Abstract
Extensive evidence indicates that the basolateral complex of the amygdala (BLA) modulates the consolidation of memories for emotionally arousing experiences, an effect that involves the activation of the glucocorticoid system. Because the BLA expresses high densities of cannabinoid CB1 receptors, the present experiments investigated whether the endocannabinoid system in the BLA influences memory consolidation and whether glucocorticoids interact with this system. The CB1 receptor agonist WIN55,212-2 (5–50 ng per 0.2 μL per side), infused bilaterally into the BLA of male Sprague–Dawley rats immediately after inhibitory avoidance training, induced dose-dependent enhancement of 48-h retention. Conversely, the CB1 receptor antagonist AM251 (0.07–0.28 ng per 0.2 μL per side) administered after training into the BLA induced inhibitory avoidance retention impairment. Furthermore, intra-BLA infusions of a low and nonimpairing dose of AM251 (0.14 ng per 0.2 μL per side) blocked the memory enhancement induced by concurrent administration of WIN55,212-2. Delayed infusions of WIN55,212-2 or AM251 administered into the BLA 3 h after training or immediate posttraining infusions of these drugs into the adjacent central amygdala did not significantly alter retention performance. Last, intra-BLA infusions of a low and otherwise nonimpairing dose of AM251 (0.14 ng per 0.2 μL per side) blocked the memory-enhancing effect induced by systemic administration of corticosterone (3 mg/kg, s.c.). These findings indicate that endocannabinoids in the BLA enhance memory consolidation and suggest that CB1 activity within this brain region is required for enabling glucocorticoid effects on memory consolidation enhancement.
Keywords: AM251; cannabinoid receptors; emotional arousal; inhibitory avoidance; WIN55,212-2
The endocannabinoid system plays an important regulatory role in several brain functions, including locomotion, emotionality, feeding, and pain control (1). Endocannabinoids—that is, anandamide and 2-arachidonoylglycerol (2-AG)—are synthesized on demand through cleavage of membrane precursors and serve as retrograde messengers at central synapses (2). They bind to the cannabinoid receptor subtype 1 (CB1 receptor) on axon terminals to regulate ion channel activity and neurotransmitter release (3). The evidence that CB1 receptors are highly expressed in the hippocampus, the prefrontal cortex, and the amygdala (4) suggests that the endocannabinoid system may be involved in regulating learning and memory. It is known that marijuana abusers experience deficits in working and short-term memory (5, 6) and that these effects depend partially on an altered activity of the hippocampus and prefrontal cortex (5, 7, 8). Animal studies, which enable a more controlled drug regimen and more constant behavioral testing, have confirmed human findings and additionally suggested that cannabinoid treatment affects memory encoding and consolidation processes (5, 9, 10).
It is well established that the basolateral complex of the amygdala (BLA; consisting of the lateral, basal, and accessory basal nuclei) is involved in mediating stress hormone effects on memory formation of emotionally arousing experiences (11, 12). CB1 receptors are highly expressed in the BLA, where they modulate synaptic transmission (13) and neuronal firing (14). Such modulating influences within the BLA may contribute to the emotionally relevant behavioral effects of cannabinoid drugs. It is well known that systemically administered cannabinoids biphasically modulate emotionality and mood states (15–17). Additionally, recent findings indicate that CB1 receptor stimulation in the BLA exerts an anxiogenic-like effect (18). Moreover, endocannabinoids within the amygdala complex facilitate memory consolidation of fear learning (19) as well as extinction of aversive memories (20). However, studies have not yet investigated whether the BLA is a critical region of the amygdala involved in mediating cannabinoid effects on memory consolidation.
Some findings indicate that endocannabinoid activity is essential for mediating some of the central effects of glucocorticoids (21, 22). In particular, it has been shown that within minutes after their administration, glucocorticoids facilitate endocannabinoid production and release in specific hypothalamic regions regulating hypothalamic–pituitary–adrenocortical axis activity (23, 24). Such interactions are of interest in light of growing evidence that glucocorticoids, in addition to inducing slow effects on gene transcription, also have a variety of rapid physiological actions (25). There is extensive evidence that glucocorticoid hormones enhance long-term consolidation of emotionally arousing experiences involving rapid actions on intracellular signaling cascades in the BLA (26, 27). Therefore, it is possible that the endocannabinoid system in the BLA mediates stress and glucocorticoid effects on memory consolidation.
The present experiments investigated whether the cannabinoid system in the BLA influences memory consolidation of emotionally arousing experiences and whether CB1 activity plays an important role in mediating glucocorticoid effects on memory enhancement. In the first experiment, different doses of the CB1 receptor agonist WIN55,212-2 were administered into the BLA immediately after aversively motivated inhibitory avoidance training. Retention was tested 48 h after the training trial. A second experiment investigated the effect of immediate posttraining intra-BLA infusions of the CB1 receptor antagonist AM251 to determine whether endogenously released cannabinoids might play a role in memory consolidation. To control for time and site specificity, other groups of rats received intra-BLA infusions of WIN55,212-2 or AM251 3 h after training or infusions of these drugs into the adjacent central nucleus of the amygdala (CeA). A third experiment used concurrent infusions of WIN55,212-2 and a nonimpairing dose of the CB1 receptor antagonist AM251 to investigate whether the memory-enhancing effects of WIN55,212-2 are mediated by a selective activation of CB1 receptors. Finally, the last experiment investigated whether CB1 activity within the BLA is required for enabling glucocorticoid-induced enhancement of memory consolidation.
