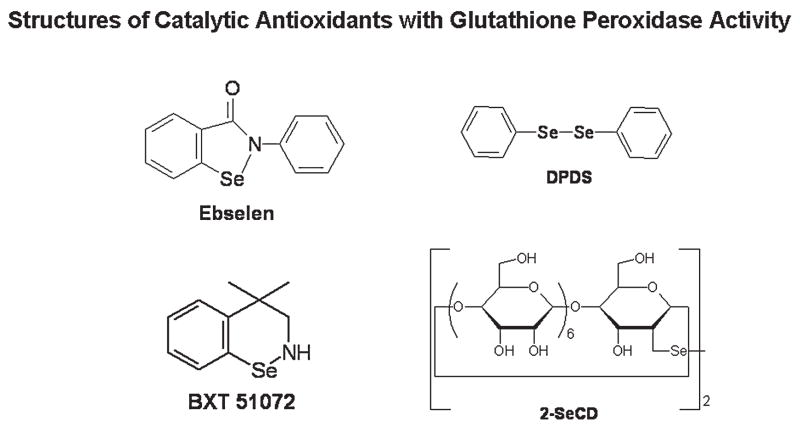
GPx enzymes are found in every compartment within the cell and tissues and are effective scavengers of cellular peroxides. The GPx mimetic class includes mono- and diselenium–containing compounds (Fig. 6). One of the best-studied GPx-like mimics is 2-phenyl-1,2-benzisoselenazol-3(2H)-one, also known as ebselen or PZ51. Ebselen was one of the first selenium-based GPx mimics developed and catalytically scavenges peroxides in the presence of reducing equivalents such as GSH, N-acetylcysteine (NAC), and dihydrolipoate (DHLA) (179). The mechanism by which this occurs is still debated and may differ under different conditions. Ebselen has also been shown to stimulate the decomposition of a number of ROS, including hypochlorous acid (HOCl) (17), singlet oxygen (174), and ONOO− (128). Ebselen can readily bind cellular thiol groups on proteins, which may complicate the interpretation of biologic effects, because many cellular proteins have reactive thiols in their catalytic domains. It has been documented that ebselen can inhibit lipoxygenases (168), NADPH oxidases (46), and nitric oxide synthases (226). All of these enzymes are also potential sources of endogenous ROS. Ebselen has been shown to be protective in a number of cell-culture systems (159, 194) and animal models of human disease (179). Ebselen is orally active and appears to be well tolerated in animals and humans. Newer analogues of ebselen have been developed, including BXT-51072, which has increased activity and potency in cell systems. These analogues [BXT series are being developed by Oxis International (http://www.oxis.com)] have been shown to be protective in a limited number of cell-culture systems and animal models of human disease (203).
FIG. 6.
Chemical structures of selenium-containing catalytic antioxidant mimetics with glutathione peroxidase activity.
A number of diselenide- and ditelluride-containing compounds have been reported to catalytically scavenge peroxides with higher GPx-like activity than ebselen (75, 86, 161, 162). Sulfur, selenium, and tellurium belong to group IV of the periodic table and have similar chemical properties. A major difference with these types of compounds is that they usually contain a diselenide bond. Earlier compounds, such as the diphenyl diselenide (DPDS), were electrophilic agents that had cytotoxic, genotoxic, and mutagenic issues (4, 163). Many previously reported diselenide compounds release free selenium during the catalytic cycle, and this may be problematic in their development as therapeutic agents. A unique aspect of a newer series of these compounds is the cyclodextrin group, which may help in directing hydrophobic peroxides toward the selenium or tellurium active site. The diselenide, 2,2′-deseleno-bis-β-cyclodextrin (2-SeCD), can scavenge a variety of peroxides including H2O2, tert-butyl hydroperoxide, and cumenyl hydroperoxide by using GSH as a cofactor (124). Only a limited number of cell-culture studies have been reported for these compounds (140, 186), and it is still unclear whether these compounds can be successfully used in animal models of lung fibrosis.
Antioxidant scavengers
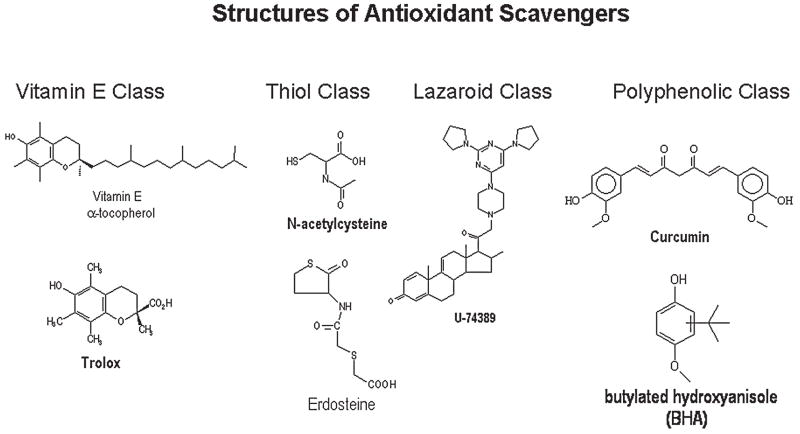
The largest categories of antioxidants are those that are reactive toward ROS, and the product of the reaction results in a less-toxic species. The naturally occurring vitamins E (α-tocopherol) and C (ascorbate) are such examples. Both the ascorbate and α-tocopherol radicals are less reactive and can be recycled by cellular reductases. Glutathione is a thiol-containing tripeptide that readily reacts with peroxides and forms a less-toxic disulfide product that is recycled by glutathione reductase. A number of synthetic compounds have been models after these endogenous antioxidants and have been shown to be protective in models of oxidative stress (Fig. 7).
FIG. 7.
Chemical structures of antioxidant scavengers.
A number of polyphenolic-based antioxidants are known, such as the water-soluble analogue of α-tocopherol, known as trolox, hindered phenols that include butylated hydroxytoluene (BHT), and various plant phenolics such as curcumin and flavonoids. These compounds are often chain-breaking antioxidants, and some have been used in the food industry as preservatives (42). In general, they require larger doses or concentrations to produce antioxidant effects in model systems because of their lower rates of reaction with ROS and their limited ability to be recycled endogenously.
Another group of compounds use a steroid nucleus substituted with antioxidant side groups and are known as lazaroids. Lazaroids are very effective at inhibiting iron-dependent lipid peroxidation (39). Lazaroids have been extensively tested as neuroprotective agents, but it is still not clear whether their neuroprotective effects are directly related to their antioxidant properties.
A large class of antioxidants is the thiol-containing compounds. The most extensively studied thiol compound is N-acetyl cysteine (NAC). NAC is a direct-acting antioxidant and can scavenge several ROS such as hypochlorous acid, peroxides, ·OH, and ONOO− (45). NAC can also serve as a cellular source of cysteine for the endogenous synthesis of GSH. NAC can suppress the activation of transcription factors such as NF-κB as a way to modulate cell-signaling pathways. A homocysteine derivative, erdosteine, is a prodrug that, when metabolized, produces an active thiol antioxidant metabolite (56). Erdosteine has beneficial effects in COPD patients (156). Amifostine is another prodrug that, when metabolized, produces an active thiol antioxidant used clinically as a radioprotective agent (37). Thiol-containing agents can also act as metal chelators and decrease oxidative stress by limiting the ability of transitional metal to participate in ROS formation. Paradoxically, some thiol-containing agents have the potential to create a more-reactive species when they react with ROS, which is often dependent on availability of oxygen and transitional metals.
ANTIOXIDANTS, OXIDATIVE STRESS AND ANIMAL MODELS OF LUNG FIBROSIS
A large majority of lung fibrosis animal models involve the overproduction of oxidants, and the fibrotic effects are potentiated in antioxidant-deficient animals (Table 2). A number of drugs are known to produce lung fibrosis in humans and animals (105, 228). Many of these drugs are chemotherapeutic agents that stimulate oxidative stress. Ionizing radiation is also a well-characterized method of producing lung fibrosis in animals, as well as a known adverse effect of cancer radiation treatment (41). A number of environmental exposures produce lung oxidative stress and fibrosis, including exposure to asbestos and silica (77, 220). In addition, known cytokines, such as TGF-β, when overproduced, result in lung fibrosis. Most of these models have been shown to stimulate lung-injury responses and oxidative stress. A major limitation with currently available animal models of lung fibrosis is that they do not closely mimic human interstitial pneumonias, and many spontaneously resolve over time (38).
Table 2.
Models of Lung Fibrosis
| Fibrinogenic agents | Species |
|---|---|
| Bleomycin | Mice, rats, hamsters, rabbits, dogs, and primates |
| Paraquat | Mice, rats, hamsters, rabbits, dogs, sheep and primates |
| Amiodarone | Mice, rats, hamsters, rabbits, dogs, and primates |
| Radiation | Mice, rats, rabbits, dogs, hamsters, sheep, and primates |
| TGF-β | Mice, rats |
| Silica | Mice, rats, hamsters, rabbits, and primates |
| Asbestos | Mice, rats, hamsters, sheep, and primates |
Fibrogenic drugs, antioxidants and oxidative stress
A number of chemotherapeutic agents can elevate intracellular ROS levels (141). The best-studied chemotherapeutic agent associated with oxidative stress and lung fibrosis is bleomycin. Bleomycin is thought to bind transitional metals and, in the presence of oxygen or H2O2, generates a strong oxidant (101, 225). The lung is thought to be a target organ because of its low levels of a cysteine protease that degrades bleomycin into an inactive form (117). Bleomycin increases ROS production in lung macrophages and alveolar type II epithelial cells in vivo (91). Bleomycin also increases lung epithelial cell apoptosis in a ROS-dependent manner (213). Bleomycin-induced cytotoxicity and lung fibrosis can be modulated by changing the intracellular levels of endogenous antioxidants (61, 84, 107, 112, 167).
Both catalytic and scavenger antioxidants have been shown to attenuate bleomycin-induced lung fibrosis in animals (Table 3). Liposomal or lecithinized delivery of SOD with or without catalase decreases bleomycin-induced lung fibrosis in rats and mice (118, 119, 193, 221). The catalytic antioxidant porphyrin MnTBAP attenuates bleomycin-induced lung fibrosis in mice (148). The administration of vitamin E has also been shown to have protective effects, and its deficiency potentiates bleomycin-induced lung fibrosis in animals (57, 58, 142, 188). Thiol-containing antioxidants have been extensively studied in the bleomycin model of lung fibrosis. The best-studied thiol-containing antioxidant is NAC. Both oral and inhaled NAC decrease lung fibrosis in rats and mice (44, 80, 129, 177). NAC also has been shown to restore lung GSH redox balance and to suppress bleomycin-induced activation of NF-κB (176). Several prodrugs, such as erdosteine and amifostine, that produce active thiol-containing metabolites have also been shown to attenuate bleomycin-induced lung fibrosis in rodents (22, 88, 144, 145, 184, 224). In addition, lazaroids are protective against bleomycin-induced lung fibrosis in rats (49, 132). A number of natural products that contain polyphenolic compounds, such as Ginko biloba extracts and curcumin, have been found to suppress lung oxidative stress and fibrosis in rats treated with bleomycin (48, 67, 93, 152, 207).
Table 3.
