Abstract
Synaptic adhesion molecules such as neuroligin are involved in synapse formation, whereas ionotropic transmitter receptors mediate fast synaptic transmission. In mutant mice deficient in the glutamate receptor δ2 subunit (δ2), the number of synapses between granule neurons (GNs) and a Purkinje neuron (PN) in the cerebellum is reduced. Here, we have examined the role of δ2 in synapse formation using culture preparations. First, we found that the size and number of GN presynaptic terminals on a PN in the primary culture prepared from knockout mice were smaller than those in control culture. Next we expressed δ2 in nonneuronal human embryonic kidney (HEK) cells and cocultured them with GNs. Punctate structures expressing marker proteins for glutamatergic presynaptic terminals were accumulated around the HEK cells. Furthermore, HEK cells expressing both δ2 and GluR1, a glutamate receptor subunit forming a functional glutamate-gated ion channel, showed postsynaptic current. Deletion of the extracellular leucine/isoleucine/valine binding protein (LIVBP) domain of δ2 abolished the induction ability, and the LIVBP domain directly fused to a transmembrane sequence was sufficient to induce presynaptic differentiation. Furthermore, a mutant GluR1 whose LIVBP domain was replaced with the δ2 LIVBP domain was sufficient by itself to establish synaptic transmission. Another member of δ glutamate receptor family δ1 also induced presynaptic differentiation. Thus, the δ glutamate receptor subfamily can induce the differentiation of glutamatergic presynaptic terminals and contribute to the establishment of synaptic transmission.
Keywords: cerebellum, granule neuron, Purkinje neuron, synapse formation
Synapse formation requires the accumulation and organization of multiple proteins on both the pre- and postsynaptic sides (1, 2). Previous studies have shown that expression of postsynaptic neuroligin in a nonneuronal cell triggers presynaptic differentiation in a contacting axon through interaction with presynaptic neurexin (3, 4). The involvement of other synaptic adhesion proteins, such as SynCAM and cadherin, in the formation or maintenance of synaptic structures has also been reported (5–7). For synaptic function, the most important postsynaptic proteins are receptors for neurotransmitters. Glutamate is the most prevalent neurotransmitter in the central nervous system, and there are ionotropic glutamate receptors (iGluRs) and metabotropic glutamate receptors on the postsynaptic membrane. iGluR opens its pore domain to allow the permeation of cations when bound by glutamate, and mediates fast excitatory synaptic transmission (8, 9).
So far, iGluR has not been shown to be directly related to synapse formation. However, in mutant mice deficient in the glutamate receptor δ2 subunit (δ2), the number of synapses between granule neurons (GNs) and a Purkinje neuron (PN) is reduced, suggesting the involvement of δ2 in synapse formation or maintenance (10, 11). δ2 is selectively expressed in cerebellar PNs and has been classified as a member of the iGluR subunit δ family with δ1 subunit, based on sequence homology. However, it was reported that glutamate binds to neither δ1 nor δ2, and whether δ1 or δ2 constitutes a functional ion channel is unknown (12–14). δ1 is expressed widely in the central nervous system of young mice, but in mature animals expression of δ1 is confined to the hippocampus, spiral ganglion, and hair cells of inner ears (12, 15).
Here, we have examined roles of δ2 and δ1 in the induction of presynaptic terminal differentiation and synapse formation using a coculture preparation of cerebellar neurons and nonneuronal human embryonic kidney (HEK) cells expressing δ2, δ1, etc. We found that the extracellular LIVBP domain of δ2 induced differentiation of glutamatergic presynaptic terminals and contributed to the establishment of synaptic transmission. We presented preliminary results in meetings and published abstracts (16, 17)
Results
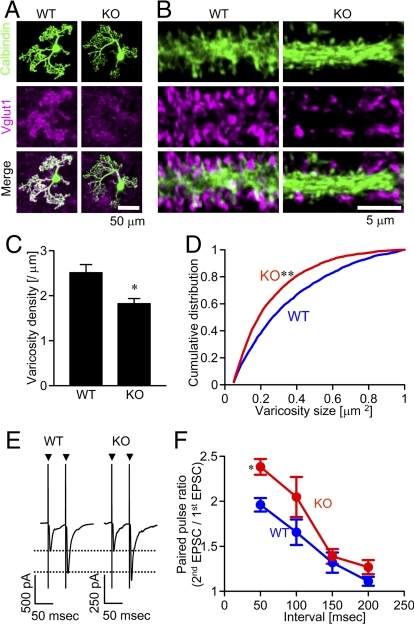
First, we asked if δ2 plays a role in synapse formation in a simplified condition without three-dimensional tissue organization and with limited intercellular interactions. Synapses between GNs and a PN were examined in a dissociated cell culture prepared from cerebella of either δ2 knockout or wild-type mice. GNs are major excitatory neurons in the cerebellum and express vesicular glutamate transporter 1 (vglut1) in presynaptic axon terminals (18). Thus, most vglut1-positive punctate structures in the immediate vicinity of PN can be regarded as presynaptic terminals of GNs. Abundant vglut1 signals on PNs labeled with anti-calbindin antibody were observed in a culture prepared from wild-type mice, whereas vglut1 signals were distributed diffusely rather than being concentrated on a PN in the knockout culture (Fig. 1 A and B). Significant differences were detected in the density and sizes of vglut1-positive puncta (Fig. 1 C and D).