Results
Posttraining Intra-BLA Infusions of the CB1 Receptor Agonist WIN55,212-2 Induce Enhancement of Inhibitory Avoidance Retention.
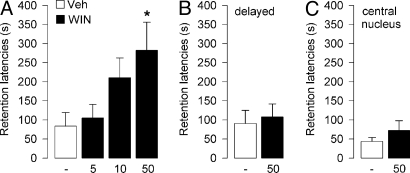
This experiment investigated whether the CB1 receptor agonist WIN55,212-2 infused into the BLA immediately after inhibitory avoidance training would modulate memory consolidation. Average step-through latencies for all groups during training, before footshock and drug treatment, were 11.3 ± 1.1 s. One-way ANOVA for training latencies revealed no significant differences between groups (F3,39 = 1.12; P = 0.35). Forty-eight-hour retention latencies of rats infused with vehicle into the BLA immediately after training were significantly longer than their entrance latencies during the training trial (P < 0.05), indicating that the rats retained memory of the shock experience. As shown in Fig. 1A, WIN55,212-2 induced dose-dependent retention enhancement. A 1-way ANOVA for retention latencies revealed a significant treatment effect (F3,39 = 3.44; P = 0.03). Posthoc comparisons indicated that retention latencies of rats given posttraining infusions of WIN55,212-2 (50 ng) were significantly longer than those of rats given vehicle (P < 0.05). Lower doses did not significantly alter retention performance.
Fig. 1.
Effects of WIN55,212-2 on retention of an inhibitory avoidance response. Step-through latencies (mean and SEM) on a 48-h retention test. (A) Immediate posttraining intra-BLA infusions of the cannabinoid agonist WIN55,212-2 (WIN; 5, 10, 50 ng per 0.2 μL) enhanced memory consolidation. *, P < 0.05 vs. vehicle (Veh; n = 10–11 per group). (B) Delayed infusions of WIN55,212-2 (WIN; 50 ng per 0.2 μL) administered into the BLA 3 h after training did not enhance memory consolidation (n = 11–12 rats per group). (C) Immediate posttraining infusions of WIN55,212-2 (WIN 50 ng per 0.2 μL) into the CeA did not enhance memory consolidation (n = 7 rats per group).
To examine whether WIN55,212-2 enhanced the consolidation phase of memory processing, other groups of rats received intra-BLA infusions of WIN55,212-2 (50 ng) or its vehicle 3 h after training. As shown in Fig. 1B, 48-h retention latencies of rats given infusions of WIN55,212-2 at 3 h after training did not differ significantly from those of rats given vehicle (P = 0.73).
Additional infusions of WIN55,212-2 were made into the adjacent CeA to investigate the site specificity of cannabinoid action. Retention latencies of rats given intra-CeA infusions of WIN55,212-2 (50 ng) immediately after training did not differ from those administered vehicle (P = 0.32; Fig. 1C).
Posttraining Intra-BLA Infusions of the CB1 Receptor Antagonist AM251 Induce Impairment of Inhibitory Avoidance Retention Performance.
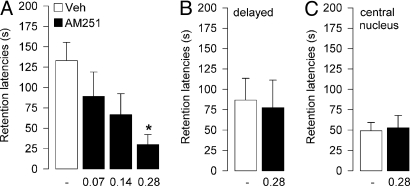
This experiment examined whether blockade of CB1 receptors in the BLA with posttraining infusions of the CB1 receptor antagonist AM251 would impair memory consolidation of inhibitory avoidance training. One-way ANOVA for training latencies before footshock and drug treatment revealed no significant differences between groups (F3,37 = 0.42; P = 0.73). Forty-eight-hour retention latencies of rats infused with vehicle into the BLA immediately after training were significantly longer than their latencies during the training trial (P < 0.001). As shown in Fig. 2A, immediate posttraining infusions of AM251 dose-dependently impaired retention (F3,37 = 3.49; P = 0.025). Posthoc comparisons indicated that retention latencies of rats given the 0.28-ng dose of AM251 were significantly shorter than those of rats given vehicle (P < 0.05; Fig. 2A). Lower doses of AM251 did not significantly impair retention.
Fig. 2.
Effects of AM251 on retention of an inhibitory avoidance response. Step-through latencies (mean and SEM) on a 48-h retention test. (A) Immediate posttraining intra-BLA infusions of the cannabinoid antagonist AM251 (0.07, 0.14, 0.28 ng per 0.2 μL) impaired memory consolidation. *, P < 0.05 vs. vehicle (Veh; n = 9–11 per group). (B) Delayed infusions of AM251 (0.28 ng per 0.2 μl) administered into the BLA 3 h after training did not impair memory consolidation (n = 10–11 rats per group). (C) Immediate posttraining infusions of AM251 (0.28 ng per 0.2 μL) into the CeA did not impair memory consolidation (n = 5–6 rats per group).