Protective Antioxidants in Animal Models of Lung Fibrosis
| Fibrogenic agent | Catalytic antioxidants | Antioxidant scavengers |
|---|---|---|
| Bleomycin | SOD, catalase, MnTBAP | Vitamin E, NAC, erdosteine, amifostine, U-74389, curcumin, ginko biloba |
| Paraquat | SOD, MnTBAP, PBN, TEMPOL | Vitamin E, GSH, NAC, erdosteine, curcumin |
| Amiodarone | PBN | Vitamin E, NAC, BHA, curcumin |
| Radiation | SOD, catalase, AEOL 10113, AEOL 10150, EUK 189 | Vitamin E, NAC, amifostine, curcumin, ginko biloba |
| TGF-β | Unknown | Unknown |
| Silica | Catalase, MnTBAP, TEMPO | GSH, NAC, U-75412, U-74389 |
| Asbestos | SOD, catalase, TEMPO | NAC |
Paraquat is a redox-active herbicide that also is known to produce fatal pulmonary fibrosis in humans (43, 202) and animals (181, 182). Paraquat is thought to redox cycle with cellular enzymes to produce the paraquat cation radical that rapidly reacts with oxygen to form O2− (3, 25, 76). Paraquat produces lung oxidative stress in animals (2, 23, 66, 113, 218) and humans (94, 135). Both catalytic and scavenger antioxidants have been shown to attenuate paraquat-induced lung injury and fibrosis in animals (see Table 3). Administration of SOD has been shown to attenuate paraquat-induced lung injury in vitro (90) and in vivo (147, 215). The manganese-containing porphyrin catalytic antioxidant MnTBAP has been shown to have protective effects against paraquat-induced injury both in vitro (55, 97) and in vivo (52). In addition, the spin-trap PBN also has protective effects against paraquat-mediated damage (170). The administration of vitamin E has protective effects, and its deficiency potentiates paraquat-induced lung injury and fibrosis in animals (18, 187). Thiol-based antioxidants also have protective effects in the paraquat model, including GSH (79, 192), NAC (83, 216, 223), and erdosteine (92). In addition, the polyphenolic compound curcumin has been reported to have protective properties against paraquat-induced lung injury (206).
The antiarrhythmic drug amiodarone produces lung fibrosis in humans (183) and animals (28). Some data suggest a role for oxidative stress in amiodarone-induced lung fibrosis. Amiodarone inhibits mitochondrial complex I and II respiration and produces mitochondrial dysfunction in lung epithelial cells and macrophages (19). In the ventilated perfused rabbit lung system, amiodarone increases the levels of ROS and oxidized glutathione (GSSG) (106). Further studies have revealed that amiodarone is metabolized to an aryl radical that may give rise to other ROS (146, 208). Both catalytic and scavenger antioxidants have been shown to attenuate amiodarone-induced lung injury and fibrosis in animals (see Table 3). PBN has been shown to directly scavenge the aryl radical produced by amiodarone (146). Vitamin E supplementation of hamsters attenuated both lung TGF-β levels and fibrosis induced by amiodarone (29, 30). The thiol-based antioxidant NAC is also effective at attenuating amiodarone-induced lung fibrosis (120). In addition, the phenolic antioxidants BHA and curcumin were effective in limiting amiodarone-induced ROS and lung injury (120, 152).
Radiation, antioxidants, and oxidative stress
Ionizing radiation produces fibrotic responses and generates hydrogen atom radical (H ), OH, and hydrated electrons (e−aq) from the ionization of water in tissues. All three of these species are highly reactive and can generate and propagate a cascade of different ROS. Whole-body radiation decreases the levels of endogenous antioxidants and increases markers of lipid oxidation in animals and humans (9, 40). Increased oxidative stress has been reported in radiation pneumonitis in humans (96) and in radiation-induced lung injury in rats (72, 209). It is interesting to note that oxidative stress is still present even weeks after the radiation exposure (59). Several animal hemithoracic irradiation models of lung fibrosis have been developed and used to screen compounds for antifibrotic effects.
Both catalytic and scavenger antioxidants have been shown to attenuate radiation-induced lung injury and fibrosis in animals (see Table 3). Radiation-induced lung fibrosis is worsened in antioxidant-deficient animals (68, 197) and attenuated in SOD-overexpression models (68, 99, 126). Manganese-containing porphyrins and salen catalytic antioxidant mimetics have also been shown to have protective effects against radiation-induced lung fibrosis (116, 153, 210). The results from studies on the administration of vitamin E and its potential to limit radiation-induced lung injury and fibrosis in animals have been controversial, with some negative findings (165, 217) and a recent positive result (16). Thiol-based antioxidants also have protective effects in the radiation-induced lung injury and fibrosis, including NAC (143) and amifostine (211). A number of flavonoids have also been used successfully to attenuate radiation-induced lung injury and fibrosis, including curcumin (200) and Ginko biloba extracts (175).
Fibrogenic cytokines and oxidative stress
A number of cytokines have been shown to stimulate fibrotic events and include TGF-β, tumor necrosis factor (TNF-α), platelet-derived growth factor (PDGF), connective tissue growth factor (CTGF), endothelin, granulocyte–macrophage colony-stimulating factor (GM-CSF), interleukin (IL-1β), IL-6, IL-10, and IL-13 (10). The best studied of these various cytokines in lung fibrosis is TGF-β. TGF-β isoforms have a number of effects on cellular responses including modulating cell growth, migration, differentiation, and apoptosis (169). TGF-β induces myofibroblast differentiation, extracellular matrix (ECM) synthesis, and inhibits ECM breakdown (227). TGF-β1 is abundant in BALF and present in fibroblastic foci biopsies from IPF subjects (24). Overexpression of TGF-β1 in animals induces a progressive lung fibrosis that is largely independent of inflammation (180). TGF-β1 produces oxidative stress by the induction of ROS production and a decrease in expression of cellular antioxidants (111). TGF-β1 induces ROS production by activation of NADPH oxidases (NOXs) and through mitochondrial dysfunction (185, 198). TGF-β1 has been shown to decrease the expression of both catalase and mitochondrial SOD2 (82). In addition, TGF-β1 has been shown to decrease cellular GSH levels through the decreased expression and activity of γ-glutamylcysteine ligase (γ-GCL), the rate-limiting step in GSH synthesis (8). Interestingly, very few studies have been reported on the effects of antioxidants in this relatively new animal model of lung fibrosis.
Fibrogenic environmental agents and oxidative stress
A number of environmental dust and fiber exposures have been associated with the development of lung fibrosis (137). Both silica and asbestos exposures produce lung fibrosis in animals (64, 104) and pneumoconiosis in humans (164). Both silica and asbestos produce injury and oxidative stress in the lungs of animals (1). In humans, a link between oxidative stress and exposure to dusts is supported by the finding of lower levels of glutathione-dependent enzymes in coal workers with pneumoconiosis (69). Numerous reports exist in the literature on the ability of both silica and asbestos exposures to increase ROS production (109, 138, 172, 204). In addition, beryllium exposure can produce lung granulomatous disease, and beryllium has recently been shown to stimulate increased production of ROS (171). A number of studies have reported an increased risk for developing IPF on various occupational and environmental exposures (13, 87, 195).
Several asbestos and silica animal models of lung fibrosis have been developed. Silica- and asbestos-induced lung fibrosis are worsened in antioxidant-deficient animals (71, 125) and attenuated in catalase-overexpression models (139). Catalytic and scavenger antioxidants have been shown to attenuate asbestos- and silica-induced lung injury and fibrosis in animals (see Table 3). Catalytic antioxidants have been shown to have protective effects against silica-induced injury, including the porphyrin MnTBAP (123) and the nitroxide TEMPO (205). Thiol-based antioxidants also have protective effects in the silica-induced injuries, including NAC and GSH (12), as well as garlic extracts that are rich in thiol-containing compounds (6). The lazaroid compounds U-75412E and U-74389G are also effective against silica-induced injury (7, 85).
ANTIOXIDANTS IN HUMAN IPF
Only a few antioxidants have actually been examined in humans with IPF. The thiol class has received the most attention to date. GSH (600 mg, twice daily for 3 days) has been given by inhalation to IPF subjects and found to increase ELF GSH levels and decrease ROS production in airway macrophages (20). NAC is an FDA-approved mucolytic drug that has been extensively used in cystic fibrosis subjects (65). Early studies examined the ability of oral NAC therapy (600 mg, 3 times daily for 5 days) to restore ELF GSH levels in IPF subjects (134). These studies found that this NAC regimen increased ELF GSH levels in IPF subjects 71% and was well tolerated. NAC has also been given to IPF subjects intravenously at 0.6-, 1.6-, and 4.8-g doses and found to elevate ELF GSH levels in IPF but not in normal subjects (133). None of these earlier studies looked at efficacy. NAC has been given to IPF subjects by inhalation (352 mg daily for 12 months), and some improvements were noted in exercise desaturation and high-resolution CT imaging; however, other lung-function tests and quality-of-life scores were not different from those with placebo (201). NAC has been given to a limited number of subjects with fibrosing alveolitis, in addition to immunosuppressive therapy (14). Oral NAC treatment (600 mg, 3 times daily for 12 weeks) increased lung ELF GSH levels 48% over baseline and was associated with improved pulmonary-function tests. A more recent multicenter randomized trial further investigated the possible benefits of oral NAC therapy in combination with azathioprine and high-dose corticosteroids in a larger cohort of IPF subjects (60). NAC treatment (600 mg, 3 times daily for 12 months) was associated with modestly improved pulmonary-function tests versus standard therapy alone. Interestingly, this study found that the NAC-treatment group had a lower rate of myelotoxicity from the immunosuppressive therapy. Although the initial studies with NAC in IPF showed only modest beneficial effects, it sets the stage for testing other antioxidants in IPF.
CONCLUSIONS
The evidence of a redox imbalance in lung fibrosis is substantial, and the rationale for testing antioxidants as potential new therapeutics for lung fibrosis is appealing. All the current animal models of lung fibrosis have clear involvement of ROS in their pathogenesis, and numerous examples of a wide array of different antioxidants attenuating fibroproliferative events are ample in the literature. These factors should continue to drive the investigation and the use of antioxidants to treat the progression of lung fibrosis and other fibroproliferative disorders in humans.
Acknowledgments
This work was supported in part by NIH grants RO1 ES012504, RO1 HL75523, and U54 ES015678, and Research Grants from Aeolus Pharmaceuticals. Dr. Day is a consultant for and holds equity in Aeolus Pharmaceuticals, which is commercially developing catalytic antioxidant mimetics as therapeutic agents.