Fig. 1.
Effects of δ2 knockout on GN presynaptic terminals. (A and B) GN terminals marked with vglut1 signal (magenta) on PNs (green, calbindin staining) in wild-type (WT) and δ2 knockout (KO) cerebellar culture. (C) The density of vglut1-positive puncta around PNs was significantly higher in wild-type culture (17 cells) than in δ2 knockout culture (25 cells) (*P < 0.05). (D) Cumulative distribution of sizes of vglut1-positive puncta on PNs (WT, 2,942 puncta; KO, 3,260 puncta) (**P < 0.01; Kolmogorov-Smirnov test). (E) Representative EPSC traces (average of 10 traces) showing paired pulse facilitation. Arrowheads indicate the stimulation. (F) PPR in δ2 knockout PNs (red) was significantly higher than that in wild-type PNs (blue) (n = 5 for each, *P < 0.05).
We next examined whether synaptic transmission was affected by δ2 knockout. A PN was whole-cell voltage-clamped and a presynaptic GN was stimulated. Double-pulse stimulation induced a pair of excitatory postsynaptic currents (EPSCs). The paired pulse ratio (PPR, second EPSC amplitude/first EPSC amplitude) reflects the presynaptic release probability of synaptic vesicles: higher PPR suggests lower release probability. PPR in δ2 knockout culture was significantly higher than that in control culture (Fig. 1 E and F) as shown previously in slice preparations (10). Thus, δ2 supports synapse formation and/or maturation in a dissociated culture preparation.
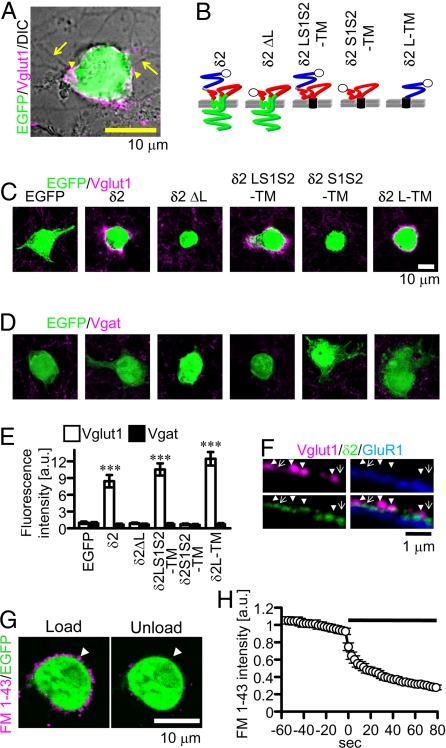
Based on the findings discussed, we hypothesized that δ2 on the postsynaptic membrane may directly interact with GN axons and thereby induce differentiation of presynaptic terminals. To address this possibility, we prepared cocultures of cerebellar neurons and HEK293T cells expressing enhanced green fluorescent protein (EGFP) together with or without δ2. Punctate signals of vglut1 were observed where GN neurites contact the HEK cells expressing δ2 and EGFP (Fig. 2A), but not those expressing only EGFP (Fig. 2C). The vglut1 signal was apposed to the concentrated δ2 signal, although the latter is not always accompanied by the former (Fig. 2F). Another synaptic vesicle protein synaptobrevin/VAMP2 and an active-zone protein bassoon were also accumulated around δ2-transfected HEK cells (supporting information (SI) Fig. S1), whereas vesicular γ-amino butyric acid (GABA) transporter (vgat), found only in inhibitory presynaptic terminals, was not accumulated (Fig. 2D). These findings suggest that δ2 expressed in HEK cells is sufficient to trigger the presynaptic terminal differentiation of GN axons but not GABAergic inhibitory neurons.
