Abstract
Mutations of MECP2 (Methyl-CpG Binding Protein 2) cause Rett syndrome. As a chromatin-associated multifunctional protein, how MeCP2 integrates external signals and regulates neuronal function remain unclear. Although neuronal activity-induced phosphorylation of MeCP2 at serine 421 (S421) has been reported, the full spectrum of MeCP2 phosphorylation together with the in vivo function of such modifications are yet to be revealed. Here, we report the identification of several MeCP2 phosphorylation sites in normal and epileptic brains from multiple species. We demonstrate that serine 80 (S80) phosphorylation of MeCP2 is critical as its mutation into alanine (S80A) in transgenic knock-in mice leads to locomotor deficits. S80A mutation attenuates MeCP2 chromatin association at several gene promoters in resting neurons and leads to transcription changes of a small number of genes. Calcium influx in neurons causes dephosphorylation at S80, potentially contributing to its dissociation from the chromatin. We postulate that phosphorylation of MeCP2 modulates its dynamic function in neurons transiting between resting and active states within neural circuits that underlie behaviors.
Keywords: MeCP2 phosphorylation, neuronal activity, Rett syndrome
The essential role of the nervous system is to receive and integrate various environmental stimuli to elicit adaptive behavioral responses. Neurons, the building blocks of the nervous system, communicate with environment via electrical and chemical signals, which trigger cellular and nuclear responses in spatiotemporally well-orchestrated events to engage behavioral outcomes. Neuronal activities at synapses trigger changes in the level of intracellular calcium, which in turn elicit downstream signaling events, leading to regulation of gene expression. These events are essential for neuronal maturation and survival (1), synaptic plasticity (2), learning, and memory (3–5). One critical aspect of these biological processes is the mechanism by which neuronal transcription factors/regulators integrate the external stimuli to trigger changes in gene expression.
Methyl-CpG Binding Protein 2 (MeCP2) appears to be one such important transcription regulator. Mutations of this X-chromosome linked gene MECP2 cause Rett syndrome (RTT), a major autism-spectrum neurological disorder affecting females (8). The clinical symptoms of RTT include deceleration of head growth, loss of speech and purposeful hand movements, irregular breathing patterns, seizures and autistic features (9, 10). Mouse genetic studies suggest that MeCP2 plays essential roles in maintaining normal neuronal functions (11, 12). Interestingly, deficiency of MeCP2 may not cause drastic permanent damage to the brain because restoring the function of MeCP2 in the symptomatic Mecp2 null mice can effectively reverse some of the major phenotypes (13).
MeCP2 is a member of the methyl-CpG binding protein family, which contains the methyl-CpG binding domain (MBD), the transcription repression domain (TRD) and the N- and C-terminal domains with unknown functions. In vitro, MeCP2 recognizes methylated CpG dinucleotides with preference of adjacent A/T sequences via the MBD and inhibits gene transcription by recruiting corepressors via its TRD domain (14, 15). In addition, MeCP2 might also be involved in regulating RNA splicing (16), chromatin compaction and nucleosome clustering (17, 18). MeCP2 is implicated in neuronal maturation (19), synaptogenesis (20), and the balance between excitatory-inhibitory neurotransmission (21–24). Most recently it was reported that MeCP2 may function both as a transcription activator and a repressor in the hypothalamus (25). Despite the ongoing intensive investigations, how MeCP2, as a multifunctional chromatin-associated protein, integrates the external stimuli and maintains neuronal functions remains unclear.
Neuronal activity has been reported to trigger phosphorylation of MeCP2 in rat neurons at S421, which was further postulated to regulate activity-dependent gene transcription and neuronal spine maturation (26). Although the study raised an interesting link between neuronal activity and posttranslational modifications of MeCP2, the dynamic spectrum of MeCP2 phosphorylation was not fully characterized and in vivo function has not been revealed. Here, we report the identification of MeCP2 phosphorylation sites in normal and epileptic brains from multiple species. Among the conserved sites, both phosphorylation at S421 and dephosphorylation at a newly identified site, S80, of MeCP2 are observed to be triggered by neuronal activity. Mutation of the S80 phosphorylation site reduces MeCP2 association with chromatin at several euchromatic gene promoters, alters transcription of several genes that are potentially important for neuronal function.
Results
Identification of MeCP2 Phosphorylation Sites in Mouse and Rat Brains.
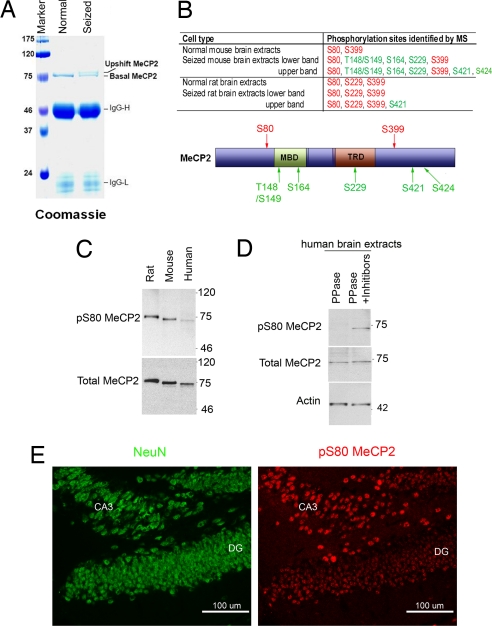
We have previously purified endogenous MECP2 from HeLa cell nuclear extracts by immunoprecipitation with a highly specific antibody recognizing full-length MeCP2 (27). Mass spectrometry analysis revealed that human MECP2 contained a single phosphorylation site at S80 (Fig. S1B). This particular serine was also highly conserved mouse and rat (Fig. S1A), suggesting that it could represent an important phosphorylation site.