ABBREVIATIONS
- AIP
acute interstitial pneumonia
- α-PBN
α-phenyl-tert-butylnitrone
- BALF
bronchoalveolar lavage fluid
- BHA
butylated hydroxyanisole
- BHT
butylated hydroxytoluene
- CTGF
connective tissue growth factor
- COP
cryptogenic organizing pneumonia
- 2-SeCD
deseleno-bis-β-cyclodextran
- DIP
desquamative interstitial pneumonia
- DAD
diffuse alveolar damage
- DHLA
dihydrolipoate
- DPDS
diphenyl diselenide
- ELF
epithelial lining fluid
- γ-GCL
γ-glutamylcysteine ligase
- GSH
glutathione
- GSSG
glutathione disulfide
- GPx
gluta-thione peroxidase
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- H2O2
hydrogen peroxide
- HO·
hydroxyl radical
- HOCl
hypochlorous acid
- IPF
idiopathic pulmonary fibrosis
- IL
interleukin
- IP
interstitial pneumonia
- LIP
lymphoid interstitial pneumonia
- MnTBAP
manganese (III) tetrakis (4-benzoic acid) porphyrins
- NAC
N-acetylcysteine
- PDGF
platelet-derived growth factor
- PMN
polymorphonuclear leukocyte
- ROS
reactive oxygen species
- RB
respiratory bronchiolitis interstitial lung disease
- O2−
superoxide
- SOD
superoxide dismutase
- TEMPO
tetramethylpiperidine N-oxyl
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- UIP
usual interstitial pneumonia
References
- 1.Abidi P, Afaq F, Arif JM, Lohani M, Rahman Q. Chrysotile-mediated imbalance in the glutathione redox system in the development of pulmonary injury. Toxicol Lett. 1999;106:31–39. doi: 10.1016/s0378-4274(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 2.Adachi J, Ishii K, Tomita M, Fujita T, Nurhantari Y, Nagasaki Y, Ueno Y. Consecutive administration of paraquat to rats induces enhanced cholesterol peroxidation and lung injury. Arch Toxicol. 2003;77:353–357. doi: 10.1007/s00204-003-0449-8. [DOI] [PubMed] [Google Scholar]
- 3.Adam A, Smith LL, Cohen GM. An assessment of the role of redox cycling in mediating the toxicity of paraquat and nitro-furantoin. Environ Health Perspect. 1990;85:113–117. doi: 10.1289/ehp.85-1568326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams WJ, Jr, Kocsis JJ, Snyder R. Acute toxicity and urinary excretion of diphenyldiselenide. Toxicol Lett. 1989;48:301–310. doi: 10.1016/0378-4274(89)90057-x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad N, Misra M, Husain MM, Srivastava RC. Metal-independent putative superoxide dismutase mimics in chemistry, biology, and medicine. Ecotoxicol Environ Saf. 1996;34:141–144. doi: 10.1006/eesa.1996.0055. [DOI] [PubMed] [Google Scholar]
- 6.Ameen M, Musthapa MS, Abidi P, Ahmad I, Rahman Q. Garlic attenuates chrysotile-mediated pulmonary toxicity in rats by altering the phase I and phase II drug metabolizing enzyme system. J Biochem Mol Toxicol. 2003;17:366–371. doi: 10.1002/jbt.10100. [DOI] [PubMed] [Google Scholar]
- 7.Antonini JM, van Dyke K, DiMatteo M, Reasor MJ. Attenuation of acute inflammatory effects of silica in rat lung by 21-aminosteroid, U74389G. Inflammation. 1995;19:9–21. doi: 10.1007/BF01534376. [DOI] [PubMed] [Google Scholar]
- 8.Arsalane K, Dubois CM, Muanza T, Begin R, Boudreau F, As-selin C, Cantin AM. Transforming growth factor-beta1 is a potent inhibitor of glutathione synthesis in the lung epithelial cell line A549: transcriptional effect on the GSH rate-limiting enzyme gamma-glutamylcysteine synthetase. Am J Respir Cell Mol Biol. 1997;17:599–607. doi: 10.1165/ajrcmb.17.5.2833. [DOI] [PubMed] [Google Scholar]
- 9.Arterbery VE, Pryor WA, Jiang L, Sehnert SS, Foster WM, Abrams RA, Williams JR, Wharam MD, Jr, Risby TH. Breath ethane generation during clinical total body irradiation as a marker of oxygen-free-radical-mediated lipid peroxidation: a case study. Free Radic Biol Med. 1994;17:569–576. doi: 10.1016/0891-5849(94)90096-5. [DOI] [PubMed] [Google Scholar]
- 10.Ask K, Martin GE, Kolb M, Gauldie J. Targeting genes for treatment in idiopathic pulmonary fibrosis: challenges and opportunities, promises and pitfalls. Proc Am Thorac Soc. 2006;3:389–393. doi: 10.1513/pats.200602-021TK. [DOI] [PubMed] [Google Scholar]
- 11.Aston K, Rath N, Naik A, Slomczynska U, Schall OF, Riley DP. Computer-aided design (CAD) of Mn(II) complexes: super-oxide dismutase mimetics with catalytic activity exceeding the native enzyme. Inorg Chem. 2001;40:1779–1789. doi: 10.1021/ic000958v. [DOI] [PubMed] [Google Scholar]
- 12.Barrett EG, Johnston C, Oberdorster G, Finkelstein JN. Anti-oxidant treatment attenuates cytokine and chemokine levels in murine macrophages following silica exposure. Toxicol Appl Pharmacol. 1999;158:211–220. doi: 10.1006/taap.1999.8716. [DOI] [PubMed] [Google Scholar]
- 13.Baumgartner KB, Samet JM, Coultas DB, Stidley CA, Hunt WC, Colby TV, Waldron JA. Occupational and environmental risk factors for idiopathic pulmonary fibrosis: a multicenter case-control study collaborating centers. Am J Epidemiol. 2000;152:307–315. doi: 10.1093/aje/152.4.307. [DOI] [PubMed] [Google Scholar]
- 14.Behr J, Maier K, Degenkolb B, Krombach F, Vogelmeier C. Antioxidative and clinical effects of high-dose N-acetylcysteine in fibrosing alveolitis: adjunctive therapy to maintenance immunosuppression. Am J Respir Crit Care Med. 1997;156:1897–1901. doi: 10.1164/ajrccm.156.6.9706065. [DOI] [PubMed] [Google Scholar]
- 15.Behr J, Maier K, Krombach F, Adelmann-Grill BC. Pathogenetic significance of reactive oxygen species in diffuse fibrosing alveolitis. Am Rev Respir Dis. 1991;144:146–150. doi: 10.1164/ajrccm/144.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Bese NS, Munzuroglu F, Uslu B, Arbak S, Yesiladali G, Sut N, Altug T, Ober A. Vitamin E protects against the development of radiation-induced pulmonary fibrosis in rats. Clin Oncol (R Coll Radiol) 2007;19:260–264. doi: 10.1016/j.clon.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 17.Biewenga GP, Bast A. Reaction of lipoic acid with ebselen and hypochlorous acid. Methods Enzymol. 1995;251:303–314. doi: 10.1016/0076-6879(95)51133-4. [DOI] [PubMed] [Google Scholar]
- 18.Block ER. Potentiation of acute paraquat toxicity by vitamin E deficiency. Lung. 1979;156:195–203. doi: 10.1007/BF02714010. [DOI] [PubMed] [Google Scholar]
- 19.Bolt MW, Card JW, Racz WJ, Brien JF, Massey TE. Disruption of mitochondrial function and cellular ATP levels by amiodarone and N-desethylamiodarone in initiation of amiodarone-induced pulmonary cytotoxicity. J Pharmacol Exp Ther. 2001;298:1280–1289. [PubMed] [Google Scholar]
- 20.Borok Z, Buhl R, Grimes GJ, Bokser AD, Hubbard RC, Holroyd KJ, Roum JH, Czerski DB, Cantin AM, Crystal RG. Effect of glutathione aerosol on oxidant-antioxidant imbalance in idiopathic pulmonary fibrosis. Lancet. 1991;338:215–216. doi: 10.1016/0140-6736(91)90350-x. [DOI] [PubMed] [Google Scholar]
- 21.Boveris A. Mitochondrial production of superoxide radical and hydrogen peroxide. Adv Exp Med Biol. 1977;78:67–82. doi: 10.1007/978-1-4615-9035-4_5. [DOI] [PubMed] [Google Scholar]
- 22.Boyaci H, Maral H, Turan G, Basyigit I, Dillioglugil MO, Yildiz F, Tugay M, Pala A, Ercin C. Effects of erdosteine on bleomycin-induced lung fibrosis in rats. Mol Cell Biochem. 2006;281:129–137. doi: 10.1007/s11010-006-0640-3. [DOI] [PubMed] [Google Scholar]
- 23.Brigelius R, Dostal LA, Horton JK, Bend JR. Alteration of the redox state of NADPH and glutathione in perfused rabbit lung by paraquat. Toxicol Ind Health. 1986;2:417–428. doi: 10.1177/074823378600200405. [DOI] [PubMed] [Google Scholar]
- 24.Broekelmann TJ, Limper AH, Colby TV, McDonald JA. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci U S A. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bus JS, Aust SD, Gibson JE. Paraquat toxicity: proposed mechanism of action involving lipid peroxidation. Environ Health Perspect. 1976;16:139–146. doi: 10.1289/ehp.7616139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cantin AM, Hubbard RC, Crystal RG. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1989;139:370–372. doi: 10.1164/ajrccm/139.2.370. [DOI] [PubMed] [Google Scholar]
- 27.Cantin AM, North SL, Hubbard RC, Crystal RG. Normal alveolar epithelial lining fluid contains high levels of glutathione. J Appl Physiol. 1987;63:152–157. doi: 10.1152/jappl.1987.63.1.152. [DOI] [PubMed] [Google Scholar]
- 28.Cantor JO, Osman M, Cerreta JM, Suarez R, Mandl I, Turino GM. Amiodarone-induced pulmonary fibrosis in hamsters. Exp Lung Res. 1984;6:1–10. doi: 10.3109/01902148409087891. [DOI] [PubMed] [Google Scholar]
- 29.Card JW, Leeder RG, Racz WJ, Brien JF, Bray TM, Massey TE. Effects of dietary vitamin E supplementation on pulmonary morphology and collagen deposition in amiodarone- and vehicle-treated hamsters. Toxicology. 1999;133:75–84. doi: 10.1016/s0300-483x(99)00009-8. [DOI] [PubMed] [Google Scholar]
- 30.Card JW, Racz WJ, Brien JF, Massey TE. Attenuation of amiodarone-induced pulmonary fibrosis by vitamin E is associated with suppression of transforming growth factor-beta1 gene expression but not prevention of mitochondrial dysfunction. J Pharmacol Exp Ther. 2003;304:277–283. doi: 10.1124/jpet.102.043208. [DOI] [PubMed] [Google Scholar]
- 31.Caselli A, Marzocchini R, Camici G, Manao G, Moneti G, Pieraccini G, Ramponi G. The inactivation mechanism of low molecular weight phosphotyrosine-protein phosphatase by H2O2. J Biol Chem. 1998;273:32554–32560. doi: 10.1074/jbc.273.49.32554. [DOI] [PubMed] [Google Scholar]
- 32.Castello P, Day BJ, Patel M. Inhibition of mitochondrial hydrogen peroxide production by lipophilic metalloporphyrins. J Pharmacol Exp Ther. doi: 10.1124/jpet.107.132134. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cederbaum AI. Microsomal generation of reactive oxygen species and their possible role in alcohol hepatotoxicity. Alcohol Alcohol Suppl. 1991;1:291–296. [PubMed] [Google Scholar]