Fig. 2.
Synapse formation on HEK cells. (A) An HEK cell transfected with EGFP (green) and δ2 in the coculture is shown. Presynaptic terminals of GNs were stained with anti-vglut1 antibody (magenta, arrowhead). Arrows indicate neurites. (B) δ2 mutant proteins. The open circle represents the HA tag. Blue, red, and green lines represent LIVBP (L), S1–S2 (S1S2), and transmembrane and intracellular domains, respectively. The black cylinder represents the transmembrane domain of platelet-derived growth factor receptor. (C and D) HEK cells transfected with EGFP alone (EGFP) or together with δ2, δ2ΔL, δ2LS1S2-TM, δ2S1S2-TM, δ2L-TM, are shown. They were stained with antibody against vglut1 (C) or vgat (D) (magenta) and shown with EGFP signal (green). (E) Total fluorescent signal intensity for vglut1 but not that for vgat around HEK cells expressing δ2, δ2LS1S2-TM, or δ2L-TM was significantly increased compared with that around HEK cells expressing only EGFP (n = 30 for each; ***P < 0.001). Each value was normalized by the total fluorescent signal intensity around HEK cells expressing only EGFP. (F) Vglut1 (magenta), δ2 (green), and GluR1 (blue) signals on a HEK cell expressing δ2 and GluR1. Only surface δ2 and GluR1 were stained with antibody against anti-HA and anti-myc, respectively. Vglut1 signal apposed to δ2 signal (arrowheads) and δ2 signal not apposed to vglut1 signal (arrows) are shown. (G) Loading (Left) and unloading (Right) of FM 1–43 (white arrowheads) around a HEK cell expressing δ2 in the coculture. (H) The time course of FM 1–43 unloading induced by electrical field stimulation (black horizontal bar; n = 20).
Next we made mutant δ2 proteins to determine the critical domain for synaptogenic activity (Fig. 2B). Deletion mutants lacking the extracellular N-terminal LIVBP domain (δ2ΔL) or the major intracellular C-terminal domain (δ2ΔC) were constructed. We also made fusion proteins in which the transmembrane segment of platelet-derived growth factor receptor (not related to δ2) was fused to the extracellular domains of δ2 (δ2LS1S2-TM, δ2S1S2-TM, and δ2L-TM). The LIVBP domain is involved in heterotetramer formation, whereas the S1−S2 domain is involved in glutamate binding in other iGluRs (19, 20). HEK cells expressing δ2ΔC accumulated vglut1 signal (data not shown), but those expressing δ2ΔL did not (Fig. 2C), suggesting a critical role of the LIVBP domain. Furthermore, HEK cells expressing δ2L-TM or δ2LS1S2-TM accumulated vglut1, synaptobrevin/VAMP2, and bassoon signals, but not vgat signal (Fig. 2 C–E and Fig. S1). In contrast, HEK cells expressing δ2S1S2-TM failed to induce presynaptic differentiation. We confirmed that all these mutant proteins were expressed on the plasma membrane (Fig. S2), negating the possibility that the failure of surface expression caused the difference. These findings suggest that the LIVBP domain is indispensable for the synaptogenic activity of δ2, whereas the intracellular C-terminal domain and S1–S2 domain are not.
The findings described so far indicate that δ2 can trigger the accumulation of presynaptic marker proteins in GN axon terminals apposed to a cell expressing δ2. However, it is unclear whether presynaptic terminal-like structures release transmitter in response to neuronal activation. We addressed this issue using styryl dye FM 1–43 (21). FM 1–43 is taken into synaptic vesicles during endocytosis and is released with neurotransmitter during exocytosis. Punctate structures on HEK cells expressing δ2 were labeled with FM 1–43 by the first electrical field stimulation, which would trigger action potential generation. The second electrical stimulation destained the puncta (Fig. 2 G and H), suggesting that δ2 induces differentiation of presynaptic terminals capable of releasing synaptic vesicles when action potentials arrive.