Using a similar immunoprecipitation protocol, we further mapped MeCP2 phosphorylation sites from normal and epileptic mouse/rat brain nuclear extracts. MeCP2 was isolated as a single polypeptide from the normal brain (marked as basal MeCP2 in Fig. 1A). Besides the basal band, a new form of MeCP2 with slower electrophoretic mobility (marked as upshift MeCP2 in Fig. 1A) was identified from the seized brain. This result is in parallel to previous findings demonstrating that an upshift form of MeCP2 can be induced when rat neurons are depolarized by extracellular potassium (26) due to phosphorylation at S421 (26).
Fig. 1.
Identification of MeCP2 phosphorylation sites. (A) Coomassie staining of the SDS/PAGE gel showed MeCP2 purified from normal and seized rat brain extracts. (B) A summary of MeCP2 phosphorylation sites identified from normal and epileptic mouse and rat brain extracts. Sites marked in red are constitutively phosphorylated and sites marked in green are only phosphorylated in seized brain samples. (C) Phosphorylated MeCP2 at S80 was detected in human, mouse and rat brain extracts and (D) the phospho-S80 band was abolished upon phosphatase treatment. (E) Immunostaining of postnatal 3-week-old mouse hippocampal dentate gyrus (DG) region with phospho-S80 MeCP2 and neuronal marker NeuN.
Both the basal and the upshift bands were subjected to mass spectrometry analysis. Mass spectrometry with ≈70% peptide coverage (Fig. S2) revealed the presence of 2 major phosphorylation sites in normal mouse brain samples: S80 and S399 (Fig. 1B). In brain extracts of epileptic mice, besides S80 and S399, there were 5 additional amino acids that could be phosphorylated: T148/S149, S164, S229, S421, and S424 (Fig. 1B). Mass spectrometry analyses on MeCP2 isolated from rat brain extracts showed 3 major sites at basal level (S80, S229 and S399) and 1 additional site (S421) upon seizure induction (Fig. 1B). It is noteworthy that phosphorylation of S421 and/or S424 only exist in the upshift band whereas the other phosphorylation sites may exist in both basal and upshift bands. These series of mass-spectrometry analyses indicate that the complex pattern of MeCP2 phosphorylation may provide a platform for regulations at different parts of the molecule.
MeCP2 Is Phosphorylated at S80 and S421 in the Brains Based on Analyses Using Phosphorylation Site-Specific Antibodies.
The data above showed that the most conserved phosphorylation site of MeCP2 was S80, whereas phosphorylation of S421 in both mouse and rat appeared to be associated with the slower migrating species of MeCP2 protein on SDS/PAGE, only in epileptic brains. S80 is physically located near the MBD, whereas S421 is at the center of the C-terminal domain (Fig. 1B). To further investigate the regulation and function of S80 and S421 phosphorylation, we created phospho-specific antibodies for S80 and S421 respectively. The specificities of these antibodies were confirmed in cell lines overexpressing wild-type MeCP2 or serine-to-alanine mutants, which abolished phosphorylation (Fig. S3 A and B). With the phospho-S80 antibody, we observed the presence of S80 phosphorylation in normal mouse, rat, and human brain extracts, which was sensitive to phosphatase treatment (Fig. 1 C and D) and was absent in S80A mutant MeCP2 knock-in transgenic mouse brain extracts (Fig. 2A). Similarly, phospho-S421 antibody detected endogenous S421 phosphorylation in normal wild-type, but not in S421AS424A mutant knock-in mouse brain extracts (Fig. 2B). It was noteworthy that although MeCP2 was widely expressed in various tissues, its protein level was highest in brain and spinal cord tissues. Consistently, MeCP2 phosphorylated at either S80 or S421 was enriched in the brain, but surprisingly not in the spinal cord (Fig. S4B). Immunohistochemical analyses of mouse brain tissue using the phospho-S80 antibody showed a relatively ubiquitous distribution of MeCP2 phosphorylated at S80 throughout the brain. In most brain regions, the staining pattern of S80 phosphorylated MeCP2 is by and large similar to that of total MeCP2 detected by the Upstate anti-C-terminal MeCP2 antibodies (Figs. 1E and Fig. S5). This finding suggests that S80 phosphorylation is a wide-spread, if not constitutive, event in brain tissues.
Fig. 2.