As shown in Fig. 2B, AM251 (0.28 ng) infused into the BLA 3 h after inhibitory avoidance training did not impair retention latencies compared with their vehicle-treated counterparts (P = 0.61). Moreover, retention latencies of animals that received intra-CeA infusions of AM251 (0.28 ng) immediately after training were not different from those given vehicle (P = 0.84; Fig. 2C).
Infusion of the CB1 Receptor Antagonist AM251 into the BLA Blocks the Memory-Enhancing Effect of the CB1 Receptor Agonist WIN55,212-2.
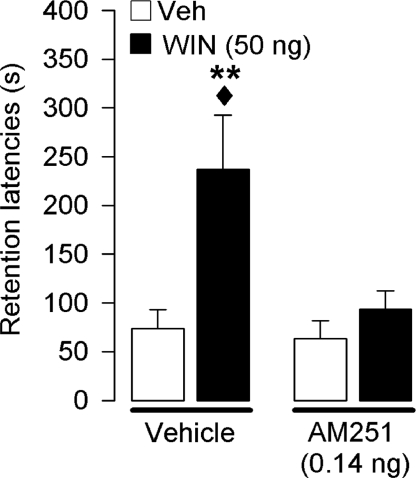
In this experiment, we examined whether the memory-enhancing effect induced by WIN55,212-2 is mediated by an activation of CB1 receptors. To address this question, we investigated whether intra-BLA infusions of the selective CB1 receptor antagonist AM251 (0.14 ng) would block the memory enhancement induced by WIN55,212-2 (50 ng). Average step-through latencies for all groups during training before footshock and drug treatment were 12.3 ± 1.1 s. A 2-way ANOVA for training latencies revealed no significant differences between groups (for all comparisons, P > 0.45). Retention latencies during the 48-h test trial of rats infused with vehicle into the BLA immediately after training were significantly longer than their latencies during the training trial (P < 0.0001). Fig. 3 shows retention latencies of rats infused concurrently with WIN55,212-2 and AM251 into the BLA immediately after training. A 2-way ANOVA for memory retention revealed a significant WIN55,212-2 × AM251 interaction effect (F1,55 = 4.13; P = 0.047). Posthoc comparisons indicated that retention latencies of rats given posttraining infusions of WIN55,212-2 were significantly longer than those given vehicle (P < 0.05). Retention latencies of rats given a nonimpairing dose of AM251 together with WIN55,212-2 were significantly shorter than those of rats treated with WIN55,212-2 alone (P < 0.05), indicating that the memory enhancement induced by WIN55,212-2 is mediated by an activation of CB1 receptors.
Fig. 3.
Effects of intra-BLA infusions of WIN55,212-2, either alone or together with AM251, on an inhibitory avoidance response. Step-through latencies (mean and SEM) on a 48-h retention test. Immediate posttraining infusions of AM251 (0.14 ng per 0.2 μL) blocked the memory-enhancing effects of concurrently administered WIN55,212-2 (WIN; 50 ng) into the BLA. **, P < 0.01 compared with the corresponding vehicle (Veh) group; ♦, P < 0.05 compared with the corresponding AM251 group (n = 14–15 per group).
Glucocorticoid Enhancement of Memory Consolidation Requires Endogenous Cannabinoids in the BLA.
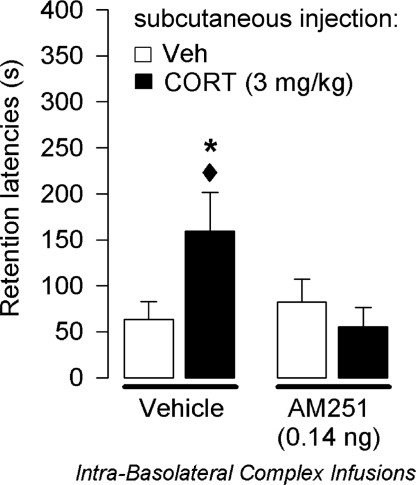
This experiment investigated whether CB1 receptor activity within the BLA is required for enabling the memory-enhancing effects induced by systemically administered corticosterone. Average step-through latencies for all groups during training before footshock and drug treatment were 12.0 ± 1.1 s. A 2-way ANOVA for training latencies revealed no significant differences between groups (P > 0.24 for all comparisons). Forty-eight-hour retention latencies of rats infused with vehicle into the BLA immediately after training were significantly longer than their latencies during the training trial (P < 0.001). As shown in Fig. 4, a 2-way ANOVA for retention latencies revealed a significant corticosterone × AM251 interaction effect (F1,40 = 5.08; P = 0.03). Posthoc comparisons indicated that corticosterone (3.0 mg/KG, s.c.) enhanced retention latencies of rats given vehicle infusions into the BLA, compared with the corresponding control group (P < 0.05). A low dose of AM251 infused into the BLA did not impair retention alone but blocked the memory-enhancing effects of corticosterone. Retention latencies of corticosterone-treated rats given AM251 into the BLA were significantly shorter than those of corticosterone-treated rats administered vehicle into the BLA (P < 0.05).
Fig. 4.