- 34.Chamulitrat W, Mason RP, Riendeau D. Nitroxide metabolites from alkylhydroxylamines and N-hydroxyurea derivatives resulting from reductive inhibition of soybean lipoxygenase. J Biol Chem. 1992;267:9574–9579. [PubMed] [Google Scholar]
- 35.Cheson BD, Curnette JT, Babior BM. The oxidative killing mechanisms of the neutrophil. Prog Clin Immunol. 1977;3:1–65. [PubMed] [Google Scholar]
- 36.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J. 2004;18:1258–1260. doi: 10.1096/fj.03-1127fje. [DOI] [PubMed] [Google Scholar]
- 37.Choi NC. Radioprotective effect of amifostine in radiation pneumonitis. Semin Oncol. 2003;30:10–17. doi: 10.1053/j.seminoncol.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 38.Chua F, Gauldie J, Laurent GJ. Pulmonary fibrosis: searching for model answers. Am J Respir Cell Mol Biol. 2005;33:9–13. doi: 10.1165/rcmb.2005-0062TR. [DOI] [PubMed] [Google Scholar]
- 39.Clark WM, Hazel JS, Coull BM. Lazaroids: CNS pharmacology and current research. Drugs. 1995;50:971–983. doi: 10.2165/00003495-199550060-00005. [DOI] [PubMed] [Google Scholar]
- 40.Clemens MR, Ladner C, Schmidt H, Ehninger G, Einsele H, Buhler E, Waller HD, Gey KF. Decreased essential antioxidants and increased lipid hydroperoxides following high-dose radiochemotherapy. Free Radic Res Commun. 1989;7:227–232. doi: 10.3109/10715768909087946. [DOI] [PubMed] [Google Scholar]
- 41.Coggle JE, Lambert BE, Moores SR. Radiation effects in the lung. Environ Health Perspect. 1986;70:261–291. doi: 10.1289/ehp.8670261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conning DM, Phillips JC. Comparative metabolism of BHA, BHT and other phenolic antioxidants and its toxicological relevance. Food Chem Toxicol. 1986;24:1145–1148. doi: 10.1016/0278-6915(86)90300-5. [DOI] [PubMed] [Google Scholar]
- 43.Copland GM, Kolin A, Shulman HS. Fatal pulmonary intra-alveolar fibrosis after paraquat ingestion. N Engl J Med. 1974;291:290–292. doi: 10.1056/NEJM197408082910607. [DOI] [PubMed] [Google Scholar]
- 44.Cortijo J, Cerda-Nicolas M, Serrano A, Bioque G, Estrela JM, Santangelo F, Esteras A, Llombart-Bosch A, Morcillo EJ. Attenuation by oral N-acetylcysteine of bleomycin-induced lung injury in rats. Eur Respir J. 2001;17:1228–1235. doi: 10.1183/09031936.01.00049701. [DOI] [PubMed] [Google Scholar]
- 45.Cotgreave IA. N-acetylcysteine: pharmacological considerations and experimental and clinical applications. Adv Pharmacol. 1997;38:205–227. [PubMed] [Google Scholar]
- 46.Cotgreave IA, Duddy SK, Kass GE, Thompson D, Moldeus P. Studies on the anti-inflammatory activity of ebselen: ebselen interferes with granulocyte oxidative burst by dual inhibition of NADPH oxidase and protein kinase C? Biochem Pharmacol. 1989;38:649–656. doi: 10.1016/0006-2952(89)90211-6. [DOI] [PubMed] [Google Scholar]
- 47.Coultas DB, Zumwalt RE, Black WC, Sobonya RE. The epidemiology of interstitial lung diseases. Am J Respir Crit Care Med. 1994;150:967–972. doi: 10.1164/ajrccm.150.4.7921471. [DOI] [PubMed] [Google Scholar]
- 48.Daba MH, Abdel-Aziz AA, Moustafa AM, Al-Majed AA, Al-Shabanah OA, El-Kashef HA. Effects of L-carnitine and Ginkgo biloba extract (EG b 761) in experimental bleomycin-induced lung fibrosis. Pharmacol Res. 2002;45:461–467. doi: 10.1006/phrs.2002.0985. [DOI] [PubMed] [Google Scholar]
- 49.Dallessio JJ, McLaughlin GE, Frank L. Reduction of bleomycin-induced acute DNA injury in the rat lung by the 21-aminosteroid, U-74389G. Crit Care Med. 1997;25:652–656. doi: 10.1097/00003246-199704000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Day BJ. Catalytic antioxidants: a radical approach to new therapeutics. Drug Discov Today. 2004;9:557–566. doi: 10.1016/S1359-6446(04)03139-3. [DOI] [PubMed] [Google Scholar]
- 51.Day BJ, Batinic-Haberle I, Crapo JD. Metalloporphyrins are potent inhibitors of lipid peroxidation. Free Radic Biol Med. 1999;26:730–736. doi: 10.1016/s0891-5849(98)00261-5. [DOI] [PubMed] [Google Scholar]
- 52.Day BJ, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced lung injury in vivo. Toxicol Appl Pharmacol. 1996;140:94–100. doi: 10.1006/taap.1996.0201. [DOI] [PubMed] [Google Scholar]
- 53.Day BJ, Fridovich I, Crapo JD. Manganic porphyrins possess catalase activity and protect endothelial cells against hydrogen peroxide-mediated injury. Arch Biochem Biophys. 1997;347:256–262. doi: 10.1006/abbi.1997.0341. [DOI] [PubMed] [Google Scholar]
- 54.Day BJ, Kariya C. A novel class of cytochrome P450 reductase redox cyclers: cationic manganoporphyrins. Toxicol Sci. 2005;85:713–719. doi: 10.1093/toxsci/kfi108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Day BJ, Shawen S, Liochev SI, Crapo JD. A metalloporphyrin superoxide dismutase mimetic protects against paraquat-induced endothelial cell injury, in vitro. J Pharmacol Exp Ther. 1995;275:1227–1232. [PubMed] [Google Scholar]
- 56.Dechant KL, Noble S. Erdosteine. Drugs. 1996;52:875–881. doi: 10.2165/00003495-199652060-00009. discussion 882. [DOI] [PubMed] [Google Scholar]
- 57.Dede S, Mert H, Mert N, Yur F, Ertekin A, Deger Y. Effects of alpha-tocopherol on serum trace and major elements in rats with bleomycin-induced pulmonary fibrosis. Biol Trace Elem Res. 2006;114:175–184. doi: 10.1385/BTER:114:1:175. [DOI] [PubMed] [Google Scholar]
- 58.Deger Y, Yur F, Ertekin A, Mert N, Dede S, Mert H. Protective effect of alpha-tocopherol on oxidative stress in experimental pulmonary fibrosis in rats. Cell Biochem Funct. 2006 doi: 10.1002/cbf.1362. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 59.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73:119–131. doi: 10.1016/j.radonc.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 60.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, MacNee W, Thomeer M, Wallaert B, Laurent F, Nicholson AG, Verbeken EK, Verschakelen J, Flower CD, Capron F, Petruzzelli S, De Vuyst P, van den Bosch JM, Rodriguez-Becerra E, Corvasce G, Lankhorst I, Sardina M, Montanari M. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. [DOI] [PubMed] [Google Scholar]
- 61.Desai VG, Lyn-Cook LE, Aidoo A, Casciano DA, Feuers RJ. Modulation of antioxidant enzymes in bleomycin-treated rats by vitamin C and beta-carotene. Nutr Cancer. 1997;29:127–132. doi: 10.1080/01635589709514613. [DOI] [PubMed] [Google Scholar]
- 62.Dickinson DA, Forman HJ. Glutathione in defense and signaling: lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 63.Doctrow SR, Huffman K, Marcus CB, Musleh W, Bruce A, Baudry M, Malfroy B. Salen-manganese complexes: combined superoxide dismutase/catalase mimics with broad pharmacological efficacy. Adv Pharmacol. 1997;38:247–269. doi: 10.1016/s1054-3589(08)60987-4. [DOI] [PubMed] [Google Scholar]
- 64.Driscoll KE, Maurer JK, Higgins J, Poynter J. Alveolar macrophage cytokine and growth factor production in a rat model of crocidolite-induced pulmonary inflammation and fibrosis. J Toxicol Environ Health. 1995;46:155–169. doi: 10.1080/15287399509532026. [DOI] [PubMed] [Google Scholar]
- 65.Duijvestijn YC, Brand PL. Systematic review of N-acetyl-cysteine in cystic fibrosis. Acta Paediatr. 1999;88:38–41. doi: 10.1080/08035259950170574. [DOI] [PubMed] [Google Scholar]
- 66.Dusinska M, Kovacikova Z, Vallova B, Collins A. Responses of alveolar macrophages and epithelial type II cells to oxidative DNA damage caused by paraquat. Carcinogenesis. 1998;19:809–812. doi: 10.1093/carcin/19.5.809. [DOI] [PubMed] [Google Scholar]
- 67.El-Khatib AS, Moustafa AM, Abdel-Aziz AA, Al-Shabanah OA, El-Kashef HA. Ginkgo biloba extract (EGb 761) modulates bleomycin-induced acute lung injury in rats. Tumori. 2001;87:417–422. doi: 10.1177/030089160108700612. [DOI] [PubMed] [Google Scholar]
- 68.Epperly MW, Epstein CJ, Travis EL, Greenberger JS. Decreased pulmonary radiation resistance of manganese superoxide dismutase (MnSOD)-deficient mice is corrected by human manganese superoxide dismutase-plasmid/liposome (SOD2-PL) intratracheal gene therapy. Radiat Res. 2000;154:365–374. doi: 10.1667/0033-7587(2000)154[0365:dprrom]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 69.Evelo CT, Bos RP, Borm PJ. Decreased glutathione content and glutathione S-transferase activity in red blood cells of coal miners with early stages of pneumoconiosis. Br J Ind Med. 1993;50:633–636. doi: 10.1136/oem.50.7.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fajer J, Borg DC, Forman A, Dolphin D, Felton RH. pi-Cation radicals and indications of metalloporphyrins. J Am Chem Soc. 1970;92:3451–3459. doi: 10.1021/ja00714a038. [DOI] [PubMed] [Google Scholar]
- 71.Fattman CL, Tan RJ, Tobolewski JM, Oury TD. Increased sensitivity to asbestos-induced lung injury in mice lacking extracellular superoxide dismutase. Free Radic Biol Med. 2006;40:601–607. doi: 10.1016/j.freeradbiomed.2005.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fleckenstein K, Zgonjanin L, Chen L, Rabbani Z, Jackson IL, Thrasher B, Kirkpatrick J, Foster WM, Vujaskovic Z. Temporal onset of hypoxia and oxidative stress after pulmonary irradiation. Int J Radiat Oncol Biol Phys. 2007;68:196–204. doi: 10.1016/j.ijrobp.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Forman HJ, Torres M, Fukuto J. Redox signaling. Mol Cell Biochem. 2002;234–235:49–62. [PubMed] [Google Scholar]