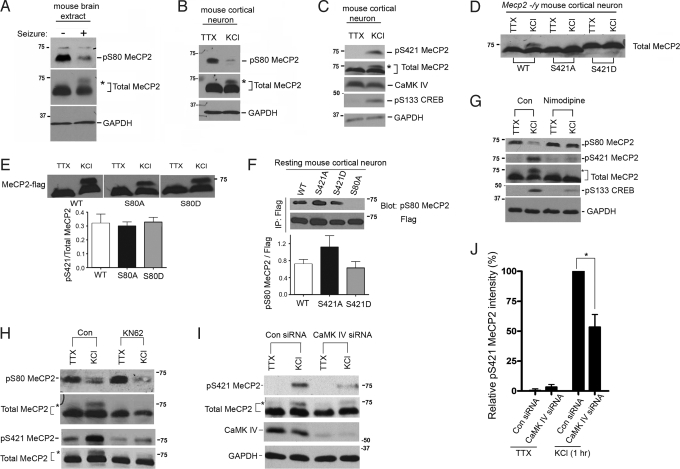
Mecp2S80A and Mecp2S421A;S424A knock-in mice showed, respectively, decreased and increased locomotor activities. (A) Phospho-S80 antibody detected a band from brain extracts of wild-type mice, but not the Mecp2S80A mice. The asterisk marks a nonspecific band. (B) Phospho-S421 antibody detected a band in normal wild-type mice brain extract, but not in brains of Mecp2S421A;S424A mice. (C) Mecp2S80A mice showed decreased locomotor activity when compared with their wild type littermates (n = 10 for wild type, n = 13 for S80A mutants, P = 0.029). Mecp2S421A;S424A mice showed increased locomotor activity when compared with their wild type littermates (n = 14 for wild type, n = 15 for S421AS424A mutants, P = 0.014).
Mecp2S80A Knock-In Mice Showed Decreased Locomotor Activity Whereas Mecp2S421A;S424A Knock-In Mice Showed Increased Locomotor Activity.
To test the in vivo functional importance of the highly conserved MeCP2 phosphorylation at S80, we generated Mecp2S80A knock-in mice that bear a mutation of S80 to alanine to abolish phosphorylation. The LSL-Mecp2S80A (LSL stands for loxP-STOP-loxP) targeting construct was made (Fig. S3C), linearized and electroporated into mouse ES cells. Southern blot analysis was performed to examine homologous recombination on both the 5′ and 3′ arms to identify correctly targeted clones (Fig. S3E). At least 2 correctly targeted ES cell lines were injected into blastocysts to generate chimeric mice. The chimeric mice were crossed with C57/B6 to obtain germ-line transmission of the LSL-Mecp2S80A allele. The LSL-Mecp2S80A mice were then crossed with mice carrying a transgenic Cre recombinase allele with germ-line activity to generate Mecp2S80A mice. Sequencing at the DNA and RNA levels demonstrated that Mecp2S80A mice encoded and transcribed a S80A mutation form of MeCP2 (Fig. S3D). Furthermore, Western blot analysis confirmed that phosphorylation at S80 was abolished in the Mecp2S80A mice (Fig. 2A). Brain extracts from multiple S80A and wild-type littermate mice showed similar MeCP2 protein levels (Fig. S4A).
We also created Mecp2S421A;S424A double mutant knock-in mice using a similar strategy. Both genomic sequencing and Western blot analyses confirmed amino acid substitutions and elimination of S421 phosphorylation (Fig. 2B and Fig. S3 D and F). Both knock-in mutant mice had normal life span (Table S1). Mecp2S80A mice were slightly overweight whereas Mecp2S421A;S424A mice had normal body weight. Interestingly, the Mecp2S80A mice showed decreased locomotor activity (62% of wild-type level), whereas Mecp2S421A;S424A mice showed increased activity when compared with their wild-type littermates (145% of wild-type level) in dark cycle running wheel assays (Fig. 2C). The S80A knock-in phenotype on locomotor activity was similar to that observed in Mecp2 null mutant mice (11, 12). The opposing locomotor phenotypes of Mecp2S80A and Mecp2S421A;S424A knock-in mice suggest that reduced locomotor activity of Mecp2S80A mice is specific to the mutation rather than reflecting some nonspecific general sickness.
Phosphorylations of MeCP2 at S80 and S421 Are Dynamically Regulated in Opposite Manners by Neuronal Activity.
To investigate the dynamic changes of MeCP2 phosphorylation in response to neuronal activity, we used both in vivo kainic acid mediated seizure inductions in adult mice and KCl mediated depolarization in in vitro cultured cortical neurons isolated from embryonic day 15 (E15) mice. In brain extracts of mice that have undergone seizures, there was a decrease in phospho-S80 and the appearance of a slower-migrating MeCP2 band, which represented a population of MeCP2 that was phosphorylated at S421 (Fig. 3A). Consistently, when E15 mouse cortical neurons were treated for 1 h with either tetrodotoxin (TTX) to inhibit spontaneous neuronal activity or 50 mM KCl to trigger membrane depolarization, S80 dephosphorylation and S421 phosphorylation were simultaneously detected in response to depolarization (Fig. 3 B and C). Both events occurred relatively quickly, as obvious changes can be detected 5–15 min post depolarization. S421A mutation eliminated the upper-shift band in KCl treated cortical neurons, whereas S421D mutant protein always migrated exactly at the upper-shifted position (Fig. 3D), consistent with the previous finding that phosphorylation of MeCP2 at S421 alters the electro-mobility of the protein (26). Interestingly, the opposing changes in S80 and S421 phosphorylation in response to membrane depolarization correlate well with the opposing phenotypes in locomotor activities of Mecp2S80A and Mecp2S421A;S424A knock-in mice. Together these observations suggest that S80 phosphorylation might be important for MeCP2 function in resting neurons, whereas S421 phosphorylation might be important in activity- induced neurons.