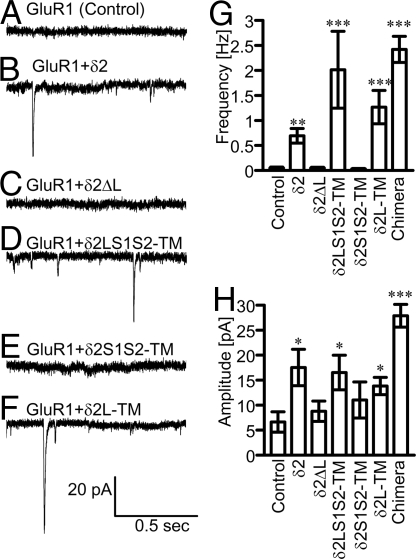
We next examined the functional synaptic transmission from GNs to δ2-expressing HEK cells electrophysiologically. Although δ2 is classified as a member of iGluRs, glutamate binding by this protein has not been detected (12). Therefore, we transfected GluR1 together with δ2 into HEK cells to record synaptic currents. GluR1 is another type of iGluR subunit belonging to the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor family consisting of GluR1–4. Whole-cell patch clamp recordings were performed on HEK cells in the presence of tetrodotoxin, bicuculline, and cyclothiazide (CTZ). CTZ prevents desensitization of GluR1 and enhances the current response (22). Miniature EPSC (mEPSC)-like currents were detected from HEK cells expressing GluR1 and δ2, δ2LS1S2-TM, or δ2L-TM (Fig. 3B, D, F, and G). In contrast, such currents were rarely recorded from HEK cells expressing GluR1 alone, or GluR1 and δ2ΔL or δ2S1S2-TM (Fig. 3 A, C, E, and G). All HEK cells expressing GluR1 showed inward currents in response to iontophoretically applied glutamate (data not shown). Glutamate-induced currents and mEPSC-like currents were both completely suppressed by 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX), an antagonist for AMPA receptors (data not shown), suggesting that the latter currents were indeed mEPSCs. mEPSC-like currents were also recorded from HEK cells expressing GluR1 GluR1, and δ2ΔL or δ2S1S2-TM at very low frequencies, and their amplitudes were significantly smaller than those recorded from HEK cells expressing GluR1 and δ2 or the mutants containing the LIVBP domain (Fig. 3H). The origin of occasional mEPSCs in HEK cells expressing only GluR1 is unknown. GluR1 might have detected glutamate released from GN terminals located around or attached to the HEK cells. A previous study showed a small number of presynaptic terminals contacting HEK cells expressing GluR4 in a coculture with GNs (23). No mEPSC-like current was recorded from HEK cells expressing only δ2. In any case, δ2 LIVBP domain expression in HEK cells robustly increased the frequency of mEPSCs, suggesting that the δ2 LIVBP domain can trigger the establishment of functional glutamatergic synaptic transmission.
Fig. 3.
Synaptic currents in HEK cells. (A–F) Representative mEPSC traces recorded from HEK cells expressing only GluR1 (control, n = 20) (A), GluR1 and δ2 (n = 20) (B), GluR1 and δ2ΔL (n = 20) (C), GluR1 and δ2LS1S2-TM (n = 15) (D), GluR1 and δ2S1S2-TM (n = 19) (E), or GluR1 and δ2L-TM (n = 16) (F) in the presence of CTZ. (G and H) The frequency (G) and amplitude (H) of mEPSCs in HEK cells (*P < 0.05; **P < 0.01; ***P < 0.001 compared with the control).