- 74.Gao F, Kinnula VL, Myllärniemi M, Oury TD. Extracellular superoxide dismutase in pulmonary fibrosis. Antiox Redox Signal. 2008;10:000–000. doi: 10.1089/ars.2007.1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao SJ, Chen M, Lin W, Luo GM, Shen JC. Kinetic studies of abzyme with glutathione peroxidase activity. Ann N Y Acad Sci. 1998;864:280–283. doi: 10.1111/j.1749-6632.1998.tb10322.x. [DOI] [PubMed] [Google Scholar]
- 76.Gray JP, Heck DE, Mishin V, Smith PJ, Hong JY, Thiruchelvam M, Cory-Slechta DA, Laskin DL, Laskin JD. Paraquat increases cyanide-insensitive respiration in murine lung epithelial cells by activating an NAD(P)H:paraquat oxidoreductase: identification of the enzyme as thioredoxin reductase. J Biol Chem. 2007;282:7939–7949. doi: 10.1074/jbc.M611817200. [DOI] [PubMed] [Google Scholar]
- 77.Gulumian M. The role of oxidative stress in diseases caused by mineral dusts and fibres: current status and future of prophylaxis and treatment. Mol Cell Biochem. 1999;196:69–77. [PubMed] [Google Scholar]
- 78.Gutteridge JM. Biological origin of free radicals, and mechanisms of antioxidant protection. Chem Biol Interact. 1994;91:133–140. doi: 10.1016/0009-2797(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 79.Hagen TM, Brown LA, Jones DP. Protection against paraquat-induced injury by exogenous GSH in pulmonary alveolar type II cells. Biochem Pharmacol. 1986;35:4537–4542. doi: 10.1016/0006-2952(86)90776-8. [DOI] [PubMed] [Google Scholar]
- 80.Hagiwara SI, Ishii Y, Kitamura S. Aerosolized administration of N-acetylcysteine attenuates lung fibrosis induced by bleomycin in mice. Am J Respir Crit Care Med. 2000;162:225–231. doi: 10.1164/ajrccm.162.1.9903129. [DOI] [PubMed] [Google Scholar]
- 81.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Maruoka S, Horie T. Transforming growth factor-beta1 induces phenotypic modulation of human lung fibroblasts to myofibroblast through a c-Jun-NH2-terminal kinase-dependent pathway. Am J Respir Crit Care Med. 2001;163:152–157. doi: 10.1164/ajrccm.163.1.2005069. [DOI] [PubMed] [Google Scholar]
- 82.Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 83.Hoffer E, Avidor I, Benjaminov O, Shenker L, Tabak A, Tamir A, Merzbach D, Taitelman U. N-acetylcysteine delays the infiltration of inflammatory cells into the lungs of paraquat-intoxicated rats. Toxicol Appl Pharmacol. 1993;120:8–12. doi: 10.1006/taap.1993.1080. [DOI] [PubMed] [Google Scholar]
- 84.Hoshino T, Nakamura H, Okamoto M, Kato S, Araya S, Nomiyama K, Oizumi K, Young HA, Aizawa H, Yodoi J. Redox-active protein thioredoxin prevents proinflammatory cytokine- or bleomycin-induced lung injury. Am J Respir Crit Care Med. 2003;168:1075–1083. doi: 10.1164/rccm.200209-982OC. [DOI] [PubMed] [Google Scholar]
- 85.Huang SH, Leonard S, Shi X, Goins MR, Vallyathan V. Anti-oxidant activity of lazaroid (U-75412E) and its protective effects against crystalline silica-induced cytotoxicity. Free Radic Biol Med. 1998;24:529–536. doi: 10.1016/s0891-5849(97)00285-2. [DOI] [PubMed] [Google Scholar]
- 86.Huang X, Dong Z, Liu J, Mao S, Xu J, Luo G, Shen J. Selenium-mediated micellar catalyst: an efficient enzyme model for glutathione peroxidase-like catalysis. Langmuir. 2007;23:1518–1522. doi: 10.1021/la061727p. [DOI] [PubMed] [Google Scholar]
- 87.Hubbard R, Lewis S, Richards K, Johnston I, Britton J. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet. 1996;347:284–289. doi: 10.1016/s0140-6736(96)90465-1. [DOI] [PubMed] [Google Scholar]
- 88.Hyde DM, Giri SN, Schiedt MJ, Krishna GA. Effects of three cysteine pro-drugs on bleomycin-induced lung fibrosis in hamsters. Pathology. 1990;22:93–101. doi: 10.3109/00313029009063787. [DOI] [PubMed] [Google Scholar]
- 89.Iles KE, Dickinson DA, Watanabe N, Iwamoto T, Forman HJ. AP-1 activation through endogenous H(2)O(2) generation by alveolar macrophages. Free Radic Biol Med. 2002;32:1304–1313. doi: 10.1016/s0891-5849(02)00840-7. [DOI] [PubMed] [Google Scholar]
- 90.Ilizarov AM, Koo HC, Kazzaz JA, Mantell LL, Li Y, Bhapat R, Pollack S, Horowitz S, Davis JM. Overexpression of manganese superoxide dismutase protects lung epithelial cells against oxidant injury. Am J Respir Cell Mol Biol. 2001;24:436–441. doi: 10.1165/ajrcmb.24.4.4240. [DOI] [PubMed] [Google Scholar]
- 91.Inghilleri S, Morbini P, Oggionni T, Barni S, Fenoglio C. In situ assessment of oxidant and nitrogenic stress in bleomycin pulmonary fibrosis. Histochem Cell Biol. 2006;125:661–669. doi: 10.1007/s00418-005-0116-7. [DOI] [PubMed] [Google Scholar]
- 92.Inglesi M, Nicola M, Fregnan GB, Bradamante S, Pagani G. Synthesis and free radical scavenging properties of the enantiomers of erdosteine. Farmaco. 1994;40:703–708. [PubMed] [Google Scholar]
- 93.Iraz M, Erdogan H, Kotuk M, Yagmurca M, Kilic T, Ermis H, Fadillioglu E, Yildirim Z. Ginkgo biloba inhibits bleomycin-induced lung fibrosis in rats. Pharmacol Res. 2006;53:310–316. doi: 10.1016/j.phrs.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 94.Ishii K, Adachi J, Tomita M, Kurosaka M, Ueno Y. Oxysterols as indices of oxidative stress in man after paraquat ingestion. Free Radic Res. 2002;36:163–168. doi: 10.1080/10715760290006493. [DOI] [PubMed] [Google Scholar]
- 95.Itoh K, Ishii T, Wakabayashi N, Yamamoto M. Regulatory mechanisms of cellular response to oxidative stress. Free Radic Res. 1999;31:319–324. doi: 10.1080/10715769900300881. [DOI] [PubMed] [Google Scholar]
- 96.Jack CI, Cottier B, Jackson MJ, Cassapi L, Fraser WD, Hind CR. Indicators of free radical activity in patients developing radiation pneumonitis. Int J Radiat Oncol Biol Phys. 1996;34:149–154. doi: 10.1016/0360-3016(95)00209-x. [DOI] [PubMed] [Google Scholar]
- 97.Kachadourian R, Flaherty MM, Crumbliss AL, Patel M, Day BJ. Synthesis and in vitro antioxidant properties of manganese(III) beta-octabromo-meso-tetrakis(4-carboxyphenyl)porphyrin. J Inorg Biochem. 2003;95:240–248. doi: 10.1016/s0162-0134(03)00135-1. [DOI] [PubMed] [Google Scholar]
- 98.Kachadourian R, Johnson CA, Min E, Spasojevic I, Day BJ. Flavin-dependent antioxidant properties of a new series of meso-N,N′-dialkyl-imidazolium substituted manganese(III) porphyrins. Biochem Pharmacol. 2004;67:77–85. doi: 10.1016/j.bcp.2003.08.036. [DOI] [PubMed] [Google Scholar]
- 99.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, Samulski TS, Dewhirst MW, Anscher MS, Vujaskovic Z. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–1066. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 100.Kanoh S, Kobayashi H, Motoyoshi K. Exhaled ethane: an in vivo biomarker of lipid peroxidation in interstitial lung diseases. Chest. 2005;128:2387–2392. doi: 10.1378/chest.128.4.2387. [DOI] [PubMed] [Google Scholar]
- 101.Kappus H, Mahmutoglu I, Kostrucha J, Scheulen ME. Liver nuclear NADPH-cytochrome P-450 reductase may be involved in redox cycling of bleomycin-Fe(III), oxy radical formation and DNA damage. Free Radic Res Commun. 1987;2:271–277. doi: 10.3109/10715768709065291. [DOI] [PubMed] [Google Scholar]
- 102.Kasai H, Allen JT, Mason RM, Kamimura T, Zhang Z. TGF-beta1 induces human alveolar epithelial to mesenchymal cell transition (EMT) Respir Res. 2005;6:56. doi: 10.1186/1465-9921-6-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kaul N, Forman HJ. Activation of NF kappa B by the respiratory burst of macrophages. Free Radic Biol Med. 1996;21:401–405. doi: 10.1016/0891-5849(96)00178-5. [DOI] [PubMed] [Google Scholar]
- 104.Kawanami O, Jiang HX, Mochimaru H, Yoneyama H, Kudoh S, Ohkuni H, Ooami H, Ferrans VJ. Alveolar fibrosis and capillary alteration in experimental pulmonary silicosis in rats. Am J Respir Crit Care Med. 1995;151:1946–1955. doi: 10.1164/ajrccm.151.6.7767544. [DOI] [PubMed] [Google Scholar]
- 105.Kehrer JP, Lee YC, Smith RD. Effect of intratracheally administered anticancer drugs on lung hydroxyproline content. Toxicol Lett. 1986;30:63–70. doi: 10.1016/0378-4274(86)90180-3. [DOI] [PubMed] [Google Scholar]
- 106.Kennedy TP, Gordon GB, Paky A, McShane A, Adkinson NF, Jr, Peters SP, Friday K, Jackman W, Sciuto AM, Gurtner GH. Amiodarone causes acute oxidant lung injury in ventilated and perfused rabbit lungs. J Cardiovasc Pharmacol. 1988;12:23–36. doi: 10.1097/00005344-198807000-00004. [DOI] [PubMed] [Google Scholar]
- 107.Kilinc C, Ozcan O, Karaoz E, Sunguroglu K, Kutluay T, Karaca L. Vitamin E reduces bleomycin-induced lung fibrosis in mice: biochemical and morphological studies. J Basic Clin Physiol Pharmacol. 1993;4:249–269. doi: 10.1515/jbcpp.1993.4.3.249. [DOI] [PubMed] [Google Scholar]
- 108.Kim DS, Collard HR, King TE., Jr Classification and natural history of the idiopathic interstitial pneumonias. Proc Am Thorac Soc. 2006;3:285–292. doi: 10.1513/pats.200601-005TK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kinnula VL. Oxidant and antioxidant mechanisms of lung disease caused by asbestos fibres. Eur Respir J. 1999;14:706–716. doi: 10.1034/j.1399-3003.1999.14c35.x. [DOI] [PubMed] [Google Scholar]
- 110.Kinnula VL, Fattman CL, Tan RJ, Oury TD. Oxidative stress in pulmonary fibrosis: a possible role for redox modulatory therapy. Am J Respir Crit Care Med. 2005;172:417–422. doi: 10.1164/rccm.200501-017PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koli K, Myllärniemi M, Keski-Oja J, Kinnula VL. Transforming growth factor-β activation in the lung: focus on fibrosis and reactive oxygen species. Antioxid Redos Signal. 2008;10:000–000. doi: 10.1089/ars.2007.1914. [DOI] [PubMed] [Google Scholar]
- 112.Kondo Y, Woo ES, Michalska AE, Choo KH, Lazo JS. Metallothionein null cells have increased sensitivity to anticancer drugs. Cancer Res. 1995;55:2021–2023. [PubMed] [Google Scholar]