Fig. 3.
Phosphorylations of MeCP2 at S80 and S421 are dynamically regulated in response to neuronal activity and do not cross-talk with each other. (A) Brain extracts of normal mice or mice that have undergone seizures induced by kainic acid were blotted first with phospho-S80 antibody and then MeCP2 full-length antibody. The asterisk marks the upper shifted band. (B and C) E15 + 3DIV cultured mouse cortical neurons were treated either with 0.5 μM tetrodotoxin (TTX) or 50 mM KCl for 1 h before harvested for protein assays. Western blot showed that upon depolarization, S80 phosphorylation decreased (B) while S421 phosphorylation increased (C) with phospho-serine 133-Creb as a positive control for depolarization. (D) E14 cultured Mecp2 −/y mouse cortical neurons were infected with lentiviruses expressing wild-type, serine 421 to alanine mutant (S421A) or serine 421 to aspartate mutant (S421D) MeCP2. Western blot was done with the antibody recognizing full-length MeCP2. (E) Flag tagged wild-type MeCP2, serine 80 to alanine mutant (S80A) and serines 80 to aspartate mutant (S80D) were expressed in E15 mouse cortical neurons by lentiviral infection. Protein lysates were blotted with flag antibody. Densitometry analysis of the upper band (phospho-S421 MeCP2) intensity versus both bands (total MeCP2) showed little difference (n = 3, 1-way ANOVA analysis, P = 0.839). (F) Similarly, neurons overexpressing flag-tagged MeCP2 were treated with TTX for 1 h before harvested for the protein lysates. Exogenous MeCP2 were immunoprecipitated with flag antibody and subjected to Western blot with phospho-S80 antibody and flag antibody respectively. Densitometry analysis of phospho-S80 intensity versus flag intensity showed that S421D did not change phospho-S80 intensity compared with wild-type MeCP2. S421A showed a slight but not significant increase of S80 phosphorylation (n = 3, 1-way ANOVA analysis, P = 0.205). (G) Nimodipine (5 μM) was applied in E15 mouse cortical neuronal culture for 1 h before depolarizing the neurons with 50 mM KCl. (H) E15 mouse cortical neurons were treated with KN62 (5 μM) for 1 h before depolarization with 50 mM KCl for 1 h and subsequently underwent Western blot. (I) SMARTPOOL CaMK IV siRNA and control siRNA (Dharmacon) were transfected into E15 mouse cortical neurons with nucleofector (Amaxa). Protein lysates were harvested 72 h after transfection and undergone Western blot. (J) Densitometry quantification of Western blots showed CaMK IV knockdown decreased S421 phosphorylation by 50% (P < 0.05, n = 3, paired t test).
To determine whether there was any cross-talk between the 2 phosphorylation events, E15 mouse cortical neurons were infected with lentiviruses expressing flag-tagged wild-type, S80 or 421 to alanine (mimics loss of phosphorylation) and S80 or 421 to aspartate (mimics phosphorylation) mutant forms of MeCP2. Western blot on the exogenous proteins showed that neither of the S80 mutants affected neuronal activity induced MeCP2 phosphorylation at S421 (Fig. 3E). In addition, we found that neither S421A nor S421D significantly altered S80 phosphorylation levels (Fig. 3F), suggesting that the phosphorylation status of S80 and S421 does not interfere with each other directly, indicating that phosphorylations at the N and C termini of MeCP2 could be regulated independently.