Effects of intra-BLA administered AM251 on systemic corticosterone-induced enhancement of an inhibitory avoidance response. Step-through latencies (mean and SEM) on a 48-h retention test. Immediate posttraining infusions of AM251 (0.14 ng per 0.2 μL) into the BLA blocked the memory enhancement induced by s.c. injections of corticosterone (CORT; 3.0 mg/kg; n = 10–12 per group). *, P < 0.05 compared with the corresponding vehicle (Veh) group; ♦, P < 0.05 compared with the corresponding CORT group.
Histology.
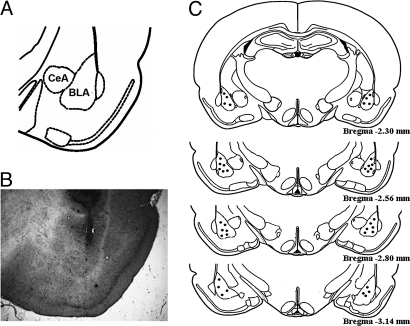
Fig. 5A is a diagram of the BLA, and Fig. 5B is a photomicrograph of a needle track terminating within the BLA. Fig. 5C illustrates infusion needle sites of 20 randomly selected rats included in the final analyses.
Fig. 5.
(A) Diagram of the rat BLA, CeA, and adjacent structures (62). (B) Representative photomicrograph (Microscope Nikon 801, original magnification ×20) of a needle track terminating in the BLA. (C) Diagrams of the rat brain sections (62) showing 40 infusion needle termination sites randomly selected from rats included in the final analyses. Only data from animals that had needle tracks terminating in the BLA and had no lesions in the surrounding BLA tissue were included in the analyses.
Discussion
The present findings provide evidence that the endocannabinoid system in the BLA is involved in modulating the consolidation of memory for inhibitory avoidance training and that CB1 activity within the BLA is essential for mediating glucocorticoid effects on long-term memory.
Bilateral posttraining infusions of the CB1 receptor agonist WIN55,212-2 into the BLA induced dose-, time-, and site-dependent enhancement of 48-h inhibitory avoidance retention performance; in contrast, bilateral posttraining blockade of CB1 receptors with the antagonist AM251 impaired retention in a dose-, time- and site-specific fashion. Furthermore, a nonimpairing dose of AM251 blocked the memory enhancement induced by coadministration of WIN55,212-2. These findings indicate that cannabinoids in the BLA are involved in the regulation of memory consolidation, plausibly via an activation of CB1 receptors. CB1 receptors are expressed at high levels in the rat amygdala, particularly the BLA (4, 13, 28). In contrast, the expression of CB1 receptors in the CeA is less clear (13, 28, 29). Our finding that cannabinoids modulate memory consolidation when administered into the BLA but not into the CeA suggests that the memory enhancement induced by intra-BLA administration of cannabinoids was mediated by influences within the BLA rather than to diffusion to the CeA. Such findings are consistent with extensive evidence that memory consolidation of emotionally arousing experiences is modulated by selective intra-BLA infusions of drugs affecting several other neuromodulatory systems (12).
Although systemically administered cannabinoids are reported to impair cognitive performance (30), there is conflicting evidence concerning the memory-modulating effects of cannabinoids administered to specific brain regions (30–32). Some studies reported that intrahippocampal infusions of cannabinoid agonists impair either the encoding or consolidation of memory of water-maze spatial, inhibitory avoidance, or object recognition training (33, 34), whereas others found enhancing effects (35). Moreover, it has been reported that the CB1 receptor antagonist AM251 infused into the hippocampus impairs memory consolidation of inhibitory avoidance training (36). Similar conflicting findings have been reported with regard to cannabinoid effects on neuroplasticity within the hippocampus (37–39). Differences in learning task, drug doses, and drug administration regimen (e.g., pretraining vs. posttraining administration) used as well as retention performance of control animals could all have contributed to these complex findings. Although only a small number of studies have examined cannabinoid effects in the amygdala complex, or in the current study the BLA, the pattern of effects appears to be more consistent. Recent findings indicate that posttraining administration of AM251 into the amygdala complex impairs memory consolidation of fear learning (19). Here, we show that a CB1 receptor agonist administered into the BLA enhances memory consolidation, whereas a CB1 receptor antagonist impairs it. Because delayed infusions of these drugs administered several hours after training were ineffective, our findings provide evidence that cannabinoids modulate time-dependent processes underlying the consolidation of memory for emotional arousing experiences. Although it is possible that AM251 under certain conditions could also act as an inverse agonist (40), the present finding that AM251 impaired retention corroborates the importance of endogenously produced cannabinoids in the modulation of cognitive processes. Moreover, the finding that endocannabinoid levels are elevated in response to stressful/alerting factors (20, 41) supports the view that endogenously stimulated CB1 activity within the BLA is involved in regulating memory enhancement of emotionally arousing experiences.
