Abstract
Fumarate and nitrate reduction regulatory (FNR) proteins are bacterial transcription factors that coordinate the switch between aerobic and anaerobic metabolism. In the absence of O2, FNR binds a [4Fe-4S]2+ cluster (ligated by Cys-20, 23, 29, 122) promoting the formation of a transcriptionally active dimer. In the presence of O2, FNR is converted into a monomeric, non-DNA-binding form containing a [2Fe-2S]2+ cluster. The reaction of the [4Fe-4S]2+ cluster with O2 has been shown to proceed via a 2-step process, an O2-dependent 1-electron oxidation to yield a [3Fe-4S]+ intermediate with release of 1 Fe2+ ion, followed by spontaneous rearrangement to the [2Fe-2S]2+ form with release of 1 Fe3+ and 2 S2− ions. Here, we show that replacement of Ser-24 by Arg, His, Phe, Trp, or Tyr enhances aerobic activity of FNR in vivo. The FNR-S24F protein incorporates a [4Fe-4S]2+ cluster with spectroscopic properties similar to those of FNR. However, the substitution enhances the stability of the [4Fe-4S]2+ cluster in the presence of O2. Kinetic analysis shows that both steps 1 and 2 are slower for FNR-S24F than for FNR. A molecular model suggests that step 1 of the FNR-S24F iron–sulfur cluster reaction with O2 is inhibited by shielding of the iron ligand Cys-23, suggesting that Cys-23 or the cluster iron bound to it is a primary site of O2 interaction. These data lead to a simple model of the FNR switch with physiological implications for the ability of FNR proteins to operate over different ranges of in vivo O2 concentrations.
Keywords: gene regulation, iron–sulfur, oxygen sensing, oxidation, transcriptional regulation
Fumarate and nitrate reduction regulatory (FNR) proteins are members of a superfamily of structurally related bacterial transcriptional factors (1). The structural framework of the founder member of the family, the Escherichia coli cAMP receptor protein (CRP), provides a versatile system for sensing and transducing environmental or metabolic signals into a physiological response (2–5). FNR proteins are O2 sensors that control the switch between aerobic and anaerobic metabolism (5–7). Based on sequence homology with CRP (8), FNR consists of a C-terminal DNA-binding domain, which recognizes specific sequences within FNR-controlled promoters (9), and an N-terminal sensory domain containing 5 cysteine residues, 4 of which, Cys-20, 23, 29, and 122, but not Cys-16, are essential for function (10, 11). The essential cysteine residues bind either a [4Fe-4S]2+ or a [2Fe-2S]2+ cluster (12–14). Under anaerobic conditions, acquisition of 1 [4Fe-4S]2+ cluster per FNR protomer causes dimerization and enhances site-specific DNA binding (15). The [4Fe-4S]2+ cluster is converted to a [2Fe-2S]2+ cluster by O2, triggering conformational changes that induce monomerization, preventing DNA binding and hence productive interactions with RNA polymerase (15–21).
Metal-centered oxidation (16) and sulfide-based oxidation (21) mechanisms have been proposed for the reaction of O2 with the FNR [4Fe-4S]2+ cluster. Recently, it was shown that the reaction of O2 with [4Fe-4S]2+ FNR occurs in 2 steps (22, 23). The first step is a second-order, 1-electron oxidation of the [4Fe-4S]2+ cluster that produces a superoxide ion, a [3Fe-4S]+ intermediate, and 1 ferrous ion. The second step is a spontaneous (first-order) decay of the [3Fe-4S]+ intermediate yielding [2Fe-2S]2+ FNR, 2 sulfides, and a ferric ion, consistent with a metal-based oxidation mechanism. The superoxide product from step 1 is thought to be recycled to O2 via hydrogen peroxide, providing a positive feedback mechanism to amplify the FNR response to O2 (22). Here, we report the reactivity of the iron–sulfur cluster of an FNR protein with a single amino acid substitution, S24F, adjacent to a Cys cluster ligand and show that the replacement inhibits both the O2-dependent and O2-independent steps of cluster conversion. This implies that a serine at position 24 allows the interaction of O2 with [4Fe-4S]2+ FNR and promotes conversion of the [3Fe-4S]+ intermediate to the [2Fe-2S]2+ product. In vivo transcription experiments indicate that other amino acids with bulky side chains at position 24 also protect the FNR iron–sulfur cluster. Molecular modeling suggests a mechanism by which Phe at position 24 protects the FNR [4Fe-4S]2+ cluster and that Cys-23 or the iron bound to it is a primary site of O2 interaction with the [4Fe-4S]2+ cluster.
Results
Replacement of Ser-24 by Phe Enhances FNR Activity Under Aerobic Conditions.
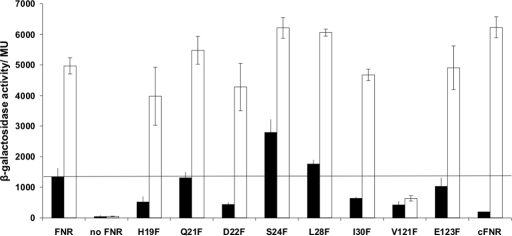
Cole and coworkers (24) showed that substitution of Ser-24 by Phe enhanced aerobic activity of FNR in vivo. It was suggested that this was most likely caused by stabilization of the FNR iron–sulfur cluster (24). Here, individual replacement of amino acid residues flanking the essential cysteine residues of FNR by Phe residues confirmed that FNR-S24F has greater aerobic activity than FNR. All other single-phenylalanine substitutions either failed to affect FNR activity significantly or inhibited both aerobic and anaerobic activity to a similar extent (Fig. 1). The amount of FNR-S24F was similar to that of FNR as judged by Western blotting [supporting information (SI) Appendix, Fig. S1]. Thus, these data suggest that the FNR iron–sulfur cluster is stabilized by the presence of Phe at position 24.