- 113.Kornbrust DJ, Mavis RD. The effect of paraquat on microsomal lipid peroxidation in vitro and in vivo. Toxicol Appl Pharmacol. 1980;53:323–332. doi: 10.1016/0041-008x(80)90433-0. [DOI] [PubMed] [Google Scholar]
- 114.Kotsonis P, Frohlich LG, Shutenko ZV, Horejsi R, Pfleiderer W, Schmidt HH. Allosteric regulation of neuronal nitric oxide synthase by tetrahydrobiopterin and suppression of auto-damaging superoxide. Biochem J. 2000;346:767–776. [PMC free article] [PubMed] [Google Scholar]
- 115.Kukreja RC, Kontos HA, Hess ML, Ellis EF. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ Res. 1986;59:612–619. doi: 10.1161/01.res.59.6.612. [DOI] [PubMed] [Google Scholar]
- 116.Langan AR, Khan MA, Yeung IW, Van Dyk J, Hill RP. Partial volume rat lung irradiation: the protective/mitigating effects of Eukarion-189, a superoxide dismutase-catalase mimetic. Radiother Oncol. 2006;79:231–238. doi: 10.1016/j.radonc.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 117.Lazo JS, Humphreys CJ. Lack of metabolism as the biochemical basis of bleomycin-induced pulmonary toxicity. Proc Natl Acad Sci U S A. 1983;80:3064–3068. doi: 10.1073/pnas.80.10.3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ledwozyw A. Protective effect of liposome-entrapped superoxide dismutase and catalase on bleomycin-induced lung injury in rats, I: antioxidant enzyme activities and lipid peroxidation. Acta Vet Hung. 1991;39:215–224. [PubMed] [Google Scholar]
- 119.Ledwozyw A. Protective effect of liposome-entrapped superoxide dismutase and catalase on bleomycin-induced lung injury in rats, II: phospholipids of the lung surfactant. Acta Physiol Hung. 1991;78:157–162. [PubMed] [Google Scholar]
- 120.Leeder RG, Brien JF, Massey TE. Investigation of the role of oxidative stress in amiodarone-induced pulmonary toxicity in the hamster. Can J Physiol Pharmacol. 1994;72:613–621. doi: 10.1139/y94-087. [DOI] [PubMed] [Google Scholar]
- 121.Lenz AG, Costabel U, Maier KL. Oxidized BAL fluid proteins in patients with interstitial lung diseases. Eur Respir J. 1996;9:307–312. doi: 10.1183/09031936.96.09020307. [DOI] [PubMed] [Google Scholar]
- 122.Liang LP, Huang J, Fulton R, Day BJ, Patel M. An orally active catalytic metalloporphyrin protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine neurotoxicity in vivo. J Neurosci. 2007;27:4326–4333. doi: 10.1523/JNEUROSCI.0019-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Liu H, Zhang H, Forman HJ. Silica induces macrophage cytokines through phosphatidylcholine-specific phospholipase C with hydrogen peroxide. Am J Respir Cell Mol Biol. 2007;36:594–599. doi: 10.1165/rcmb.2006-0297OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu J, Luo G, Ren X, Mu Y, Bai Y, Shen J. A bis-cyclodextrin diselenide with glutathione peroxidase-like activity. Biochim Biophys Acta. 2000;1481:222–228. doi: 10.1016/s0167-4838(00)00130-8. [DOI] [PubMed] [Google Scholar]
- 125.Lombard-Gillooly K, Hubbard AK. Modulation of silica-induced lung injury by reducing lung non-protein sulfhydryls with buthionine sulfoximine. Toxicol Lett. 1993;66:305–315. doi: 10.1016/0378-4274(93)90012-m. [DOI] [PubMed] [Google Scholar]
- 126.Malaker K, Das RM. Effect of superoxide dismutase on early radiation injury of lungs in the rat. Mol Cell Biochem. 1988;84:141–145. doi: 10.1007/BF00421048. [DOI] [PubMed] [Google Scholar]
- 127.Mannino DM, Etzel RA, Parrish RG. Pulmonary fibrosis deaths in the United States, 1979–1991: an analysis of multiple-cause mortality data. Am J Respir Crit Care Med. 1996;153:1548–1552. doi: 10.1164/ajrccm.153.5.8630600. [DOI] [PubMed] [Google Scholar]
- 128.Masumoto H, Sies H. The reaction of ebselen with peroxynitrite. Chem Res Toxicol. 1996;9:262–267. doi: 10.1021/tx950115u. [DOI] [PubMed] [Google Scholar]
- 129.Mata M, Ruiz A, Cerda M, Martinez-Losa M, Cortijo J, Santangelo F, Serrano-Mollar A, Llombart-Bosch A, Morcillo EJ. Oral N-acetylcysteine reduces bleomycin-induced lung damage and mucin Muc5ac expression in rats. Eur Respir J. 2003;22:900–905. doi: 10.1183/09031936.03.00018003. [DOI] [PubMed] [Google Scholar]
- 130.McCord JM. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985;312:159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- 131.McCord JM, Fridovich I. The biology and pathology of oxygen radicals. Ann Intern Med. 1978;89:122–127. doi: 10.7326/0003-4819-89-1-122. [DOI] [PubMed] [Google Scholar]
- 132.McLaughlin GE, Frank L. Effects of the 21-aminosteroid, U74389F, on bleomycin-induced pulmonary fibrosis in rats. Crit Care Med. 1994;22:313–319. doi: 10.1097/00003246-199402000-00024. [DOI] [PubMed] [Google Scholar]
- 133.Meyer A, Buhl R, Kampf S, Magnussen H. Intravenous N-acetylcysteine and lung glutathione of patients with pulmonary fibrosis and normals. Am J Respir Crit Care Med. 1995;152:1055–1060. doi: 10.1164/ajrccm.152.3.7663783. [DOI] [PubMed] [Google Scholar]
- 134.Meyer A, Buhl R, Magnussen H. The effect of oral N-acetyl-cysteine on lung glutathione levels in idiopathic pulmonary fibrosis. Eur Respir J. 1994;7:431–436. doi: 10.1183/09031936.94.07030431. [DOI] [PubMed] [Google Scholar]
- 135.Minakata K, Suzuki O, Saito S, Harada N. Ascorbate radical levels in human sera and rat plasma intoxicated with paraquat and diquat. Arch Toxicol. 1993;67:126–130. doi: 10.1007/BF01973683. [DOI] [PubMed] [Google Scholar]
- 136.Mitchell JB, Krishna MC, Kuppusamy P, Cook JA, Russo A. Protection against oxidative stress by nitroxides. Exp Biol Med (Maywood) 2001;226:620–621. doi: 10.1177/153537020222600703. [DOI] [PubMed] [Google Scholar]
- 137.Mossman BT, Janssen YM, Marsh JP, Sesko A, Shatos MA, Doherty J, Adler KB, Hemenway D, Mickey R, Vacek, et al. Development and characterization of a rapid-onset rodent inhalation model of asbestosis for disease prevention. Toxicol Pathol. 1991;19:412–418. doi: 10.1177/0192623391019004-110. [DOI] [PubMed] [Google Scholar]
- 138.Mossman BT, Marsh JP. Evidence supporting a role for active oxygen species in asbestos-induced toxicity and lung disease. Environ Health Perspect. 1989;81:91–94. doi: 10.1289/ehp.898191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mossman BT, Marsh JP, Sesko A, Hill S, Shatos MA, Doherty J, Petruska J, Adler KB, Hemenway D, Mickey R, Vacek P, Kagan E. Inhibition of lung injury, inflammation, and interstitial pulmonary fibrosis by polyethylene glycol-conjugated catalase in a rapid inhalation model of asbestosis. Am Rev Respir Dis. 1990;141:1266–1271. doi: 10.1164/ajrccm/141.5_Pt_1.1266. [DOI] [PubMed] [Google Scholar]
- 140.Mu Y, Lv S, Ren X, Jin G, Liu J, Yan G, Li W, Shen J, Luo G. UV-B induced keratinocyte apoptosis is blocked by 2-selenium-bridged beta-cyclodextrin, a GPX mimic. J Photochem Photobiol B. 2003;69:7–12. doi: 10.1016/s1011-1344(02)00386-x. [DOI] [PubMed] [Google Scholar]
- 141.Muller I, Niethammer D, Bruchelt G. Anthracycline-derived chemotherapeutics in apoptosis and free radical cytotoxicity [Review] Int J Mol Med. 1998;1:491–494. doi: 10.3892/ijmm.1.2.491. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura H, Sato S, Takahashi K. Effects of vitamin E deficiency on bleomycin-induced pulmonary fibrosis in the hamster. Lung. 1988;166:161–176. doi: 10.1007/BF02714044. [DOI] [PubMed] [Google Scholar]
- 143.Neal R, Matthews RH, Lutz P, Ercal N. Antioxidant role of N-acetyl cysteine isomers following high dose irradiation. Free Radic Biol Med. 2003;34:689–695. doi: 10.1016/s0891-5849(02)01372-2. [DOI] [PubMed] [Google Scholar]
- 144.Nici L, Calabresi P. Amifostine modulation of bleomycin-induced lung injury in rodents. Semin Oncol. 1999;26:28–33. [PubMed] [Google Scholar]
- 145.Nici L, Santos-Moore A, Kuhn C, Calabresi P. Modulation of bleomycin-induced pulmonary toxicity in the hamster by the antioxidant amifostine. Cancer. 1998;83:2008–2014. [PubMed] [Google Scholar]
- 146.Nicolescu AC, Comeau JL, Hill BC, Bedard LL, Takahashi T, Brien JF, Racz WJ, Massey TE. Aryl radical involvement in amiodarone-induced pulmonary toxicity: investigation of protection by spin-trapping nitrones. Toxicol Appl Pharmacol. 2007;220:60–71. doi: 10.1016/j.taap.2006.12.031. [DOI] [PubMed] [Google Scholar]
- 147.Ogata H, Luo XX, Xu X. Comparison of effects of margarite extract and recombinant human superoxide dismutase on paraquat-induced superoxide anion radicals in rat lung. Circ Shock. 1994;43:161–165. [PubMed] [Google Scholar]
- 148.Oury TD, Thakker K, Menache M, Chang LY, Crapo JD, Day BJ. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am J Respir Cell Mol Biol. 2001;25:164–169. doi: 10.1165/ajrcmb.25.2.4235. [DOI] [PubMed] [Google Scholar]
- 149.Pasternack RFSW. Catalysis of the disproportionation of super-oxide by metalloporphyrins. J Inorgan Biochem. 1979;11:261–267. doi: 10.1016/s0162-0134(00)80022-7. [DOI] [PubMed] [Google Scholar]
- 150.Phan SH, Fantone JC. Inhibition of bleomycin-induced pulmonary fibrosis by lipopolysaccharide. Lab Invest. 1984;50:587–591. [PubMed] [Google Scholar]
- 151.Psathakis K, Mermigkis D, Papatheodorou G, Loukides S, Panagou P, Polychronopoulos V, Siafakas NM, Bouros D. Exhaled markers of oxidative stress in idiopathic pulmonary fibrosis. Eur J Clin Invest. 2006;36:362–367. doi: 10.1111/j.1365-2362.2006.01636.x. [DOI] [PubMed] [Google Scholar]