To gain more insights into the upstream signals regulating MeCP2 phosphorylation, we applied nimodipine, an L-type voltage gated calcium channel (L-VGCC) antagonist, to mouse cortical neuronal cultures. Nimodipine effectively blocked both S80 dephosphorylation and S421 phosphorylation induced by membrane depolarization, suggesting that calcium influx through L-VGCC was the common upstream trigger for both events (Fig. 3G). To further identify the kinases that phosphorylate MeCP2 at S80 and/or S421 sites, we treated cortical neuronal culture with well-characterized kinase inhibitors before membrane depolarization and assessed the effect using Western blot with site-specific phosphorylation antibodies. Pretreatment for 1 h before depolarization with KN-62, an inhibitor of CaMK II and CaMK IV, effectively blocked S421 phosphorylation induction without affecting S80 dephosphorylation (Fig. 3H). Both CaMK II and CaMK IV are serine/threonine kinases that are essential mediators of neuronal activity dependent synaptic plasticity (28). The expression of different CaMK II isoforms is spatiotemporally regulated during development (29). Immunoblotting of mouse brain tissues at different ages showed that total CaMK II protein level was almost undetectable at E15. Consistent with a role of CaMK II in neuronal maturation, the protein level of CaMK II rapidly increased during postnatal 3 weeks (Fig. S6). Therefore, it is unlikely that CaMK II was the major mediator of MeCP2 S421 phosphorylation in cultured E15 mouse cortical neurons. In contrast, CaMK IV was highly expressed in both embryonic and postnatal brains. In addition, as a protein that predominantly functions in the nuclei of neurons, CaMK IV can phosphorylate CREB and CBP in response to neuronal activity (30, 31). To test whether CaMK IV may mediate phosphorylation of MeCP2 S421, we used CaMK IV siRNA to knockdown endogenous CaMK IV and observed a 50% decrease in S421 phosphorylation (Fig. 3 I and J). This indicates that CaMK IV plays an important role in neuronal activity induced MeCP2 S421 phosphorylation in mouse embryonic cortical neurons. Although CaMK II protein was barely detectable in embryonic cortical neurons, we could not exclude the possibility that MeCP2 S421 phosphorylation in a later postnatal stage may involve other kinases including CaMK II as reported in rat hippocampal neuronal cultures (26). On the contrary, the inhibitors for CDC2, CDK5, PKC, MAPK or PI3K alone failed to significantly alter levels of either S80 or S421 phosphorylation (data not shown). The identity of S80 kinase requires further investigation.
MeCP2 Phosphorylation at S80 Plays Important Roles in Its Chromatin Association Properties.
To elucidate the functional consequence of MeCP2 S80 phosphorylation, we first sought to examine whether phosphorylation of S80 may affect the subcellular localization of MeCP2 at heterochromatic regions indicated by DAPI puncta staining in mouse cortical neurons as reported in refs. 32 and 33. Our results (Fig. S7) suggest that MeCP2 phosphorylation might not affect its overall subcellular localization.
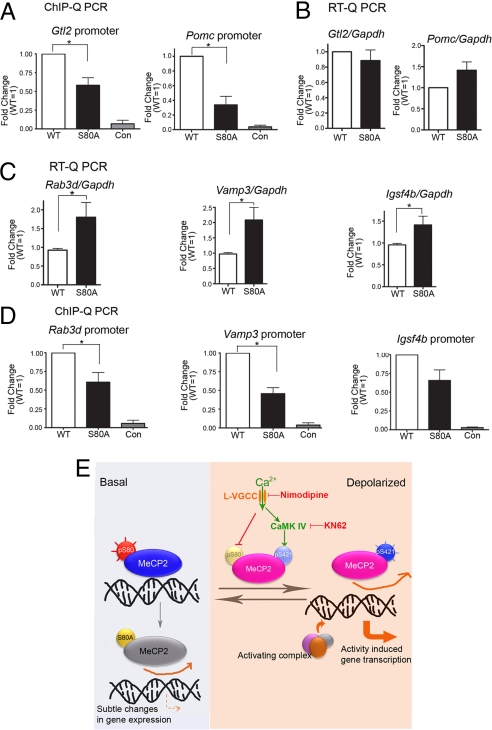
To test the possibility that MeCP2 association with chromatin at specific euchromatic gene promoters may be influenced by phosphorylation and dephosphorylation of MeCP2 at S80, chromatin immunoprecipitation (ChIP) assays were used to assess MeCP2 chromatin association in resting neurons. In E15.5 mouse cortical neurons expressing HA or flag-tagged wild-type or site-specific phosphorylation mutant MeCP2, anti-HA or anti-flag antibody was used for ChIP followed by quantitative PCR (ChIP-qPCR) analyses with 2 known high affinity MeCP2 bound gene promoters including Pomc (Martinowich and Sun, unpublished observation) and Gtl2 (34). Comparing with nontagged samples, ChIP with flag or HA antibody showed specific enrichment and very similar immunoprecipitation efficiency of wild type and S80A MeCP2 (Fig. S8A). In addition, we normalized the ChIP signals with expression levels of tagged MeCP2 to more faithfully reveal the chromatin association affinity of wild-type versus mutant MeCP2. Because more MeCP2 phosphorylation sites may be involved upon KCl depolarization, without knowing whether the phosphorylation events at each individual site are interdependent, it could be difficult to delineate the effect of a single phosphorylation site mutation on MeCP2 chromatin association under depolarization condition. Therefore, we focused our analyses in TTX treated resting neurons in which S80 was phosphorylated. As shown in Fig. 4A, with either HA or flag antibody, at both Pomc and Gtl2 promoters, mutation of S80 to alanine led to a decrease in chromatin binding affinity of MeCP2 by 50–60%, whereas the expression levels of tagged wild type and S80A mutant MeCP2 were approximately the same (Fig. S9C). The result suggests that S80 may be important for maintaining chromatin association of the protein, and therefore posttranslational modifications of S80 may play important roles in MeCP2 function. However, the decreased binding of MeCP2 serine 80 to alanine mutant on Pomc and Gtl2 promoters did not lead to obvious changes in expression levels of these genes (Fig. 4B), suggesting that reduced MeCP2-chromatin association might not always be sufficient to induce the gene transcription. According to published study, S421A mutation enhances MeCP2 association with its target gene in depolarized neurons (26). Thus, the opposing effects of S80A and S421A mutations in MeCP2 chromatin association correlate well with the knock-in mice phenotype, even though the detailed mechanisms underlying the behavioral phenotypes remain to be determined.
