Abstract
Antigen recognition alone is insufficient for the activation of adaptive immune responses mediated by conventional lymphocytes. Additional signals that indicate the origin of the antigen are also required. These signals are generally provided by the innate immune system upon recognition of conserved microbial structures by a variety of pattern recognition receptors (PRRs). The Toll-like receptors (TLRs) are the best-characterized family of PRRs and control the activation of adaptive immune responses to a variety of immunizations and infections. However, recent studies have questioned the role of TLRs in the induction of antibody responses and, thus, this issue has become controversial. In contrast to earlier studies supporting a role for TLRs in antibody responses, these studies used haptenated antigens rather than native antigens for immunization, but did not consider the potential effect of antigen haptenation on immunogenicity. Here, we show that commonly used haptenated proteins, unlike native proteins, are inherently immunogenic. This immunogenicity is TLR-independent, but the T and B cell responses induced are primarily hapten-specific, rather than protein-specific. Thus, although haptens have immunostimulatory activity, it is distinct from classical adjuvants, which induce immune responses directed at the admixed antigens. Our results thus highlight an unappreciated and unique immunogenicity of haptenated proteins, and provide an experimental explanation for a seeming discrepancy between published results.
Keywords: adjuvant, antibody, innate immunity, Toll-like receptor
Innate immune recognition is mediated by several families of pattern recognition receptors (PRRs), including the Toll-like receptors (TLRs), NOD-like receptors (NLRs), Dectin-1 and related C-type lectin receptors, and retinoic acid inducible gene-I (RIG-I) and melanoma differentiation-associated gene-5 (MDA-5) (1). Each family of PRRs is specialized to deal with particular classes of pathogens. For example, Dectin-1 detects the fungal cell wall component β-glucan (2) and induces phagocytosis and activation of the T helper-17 (Th17) arm of adaptive immunity (3, 4). Nucleotide-binding oligomerization domain containing 1 (NOD1) and NOD2 proteins detect peptidoglycan fragments in the cytosol and contribute to both innate and adaptive responses (5). RIG-I and MDA-5 recognize viral RNA and trigger IFN production and antiviral immunity, including activation of cytotoxic T lymphocytes (CTLs) (6, 7). TLRs detect a variety of molecular structures derived from bacterial, viral and fungal pathogens and lead to the activation of T and B cells (8). Each of these families of PRRs is capable of activating the appropriate class of the adaptive immune response. Therefore, the combination of immunostimulatory components (i.e., PRR ligands) contained in a given pathogen determines the relative requirement for any particular PRR in activation of adaptive immunity. Most pathogens contain ligands for more than 1 family of PRRs, which complicates analyses of the roles of different PRR families. However, analysis of the specific mechanisms of activation of the adaptive immune response can be facilitated by the use of model antigens and simple adjuvants, whereby targeting of a particular class of PRRs, using their corresponding microbial ligands, provides an adjuvant activity and confers immunogenicity to otherwise nonimmunogenic protein antigens.
In previous studies, we investigated the role of the TLR signaling pathway in the induction of adaptive immune responses, using mice deficient in the critical TLR signaling adaptor Myeloid Differentiation primary response gene 88 (MyD88) (9, 10). To isolate the contribution of TLRs we used a single defined TLR ligand, Lipopolysaccharide (LPS), as an adjuvant, and the native proteins ovalbumin (OVA) or human serum albumin (HSA), as model antigens. incomplete Freund's adjuvant (IFA) and aluminum hydroxide, which contain low levels of contaminating PRR ligands, were used simply for their depot effect, which is important for soluble antigens. Using this system, we demonstrated that optimal CD4+ T cell responses and T-dependent antibody responses require intact TLR signaling; furthermore, we showed that optimal T-dependent antibody responses to OVA and HSA not only require TLR signaling in the antigen presenting dendritic cells, but also depend on TLR signaling in B cells (10). Notably, the contribution of TLRs to antibody responses depended on the antibody isotype and was restricted to IgM, IgG1 and IgG2 classes, whereas the IgE response was not dependent on TLRs (10, 11). The role of a B cell intrinsic TLR signal in antibody production was also demonstrated in the human system (12) and in the murine system in response to viral infection (13, 14) or virus-like particles (15), and in autoimmunity (16–18).
Despite a large body of evidence linking TLRs and adaptive immunity, 2 recent reports have questioned the role of TLRs in antibody production based on analysis of antibody responses in MyD88 and TRIF (TIR-domain-containing adapter-inducing IFN-β) double-deficient mice or mice with a B cell-specific deficiency in MyD88 (19–21). The reasons for this discrepancy are not clear, creating considerable confusion with regard to the involvement of TLRs in the control of antibody responses.