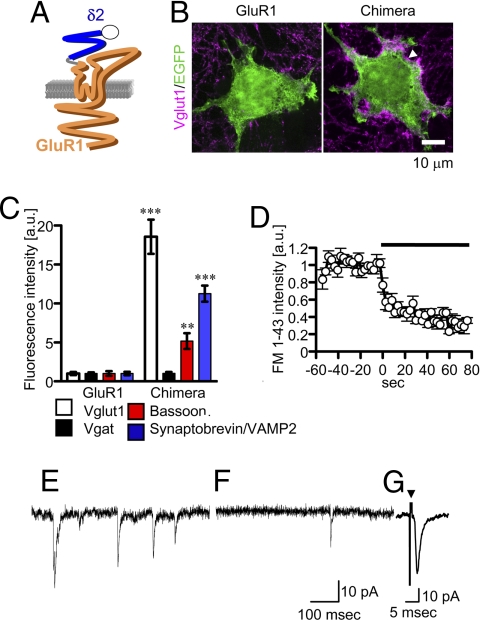
To further confirm the pivotal role of the LIVBP domain of δ2 in the induction of presynaptic differentiation, a δ2/GluR1 chimera whose LIVBP domain was replaced with that of δ2 was constructed (Fig. 4A). When expressed in HEK cells, the chimera was transported to the cell surface (Fig. S3) and induced the accumulation of vglut1, synaptobrevin/VAMP2, and bassoon, but not that of vgat (Fig. 4 B and C). FM 1–43 was efficiently loaded and unloaded around HEK293T cells expressing the chimera by electrical stimulation (Fig. 4D). These cells also showed glutamate-induced inward currents and mEPSCs in the presence of CTZ (Figs. 3E and 4E). Furthermore, it was possible to record mEPSCs in these cells even in the absence of CTZ (Fig. 4F; frequency, 1.1 ± 0.2 Hz; amplitude, 23.3 ± 2.0 pA, n = 19), presumably because of the efficient accumulation of functional iGluRs on the HEK cell membrane apposed to GN presynaptic terminals. We suppose that the δ2 LIVBP domain of the chimera triggered formation of GN presynaptic terminals and that the S1–S2 domain was bound by glutamate released from terminals and opened the pore domain of the chimera. mEPSCs were rarely detected without CTZ in the HEK cells expressing δ2 and GluR1. GluR1 was not particularly concentrated on the HEK cell membrane apposed to presynaptic terminals (Fig. 2F). We also recorded evoked EPSCs in HEK cells expressing the chimera by electrically stimulating a nearby GN (Fig. 4G), confirming that the arrival of an action potential at the presynaptic terminal triggered glutamate release. Thus, the δ2/GluR1 chimera is an interesting and useful molecule equipped with both the synaptogenic activity and glutamate sensitivity.
Fig. 4.
GluR1/δ2 chimera. (A) In the chimera, the LIVBP domain of GluR1 (orange) was replaced with that of δ2 (blue). (B and C) Vglut1 signals (white arrow) were accumulated around HEK cells expressing the chimera. (**P < 0.01; ***P < 0.001 compared with the control, GluR1) (D) The time course of FM 1–43 unloading (n = 5) induced by electrical stimulation (black bar). (E) A representative mEPSC trace recorded from a HEK cell expressing the chimera in the presence of CTZ (n = 19). (F and G) Representative mEPSC (F) and evoked EPSC (G) traces (average of 13 traces) recorded from HEK cells expressing the chimera without CTZ. An arrowhead indicates the stimulation.
Finally, we examined whether δ1, another member of the iGluR δ family, could also trigger synapse formation. Vglut1, VAMP2, and bassoon were accumulated around HEK cells expressing δ1, whereas vgat was not (Fig. S4). Staining and destaining of FM 1–43 by electrical stimulations were also observed around δ1-expressing HEK cells. Furthermore, HEK cells transfected with δ1 and GluR1 showed mEPSC-like currents at high frequencies (Fig. S5). These findings suggest that δ1 can also induce the differentiation of glutamatergic presynaptic terminals.
Discussion
We showed here that iGluR subunits δ2 and δ1 can trigger the differentiation of GN presynaptic terminals and contribute to the establishment of functional synaptic transmission. The extracellular LIVBP domain of δ2 plays an essential role as indicated by our findings that the deletion of the LIVBP domain abolished the induction ability, and the LIVBP domain directly fused to a transmembrane sequence was sufficient to establish synaptic transmission. Furthermore, a mutant GluR1 whose LIVBP domain was replaced with the δ2 LIVBP domain was sufficient to establish synaptic transmission. Similar findings in line with our conclusion were published recently (24). One straightforward explanation is that δ2 binds to some presynaptic molecules directly through the LIVBP domain and this interaction triggers the presynaptic differentiation. However, it is also possible that δ2 or δ1 influences GN axons indirectly through extracellular or postsynaptic molecules. The involvement of secreted molecules in synapse formation has been reported (2, 25, 26). It is also known that GluR2, an AMPA-type iGluR subunit, interacts with a cell adhesion molecule, N-cadherin, on the postsynaptic membrane (27). Future studies to identify binding partners of δ2 and/or δ1 will be needed to unravel the molecular mechanism of how δ2 and δ1 contribute to synapse formation, maintenance, and/or function.