Fig. 1.
Activity of FNR proteins with Phe substitutions at residues adjacent to essential Cys ligands. Plasmid pGS24 was subjected to QuikChange site-directed mutagenesis (Stratagene) to create Phe substitutions and introduced to JRG1728 (fnr lac) containing pFF-41.5, a plasmid with an FNR-activated promoter fused to lacZ. The activity of chromosomally encoded FNR (cFNR) is shown for comparison. Cultures were grown in L broth under aerobic (filled bars) or anaerobic (open bars) conditions. The mean β-galactosidase activities in Miller units (MU) and standard deviations are shown (n = 9). The line indicates the amount of aerobic activity associated with wild-type FNR overproduced from pGS24.
Reconstitution of the FNR-S24F Iron–Sulfur Cluster.
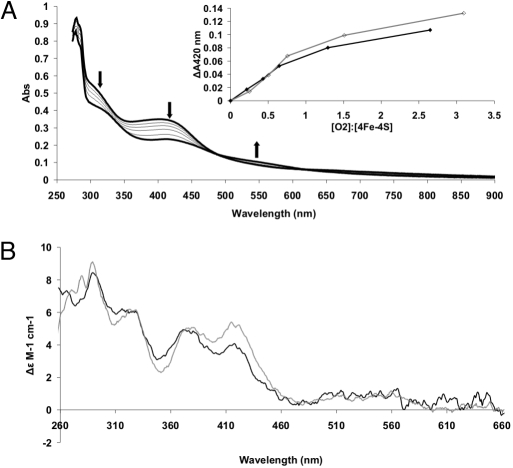
Isolation of FNR-S24F was achieved as described for wild-type FNR (16). The protein from aerobic cultures was colored, consistent with the presence of an iron–sulfur cluster more stable to O2. Anaerobic reconstitution of the FNR-S24F iron–sulfur cluster resulted in a protein that was EPR-silent, with UV-visible and CD spectra similar to those of anaerobic FNR (Fig. 2), containing 3.4–3.9 Fe per monomer of protein. Thus, the presence of Phe at position 24 does not impair the ability of FNR to bind an iron–sulfur cluster with properties very similar to the [4Fe-4S] cluster acquired by wild-type FNR under the same conditions.
Fig. 2.
Spectroscopic properties of reconstituted anaerobic FNR-S24F. (A) UV-visible spectra of 21.9 μM FNR-S24F cluster during a titration with O2 as air-saturated buffer. The upper spectrum, with absorbance maxima at 320 and 405 nm, is the anaerobic sample before the addition of O2 and is indicative of the presence of a [4Fe-4S] cluster. Subsequent spectra show changes upon the addition of 5, 10, 15, 29, and 51 μM O2. After each O2 addition, the incubations were continued for 90 min before collecting the absorbance spectra to ensure that the reactions were complete. Arrows show direction of absorbance change. The final spectrum, with absorbance maxima at 320, 420, and 500–600 nm, is characteristic of a [2Fe-2S] cluster. (Inset) Relationship between the O2:[4Fe-4S]2+ ratio and the change in absorbance at 420 nm for FNR (gray line) and FNR-S24F (black line). (B) Comparison of the CD spectra of FNR (gray line) with FNR-S24F (black line).
Reaction of FNR-S24F Iron–Sulfur Cluster with O2.
Titration of the [4Fe-4S]2+ cluster of FNR-S24F with air-saturated buffer at room temperature caused decreases in absorbance at 420 nm and increases ≈550 nm, as reported for FNR (Fig. 2A and ref. 16), with the final spectrum indicating that the cluster has undergone conversion to a [2Fe-2S]2+ form. Plotting ΔA420 against the O2:[4Fe-4S]2+ ratio for FNR and FNR-S24F resulted in superimposable curves, suggesting that both clusters react in the same way (Fig. 2A). In the data shown, clean end points to the titrations were not obtained because the prolonged incubation period required for reaction of the FNR-S24F [4Fe-4S] cluster with O2 was sufficient to allow partial degradation of the [2Fe-2S] cluster to contribute to the ΔA420 readings; shorter incubations with FNR resulted in titrations entirely consistent with previous data (16).
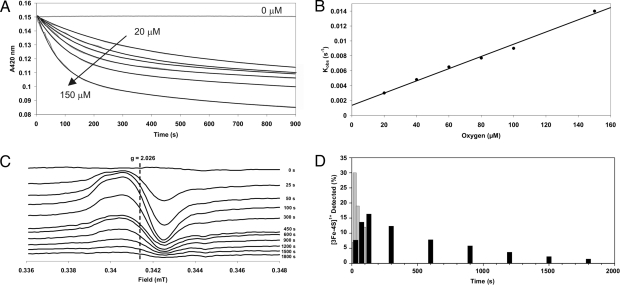
The first step of the FNR cluster conversion, from [4Fe-4S]2+ to [3Fe-4S]+, has been shown to be O2-dependent, whereas the second step, to [2Fe-2S]2+, is a thermally driven reaction (22). We have tested the FNR-S24F protein to ascertain whether it undergoes a similar 2-step mechanism and, if so, which steps have been altered by the amino acid substitution. Reconstituted FNR-S24F protein was exposed to different O2:[4Fe-4S]2+ ratios, and the 420-nm-decays were measured (Fig. 3A). The data were best fitted with double exponential functions. With a 10-fold molar excess of O2 to cluster the observed pseudo-first-order rate constants (kobs) were 0.009 s−1 and 0.040 s−1 for FNR-S24F and FNR, respectively. A plot of kobs against O2 concentration reveals a linear relationship, suggesting that the conversion from a [4Fe-4S]2+ cluster to the [3Fe-4S]+ intermediate is O2-dependent (Fig. 3B) and gives an apparent second-order rate constant, k1, of ≈80 M−1 s−1 for FNR-S24F (Fig. 3B) and ≈270 M−1 s−1 for FNR. The conversion of the FNR-S24F [3Fe-4S]+ to [2Fe-2S]2+ was, like that of FNR, O2-independent with a rate constant, k2, of ≈5.0 × 10−4 s−1, ≈3.4-fold lower than that observed for wild-type FNR (≈1.7 × 10−3 s−1).