Fig. 4.
MeCP2 S80A mutation attenuated chromatin association affinity at candidate gene promoters and caused subtle gene expression changes. E15 mouse cortical neurons were infected with lentiviruses overexpressing flag-tagged or HA-tagged wild-type and S80A MeCP2. (A and D) Chromatin immunoprecipitation followed by quantitative PCR showed that at Gtl2 and Pomc promoters (A) and Rab3d, Vamp3, and Igsf4b promoters (D), serine 80 to alanine mutation weakened MeCP2 association with chromatin. IP/WCE values have been normalized with flag protein expression level (t test results: Gtl2 n = 6, P = 0.008; Pomc n = 7, P = 0.005; Vamp3 n = 3, P = 0.002; Rab3d n = 3, P = 0.046). (B and C) RNA was extracted from E15 + 3DIV Mecp2 −/y mouse cortical neurons infected with lentiviruses expressing wild-type and S80A MeCP2 respectively and subjected to quantitative RT-PCR. Among the candidate binding targets, Igsf4b, Rab3d and Vamp3 (C) showed significant up-regulation in MeCP2 S80A samples compared with wild-type samples (n = 5, P < 0.05, t test), whereas Gtl2 and Pomc (B) did not show obvious changes in gene transcription. (E) A hypothetical model of how MeCP2 phosphorylation and dephosphorylation may be part of the neuronal switch mechanism between resting and activated states.
MeCP2 S80A Mutation Causes Subtle Changes of Gene Expression in Resting Neurons.
Because MeCP2 S80A mutant attenuated chromatin association on specific gene promoters and Mecp2S80A knock-in transgenic mice have clear behavioral phenotypes, we decided to determine whether MeCP2 S80A mutation might affect gene transcription. E15.5 Mecp2 null mouse cortical neurons were infected with lentiviruses expressing wild-type or S80A MeCP2. Western blot analysis showed that protein levels of exogenous wild-type and mutant MeCP2 were largely comparable, and was only 1–1.5-fold of that of endogenous MeCP2 in wild-type cortical neurons (Fig. S9C). RNAs harvested from TTX treated resting neurons were subjected to gene expression profiling. As shown in Fig. S9 A and B, only a small group of genes (149 genes up-regulated and 56 genes down-regulated, FDR <0.05) (Tables S2–S3), including some genes potentially related to synaptic function, were dysregulated in S80A mutant rescued neurons when compared with wild-type MeCP2 rescued neurons, consistent with the notion that loss of MeCP2 affects specific genes rather than global changes in gene transcription (35). Among these genes, Rab3d and Vamp3 were validated as up-regulated upon S80A mutation, both of which were likely to be involved in synaptic transmission (Fig. 4C) (6, 36). Igsf4b (Cadm3/Necl-1), a neural-tissue specific cell adhesion molecule, was also up-regulated in S80A mutant rescued neurons (Fig. 4C) (7). S80A mutation also attenuated MeCP2 binding to Rab3d, Vamp3 and Igsf4b promoters (Fig. 4D and Fig. S8B). Based on what were observed with Rab3d, Vamp3, Pomc, Gtl2 and Igsf4b promoters and their mRNA levels, it is clear that MeCP2 association with chromatin is not always a major predictor for gene transcription of all its binding targets. However, transcription changes in relatively small amount of genes might still lead to neurological phenotypes.
Discussion
MeCP2 binds to methylated DNA and has been considered as a classic epigenetic factor that regulates gene expression. The revelation that it undergoes dynamic phosphorylation at multiple sites upon neuronal activity suggests that MeCP2 is regulated by multiple signaling pathways. Specifically, in this study, we identified multiple MeCP2 phosphorylation sites from samples of different species. Among them, S80 phosphorylation is highly conserved in all of the samples. Mecp2S80A knock-in mouse studies provide the first in vivo evidence that S80 and/or phosphorylation of S80 has important neurobiological functions as indicated by the significant reduction of locomotor activities, a phenotype reminiscent of Mecp2 null mice and RTT patients.
Although it is known that neuronal activity induces MeCP2 phosphorylation (at S421), we found that S80 phosphorylation was negatively regulated by neuronal activity. Moreover, dephosphorylation of S80 also contributes to the attenuated association of MeCP2 with some of its target chromatin regions. The opposing regulation of S80 and S421 phosphorylation by neuronal activity suggests that S80 phosphorylation is primarily associated with MeCP2 function in resting neurons whereas S421 phosphorylation is associated with that in depolarized neurons. As summarized in Fig. 4E, when a neuron transits between resting and activated states in functioning neural circuits, its gene transcription program also shifts between these two states. Activity dependent regulation of MeCP2 phosphorylation might be part of the switch mechanism controlling such transitions. S80 phosphorylation may allow MeCP2 to rebind to chromatin when cells enter the resting state, whereas S421 phosphorylation allows MeCP2 to dissociate from chromatin when cells are depolarized. It is likely that S80A mutation of MeCP2 will interfere with the normal regulatory loop of MeCP2 function and transcription regulation in neurons between resting and depolarized states, and therefore leading to behavioral phenotypes. It is also possible that some gene expression changes upon S80A mutation at basal level may indirectly further affect the kinetics of neuronal activity dependent gene expression.