Endocannabinoids function as diffusible and short-lived modulators that may transmit signals retrogradely from postsynaptic to presynaptic neurons (3). Depolarization of a postsynaptic pyramidal cell, resulting in a significant increment in intracellular Ca2+ concentration, triggers the release of endocannabinoids, which can freely cross the lipid membrane to act retrogradely on presynaptic CB1 receptors. Activation of CB1 receptors decreases GABA release (42–44) via a rapid inhibition of Ca2+ entry into the terminals (45, 46). Thus, a depolarization of pyramidal cells produces a short time window when inhibition from a specific source is reduced and excitability is increased, an effect that has been shown to facilitate long-term potentiation of glutamatergic synaptic inputs (47). Interestingly, it has been reported that the amygdaloid GABAergic system is involved in the modulation of memory storage (48). Posttraining i.p. or intra-BLA administration of the GABAA receptor antagonist bicuculline enhances retention of emotional memory, whereas posttraining intraamygdala administration of the GABAA receptor agonist muscimol impairs memory consolidation (49, 50). Furthermore, it has been shown that inhibition of GABAergic activity within the BLA enhances memory consolidation by increasing noradrenergic transmission within this brain region (51), a neurotransmitter critically involved in mediating emotional arousal effects on memory consolidation (11, 12). In this scenario, our results suggest that intra-BLA administration of WIN55,212-2 may enhance memory consolidation by locally inhibiting GABA release and thereby stimulating noradrenergic activity.
Our findings further indicated that blockade of CB1 activity in the BLA prevents corticosterone effects on memory consolidation. Glucocorticoid hormones are known to enhance long-term consolidation of emotionally arousing events via often nongenomic interactions with norepinephrine and its intracellular signaling systems within the BLA (27, 52). For example, it has been shown that a blockade of noradrenergic neurotransmission in the BLA with specific adrenoceptor antagonists prevents glucocorticoid effects on memory consolidation (53–55). Previous studies indicated that glucocorticoids may also interact with the endocannabinoid system. Bidirectional and functional relationships between glucocorticoids and the endocannabinoid system have been demonstrated (22, 56–58). Exogenous cannabinoids activate the hypothalamic–pituitary–adrenocortical axis to induce a neuroendocrine stress response (59). Glucocorticoids, in turn, secreted in response to stressful experiences, rapidly increase endocannabinoid release in the hypothalamic supraoptic nucleus and paraventricular nucleus (23, 60). Hill et al. (24) have further shown that chronic corticosterone treatment increases the levels of the endocannabinoid 2-AG in the rat amygdala. Such findings suggest that glucocorticoids might enhance memory consolidation at least in part by stimulating endocannabinoid neurotransmission. As discussed above, endocannabinoids may then increase BLA activity by decreasing GABAergic neurotransmission, leading to increased noradrenergic activity within the BLA. Interestingly, a recent study indicated that glucocorticoids enhance the excitability of BLA neurons by decreasing the impact of GABAergic influences (61).
In summary, the present findings indicate that endocannabinoids in the BLA modulate memory consolidation of emotionally arousing experiences and suggest that CB1 activity within this brain region is critically involved in mediating glucocorticoid effects on memory consolidation.
Materials and Methods
Subjects.
Male Sprague–Dawley rats (280–320 g at the time of surgery; Charles River Laboratories) were housed individually and maintained in a temperature-controlled environment (20 °C ± 1 °C) under a 12-h light/12-h dark cycle (0700–1900 h lights on) with food and water available ad libitum. All procedures were performed in compliance with the guidelines of the U.S. National Institutes of Health and the Italian Ministry of Health (D.L. 116/92), the Declaration of Helsinki, and the Guide for the Care and Use of Mammals in Neuroscience and Behavioral Research (National Research Council, 2004).
Surgery.
The rats were anesthetized with sodium pentobarbital (50 mg/kg, i.p.) and given atropine sulfate (0.1 mg, i.p.) to maintain respiration as well as 3 mL of saline (s.c.) to facilitate clearance of these drugs and prevent dehydration. The rats were then placed in a stereotaxic frame (Kopf Instruments), and 2 stainless-steel guide cannulae (15 mm; 23 gauge) were implanted bilaterally, with the cannula tips 2 mm above the BLA [coordinates: anteroposterior (AP), −2.8 mm from bregma; mediolateral (ML), ±5.0 mm from the midline; dorsoventral (DV), −6.5 mm from skull surface; and incisor bar −3.3 mm from interaural] according to the atlas of Paxinos and Watson (62). Other rats received bilateral guide cannulae (15 mm; 23 gauge), with the tips located 2.0 mm above the CeA (coordinates: AP, −2.2 mm; ML, ±4.3 mm; and DV, −6.0 mm). The cannulae were affixed to the skull with 2 anchoring screws and dental cement. Stylets (15-mm-long 00 insect dissection pins) were inserted into each cannula to maintain patency. After surgery, the rats were allowed to recover for 10 days before training. The rats were handled 1 min per day for 3 days before training.
Inhibitory Avoidance Apparatus and Experimental Procedures.
The inhibitory avoidance apparatus consisted of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top, and 6.4 cm wide at the bottom) divided into 2 compartments separated by a sliding door. The starting compartment (31 cm long) was made of opaque white plastic and illuminated by a lamp; the shock compartment (60 cm long) was made of 2 dark, electrifiable metal plates and was not illuminated. Training and testing occurred between 1000 and 1600 h and were conducted in a sound- and light-attenuated room. For training, the rats were placed into the starting compartment facing away from the door and were permitted to explore the apparatus. After the rats stepped completely into the dark compartment, the sliding door was closed, and a single footshock (0.6 mA) was delivered for 1 s. The animals were removed from the shock compartment 15 s after termination of the footshock. Retention was tested 48 h later. On the retention test trial, the rats were placed into the starting compartment, and the latency to reenter the shock compartment with all 4 paws (maximum latency of 600 s) was recorded. Longer latencies were interpreted as indicating better retention. Immediately after the training and testing of each animal, the apparatus was cleaned with a 70% ethanol solution.