Studies producing conflicting data on the role of TLRs in antibody responses differ notably in the type of antigen used for immunization. Although studies supporting a role for TLRs (9, 10) used unmodified native protein antigens, studies that failed to find such a role (19–21) used antigens that were chemically modified by haptenation—a procedure involving the conjugation of multiple small chemical moieties to a carrier protein. Haptenation is a widely used approach to study antibody responses. Antibody responses to haptenated proteins are T cell dependent (22) and, thus, protein haptenation is thought to simply provide defined epitopes for the measurement of antibody titers and affinities without altering the requirements for B cell responses.
However, we show here that certain haptenated proteins are highly immunogenic, whereas native proteins are nonimmunogenic. Unlike native proteins mixed with adjuvant, which induced strong anti-protein responses, haptenated proteins induced strong hapten-specific responses, but weak protein-specific responses. This unique immunogenicity was TLR-independent, and CD4+ T cell and IgG1 responses to haptenated antigens were therefore largely MyD88-independent. Haptenated proteins therefore possess a unique, hapten-focused immunogenicity that affects the requirements for adaptive immune activation. Thus, studies questioning the role of TLRs in antibody responses reached their conclusions because of the unappreciated effects of protein haptenation on immunogenicity. This created a controversy in the field regarding the role of TLRs in antibody responses. Our data reconfirm the importance of TLRs in antibody responses to native proteins plus TLR ligands, provide a simple explanation for a seeming discrepancy in datasets and highlight the unique immunogenicity of haptenated antigens.
Results
Haptenated Proteins in IFA Induce Robust IgG1 Responses in MyD88-Deficient Mice.
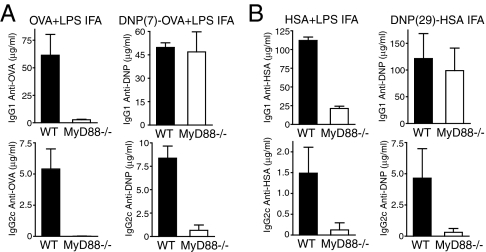
The most obvious difference between studies supporting a role for TLRs in antibody responses and those arguing against a role for TLRs is in the type of antigen used for immunization; studies that found a role for TLRs in antibody responses used native protein antigens or live pathogens, whereas studies arguing against a role for TLRs used proteins modified by haptenation. To determine whether this experimental difference was responsible for the discrepancy in datasets, we directly compared the role of TLRs in antibody responses to haptenated versus native proteins in response to immunization with antigen emulsified in the depot adjuvant IFA. WT C57BL/6 and MyD88-deficient mice (23) were immunized with the haptenated proteins dinitrophenyl (DNP)-HSA (on average 29 molecules of DNP per HSA) or DNP-OVA (on average 7 molecules of DNP per OVA), or native HSA or OVA with or without LPS, and antibody titers in the serum were measured after 14 days. As we demonstrated in ref. 10, MyD88-deficient mice immunized with native protein (either HSA or OVA) exhibited reduced titers of antigen specific IgG1 and IgG2c as compared with WT mice (Fig. 1 A and B). In contrast, DNP-HSA (Fig. 1B) and DNP-OVA (Fig. 1A) induced robust IgG1 responses in MyD88-deficient mice, whereas IgG2c production remained largely MyD88 dependent.
Fig. 1.
Haptenated proteins in IFA induce robust IgG1 responses in MyD88-deficient mice. Age matched MyD88-deficient and WT C57BL/6 mice were immunized in the footpad with OVA or DNP(7)-OVA plus LPS (A) or with low endotoxin HSA plus LPS or DNP(29)-HSA (B) emulsified in IFA. HSA-, OVA- or DNP-specific serum antibody titers were measured by ELISA on day 14 after immunization.
Haptenated Proteins in Aluminum Hydroxide Induce MyD88-Independent IgG1 Responses.
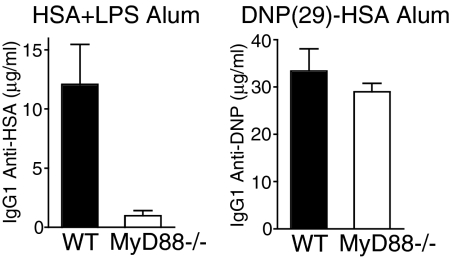
We next examined whether native versus haptenated proteins in aluminum hydroxide (alum) exhibited differential requirements for TLR signaling. We examined the IgG1 response to HSA plus LPS versus DNP-HSA in alum in WT C57BL/6 and MyD88-deficient mice. In line with what we observed for IFA, the IgG1 response to HSA plus LPS in alum was defective in MyD88-deficient mice, whereas the IgG1 response to DNP-HSA in alum was normal in MyD88-deficient mice (Fig. 2).
Fig. 2.
Haptenated proteins in aluminum hydroxide adjuvant induce robust IgG1 responses in MyD88-deficient mice. WT C57BL/6 and MyD88-deficient mice were immunized i.p. with either HSA plus LPS or DNP(29)-HSA in alum. Serum HSA- or DNP-specific IgG1 was measured on day 14 after immunization by ELISA.