Fig. 3.
The O2 dependence and rate of the FNR-S24F [4Fe-4S]2+ cluster conversion upon addition of O2. (A) Reaction of ≈20 μM FNR-S24F with different concentrations of O2 (0, 20, 40, 60, 80, 100, and 150 μM) measured by optical absorbance at 420 nm. Data are shown in gray, and double-exponential function fits are plotted as black lines. Data are the average of at least three experiments. (B) First observed (pseudo-first-order) rate constants, from A, as a function of O2 concentration. (C) Changes in EPR spectra of 20.4 μM FNR-S24F cluster during reaction with 200 μM O2. The reaction was performed in a sealed anaerobic cuvette and initiated by the injection of air-saturated buffer. Samples were transferred to EPR tubes and immediately frozen. EPR parameters: temperature, 15 K; microwave power, 2.0 mW; frequency, 9.7 GHz; modulation, 5 G. (D) Signal intensity of the [3Fe-4S]+ intermediate (expressed as a percentage of the original [4Fe-4S]2+ cluster concentration) as a function of time for FNR (gray bars) and the FNR-S24F mutant protein (black bars).
EPR spectroscopy was used to determine whether the conversion of the FNR-S24F cluster proceeds via a [3Fe-4S]+ intermediate. A 10-fold molar excess of O2 was added to a sample of reconstituted 20 μM FNR-S24F, and samples were removed and rapidly frozen in EPR tubes. As expected, before the addition of O2 the cluster was EPR silent, but upon exposure to O2 a signal centered at g = ≈2.02, characteristic of the [3Fe-4S]+ cluster, increased in intensity up to ≈130 s after O2 exposure, before decreasing again (Fig. 3 C and D). These data are consistent with [4Fe-4S]2+ to [3Fe-4S]+ and [3Fe-4S]+ to [2Fe-2S]2+ conversion rates slower than those observed with the wild-type protein, and with the second step being ≈1 order of magnitude slower than the first. Thus, it appears that Phe at position 24 protects the FNR [4Fe-4S]2+ cluster from O2 and also inhibits the O2-independent conversion of the [3Fe-4S]+ intermediate to the [2Fe-2S]2+ product. This implies that Ser-24 is significant in both steps of cluster conversion.
Bulky Amino Acid Side Chains at Position 24 Stabilize the FNR Iron–Sulfur Cluster.
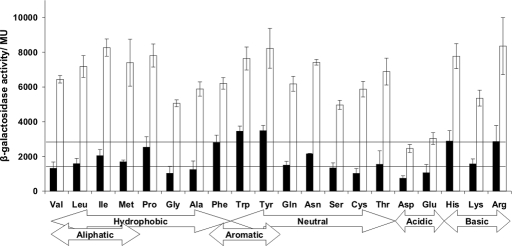
To discover whether other substitutions at position 24 could influence the reactivity of the FNR iron–sulfur cluster, 19 variants were created and tested for the ability to activate an FNR-dependent promoter under aerobic conditions. Replacement of Ser-24 by Arg, His, Phe, Trp, or Tyr enhanced FNR activity >2-fold under aerobic conditions (Fig. 4). In addition, these variants significantly affected the growth of aerobic E. coli cultures, resulting in small colonies on agar plates. These data support the proposal that a bulky amino acid side chain at position 24 protects the FNR iron–sulfur cluster from O2 and also suggest that enhanced FNR activity is detrimental during aerobic growth of E. coli.
Fig. 4.
Activity of E. coli FNR proteins with substitutions at residue 24. Plasmid pGS24 was subjected to QuikChange site-directed mutagenesis (Stratagene), and the plasmids expressing the indicated FNR variants were introduced to JRG1728 (fnr lac) containing pFF-41.5, a plasmid with an FNR-activated promoter fused to lacZ. Cultures were grown in L broth either aerobically (filled bars) or anaerobically (open bars). The mean β-galactosidase activities in MU ± SD are shown (n = 9). The horizontal lines indicate the amount of aerobic activity associated with overproduced wild-type FNR (Ser-24) and FNR-S24F.