- 152.Punithavathi D, Venkatesan N, Babu M. Curcumin inhibition of bleomycin-induced pulmonary fibrosis in rats. Br J Pharmacol. 2000;131:169–172. doi: 10.1038/sj.bjp.0703578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rabbani ZN, Batinic-Haberle I, Anscher MS, Huang J, Day BJ, Alexander E, Dewhirst MW, Vujaskovic Z. Long-term administration of a small molecular weight catalytic metalloporphyrin antioxidant, AEOL 10150, protects lungs from radiation-induced injury. Int J Radiat Oncol Biol Phys. 2007;67:573–580. doi: 10.1016/j.ijrobp.2006.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 155.Rahman I, Biswas SK, Kode A. Oxidant and antioxidant balance in the airways and airway diseases. Eur J Pharmacol. 2006;533:222–239. doi: 10.1016/j.ejphar.2005.12.087. [DOI] [PubMed] [Google Scholar]
- 156.Rahman I, Kilty I. Antioxidant therapeutic targets in COPD. Curr Drug Targets. 2006;7:707–720. doi: 10.2174/138945006777435254. [DOI] [PubMed] [Google Scholar]
- 157.Rahman I, Skwarska E, Henry M, Davis M, O’Connor CM, FitzGerald MX, Greening A, MacNee W. Systemic and pulmonary oxidative stress in idiopathic pulmonary fibrosis. Free Radic Biol Med. 1999;27:60–68. doi: 10.1016/s0891-5849(99)00035-0. [DOI] [PubMed] [Google Scholar]
- 158.Rahman I, Yang SR, Biswas SK. Current concepts of redox signaling in the lungs. Antioxid Redox Signal. 2006;8:681–689. doi: 10.1089/ars.2006.8.681. [DOI] [PubMed] [Google Scholar]
- 159.Ramakrishnan N, Kalinich JF, McClain DE. Ebselen inhibition of apoptosis by reduction of peroxides. Biochem Pharmacol. 1996;51:1443–1451. doi: 10.1016/0006-2952(96)00084-6. [DOI] [PubMed] [Google Scholar]
- 160.Reddy JK, Rao MS. Oxidative DNA damage caused by persistent peroxisome proliferation: its role in hepatocarcinogenesis. Mutat Res. 1989;214:63–68. doi: 10.1016/0027-5107(89)90198-x. [DOI] [PubMed] [Google Scholar]
- 161.Ren X, Liu J, Luo G, Zhang Y, Luo Y, Yan G, Shen J. A novel selenocystine-beta-cyclodextrin conjugate that acts as a glutathione peroxidase mimic. Bioconjug Chem. 2000;11:682–687. doi: 10.1021/bc0000076. [DOI] [PubMed] [Google Scholar]
- 162.Ren X, Xue Y, Zhang K, Liu J, Luo G, Zheng J, Mu Y, Shen J. A novel dicyclodextrinyl ditelluride compound with antioxidant activity. FEBS Lett. 2001;507:377–380. doi: 10.1016/s0014-5793(01)03011-3. [DOI] [PubMed] [Google Scholar]
- 163.Rosa RM, Sulzbacher K, Picada JN, Roesler R, Saffi J, Brendel M, Henriques JA. Genotoxicity of diphenyl diselenide in bacteria and yeast. Mutat Res. 2004;563:107–115. doi: 10.1016/j.mrgentox.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 164.Ross MH, Murray J. Occupational respiratory disease in mining. Occup Med (Lond) 2004;54:304–310. doi: 10.1093/occmed/kqh073. [DOI] [PubMed] [Google Scholar]
- 165.Rostock RA, Stryker JA, Abt AB. Evaluation of high-dose vitamin E as a radioprotective agent. Radiology. 1980;136:763–766. doi: 10.1148/radiology.136.3.7403557. [DOI] [PubMed] [Google Scholar]
- 166.Rottoli P, Magi B, Cianti R, Bargagli E, Vagaggini C, Nikiforakis N, Pallini V, Bini L. Carbonylated proteins in bronchoalveolar lavage of patients with sarcoidosis, pulmonary fibrosis associated with systemic sclerosis and idiopathic pulmonary fibrosis. Proteomics. 2005;5:2612–2618. doi: 10.1002/pmic.200401206. [DOI] [PubMed] [Google Scholar]
- 167.Russo A, Mitchell JB, McPherson S, Friedman N. Alteration of bleomycin cytotoxicity by glutathione depletion or elevation. Int J Radiat Oncol Biol Phys. 1984;10:1675–1678. doi: 10.1016/0360-3016(84)90526-1. [DOI] [PubMed] [Google Scholar]
- 168.Safayhi H, Tiegs G, Wendel A. A novel biologically active seleno-organic compound V: inhibition by ebselen (PZ 51) of rat peritoneal neutrophil lipoxygenase. Biochem Pharmacol. 1985;34:2691–2694. doi: 10.1016/0006-2952(85)90569-6. [DOI] [PubMed] [Google Scholar]
- 169.Sanchez-Capelo A. Dual role for TGF-beta1 in apoptosis. Cytokine Growth Factor Rev. 2005;16:15–34. doi: 10.1016/j.cytogfr.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 170.Sata T, Kubota E, Misra HP, Mojarad M, Pakbaz H, Said SI. Paraquat-induced lung injury: prevention by N-tert-butyl-alpha-phenylnitrone, a free-radical spin-trapping agent. Am J Physiol. 1992;262:L147–L152. doi: 10.1152/ajplung.1992.262.2.L147. [DOI] [PubMed] [Google Scholar]
- 171.Sawyer RT, Dobis DR, Goldstein M, Velsor L, Maier LA, Fontenot AP, Silveira L, Newman LS, Day BJ. Beryllium-stimulated reactive oxygen species and macrophage apoptosis. Free Radic Biol Med. 2005;38:928–937. doi: 10.1016/j.freeradbiomed.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 172.Schapira RM, Ghio AJ, Effros RM, Morrisey J, Almagro UA, Dawson CA, Hacker AD. Hydroxyl radical production and lung injury in the rat following silica or titanium dioxide instillation in vivo. Am J Respir Cell Mol Biol. 1995;12:220–226. doi: 10.1165/ajrcmb.12.2.7865220. [DOI] [PubMed] [Google Scholar]
- 173.Schwartz DA, Van Fossen DS, Davis CS, Helmers RA, Dayton CS, Burmeister LF, Hunninghake GW. Determinants of progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1994;149:444–449. doi: 10.1164/ajrccm.149.2.8306043. [DOI] [PubMed] [Google Scholar]
- 174.Scurlock R, Rougee M, Bensasson RV, Evers M, Dereu N. Deactivation of singlet molecular oxygen by organo-selenium compounds exhibiting glutathione peroxidase activity and by sulfur-containing homologs. Photochem Photobiol. 1991;54:733–736. doi: 10.1111/j.1751-1097.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- 175.Sener G, Kabasakal L, Atasoy BM, Erzik C, Velioglu-Ogunc A, Cetinel S, Gedik N, Yegen BC. Ginkgo biloba extract protects against ionizing radiation-induced oxidative organ damage in rats. Pharmacol Res. 2006;53:241–252. doi: 10.1016/j.phrs.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 176.Serrano-Mollar A, Closa D, Prats N, Blesa S, Martinez-Losa M, Cortijo J, Estrela JM, Morcillo EJ, Bulbena O. In vivo anti-oxidant treatment protects against bleomycin-induced lung damage in rats. Br J Pharmacol. 2003;138:1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shahzeidi S, Sarnstrand B, Jeffery PK, McAnulty RJ, Laurent GJ. Oral N-acetylcysteine reduces bleomycin-induced collagen deposition in the lungs of mice. Eur Respir J. 1991;4:845–852. [PubMed] [Google Scholar]
- 178.Sharpe MA, Ollosson R, Stewart VC, Clark JB. Oxidation of nitric oxide by oxomanganese-salen complexes: a new mechanism for cellular protection by superoxide dismutase/catalase mimetics. Biochem J. 2002;366:97–107. doi: 10.1042/BJ20020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med. 1993;14:313–323. doi: 10.1016/0891-5849(93)90028-s. [DOI] [PubMed] [Google Scholar]
- 180.Sime PJ, Xing Z, Graham FL, Csaky KG, Gauldie J. Aden-ovector-mediated gene transfer of active transforming growth factor-beta1 induces prolonged severe fibrosis in rat lung. J Clin Invest. 1997;100:768–776. doi: 10.1172/JCI119590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Smith P. A light- and electron-microscope study of the pulmonary lesions induced in rats by paraquat. J Pathol. 1971;104:vii. [PubMed] [Google Scholar]
- 182.Smith P, Heath D, Kay JM. The pathogenesis and structure of paraquat-induced pulmonary fibrosis in rats. J Pathol. 1974;114:57–67. doi: 10.1002/path.1711140202. [DOI] [PubMed] [Google Scholar]
- 183.Sobol SM, Rakita L. Pneumonitis and pulmonary fibrosis associated with amiodarone treatment: a possible complication of a new antiarrhythmic drug. Circulation. 1982;65:819–824. doi: 10.1161/01.cir.65.4.819. [DOI] [PubMed] [Google Scholar]
- 184.Sogut S, Ozyurt H, Armutcu F, Kart L, Iraz M, Akyol O, Ozen S, Kaplan S, Temel I, Yildirim Z. Erdosteine prevents bleomycin-induced pulmonary fibrosis in rats. Eur J Pharmacol. 2004;494:213–220. doi: 10.1016/j.ejphar.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 185.Sturrock A, Cahill B, Norman K, Huecksteadt TP, Hill K, Sanders K, Karwande SV, Stringham JC, Bull DA, Gleich M, Kennedy TP, Hoidal JR. Transforming growth factor-beta1 induces Nox4 NAD(P)H oxidase and reactive oxygen species-dependent proliferation in human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L661–L673. doi: 10.1152/ajplung.00269.2005. [DOI] [PubMed] [Google Scholar]
- 186.Sun Y, Mu Y, Ma S, Gong P, Yan G, Liu J, Shen J, Luo G. The molecular mechanism of protecting cells against oxidative stress by 2-selenium-bridged beta-cyclodextrin with glutathione peroxidase activity. Biochim Biophys Acta. 2005;1743:199–204. doi: 10.1016/j.bbamcr.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 187.Suntres ZE, Shek PN. Intratracheally administered liposomal alpha-tocopherol protects the lung against long-term toxic effects of paraquat. Biomed Environ Sci. 1995;8:289–300. [PubMed] [Google Scholar]
- 188.Suntres ZE, Shek PN. Protective effect of liposomal alpha-tocopherol against bleomycin-induced lung injury. Biomed Environ Sci. 1997;10:47–59. [PubMed] [Google Scholar]
- 189.Suzuki YJ, Forman HJ, Sevanian A. Oxidants as stimulators of signal transduction. Free Radic Biol Med. 1997;22:269–285. doi: 10.1016/s0891-5849(96)00275-4. [DOI] [PubMed] [Google Scholar]
- 190.Swartz HM. Principles of the metabolism of nitroxides and their implications for spin trapping. Free Radic Res Commun. 1990;9:399–405. doi: 10.3109/10715769009145700. [DOI] [PubMed] [Google Scholar]
- 191.Szabo C, Day BJ, Salzman AL. Evaluation of the relative contribution of nitric oxide and peroxynitrite to the suppression of mitochondrial respiration in immunostimulated macrophages using a manganese mesoporphyrin superoxide dismutase mimetic and peroxynitrite scavenger. FEBS Lett. 1996;381:82–86. doi: 10.1016/0014-5793(96)00087-7. [DOI] [PubMed] [Google Scholar]