It appears that phosphorylation of S80 and S421 at the 2 termini of the MeCP2 molecule could be modular and do not interfere with each other. However, in addition to S421, we found 4 additional sites, phosphorylation of which may be induced by neuronal activity. Whether the other phosphorylation sites coexist on the same molecule, function in the same brain region, or exert their functions in coordinated or antagonizing manners will need further characterization. It is tempting to speculate that the intricacy of assorted pathways and networks all converging on MeCP2 may confer multiple functional regulations of this molecule, which well fit in the context of MeCP2 being a central sensor for different neuronal stimuli and an executor to mediate different responses.
Since MeCP2 is a protein tightly bound to the chromatin, an attractive hypothesis would be that its genome-wide localization may be regulated by phosphorylation in response to neuronal activity. Our data demonstrate that S80A decreases MeCP2 chromatin association at several euchromatic candidate gene promoters in resting neurons without affecting MeCP2 localization to heterochromatic regions or reducing MeCP2 MBD domain binding to naked methylated DNA oligos (Fig. S10), suggesting that phosphorylation/dephosphorylation of MeCP2 may only fine-tune its association with a subset of chromatin regions. Whether specific site phosphorylation/dephosphorylation regulates MeCP2 association with different chromatin regions needs further investigations with genome-wide approaches.
Our gene expression data suggest that in relatively homogenous cortical resting neurons, prevention of MeCP2 phosphorylation at S80 does not disturb global transcription. In addition, a number of direct MeCP2 bound target genes such as Gtl2 and Pomc, do not become effectively derepressed upon S80A mutation. These observations suggest that MeCP2 may not be a major determinant for transcription of all its target genes. However, some of the MeCP2 direct target genes that are potentially related to synaptic function are indeed up-regulated upon S80A mutation, which might contribute to neurological outcomes. Further brain regional specific gene expression analyses need to be carried out to reveal the molecular mechanisms underlying the locomotor deficits observed in Mecp2S80A knock-in mice.
Taken together, our results suggest that mouse MeCP2 can be dynamically regulated via dephosphorylation and phosphorylation at multiple sites induced by neuronal activity. In addition, using gene knock-in approaches, we revealed that one of the most conserved phosphorylaton sites, S80, was critically involved in neuro-behavioral regulation in vivo. Future studies addressing the functional relevance of phosphorylation regulation at each individual site through knock-in approaches will be needed to reveal whether MeCP2, via phosphorylation regulation at different sites, serves as an activity sensor or signaling integrator in neurons. Ultimately, elucidating how posttranslational modifications may affect MeCP2 function will advance our understanding of MeCP2 normal function and the etiology of Rett syndrome.
Materials and Methods
Antibodies.
The polyclonal MeCP2 full-length antibody was generated as described in ref. 27. The polyclonal phospho-serine 80 and phospho-serine 421 MeCP2 antibodies were raised in rabbits (Abgent and Covance). The other commercial available antibodies are listed in SI Materials and Methods.
Immunoprecipitation of Endogenous MeCP2 and Mass Spectrometry Analysis.
Endogenous MeCP2 was purified from mouse/rat brain tissue nuclear extracts by immunoprecipitation as described in ref. 27. The MeCP2 bands from Coommassie stained gel were cut and sent for mass spectrometry analysis (W.M. Keck Biomedical Mass Spectrometry Lab, University of Virginia).
Generation and Initial Analysis of the Mecp2S80A and Mecp2S421A;S424A Mice.
Detailed description of the generation and locomotor activity analyses of the knock-in mice can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank Dr. Adrian Bird for his comments on the manuscript, members of Sun Lab for helpful discussion, and Wenyu Zhu for technical assistance. This work was supported by National Institutes of Health Grant R56MH082068 (to Y.E.S.), Intramural Research Program of the National Institute on Aging Grant Z01 AG000668-06 (to W.W.), and grants from Rett Syndrome Research Foundation and International Rett Syndrome Foundation (to W.W., Y.E.S., Q.C. and R.J.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 4577.
This article contains supporting information online at www.pnas.org/cgi/content/full/0811648106/DCSupplemental.