Discussion
To function as an O2-sensing transcription factor, the sensitivity of the FNR [4Fe-4S]2+ cluster must have evolved to operate within an optimum range of O2 concentration, and, moreover, this range may well be different for different bacteria. Our current understanding is that FNR is continually supplied with [4Fe-4S]2+ clusters by the iron–sulfur cluster biosynthetic machinery (25–27), but O2 converts the [4Fe-4S]2+ cluster to a [3Fe-4S]+ cluster that spontaneously disassembles to yield a [2Fe-2S]2+ cluster with concomitant inactivation of FNR (12, 15, 22, 23). The properties of FNR-S24F described here are entirely consistent with this view of the FNR switch. It is implicit in this that the relative rates of cluster incorporation and degradation must be finely balanced; if the rate of degradation in the presence of O2 is too slow, “anaerobic” genes will be transcribed needlessly. The enhanced aerobic activity associated with FNR-S24F indicates that the amino acid residue adjacent to the iron ligand Cys-23 influences the reactivity of the iron–sulfur cluster toward O2, and the in vitro rate measurements indicate that this is because of a decrease in the rate of O2-dependent [4Fe-4S]2+ to [3Fe-4S]+ conversion. Thus, the key feature of the FNR switch is the reaction of the [4Fe-4S] cluster with O2. Measurement of aerobic activation from pFF-41.5 in the presence of chromosomally encoded FNR suggests that FNR is on average 2.5% active under aerobic conditions [150 ± 8.6 Miller units (MU) under aerobic conditions and 6,177 ± 343 MU under anaerobic conditions; Fig. 1, labeled cFNR]. Knowing that the intracellular concentration of FNR is ≈6 μM (21), the concentration of FNR in the off state under aerobic conditions is ≈5.85 μM. Thus, a model can be constructed (Fig. 5A) that predicts that FNR is 50% active (3 μM active FNR) at 6 μM O2, consistent with in vivo measurements (28, 29). The importance of controlling the concentration of FNR by negative autoregulation and proteolysis (31) is evident; the model predicts that a 3-fold increase in FNR concentration results in 3 μM active FNR at 30 μM O2, an O2 concentration at which FNR is normally <10% active (28). This is supported by the data in Fig. 1 in which increasing FNR concentration by expression from a plasmid (filled bar labeled FNR) leads to an ≈10-fold increase in FNR activity under aerobic conditions compared with chromosomally encoded FNR (filled bar labeled cFNR). The model also emphasizes the importance of regulating iron–sulfur cluster supply, mainly through the action of IscR, for appropriate O2 sensing (32). Furthermore, the 3.4-fold lower k1 for FNR-S24F reaction with O2 means that 50% activity is reached at 20 μM O2 (compared with 6 μM O2 for FNR); hence, a single amino acid substitution (S24F) is capable of significantly altering the dynamic range of the FNR response.
Fig. 5.
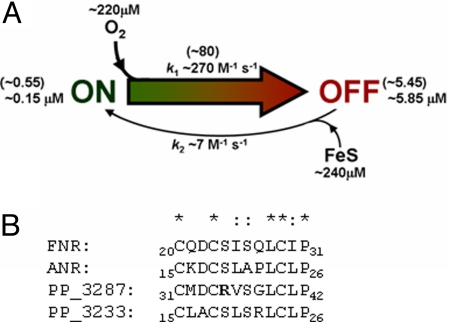
Model of the reaction of FNR with oxygen and the importance of Ser-24. (A) Simple model of the FNR switch. FNR exists in two functional states: on, corresponding to the DNA-binding form (i.e., [4Fe-4S] FNR), and off, corresponding to the non-DNA-binding forms (i.e., [3Fe-4S], [2Fe-2S], and apo forms). The concentration of FNR in the cell is ≈6 μM and is relatively constant in the presence and absence of O2 (21). An FNR-dependent reporter gene suggests that chromosomally encoded FNR is 2.5% active under fully aerobic conditions (Fig. 1, columns labeled cFNR), and thus the concentration of FNR in the off state under these conditions is ≈5.85 μM. The on to off switch (k1 ≈270 M−1 s−1) is O2-dependent. At a fully aerobic steady state (≈220 μM O2), [on] = k2/k1 [off], or (0.15 × 10−6 = k2/270 × 5.85 × 10−6), hence k2 = ≈7 M−1 s−1. The switch from off to on (k2) requires the incorporation of a [4Fe-4S] cluster, which depends on the supply of iron–sulfur clusters (FeS) from the biosynthetic machinery. Time-resolved transcriptomic studies suggest that the maximum FNR response during the switch from aerobic to anoxic growth occurs within 5–10 min (30), suggesting that the rate of off to on (vON) is ≈10 × 10−9 M s−1. This information can be used to calculate a nominal concentration of FeS available for incorporation into FNR, vON = k2 [off] [FeS], giving [FeS] = ≈240 μM. The model can be used to calculate FNR activity at different environmental O2 concentrations, i.e., 50% FNR activity at ≈6 μM O2 and 95% activity at ≈0.3 μM O2. The values in parentheses are those specifically for FNR-S24F. (B) Alignment of amino acid sequences near the N-terminal cysteine residues of E. coli FNR and three FNR proteins from P. putida (ANR, PP_3287 and PP_3233). *, conserved residues; :, similar residues. The Arg (R) residue proposed to alter the O2 sensitivity of PP_3387 relative to FNR/ANR is indicated in bold.
Alignment of 209 proteins that possess the E. coli essential cysteine signature (Cys20XXCys23XXXXXCys29 and Cys122) reveals that 119 have Ser at the position equivalent to Ser-24 in E. coli K-12. However, residues other than Ser (Ala, Arg, Asn, Gln, Gly, His, Leu, Met, Thr, Val, but not aromatic or acidic residues) are found at position 24 (or its equivalent location) in other FNR proteins. It is notable that for both E. coli (see above) and gonococcal (24) FNR proteins, the presence of amino acid residues with bulky side chains (Arg, His, Phe, Trp, or Tyr) results in significant aerobic activity. This suggests that Ser-24 is important for E. coli-type FNR proteins to respond within the appropriate physiological range of O2 concentrations and raises the possibility that evolution has selected other residues in different subclasses of FNR proteins to tune their O2 response to their environmental niche. It is, therefore, notable that Arg is found in the equivalent of position 24 of some FNR proteins. For example, Pseudomonas putida KT2440 has three FNR-type proteins (Fig. 5B); two of these have Ser, whereas the third (PP_3287) has Arg at the equivalent of position 24. The work described here suggests that FNR proteins from P. putida operate across different O2 ranges to optimize gene expression patterns.