- 192.Szabo L, Matkovics B, Barabas K, Oroszlan G. Effects of various thiols on paraquat toxicity. Comp Biochem Physiol C. 1986;83:149–153. doi: 10.1016/0742-8413(86)90028-9. [DOI] [PubMed] [Google Scholar]
- 193.Tamagawa K, Taooka Y, Maeda A, Hiyama K, Ishioka S, Yamakido M. Inhibitory effects of a lecithinized superoxide dismutase on bleomycin-induced pulmonary fibrosis in mice. Am J Respir Crit Care Med. 2000;161:1279–1284. doi: 10.1164/ajrccm.161.4.9906099. [DOI] [PubMed] [Google Scholar]
- 194.Tamasi V, Jeffries JM, Arteel GE, Falkner KC. Ebselen augments its peroxidase activity by inducing nrf-2-dependent transcription. Arch Biochem Biophys. 2004;431:161–168. doi: 10.1016/j.abb.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 195.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 196.Terasaki Y, Akuta T, Terasaki M, Sawa T, Mori T, Okamoto T, Ozaki M, Takeya M, Akaike T. Guanine nitration in idiopathic pulmonary fibrosis and its implication for carcinogenesis. Am J Respir Crit Care Med. 2006;174:665–673. doi: 10.1164/rccm.200510-1580OC. [DOI] [PubMed] [Google Scholar]
- 197.Thanislass J, Raveendran M, Devaraj H. Buthionine sulfoximine-induced glutathione depletion: its effect on antioxidants, lipid peroxidation and calcium homeostasis in the lung. Biochem Pharmacol. 1995;50:229–234. doi: 10.1016/0006-2952(95)00123-h. [DOI] [PubMed] [Google Scholar]
- 198.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem. 1995;270:30334–30338. doi: 10.1074/jbc.270.51.30334. [DOI] [PubMed] [Google Scholar]
- 199.Thannickal VJ, Toews GB, White ES, Lynch JP, 3rd, Martinez FJ. Mechanisms of pulmonary fibrosis. Annu Rev Med. 2004;55:395–417. doi: 10.1146/annurev.med.55.091902.103810. [DOI] [PubMed] [Google Scholar]
- 200.Thresiamma KC, George J, Kuttan R. Protective effect of curcumin, ellagic acid and bixin on radiation induced toxicity. Indian J Exp Biol. 1996;34:845–847. [PubMed] [Google Scholar]
- 201.Tomioka H, Kuwata Y, Imanaka K, Hashimoto K, Ohnishi H, Tada K, Sakamoto H, Iwasaki H. A pilot study of aerosolized N-acetylcysteine for idiopathic pulmonary fibrosis. Respirology. 2005;10:449–455. doi: 10.1111/j.1440-1843.2005.00725.x. [DOI] [PubMed] [Google Scholar]
- 202.Toner PG, Vetters JM, Spilg WG, Harland WA. Fine structure of the lung lesion in a case of paraquat poisoning. J Pathol. 1970;102:182–185. doi: 10.1002/path.1711020311. [DOI] [PubMed] [Google Scholar]
- 203.Tuvlin JA, Kane SV. Novel therapies in the treatment of ulcerative colitis. Expert Opin Investig Drugs. 2003;12:483–490. doi: 10.1517/13543784.12.3.483. [DOI] [PubMed] [Google Scholar]
- 204.Vallyathan V, Castranova V, Pack D, Leonard S, Shumaker J, Hubbs AF, Shoemaker DA, Ramsey DM, Pretty JR, McLaurin JL, et al. Freshly fractured quartz inhalation leads to enhanced lung injury and inflammation: potential role of free radicals. Am J Respir Crit Care Med. 1995;152:1003–1009. doi: 10.1164/ajrccm.152.3.7663775. [DOI] [PubMed] [Google Scholar]
- 205.Vallyathan V, Leonard S, Kuppusamy P, Pack D, Chzhan M, Sanders SP, Zweir JL. Oxidative stress in silicosis: evidence for the enhanced clearance of free radicals from whole lungs. Mol Cell Biochem. 1997;168:125–132. doi: 10.1023/a:1006850920080. [DOI] [PubMed] [Google Scholar]
- 206.Venkatesan N. Pulmonary protective effects of curcumin against paraquat toxicity. Life Sci. 2000;66:PL21–L28. doi: 10.1016/s0024-3205(99)00576-7. [DOI] [PubMed] [Google Scholar]
- 207.Venkatesan N, Punithavathi V, Chandrakasan G. Curcumin protects bleomycin-induced lung injury in rats. Life Sci. 1997;61:PL51–PL58. doi: 10.1016/s0024-3205(97)00443-8. [DOI] [PubMed] [Google Scholar]
- 208.Vereckei A, Blazovics A, Gyorgy I, Feher E, Toth M, Szenasi G, Zsinka A, Foldiak G, Feher J. The role of free radicals in the pathogenesis of amiodarone toxicity. J Cardiovasc Electrophysiol. 1993;4:161–177. doi: 10.1111/j.1540-8167.1993.tb01220.x. [DOI] [PubMed] [Google Scholar]
- 209.Vujaskovic Z, Anscher MS, Feng QF, Rabbani ZN, Amin K, Samulski TS, Dewhirst MW, Haroon ZA. Radiation-induced hypoxia may perpetuate late normal tissue injury. Int J Radiat Oncol Biol Phys. 2001;50:851–855. doi: 10.1016/s0360-3016(01)01593-0. [DOI] [PubMed] [Google Scholar]
- 210.Vujaskovic Z, Batinic-Haberle I, Rabbani ZN, Feng QF, Kang SK, Spasojevic I, Samulski TV, Fridovich I, Dewhirst MW, Anscher MS. A small molecular weight catalytic metalloporphyrin antioxidant with superoxide dismutase (SOD) mimetic properties protects lungs from radiation-induced injury. Free Radic Biol Med. 2002;33:857–863. doi: 10.1016/s0891-5849(02)00980-2. [DOI] [PubMed] [Google Scholar]
- 211.Vujaskovic Z, Feng QF, Rabbani ZN, Samulski TV, Anscher MS, Brizel DM. Assessment of the protective effect of amifostine on radiation-induced pulmonary toxicity. Exp Lung Res. 2002;28:577–590. doi: 10.1080/01902140290096791. [DOI] [PubMed] [Google Scholar]
- 212.Waghray M, Cui Z, Horowitz JC, Subramanian IM, Martinez FJ, Toews GB, Thannickal VJ. Hydrogen peroxide is a diffusible paracrine signal for the induction of epithelial cell death by activated myofibroblasts. FASEB J. 2005;19:854–856. doi: 10.1096/fj.04-2882fje. [DOI] [PubMed] [Google Scholar]
- 213.Wallach-Dayan SB, Izbicki G, Cohen PY, Gerstl-Golan R, Fine A, Breuer R. Bleomycin initiates apoptosis of lung epithelial cells by ROS but not by Fas/FasL pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L790–L796. doi: 10.1152/ajplung.00300.2004. [DOI] [PubMed] [Google Scholar]
- 214.Walter N, Collard HR, King TE., Jr Current perspectives on the treatment of idiopathic pulmonary fibrosis. Proc Am Thorac Soc. 2006;3:330–338. doi: 10.1513/pats.200602-016TK. [DOI] [PubMed] [Google Scholar]
- 215.Wasserman B, Block ER. Prevention of acute paraquat toxicity in rats by superoxide dismutase. Aviat Space Environ Med. 1978;49:805–809. [PubMed] [Google Scholar]
- 216.Wegener T, Sandhagen B, Chan KW, Saldeen T. N-acetyl-cysteine in paraquat toxicity: toxicological and histological evaluation in rats. Ups J Med Sci. 1988;93:81–89. doi: 10.1517/03009734000000041. [DOI] [PubMed] [Google Scholar]
- 217.Wiegman EM, van Gameren MM, Kampinga HH, Szabo BG, Coppes RP. Post-irradiation dietary vitamin E does not affect the development of radiation-induced lung damage in rats. Radiother Oncol. 2004;72:67–70. doi: 10.1016/j.radonc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 218.Wilhelm J, Frydrychova M, Vizek M. Hydrogen peroxide in the breath of rats: the effects of hypoxia and paraquat. Physiol Res. 1999;48:445–449. [PubMed] [Google Scholar]
- 219.Xaubet A, Marin-Arguedas A, Lario S, Ancochea J, Morell F, Ruiz-Manzano J, Rodriguez-Becerra E, Rodriguez-Arias JM, Inigo P, Sanz S, Campistol JM, Mullol J, Picado C. Transforming growth factor-beta1 gene polymorphisms are associated with disease progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2003;168:431–435. doi: 10.1164/rccm.200210-1165OC. [DOI] [PubMed] [Google Scholar]
- 220.Yamano Y, Kagawa J, Hanaoka T, Takahashi T, Kasai H, Tsugane S, Watanabe S. Oxidative DNA damage induced by silica in vivo. Environ Res. 1995;69:102–107. doi: 10.1006/enrs.1995.1031. [DOI] [PubMed] [Google Scholar]
- 221.Yamazaki C, Hoshino J, Hori Y, Sekiguchi T, Miyauchi S, Mizuno S, Horie K. Effect of lecithinized-superoxide dismutase on the interstitial pneumonia model induced by bleomycin in mice. Jpn J Pharmacol. 1997;75:97–100. doi: 10.1254/jjp.75.97. [DOI] [PubMed] [Google Scholar]
- 222.Yang IV, Burch LH, Steele MP, Savov JD, Hollingsworth JW, McElvania-Tekippe E, Berman KG, Speer MC, Sporn TA, Brown KK, Schwarz MI, Schwartz DA. Gene expression profiling of familial and sporadic interstitial pneumonia. Am J Respir Crit Care Med. 2007;175:45–54. doi: 10.1164/rccm.200601-062OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Yeh ST, Guo HR, Su YS, Lin HJ, Hou CC, Chen HM, Chang MC, Wang YJ. Protective effects of N-acetylcysteine treatment post acute paraquat intoxication in rats and in human lung epithelial cells. Toxicology. 2006;223:181–190. doi: 10.1016/j.tox.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 224.Yildirim Z, Kotuk M, Iraz M, Kuku I, Ulu R, Armutcu F, Ozen S. Attenuation of bleomycin-induced lung fibrosis by oral sulfhydryl containing antioxidants in rats: erdosteine and N-acetylcysteine. Pulmon Pharmacol Ther. 2005;18:367–373. doi: 10.1016/j.pupt.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 225.Yu TW, Anderson D. Reactive oxygen species-induced DNA damage and its modification: a chemical investigation. Mutat Res. 1997;379:201–210. doi: 10.1016/s0027-5107(97)00141-3. [DOI] [PubMed] [Google Scholar]
- 226.Zembowicz A, Hatchett RJ, Radziszewski W, Gryglewski RJ. Inhibition of endothelial nitric oxide synthase by ebselen: prevention by thiols suggests the inactivation by ebselen of a critical thiol essential for the catalytic activity of nitric oxide synthase. J Pharmacol Exp Ther. 1993;267:1112–1118. [PubMed] [Google Scholar]
- 227.Zhang K, Phan SH. Cytokines and pulmonary fibrosis. Biol Signals. 1996;5:232–239. doi: 10.1159/000109195. [DOI] [PubMed] [Google Scholar]
- 228.Zimmerman MS, Ruckdeschel JC, Hussain M. Chemotherapy-induced interstitial pneumonitis during treatment of small cell anaplastic lung cancer. J Clin Oncol. 1984;2:396–405. doi: 10.1200/JCO.1984.2.5.396. [DOI] [PubMed] [Google Scholar]
- 229.Zollner S, Haseloff RF, Kirilyuk IA, Blasig IE, Rubanyi GM. Nitroxides increase the detectable amount of nitric oxide released from endothelial cells. J Biol Chem. 1997;272:23076–23080. doi: 10.1074/jbc.272.37.23076. [DOI] [PubMed] [Google Scholar]