References
- 1.West AE, et al. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci USA. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soderling TR. CaM-kinases: Modulators of synaptic plasticity. Curr Opin Neurobiol. 2000;10:375–380. doi: 10.1016/s0959-4388(00)00090-8. [DOI] [PubMed] [Google Scholar]
- 3.Frankland PW, O'Brien C, Ohno M, Kirkwood A, Silva AJ. Alpha-CaMKII-dependent plasticity in the cortex is required for permanent memory. Nature. 2001;411:309–313. doi: 10.1038/35077089. [DOI] [PubMed] [Google Scholar]
- 4.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42:961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei F, et al. Calcium calmodulin-dependent protein kinase IV is required for fear memory. Nat Neurosci. 2002;5:573–579. doi: 10.1038/nn0602-855. [DOI] [PubMed] [Google Scholar]
- 6.Deak F, Shin OH, Kavalali ET, Sudhof TC. Structural determinants of synaptobrevin 2 function in synaptic vesicle fusion. J Neurosci. 2006;26:6668–6676. doi: 10.1523/JNEUROSCI.5272-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kakunaga S, et al. Nectin-like molecule-1/TSLL1/SynCAM3: A neural tissue-specific immunoglobulin-like cell–cell adhesion molecule localizing at non-junctional contact sites of presynaptic nerve terminals, axons and glia cell processes. J Cell Sci. 2005;118(Pt 6):1267–1277. doi: 10.1242/jcs.01656. [DOI] [PubMed] [Google Scholar]
- 8.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 9.Rett A. On a unusual brain atrophy syndrome in hyperammonemia in childhood. Wien Med Wochenschr. 1966;116:723–726. in German. [PubMed] [Google Scholar]
- 10.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett's syndrome: Report of 35 cases. Ann Neurol. 1983;14:471–479. doi: 10.1002/ana.410140412. [DOI] [PubMed] [Google Scholar]
- 11.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 12.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 13.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological defects in a mouse model of Rett syndrome. Science. 2007;315:1143–1147. doi: 10.1126/science.1138389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 15.Klose RJ, et al. DNA binding selectivity of MeCP2 due to a requirement for A/T sequences adjacent to methyl-CpG. Mol Cell. 2005;19:667–678. doi: 10.1016/j.molcel.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 16.Young JI, et al. Regulation of RNA splicing by the methylation-dependent transcriptional repressor methyl-CpG binding protein 2. Proc Natl Acad Sci USA. 2005;102:17551–17558. doi: 10.1073/pnas.0507856102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikitina T, et al. Multiple modes of interaction between the methylated DNA binding protein MeCP2 and chromatin. Mol Cell Biol. 2007;27:864–877. doi: 10.1128/MCB.01593-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Georgel PT, et al. Chromatin compaction by human MeCP2. Assembly of novel secondary chromatin structures in the absence of DNA methylation. J Biol Chem. 2003;278:32181–32188. doi: 10.1074/jbc.M305308200. [DOI] [PubMed] [Google Scholar]
- 19.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 20.Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J Neuropathol Exp Neurol. 2005;64:537–544. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- 21.Medrihan L, et al. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–121. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- 22.Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol. 2006;16:710–716. doi: 10.1016/j.cub.2006.02.062. [DOI] [PubMed] [Google Scholar]
- 23.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dani VS, et al. Reduced cortical activity due to a shift in the balance between excitation and inhibition in a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2005;102:12560–12565. doi: 10.1073/pnas.0506071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu K, Nan X, Bird A, Wang W. Testing for association between MeCP2 and the brahma-associated SWI/SNF chromatin-remodeling complex. Nat Genet. 2006;38:962–964. doi: 10.1038/ng0906-962. author reply 964–967. [DOI] [PubMed] [Google Scholar]
- 28.Wayman GA, Lee YS, Tokumitsu H, Silva A, Soderling TR. Calmodulin-kinases: Modulators of neuronal development and plasticity. Neuron. 2008;59:914–931. doi: 10.1016/j.neuron.2008.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayer KU, Lohler J, Schulman H, Harbers K. Developmental expression of the CaM kinase II isoforms: Ubiquitous gamma- and delta-CaM kinase II are the early isoforms and most abundant in the developing nervous system. Brain Res Mol Brain Res. 1999;70:147–154. doi: 10.1016/s0169-328x(99)00131-x. [DOI] [PubMed] [Google Scholar]
- 30.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 31.Impey S, et al. Phosphorylation of CBP mediates transcriptional activation by neural activity and CaM kinase IV. Neuron. 2002;34:235–244. doi: 10.1016/s0896-6273(02)00654-2. [DOI] [PubMed] [Google Scholar]
- 32.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 33.Ballas N, Grunseich C, Lu DD, Speh JC, Mandel G. REST and its corepressors mediate plasticity of neuronal gene chromatin throughout neurogenesis. Cell. 2005;121:645–657. doi: 10.1016/j.cell.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 34.Jordan C, Li HH, Kwan HC, Francke U. Cerebellar gene expression profiles of mouse models for Rett syndrome reveal novel MeCP2 targets. BMC Med Genet. 2007;8:36. doi: 10.1186/1471-2350-8-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tudor M, Akbarian S, Chen RZ, Jaenisch R. Transcriptional profiling of a mouse model for Rett syndrome reveals subtle transcriptional changes in the brain. Proc Natl Acad Sci USA. 2002;99:15536–15541. doi: 10.1073/pnas.242566899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schluter OM, Basu J, Sudhof TC, Rosenmund C. Rab3 superprimes synaptic vesicles for release: Implications for short-term synaptic plasticity. J Neurosci. 2006;26:1239–1246. doi: 10.1523/JNEUROSCI.3553-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.