Our findings provide an explanation of why the number of GN-PN synapses is reduced in δ2 knockout mice. However, it should be noted that there are some GN-PN synapses in the mutant mice. Furthermore, basket, stellate, and Golgi neurons, which do not express δ2, receive synaptic inputs from GNs (28). Therefore, δ2 is dispensable, and other synaptic adhesion molecules, such as neuroligin, also appear to contribute to presynaptic differentiation of GNs. What, then, is the specific role of δ2? δ2 is selectively expressed on the PN postsynaptic membrane apposed to GN presynaptic terminals, and GN-PN synapses are presumably the most abundant synapses in the central nervous system: more than 100,000 GN presynaptic terminals contact on a PN (28). It is known that the induction conditions and the mechanism of synaptic plasticity at GN-PN synapses are different from those in the hippocampus (29, 30). δ2 might contribute to the expression of the specific properties of an enormous number of GN-PN synapses.
Finally, what is the role of δ1? δ1 is expressed widely in young mice, but the distribution of δ1 expression is restricted in mature animals (12, 15). Thus, δ1 might play some roles in the synapse formation during development, although the behavioral and neurological abnormalities in δ1 knockout mice reported so far are confined to mild defects in the auditory ability (15).
Materials and Methods
Cell Culture.
Cerebella were dissected out from newborn mouse pups and incubated in Ca2+ and Mg2+-free HBSS containing 0.1% trypsin and 0.05% DNase for 15 min at 37 °C (31). Neurons were dissociated by trituration and seeded on polyD-lysine-coated coverslips in DMEM/F12-based medium containing 2% FBS. The next day, 75% of the medium was replaced with serum-free medium. Thereafter, a half of medium was replaced with serum-free medium every week. To inhibit glial proliferation, cytosine β-D-arabinofuranoside (5 μM) was added to the medium from 4 days after dissociation. HEK cells were transfected using Lipofectamine 2000 (Invitrogen) and added to the cerebellar culture on day 14 of their in vitro culturing.
Expression Vectors.
The expression vector of HA-δ2 was constructed as described (31). Mutant δ2 cDNAs (δ2ΔC and δ2ΔL) were cloned by PCR and inserted into pCAGplay. The expression vector of HA-GluR1 or myc-GluR1 was subcloned from mouse GluR1 flip cDNA. HA or myc tag-encoding sequence was ligated and inserted into pCAGplay. The δ2/GluR1 chimera sequence was generated by extended PCR and inserted into pCAGplay. The transmembrane sequence of platelet-derived growth factor was obtained from pDisplay (Clontech) by PCR and inserted into pCAGplay (pCAGplay-TM). The fragments of δ2LS1S2 and δ2S1S2 were generated as described (32). These fragments were inserted between the CAG promoter sequence and transmembrane sequence of pCAGplay-TM to generate δ2LS1S2-TM and δ2S1S2-TM, respectively. The LIVBP sequence of δ2 was generated by PCR and inserted between the CAG sequence and transmembrane sequence of pCAGplay-TM. The mouse δ1 cDNA was cloned from a library prepared from the hippocampus and inserted into pCR 4Blunt-TOPO (Invitrogen) and then into pCAGplay. The fragment of CMV promoter-EGFP excised from pEGFP-N1 (Clontech) was inserted into the expression vectors described previously. The DsRed2 sequence of pDsRed2 (Clontech) was replaced with the EGFP sequence and inserted into the expression vector of GluR1. DsRed2 was used to confirm coexpression of GluR1 with δ2.
Electrophysiology.
Whole-cell patch-clamp recording was performed in the external solution containing the following (in mM): 145 NaCl, 5 KOH, 2 CaCl2, 1 MgCl2, 10 Hepes, and 10 glucose (pH 7.3) at room temperature (20–24 °C). It also contained 1 μM tetrodotoxin (Wako) and 20 μM bicuculline (Tocris) in mEPSC recordings. Tetrodotoxin blocks voltage-gated Na+ channel and suppresses action potentials, and bicuculline inhibits ionotropic GABAΑ receptor. In some experiments, 100 μM CTZ (Tocris) was added to prevent desensitization. Evoked EPSCs were recorded in the presence of 20 μM bicuculline by stimulating a nearby GN with a glass pipette filled with the external solution. Patch pipettes were filled with the internal solution containing the following (in mM): 147 CsCl, 10 CsOH, 5 EGTA, and 10 Hepes (pH 7.3). In some experiments, 5 mM QX-314 (Tocris) was added to the internal solution to prevent action potential generation. The electrode resistance was 2–6 MΩ. The membrane potential was held at −70 mV. Only recording with an input resistance of >100 MΩ and series resistance of <25 MΩ was accepted. Recording was performed with an EPC-9 amplifier (HEKA), and the recorded current was digitally filtered at 1.5 kHz. Mini Analysis (Synaptosoft) was used to analyze mEPSCs. The method for iontophoretic application of glutamate was described previously (31).