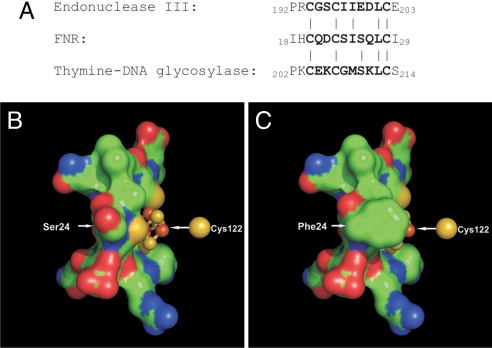
The lack of an FNR structure is a barrier to understanding the role of the Ser-24 in modulating the O2 sensitivity of the FNR [4Fe-4S] cluster. A search of the RCSB protein databank using PSIPRED software (33) yielded two structures with iron–sulfur clusters ligated by an FNR-like CysXXCysXXXXXCys motif. These proteins, a thymine-DNA glycosylase (34) and endonuclease III (35, 36), contain redox-inactive, stable [4Fe-4S] clusters that probably fulfill structural roles (37). In both proteins the fourth Cys is close to the others in the primary structure, whereas in FNR the fourth ligand (Cys-122) is more remote, presumably operating across an intramolecular interface between the main body of the protein and the N-terminal region. Comparison of the amino acid sequences equivalent to those linking Cys-23 and Cys-29 of FNR shows good conservation of side-chain properties (polarity, hydrophobicity) except at the position corresponding to 24 in FNR (Fig. 6A). In endonuclease III and thymine-DNA glycosylase, the position equivalent to Ser-24 in FNR is occupied by nonpolar residues, Ile and Gly, respectively (34, 35). Thus, we modeled residues Cys-20–Cys-29 of FNR with a [4Fe-4S] cluster bound by using SWISS-Model (38–42) and endonuclease III as a template. The model predicts that Ser-24 is positioned such that there is solvent access to the iron–sulfur cluster close to Cys-23 (Fig. 6B). Here, it has been shown that replacement of Ser-24 by the bulky nonpolar amino acid Phe significantly stabilizes the FNR [4Fe-4S] cluster, and the model predicts that the Phe side chain sits in close proximity to the sulfur atom of Cys-23, shielding it and the iron bound by Cys-23 (Fig. 6C). It is also likely that the S24F substitution affects the redox potential of the cluster [4Fe-4S]3+/2+ couple and that this in turn contributes to the observed difference in the rate of the initial reaction with O2. At present it is unknown exactly how replacement of Ser-24 by Phe inhibits the O2-independent [3Fe-4S]+ to [2Fe-2S]2+ conversion, but a bulky side chain such as Phe could slow the conformational changes required to rearrange the cysteine ligands from a tetrahedral array required to ligate a [4Fe-4S] cluster to a planar one to bind the flat [2Fe-2S]2+ cluster.
Fig. 6.
Model of the [4Fe-4S] binding region of FNR and FNR-S24F. (A) Alignment of amino acid sequences of the modeled region from FNR, endonuclease III, and thymine-DNA glycosylase. Bold type denotes the central Cys motif, connecting lines indicate residue identities, and numbers are the residue number in the full primary sequence of the respective molecules. (B and C) Predicted structure of FNR (B) and FNR-S24F (C) cluster regions produced in SWISS-Model by using the structure of endonuclease III (PDB code 2abk) as a template. Also represented is the sulfur atom of Cys-122 that binds the 4th iron atom of the iron-sulfur cluster. Images were produced by using PyMOL and show identical views. Note that the cluster ligand Cys-23 that is exposed in the FNR model (B) is shielded in the FNR-S24F model (C).
In summary, we have demonstrated a 2-step mechanism for the FNR [4Fe-4S]2+ to [2Fe-2S]2+ cluster conversion via a [3Fe-4S]+ intermediate, shown that the residue at position 24 in FNR modulates the reaction of the [4Fe-4S]2+ cluster with O2, and formulated a plausible molecular model that suggests that O2 interacts with the FNR [4Fe-4S]2+ cluster in the vicinity of Cys-23. Thus, our work provides insight into the mechanism of the iron–sulfur cluster rearrangements that switch FNR between active and inactive forms and implies that residues equivalent to position 24 in E. coli FNR, inter alia, play a key role in determining the sensitivity of the switch, thereby matching FNR activity to a range of O2 concentrations that require a response in the environmental niche inhabited by the bacterium.
Materials and Methods
In Vivo Transcription Assays.