Haptenated Proteins Induce MyD88-Independent CD4+ T Cell Responses.
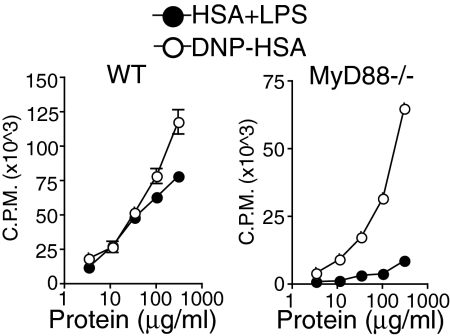
Antibody responses can be characterized as either T-dependent or T-independent based on the requirement for T cell help. Highly multivalent antigens and antigens that specifically induce innate immune pathways in B cells can induce T-independent antibody responses. However, antibody responses to native proteins and haptenated proteins alike are T cell-dependent (22). We have shown previously that T cell responses to native antigen plus LPS emulsified in IFA are TLR-dependent (refs. 9 and 24 and Fig. 3). We wished to examine whether CD4+ T cell responses to haptenated antigens are also TLR-signaling dependent. Therefore, we compared CD4+ T cell responses to immunizations with HSA plus LPS or DNP-HSA in IFA in WT C57BL/6 and MyD88-deficient mice. As reported in refs. 9 and 10, CD4+ T cell responses to HSA plus LPS in MyD88-deficient mice were severely reduced as compared with WT mice (Fig. 3 and refs. 9 and 10). In contrast, compared with WT mice, DNP-HSA induced a robust, albeit slightly reduced, CD4+ T cell response in MyD88-deficient mice (Fig. 3).
Fig. 3.
Haptenated proteins in IFA induce a CD4+ T cell response in MyD88-deficient mice. MyD88-deficient and WT C57BL/6 mice were immunized in the footpad with either HSA and LPS or DNP (29)-HSA in IFA. CD4+ T cells were isolated from draining lymph nodes on day 8 after immunization and restimulated in vitro with irradiated splenocytes as antigen presenting cells and titrating doses of antigen. Proliferation was measured by incorporation of 3H-Thymidine during the last 16 h of a 72-h stimulation (cpm).
Haptenated Proteins Can Induce Adaptive Immune Responses in Mice Completely Deficient in TLR-Signaling.
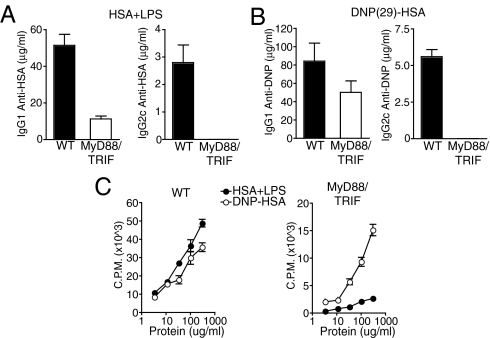
TLRs use both MyD88- and TRIF-dependent signaling pathways (8). TLR-dependent signaling thus remains partially intact in MyD88-deficient mice. Therefore, we also examined the induction of adaptive immune responses to haptenated protein in MyD88 and TRIF double-deficient mice, which lack all known TLR-dependent signaling. As was observed for MyD88-deficient mice, MyD88 and TRIF double-deficient mice showed defects in their CD4+ T cell and IgG1 responses to HSA and LPS, but exhibited robust responses to DNP-HSA, again with the only exception being the IgG2c response, which remained largely MyD88- and TRIF-dependent under both conditions (Fig. 4).
Fig. 4.
Haptenated proteins induce robust adaptive responses in MyD88 and TRIF double-deficient mice. MyD88 and TRIF double-deficient and WT C57BL/6 mice were immunized with HSA plus LPS (A and C) or DNP(29)-HSA (B and C) in IFA in the footpad. OVA-specific (A) or DNP-specific (B) serum antibody titers were measured by ELISA on day 14 after immunization. (C) CD4+ T cells were isolated from the draining lymph nodes of immunized MyD88 and TRIF double-deficient and WT C57BL/6 mice on day 8 after immunization and restimulated with titrating doses of antigen and irradiated splenocytes for 3 days. T cell proliferation was measured by [3H]thymidine incorporation during the last 16 h of culture (cpm).
Haptenation Confers a Hapten-Specific Immunogenicity on Nonimmunogenic Protein Antigens.