Drugs and Infusion Procedures.
The CB1 receptor agonist WIN55,212-2 (5, 10, and 50 ng per 0.2 μL per side) and the CB1 receptor antagonist AM251 (0.07, 0.14, and 0.28 ng per 0.2 μL per side) were dissolved in a vehicle containing 10% DMSO and 90% saline and administered into the BLA immediately after inhibitory avoidance training. To control for time specificity, WIN55,212-2 (50 ng per 0.2 μL per side) and AM251 (0.28 ng per 0.2 μL per side) were administered 3 h after training into the BLA of different groups of rats. To control for site specificity, in other rats these drugs were infused into the CeA immediately after training. Some studies indicated that WIN55,212-2, in addition to activating CB1 receptors, also binds to CB2 receptors (63). Therefore, in a third experiment, WIN55,212-2 (50 ng) was concurrently infused into the BLA immediately after inhibitory avoidance training, either alone or together with a nonimpairing dose of the CB1 receptor antagonist AM251 (0.14 ng) in a total volume of 0.2 μL per side to examine whether the effects of WIN55,212-2 are mediated via a selective activation of CB1 receptors. For the last experiment, the glucocorticoid corticosterone (3 mg/kg; dissolved in 5% ethanol in saline) or its vehicle was administered s.c. immediately following a posttraining intra-BLA infusion of AM251 (0.14 ng per 0.2 μL per side).
Bilateral infusions of drug or an equivalent volume of vehicle into the BLA or CeA were made by using 30-gauge injection needles connected to 10-μL Hamilton microsyringes by polyethylene (PE-20) tubing. The injection needles protruded 2.0 mm beyond the cannula tips, and a 0.2-μL injection volume per hemisphere was infused at the rate of 0.37 μL/min by an automated syringe pump (KD Instruments). The infusion volume was based on findings that this volume of an excitotoxin administered at identical injection sites produced selective lesions of either the BLA or CeA (64). The injection needles were retained within the cannulae for 20 s following drug infusion to maximize diffusion and to prevent backflow of drug along the cannula track. All drugs were purchased from Sigma–Aldrich and freshly prepared before each experiment.
Histology.
The rats were anesthetized with an overdose of sodium pentobarbital (100 mg/kg, i.p.) and perfused intracardially with a 0.9% saline solution. The brains were then removed and immersed in a 4% formaldehyde solution. At least 48 h before sectioning, the brains were transferred to a 20% sucrose solution in saline for cryoprotection. Coronal sections of 35 μm were cut on a cryostat, mounted on gelatin-coated slides, and stained with cresyl violet. The location of infusion needle tips was determined by examining the sections under a light microscope according to the atlas plates of Paxinos and Watson (62). Only animals with needle tips located within the boundaries of the BLA or CeA and no damage to the target tissues were included in the final analysis.
Statistics.
Training and retention latencies of rats given immediate posttraining infusions of WIN55,212-2 or AM251 into the BLA were analyzed with 1-way ANOVAs. The interactions between concurrent intra-BLA infusions of WIN55,212-2 and AM251 and between intra-BLA infusions of AM251 and s.c. corticosterone were analyzed with 2-way ANOVAs. The source of the detected significances was determined by Tukey–Kramer posthoc tests. Differences in retention latencies for the delayed intra-BLA infusions or for immediate posttraining infusion into the CeA were analyzed with unpaired t tests. To determine whether learning had occurred, paired t tests were used to compare the training and retention latencies of the vehicle groups. Data are expressed as mean ± SEM. P values of less than 0.05 were considered statistically significant. The number of rats per group is indicated in the figure legends.
Acknowledgments.
We thank Daniela Valeri, Angela Saraceno, Alessandra Sordi, and Elisa Torre for technical assistance. This research was supported by Fondo per gli Investimenti della Ricerca di Base 2006 (to V.C.) from the Ministero dell'Istruzione, dell' Università e della Ricerca (Rome).
Footnotes
The authors declare no conflict of interest.
See Commentary on page 4579.