Immunocytochemistry.
Cultured cells were fixed in PBS containing 4% paraformaldehyde and 4% sucrose for 10 min at room temperature. After permeabilization in PBS containing 0.5% Tween 20, samples were processed for immunofluorescent staining. After washing, the coverslip was mounted with glycerol-based medium AntiFade (Invitrogen). Images were captured with an FV1000 confocal laser scanning microscope (Olympus) or an LSM510 confocal laser scanning microscope (Zeiss). The image treated with a large median filter (1.5 μm2) was subtracted from the original image to improve the separation of vglut1-postive puncta. Then, signals below an arbitrarily set threshold were removed, and the number and sizes of vglut1-positive puncta were measured. To quantify the amount of presynaptic protein around transfected HEK cells, the total fluorescence intensity on the rim of each cell was measured. Conditions to capture images and the threshold were kept constant throughout a series of experiments. The primary and secondary antibodies used were monoclonal anti-calbindin D-28K (Swant), guinea pig anti-vglut1 (Chemicon), rabbit anti-vgat (Synaptic Systems), monoclonal anti-bassoon (Stressgen), monoclonal anti-synaptobrevin/VAMP2 (Synaptic Systems), monoclonal anti-HA (Roche), rabbit anti-myc (Abcam), and Alexa 350-, 488-, or 568- conjugated goat anti-mouse, rabbit, or guinea pig Ig antibodies (Invitrogen). Images were analyzed with ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/).
FM 1–43 Imaging.
Presynaptic terminals were loaded with FM 1–43 (20 μM; Invitrogen) by field electrical stimulation through platinum wires. Five pulses (15 V, 500 μsec, 50 Hz) were applied 100 times at 1 Hz. After loading, ADVASEP-7 (1 mM; Biotium) was added for 5 min to reduce background staining. Then, the second field stimulation (8,000 pulses, 100 Hz) was applied to unload FM 1–43. Images were captured using an FV 1000 confocal microscope.
Statistics.
All data were expressed as mean ± SEM. One-way analysis of variance with Steel-Dwass's multiple comparison tests or one-tailed unpaired t test was used to detect significant differences unless otherwise stated.
Supplementary Material
Acknowledgments.
We thank S. Kawaguchi, Y. Tagawa, and E. Nakajima for comments on the manuscript, and M. Mishina for δ2 knockout mice, δ2, and GluR1 cDNA. This work was supported by grants-in-aid for scientific research in Japan to T.H., by a fellowship from Japan Society for the Promotion of Science to T.K., and by Global COE Program A06 of Kyoto University.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900892106/DCSupplemental.
References
- 1.Waites CL, Craig AM, Garner CC. Mechanisms of vertebrate synaptogenesis. Annu Rev Neurosci. 2005;28:251–274. doi: 10.1146/annurev.neuro.27.070203.144336. [DOI] [PubMed] [Google Scholar]
- 2.Fox MA, Umemori H. Seeking long-term relationship: Axon and target communicate to organize synaptic differentiation. J Neurochem. 2006;97:1215–1231. doi: 10.1111/j.1471-4159.2006.03834.x. [DOI] [PubMed] [Google Scholar]
- 3.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 4.Fabrichny IP, et al. Structural analysis of the synaptic protein neuroligin and its β-neurexin complex: Determinants for folding and cell adhesion. Neuron. 2007;56:979–991. doi: 10.1016/j.neuron.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synaptic assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 6.Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–2419. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- 7.Takeichi M. The cadherin superfamily in neuronal connections and interactions. Nat Rev Neurosci. 2007;8:11–20. doi: 10.1038/nrn2043. [DOI] [PubMed] [Google Scholar]
- 8.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 9.Mayer ML. Glutamate receptor ion channels. Curr Opin Neurobiol. 2005;15:282–288. doi: 10.1016/j.conb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kashiwabuchi N, et al. Impairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR δ2 mutant mice. Cell. 1995;81:245–252. doi: 10.1016/0092-8674(95)90334-8. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara H, et al. Impaired parallel fiber→Purkinje cell synapse stabilization during cerebellar development of mutant mice lacking the glutamate receptor δ2 subunit. J Neurosci. 2007;17:9613–9623. doi: 10.1523/JNEUROSCI.17-24-09613.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomeli H, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- 13.Araki K, et al. Selective expression of the glutamate receptor channel δ2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. doi: 10.1006/bbrc.1993.2614. [DOI] [PubMed] [Google Scholar]