Cultures of E. coli JRG1728 (fnr lac) containing the reporter plasmid pFF-41.5 and pBR322 derivatives expressing FNR proteins with single Phe substitutions at positions flanking essential cysteine residues [pGS24 (FNR), pGS2139 (FNR-H19F), pGS2140 (FNR-Q21F), pGS2140A (FNR-D22F), pGS2140B (FNR-S24F), pGS2141 (FNR-L28F), pGS2142 (FNR-I30F), pGS2143 (FNR-V121F), or pGS2144 (FNR-E123F)] or different amino acids at position 24 (pGS2193–2210) were grown in L broth supplemented with appropriate antibiotics (100 μg mL−1 ampicillin, 25 μg mL−1 tetracycline, 30 μg mL−1 chloramphenicol) under aerobic (25-mL universal tubes containing 5 mL of medium shaken at 250 rpm) or anaerobic (sealed bottles filled to the neck) conditions at 37 °C overnight. β-Galactosidase activities were then measured for triplicate cultures (43).
Purification of Reconstituted [4Fe-4S] FNR.
GST-FNR and GST-FNR-S24F fusion proteins were overproduced in aerobically grown E. coli BL21λDE3 cultures containing the expression plasmids pGS572 (FNR) and pGS2120 (FNR-S24F) and were purified under anaerobic conditions in 25 mM Hepes, 2.5 mM CaCl2, 100 mM NaCl, 500 mM KCl, 100 mM NaNO3 (pH 7.5) buffer, as described (16, 44). Under these conditions, O2 was undetectable in anaerobic buffers (16). The FNR proteins were released by using thrombin, and [4Fe-4S] clusters were reconstituted under anaerobic conditions, as described (16, 44).
Quantitative Procedures.
Apo-FNR protein concentrations and iron contents of reconstituted proteins were determined as described in ref. 16; and based on the analyses, [4Fe-4S] FNR samples exhibited ε405 nm values of 16,220 ± 135 M−1 cm−1 (45).
Spectroscopy.
Absorbance measurements were made with a Cary UV-visible spectrophotometer. CD measurements were made with a Jasco J-810 spectropolarimeter. EPR measurements were made with an X-band Bruker EMX EPR spectrometer equipped with a TE-102 microwave cavity and an ESR-900 helium flow cryostat (Oxford Instruments). To study the initial products of FNR-S24F reaction with O2, an aliquot of [4Fe-4S] FNR-S24F (≈20 μM final concentration) was mixed with an aliquot of aerobic buffer (219.5 μM O2, 21 °C, final concentration ≈200 μM O2), and aliquots were loaded into EPR tubes and frozen rapidly to 77 K at the indicated times.
Kinetic Measurements.
Reactions were initiated by injection of air-saturated buffer (≈212 μM O2) into sealed anaerobic cuvettes containing [4Fe-4S] FNR (10 μM final concentration, 1-mL final volume) at 25 °C. The dead time of mixing was ≈5 s. Changes in absorbance at 420 nm were used to monitor the conversion of the cluster. The rate constants are the averages of at least three repeats. Measurements by Clark O2 electrode, Winkler titration (16), and an OxyMicro probe (World Precision Instruments) indicated that O2 loss to the headspace was insignificant under the conditions used (SI Appendix, Figs. S2 and S3).
Data Analysis.
The conversion of [4Fe-4S] FNR to [2Fe-2S] FNR was followed under pseudo-first-order conditions (with O2 in excess) by measuring absorbance changes at 420 nm. Absorbance datasets were fitted by using SigmaPlot 9.0 to the double exponential function y = y0 + ae−bx + ce−dx, where b and d are the 2 first-order rate constants, a and c are the amplitudes, and y0 is the offset. Observed rate constants (kobs) obtained from the fits (corresponding to b in the above expression, i.e., corresponding to the initial, O2-dependent reaction) were plotted against the corresponding initial concentration of O2 to obtain the apparent second-order rate constant.
3D Structure Modeling.
Prediction of the 3D structure of the cluster-containing region of FNR was achieved by using the program SWISS-Model (38–42). The endonuclease III structure was used as a template by submitting amino acid sequence YTCIARKPRCQDC(S/F)ASQLCEYKEKVD, a hybrid sequence of 10 residues of FNR/FNR-S24F from Cys-20 to Cys-29 (in bold), flanked by the 9 N-terminal residues preceding Cys-194 and the 7 C-terminal residues preceding Cys-203 of endonuclease III. The resulting protein database file was edited, removing nonsimilar residues, and images were created by using the program PyMOL (46), resulting in a model of residues PRCQDC(S/F)ASQLCE and the iron–sulfur cluster.
Supplementary Material
Acknowledgments.
We thank Timothy Overton (University of Birmingham, U.K.), Ian Davies (World Precision Instruments), Mark Shepherd, Kelly Davidge, Jayne-Louise Wilson, Robert K. Poole, and Ruth Roberts (University of Sheffield). This work was supported by the Biotechnology and Biological Sciences Research Council, U.K., by the Wellcome Trust, and by the SysMO initiative (www.sysmo.net).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804943106/DCSupplemental.