References
- 1.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hashimotodani Y, Ohno-Shosaku T, Kano M. Endocannabinoids and synaptic function in the CNS. Neuroscientist. 2007;13:127–137. doi: 10.1177/1073858406296716. [DOI] [PubMed] [Google Scholar]
- 3.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 4.Herkenham M, et al. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riedel G, Davies SN. Cannabinoid function in learning, memory and plasticity. Handb Exp Pharmacol. 2005:445–477. doi: 10.1007/3-540-26573-2_15. [DOI] [PubMed] [Google Scholar]
- 6.Ranganathan M, D'Souza DC. The acute effects of cannabinoids on memory in humans: A review. Psychopharmacology (Berl) 2006;188:425–444. doi: 10.1007/s00213-006-0508-y. [DOI] [PubMed] [Google Scholar]
- 7.Block RI, et al. Effects of frequent marijuana use on memory-related regional cerebral blood flow. Pharmacol Biochem Behav. 2002;72:237–250. doi: 10.1016/s0091-3057(01)00771-7. [DOI] [PubMed] [Google Scholar]
- 8.Nestor L, Roberts G, Garavan H, Hester R. Deficits in learning and memory: Parahippocampal hyperactivity and frontocortical hypoactivity in cannabis users. Neuroimage. 2008;40:1328–1339. doi: 10.1016/j.neuroimage.2007.12.059. [DOI] [PubMed] [Google Scholar]
- 9.Clarke JR, et al. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem. 2008;90:374–381. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Robinson L, et al. Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 2008;198:551–563. doi: 10.1007/s00213-007-1012-8. [DOI] [PubMed] [Google Scholar]
- 11.McGaugh JL. Memory–a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- 12.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 13.Katona I, et al. Distribution of CB1 cannabinoid receptors in the amygdala and their role in the control of GABAergic transmission. J Neurosci. 2001;21:9506–9518. doi: 10.1523/JNEUROSCI.21-23-09506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pistis M, et al. Cannabinoids modulate neuronal firing in the rat basolateral amygdala: Evidence for CB1- and non-CB1-mediated actions. Neuropharmacology. 2004;46:115–125. doi: 10.1016/j.neuropharm.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Gobbi G, et al. Antidepressant-like activity and modulation of brain monoaminergic transmission by blockade of anandamide hydrolysis. Proc Natl Acad Sci USA. 2005;102:18620–18625. doi: 10.1073/pnas.0509591102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Witkin JM, Tzavara ET, Nomikos GG. A role for cannabinoid CB1 receptors in mood and anxiety disorders. Behav Pharmacol. 2005;16:315–331. doi: 10.1097/00008877-200509000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Bortolato M, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- 18.Rubino T, et al. CB1 receptor stimulation in specific brain areas differently modulate anxiety-related behaviour. Neuropharmacology. 2008;54:151–160. doi: 10.1016/j.neuropharm.2007.06.024. [DOI] [PubMed] [Google Scholar]
- 19.Bucherelli C, Baldi E, Mariottini C, Passani MB, Blandina P. Aversive memory reactivation engages in the amygdala only some neurotransmitters involved in consolidation. Learn Mem. 2006;13:426–430. doi: 10.1101/lm.326906. [DOI] [PubMed] [Google Scholar]
- 20.Marsicano G, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 21.Weidenfeld J, Feldman S, Mechoulam R. Effect of the brain constituent anandamide, a cannabinoid receptor agonist, on the hypothalamo-pituitary-adrenal axis in the rat. Neuroendocrinology. 1994;59:110–112. doi: 10.1159/000126646. [DOI] [PubMed] [Google Scholar]
- 22.Barna I, Zelena D, Arszovszki AC, Ledent C. The role of endogenous cannabinoids in the hypothalamo-pituitary-adrenal axis regulation: in vivo and in vitro studies in CB1 receptor knockout mice. Life Sci. 2004;75:2959–2970. doi: 10.1016/j.lfs.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 23.Di S, Malcher-Lopes R, Halmos KC, Tasker JG. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hill MN, Ho WS, Meier SE, Gorzalka BB, Hillard CJ. Chronic corticosterone treatment increases the endocannabinoid 2-arachidonylglycerol in the rat amygdala. Eur J Pharmacol. 2005;528:99–102. doi: 10.1016/j.ejphar.2005.10.058. [DOI] [PubMed] [Google Scholar]
- 25.Dallman MF. Fast glucocorticoid actions on brain: Back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 26.McGaugh JL, Roozendaal B. Role of adrenal stress hormones in forming lasting memories in the brain. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 27.Roozendaal B. 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 28.Tsou K, et al. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 29.Cota D, et al. Requirement of cannabinoid receptor type 1 for the basal modulation of hypothalamic-pituitary-adrenal axis function. Endocrinology. 2007;148:1574–1581. doi: 10.1210/en.2005-1649. [DOI] [PubMed] [Google Scholar]
- 30.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 31.Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: Animal studies. Curr Drug Targets CNS Neurol Disord. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- 32.Pamplona FA, Takahashi RN. WIN 55212–2 impairs contextual fear conditioning through the activation of CB1 cannabinoid receptors. Neurosci Lett. 2006;397:88–92. doi: 10.1016/j.neulet.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Barros DM, et al. Interactions between anandamide-induced anterograde amnesia and post-training memory modulatory systems. Brain Res. 2004;1016:66–71. doi: 10.1016/j.brainres.2004.04.067. [DOI] [PubMed] [Google Scholar]