- 14.Hirano T. Cerebellar regulation mechanisms learned from studies on GluRδ2. Mol Neurobiol. 2006;33:1–16. doi: 10.1385/MN:33:1:001. [DOI] [PubMed] [Google Scholar]
- 15.Gao J, et al. Orphan glutamate receptor δ1 subunit required for high-frequency hearing. Mol Cell Biol. 2007;27:4500–4512. doi: 10.1128/MCB.02051-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuroyanagi T, Hirano T. Glutamate receptor δ2 subunit induces presynaptic differentiation of a cerebellar granule neuron. Neurosci Res 58 Suppl. 2007;1:S132. [Google Scholar]
- 17.Kuroyanagi T, Hirano T. Induction of presynaptic differentiation by glutamate receptor δ2 subunit; 38th Annual Meeting of the Society for Neuroscience; November 15–19; Washington, DC. 2008. p. 333.12. abstr. [Google Scholar]
- 18.Hioki H, et al. Differential distribution of vesicular glutamate transporters in the rat cerebellar cortex. Neurosci. 2003;117:1–6. doi: 10.1016/s0306-4522(02)00943-0. [DOI] [PubMed] [Google Scholar]
- 19.Leuschner WD, Hoch W. Subtype-specific assembly of alpha-amino-3-hydroxy-5-methyl-4-isxazole propionic acid receptor subunits is mediated by their N-terminal domains. J Biol Chem. 1999;274:16907–16916. doi: 10.1074/jbc.274.24.16907. [DOI] [PubMed] [Google Scholar]
- 20.Ayalon G, Sagev E, Elgavish S, Stern-Bach Y. Two regions in the N-terminal domain of ionotropic glutamate receptor 3 form the subunit oligomerization interfaces that control subtype-specific receptor assembly. J Biol Chem. 2005;280:15053–15060. doi: 10.1074/jbc.M408413200. [DOI] [PubMed] [Google Scholar]
- 21.Kay AR, et al. Imaging synaptic activity in intact brain and slices with FM 1–43 in C. elegans, lamprey, and rat. Neuron. 1999;24:809–817. doi: 10.1016/s0896-6273(00)81029-6. [DOI] [PubMed] [Google Scholar]
- 22.Fucile S, Miledi R, Eusebi F. Effects of cyclothiazide on GluR1/AMPA receptors. Proc Natl Acad Sci USA. 2006;103:2943–2947. doi: 10.1073/pnas.0511063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90:3950–3957. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- 24.Uemura T, Mishina M. The amino-terminal domain of glutamate receptor δ2 triggers presynaptic differentiation. Biochem Biophys Res Commun. 2008;377:1315–1319. doi: 10.1016/j.bbrc.2008.10.170. [DOI] [PubMed] [Google Scholar]
- 25.O'Brien R, et al. Synaptically targeted narp plays an essential role in the aggregation of AMPA receptors at excitatory synapses in cultured spinal neurons. J Neurosci. 2002;22:4487–4498. doi: 10.1523/JNEUROSCI.22-11-04487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirai H, et al. Cbln1 is essential for synaptic integrity and plasticity in the cerebellum. Nat Neurosci. 2005;8:1534–1541. doi: 10.1038/nn1576. [DOI] [PubMed] [Google Scholar]
- 27.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78:272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 29.Jorntell H, Hansel C. Synaptic memories upside down: Bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron. 2006;52:227–238. doi: 10.1016/j.neuron.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Massey PV, Bashir ZI. Long-term depression: Multiple forms and implications for brain function. Trends Neurosci. 2007;30:176–184. doi: 10.1016/j.tins.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 31.Yawata S, Tsuchida H, Kengaku M, Hirano T. Membrane-proximal region of glutamate receptor δ2 subunit is critical for long-term depression and interaction with protein interacting with C kinase 1 in a cerebellar Purkinje neuron. J Neurosci. 2006;26:3626–3633. doi: 10.1523/JNEUROSCI.4183-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naur P, et al. Ionotropic glutamate-like receptor δ2 binds D-serine and glycine. Proc Natl Acad Sci USA. 2007;104:14116–141221. doi: 10.1073/pnas.0703718104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.