References
- 1.Korner H, Sofia HJ, Zumft WG. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: Exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol Rev. 2003;27:559–592. doi: 10.1016/S0168-6445(03)00066-4. [DOI] [PubMed] [Google Scholar]
- 2.Green J, Scott C, Guest JR. Functional versatility in the CRP-FNR superfamily of transcription factors: FNR and FLP. Adv Microb Physiol. 2001;44:1–34. doi: 10.1016/s0065-2911(01)44010-0. [DOI] [PubMed] [Google Scholar]
- 3.Spiro S. The FNR family of transcriptional regulators. Antonie Van Leeuwenhoek. 1994;66:23–36. doi: 10.1007/BF00871630. [DOI] [PubMed] [Google Scholar]
- 4.Schultz SC, Shields GC, Steitz TA. Crystal structure of a CAP–DNA complex: The DNA is bent by 90 degrees. Science. 1991;253:1001–1007. doi: 10.1126/science.1653449. [DOI] [PubMed] [Google Scholar]
- 5.Green J, Paget MS. Bacterial redox sensors. Nat Rev Microbiol. 2004;2:954–966. doi: 10.1038/nrmicro1022. [DOI] [PubMed] [Google Scholar]
- 6.Guest JR. The Leeuwenhoek Lecture, 1995. Adaptation to life without oxygen. Philos Trans R Soc London B. 1995;350:189–202. doi: 10.1098/rstb.1995.0152. [DOI] [PubMed] [Google Scholar]
- 7.Guest JR. Oxygen-regulated gene expression in Escherichia coli. The 1992 Marjory Stephenson Prize Lecture. J Gen Microbiol. 1992;138:2253–2263. doi: 10.1099/00221287-138-11-2253. [DOI] [PubMed] [Google Scholar]
- 8.Shaw DJ, Rice DW, Guest JR. Homology between CAP and FNR, a regulator of anaerobic respiration in Escherichia coli. J Mol Biol. 1983;166:241–247. doi: 10.1016/s0022-2836(83)80011-4. [DOI] [PubMed] [Google Scholar]
- 9.Spiro S, et al. Interconversion of the DNA-binding specificities of two related transcription regulators, CRP and FNR. Mol Microbiol. 1990;4:1831–1838. doi: 10.1111/j.1365-2958.1990.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 10.Sharrocks AD, Green J, Guest JR. In vivo and in vitro mutants of FNR the anaerobic transcriptional regulator of E. coli. FEBS Lett. 1990;270:119–122. doi: 10.1016/0014-5793(90)81248-m. [DOI] [PubMed] [Google Scholar]
- 11.Melville SB, Gunsalus RP. Mutations in fnr that alter anaerobic regulation of electron transport-associated genes in Escherichia coli. J Biol Chem. 1990;265:18733–18736. [PubMed] [Google Scholar]
- 12.Khoroshilova N, Popescu C, Munck E, Beinert H, Kiley PJ. Iron–sulfur cluster disassembly in the FNR protein of Escherichia coli by O2: [4Fe-4S] to [2Fe-2S] conversion with loss of biological activity. Proc Natl Acad Sci USA. 1997;94:6087–6092. doi: 10.1073/pnas.94.12.6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiley PJ, Beinert H. Oxygen sensing by the global regulator, FNR: The role of the iron–sulfur cluster. FEMS Microbiol Rev. 1999;22:341–352. doi: 10.1111/j.1574-6976.1998.tb00375.x. [DOI] [PubMed] [Google Scholar]
- 14.Green J, et al. Reconstitution of the [4Fe-4S] cluster in FNR and demonstration of the aerobic–anaerobic transcription switch in vitro. Biochem J. 1996;316:887–892. doi: 10.1042/bj3160887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazazzera BA, Beinert H, Khoroshilova N, Kennedy MC, Kiley PJ. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J Biol Chem. 1996;271:2762–2768. doi: 10.1074/jbc.271.5.2762. [DOI] [PubMed] [Google Scholar]
- 16.Crack J, Green J, Thomson AJ. Mechanism of oxygen sensing by the bacterial transcription factor fumarate–nitrate reduction (FNR) J Biol Chem. 2004;279:9278–9286. doi: 10.1074/jbc.M309878200. [DOI] [PubMed] [Google Scholar]
- 17.Khoroshilova N, Beinert H, Kiley PJ. Association of a polynuclear iron–sulfur center with a mutant FNR protein enhances DNA binding. Proc Natl Acad Sci USA. 1995;92:2499–2503. doi: 10.1073/pnas.92.7.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazazzera BA, Bates DM, Kiley PJ. The activity of the Escherichia coli transcription factor FNR is regulated by a change in oligomeric state. Genes Dev. 1993;7:1993–2005. doi: 10.1101/gad.7.10.1993. [DOI] [PubMed] [Google Scholar]
- 19.Popescu CV, Bates DM, Beinert H, Munck E, Kiley PJ. Mössbauer spectroscopy as a tool for the study of activation/inactivation of the transcription regulator FNR in whole cells of Escherichia coli. Proc Natl Acad Sci USA. 1998;95:13431–13435. doi: 10.1073/pnas.95.23.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jordan PA, Thomson AJ, Ralph ET, Guest JR, Green J. FNR is a direct oxygen sensor having a biphasic response curve. FEBS Lett. 1997;416:349–352. doi: 10.1016/s0014-5793(97)01219-2. [DOI] [PubMed] [Google Scholar]
- 21.Sutton VR, Mettert EL, Beinert H, Kiley PJ. Kinetic analysis of the oxidative conversion of the [4Fe-4S]2+ cluster of FNR to a [2Fe-2S]2+ cluster. J Bacteriol. 2004;186:8018–8025. doi: 10.1128/JB.186.23.8018-8025.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crack JC, Green J, Cheesman MR, Le Brun NE, Thomson AJ. Superoxide-mediated amplification of the oxygen-induced switch from [4Fe-4S] to [2Fe-2S] clusters in the transcriptional regulator FNR. Proc Natl Acad Sci USA. 2007;104:2092–2097. doi: 10.1073/pnas.0609514104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crack JC, et al. Influence of the environment on the [4Fe-4S]2+ to [2Fe-2S]2+ cluster switch in the transcriptional regulator FNR. J Am Chem Soc. 2008;130:1749–1758. doi: 10.1021/ja077455+. [DOI] [PubMed] [Google Scholar]