- 34.Robinson L, et al. Hippocampal endocannabinoids inhibit spatial learning and limit spatial memory in rats. Psychopharmacology (Berl) 2007;198:551–563. doi: 10.1007/s00213-007-1012-8. [DOI] [PubMed] [Google Scholar]
- 35.de Oliveira Alvares L, Genro BP, Diehl F, Quillfeldt JA. Differential role of the hippocampal endocannabinoid system in the memory consolidation and retrieval mechanisms. Neurobiol Learn Mem. 2008;90:1–9. doi: 10.1016/j.nlm.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 36.de Oliveira Alvares L, et al. Amnestic effect of intrahippocampal AM251, a CB1-selective blocker, in the inhibitory avoidance, but not in the open field habituation task, in rats. Neurobiol Learn Mem. 2005;83:119–124. doi: 10.1016/j.nlm.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 37.de Oliveira Alvares L, et al. AM251, a selective antagonist of the CB1 receptor, inhibits the induction of long-term potentiation and induces retrograde amnesia in rats. Brain Res. 2006;1075:60–67. doi: 10.1016/j.brainres.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 38.Paton GS, Pertwee RG, Davies SN. Correlation between cannabinoid mediated effects on paired pulse depression and induction of long term potentiation in the rat hippocampal slice. Neuropharmacology. 1998;37:1123–1130. doi: 10.1016/s0028-3908(98)00096-3. [DOI] [PubMed] [Google Scholar]
- 39.Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN55212-2: Reversal by SR141716 A, a selective antagonist of CB1 cannabinoid receptors. Naunyn Schmiedebergs Arch Pharmacol. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- 40.de Oliveira Alvares L, et al. Opposite action of hippocampal CB1 receptors in memory reconsolidation and extinction. Neuroscience. 2008;154:1648–1655. doi: 10.1016/j.neuroscience.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Hohmann AG, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 42.Katona I, et al. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hajos N, Freund TF. Distinct cannabinoid sensitive receptors regulate hippocampal excitation and inhibition. Chem Phys Lipids. 2002;121:73–82. doi: 10.1016/s0009-3084(02)00149-4. [DOI] [PubMed] [Google Scholar]
- 44.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 45.Wilson RI, Kunos G, Nicoll RA. Presynaptic specificity of endocannabinoid signaling in the hippocampus. Neuron. 2001;31:453–462. doi: 10.1016/s0896-6273(01)00372-5. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABAA synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci. 2002;5:723–724. doi: 10.1038/nn879. [DOI] [PubMed] [Google Scholar]
- 48.McGaugh JL. Memory consolidation and the amygdala: A systems perspective. Trends Neurosci. 2002;25:456. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 49.Brioni JD, Nagahara AH, McGaugh JL. Involvement of the amygdala GABAergic system in the modulation of memory storage. Brain Res. 1989;487:105–112. doi: 10.1016/0006-8993(89)90945-1. [DOI] [PubMed] [Google Scholar]
- 50.Castellano C, Brioni JD, Nagahara AH, McGaugh JL. Post-training systemic and intra-amygdala administration of the GABA-B agonist baclofen impairs retention. Behav Neural Biol. 1989;52:170–179. doi: 10.1016/s0163-1047(89)90285-9. [DOI] [PubMed] [Google Scholar]
- 51.Hatfield T, Spanis C, McGaugh JL. Response of amygdalar norepinephrine to footshock and GABAergic drugs using in vivo microdialysis and HPLC. Brain Res. 1999;835:340–345. doi: 10.1016/s0006-8993(99)01566-8. [DOI] [PubMed] [Google Scholar]
- 52.Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- 53.Roozendaal B, Quirarte GL, McGaugh JL. Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor–cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 54.Roozendaal B, de Quervain DJ, Schelling G, McGaugh JL. A systemically administered beta-adrenoceptor antagonist blocks corticosterone-induced impairment of contextual memory retrieval in rats. Neurobiol Learn Mem. 2004;81:150–154. doi: 10.1016/j.nlm.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 55.Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci USA. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rademacher DJ, et al. Effects of acute and repeated restraint stress on endocannabinoid content in the amygdala, ventral striatum, and medial prefrontal cortex in mice. Neuropharmacology. 2008;54:108–116. doi: 10.1016/j.neuropharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 57.Patel S, Roelke CT, Rademacher DJ, Cullinan WE, Hillard CJ. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology. 2004;145:5431–5438. doi: 10.1210/en.2004-0638. [DOI] [PubMed] [Google Scholar]
- 58.Hill MN, et al. Prolonged glucocorticoid treatment decreases cannabinoid CB1 receptor density in the hippocampus. Hippocampus. 2008;18:221–226. doi: 10.1002/hipo.20386. [DOI] [PubMed] [Google Scholar]
- 59.Steiner MA, Wotjak CT. Role of the endocannabinoid system in regulation of the hypothalamic-pituitary-adrenocortical axis. Prog Brain Res. 2008;170:397–432. doi: 10.1016/S0079-6123(08)00433-0. [DOI] [PubMed] [Google Scholar]
- 60.Di S, Malcher-Lopes R, Marcheselli VL, Bazan NG, Tasker JG. Rapid glucocorticoid-mediated endocannabinoid release and opposing regulation of glutamate and gamma-aminobutyric acid inputs to hypothalamic magnocellular neurons. Endocrinology. 2005;146:4292–4301. doi: 10.1210/en.2005-0610. [DOI] [PubMed] [Google Scholar]
- 61.Duvarci S, Paré D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 2007. [DOI] [PubMed] [Google Scholar]
- 63.Pertwee RG, Ross RA. Cannabinoid receptors and their ligands. Prostaglandins Leukot Essent Fatty Acids. 2002;66:101–121. doi: 10.1054/plef.2001.0341. [DOI] [PubMed] [Google Scholar]
- 64.Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]