- 24.Overton T, et al. Transcription activation at Escherichia coli FNR-dependent promoters by the gonococcal FNR protein: Effects of a novel S18F substitution and comparisons with the corresponding substitution in E. coli FNR. J Bacteriol. 2003;185:4734–4747. doi: 10.1128/JB.185.16.4734-4747.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dibden DP, Green J. In vivo cycling of the Escherichia coli transcription factor FNR between active and inactive states. Microbiology. 2005;151:4063–4070. doi: 10.1099/mic.0.28253-0. [DOI] [PubMed] [Google Scholar]
- 26.Bates DM, et al. Substitution of leucine 28 with histidine in the Escherichia coli transcription factor FNR results in increased stability of the [4Fe-4S]2+ cluster to oxygen. J Biol Chem. 2000;275:6234–6240. doi: 10.1074/jbc.275.9.6234. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz CJ, Djaman O, Imlay JA, Kiley PJ. The cysteine desulfurase, IscS, has a major role in in vivo Fe-S cluster formation in Escherichia coli. Proc Natl Acad Sci USA. 2000;97:9009–9014. doi: 10.1073/pnas.160261497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker S, Holighaus G, Gabrielczyk T, Unden G. O2 as the regulatory signal for FNR-dependent gene regulation in Escherichia coli. J Bacteriol. 1996;178:4515–4521. doi: 10.1128/jb.178.15.4515-4521.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marshall FA, et al. A novel promoter architecture for microaerobic activation by the anaerobic transcription factor FNR. Mol Microbiol. 2001;39:747–753. doi: 10.1046/j.1365-2958.2001.02262.x. [DOI] [PubMed] [Google Scholar]
- 30.Partridge JD, et al. Transition of Escherichia coli from aerobic to micro-aerobic conditions involves fast and slow reacting regulatory components. J Biol Chem. 2007;282:11230–11237. doi: 10.1074/jbc.M700728200. [DOI] [PubMed] [Google Scholar]
- 31.Mettert EL, Kiley PJ. Contributions of the [4Fe-4S]-FNR and integration host factor to fnr transcriptional regulation. J Bacteriol. 2007;189:3036–3043. doi: 10.1128/JB.00052-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giel JL, Rodionov D, Lui M, Blattner FR, Kiley PJ. IscR-dependent gene expression links iron–sulfur cluster assembly to the control of O2-regulated genes in Escherichia coli. Mol Microbiol. 2006;60:1058–1075. doi: 10.1111/j.1365-2958.2006.05160.x. [DOI] [PubMed] [Google Scholar]
- 33.McGuffin LJ, Bryson K, Jones DT. The PSIPRED protein structure prediction server. Bioinformatics. 2000;16:404–405. doi: 10.1093/bioinformatics/16.4.404. [DOI] [PubMed] [Google Scholar]
- 34.Mol CD, Arvai AS, Begley TJ, Cunningham RP, Tainer JA. Structure and activity of a thermostable thymine-DNA glycosylase: Evidence for base twisting to remove mismatched normal DNA bases. J Mol Biol. 2002;315:373–384. doi: 10.1006/jmbi.2001.5264. [DOI] [PubMed] [Google Scholar]
- 35.Thayer MM, Ahern H, Xing D, Cunningham RP, Tainer JA. Novel DNA binding motifs in the DNA repair enzyme endonuclease III crystal structure. EMBO J. 1995;14:4108–4120. doi: 10.1002/j.1460-2075.1995.tb00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuo CF, et al. Atomic structure of the DNA repair [4Fe-4S] enzyme endonuclease III. Science. 1992;258:434–440. doi: 10.1126/science.1411536. [DOI] [PubMed] [Google Scholar]
- 37.Thomson AJ. Cross-linked by a cluster. Curr Biol. 1993;3:173–174. doi: 10.1016/0960-9822(93)90264-o. [DOI] [PubMed] [Google Scholar]
- 38.Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: A web-based environment for protein structure homology modeling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- 39.Kopp J, Schwede T. The SWISS-MODEL repository of annotated three-dimensional protein structure homology models. Nucleic Acids Res. 2004;32:D230–D234. doi: 10.1093/nar/gkh008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwede T, Kopp J, Guex N, Peitsch MC. SWISS-MODEL: An automated protein homology-modeling server. Nucleic Acids Res. 2003;31:3381–3385. doi: 10.1093/nar/gkg520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guex N, Peitsch MC. SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 1997;18:2714–2723. doi: 10.1002/elps.1150181505. [DOI] [PubMed] [Google Scholar]
- 42.Peitsch MC. Protein modeling by E-mail Bio/Technology. 1995;13:658–660. [Google Scholar]
- 43.Miller JH. Experiments in Molecular Genetics. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1972. [Google Scholar]
- 44.Crack JC, Le Brun NE, Thomson AJ, Green J, Jervis AJ. Reactions of nitric oxide and oxygen with FNR (regulator of fumarate and nitrate reduction), a global transcriptional regulator, during anaerobic growth of. Escherichia coli. Methods Enzymol. 2008;437:191–209. doi: 10.1016/S0076-6879(07)37011-0. [DOI] [PubMed] [Google Scholar]
- 45.Crack JC, Green J, Le Brun NE, Thomson AJ. Detection of sulfide release from the oxygen-sensing [4Fe-4S] cluster of FNR. J Biol Chem. 2006;281:18909–18913. doi: 10.1074/jbc.C600042200. [DOI] [PubMed] [Google Scholar]
- 46.DeLano WL. The PyMOL Molecular Graphics System. Palo Alto, CA: DeLano Scientific; 2002. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.