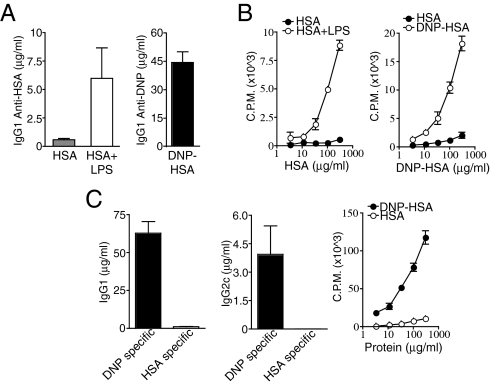
The protein antigen HSA, when purified to be devoid of contaminating TLR ligands, fails to induce a robust adaptive immune response when combined with a suitably TLR-ligand free depot adjuvant such as alum (Fig. 5 A and B and refs. 9 and 10). Addition of the TLR4 ligand LPS to TLR-ligand free HSA is sufficient to induce robust CD4+ T cell and antibody responses to HSA (Fig. 5 A and B and refs. 9 and 10). We therefore asked whether haptenation of a pure (TLR-ligand free) protein antigen would also be sufficient to induce adaptive immunity. We first examined the antibody response to DNP-conjugated, endotoxin-free HSA in alum. DNP-HSA in alum, unlike endotoxin-free HSA, induced a robust IgG1 response (Fig. 5A). Endotoxin-free HSA in IFA also fails to induce robust CD4+ T cell responses (Fig. 5B and ref. 9). Therefore, we examined the CD4+ T cell response to endotoxin-free DNP-HSA in IFA. Endotoxin-free DNP-HSA in IFA, unlike endotoxin-free HSA in IFA, induced a robust CD4+ T cell response (Fig. 5B).
Fig. 5.
Protein haptenation imparts a hapten-focused immunogenicity on a nonimmunogenic protein. (A) WT C57BL/6 mice were immunized i.p. with low-endotoxin HSA, HSA plus LPS, or low-endotoxin DNP(29)-HSA in alum. Serum DNP-specific or HSA-specific IgG1 and IgG2c titers were measured by ELISA on day 14 after immunization. (B) WT C57BL/6 mice were immunized in the footpad with HSA, HSA plus LPS, or low-endotoxin DNP(29)-HSA in IFA and day 8 CD4+ T cell responses to titrating doses of either DNP-HSA or HSA were measured by [3H]thymidine incorporation for the last 12–16 h of a 72-h restimulation (cpm). (C) WT C57BL/6 mice were immunized in the footpad with DNP(29)-HSA in IFA. On day 14 after immunization, DNP-specific and HSA-specific IgG1 and IgG2c titers were measured by ELISA. On day 8 after immunization, CD4+ T cells were isolated from draining lymph nodes and restimulated with titrating doses of either DNP-HSA or HSA and irradiated splenocytes. Proliferation was measured by [3H]thymidine incorporation for the last 12–16 h of a 72-h stimulation.
Notably, unlike HSA plus LPS, DNP-HSA in IFA did not induce robust Ab responses or CD4+ T cell responses to nonhaptenated HSA. Instead, immunizations with the haptenated protein antigen DNP-HSA induced high-titers of DNP-specific antibodies, and low titers of HSA-specific antibodies (Fig. 5C). In addition, CD4+ T cell responses induced by DNP-HSA were directed primarily toward dinitrophenylated peptides as DNP-HSA elicited a strong CD4+ T cell restimulation response whereas HSA elicited a very weak restimulation response in mice previously immunized with haptenated protein (Fig. 5C). Shimizu et al. (25) recently reported similar results using the hapten oxazalone. Thus, haptenated proteins do not act like classical PRR stimulating adjuvants, such as TLR-ligands, which induce responses to the proteins with which they are mixed, but instead induce qualitatively different responses that are predominantly hapten-specific.
Discussion
The use of haptenated proteins in immunology dates back to the early 20th century when Karl Landsteiner performed his pioneering work on the specificity of serological responses (26). Haptenated proteins have since been widely used to provide defined epitopes for the measurement of antibody titers and affinities. Although protein-haptenation is thought to do little more than create epitopes for B cell recognition, we show here that certain haptenated proteins can induce adaptive immune responses under conditions where native proteins fail to induce such responses; thus, haptenation does more than simply create epitopes for antigen receptor recognition.
In this study, we compared the innate requirements for induction of adaptive immune responses to either native or haptenated proteins. We found that TLR-signaling was required for optimal IgG1, IgG2c and CD4+ T cell responses to immunizations with the native proteins HSA and OVA plus LPS emulsified in the depot adjuvant IFA, whereas TLR signaling-deficient mice exhibited robust IgG1 and CD4+ T cell responses to immunizations with the haptenated proteins DNP-OVA and DNP-HSA emulsified in IFA. IgG2c responses remained largely MyD88-dependent regardless of the antigen used for immunization. Haptenated proteins induced robust IgG1 responses in MyD88-deficient mice in response to immunizations performed in multiple common adjuvants, including both IFA and alum, suggesting that this phenomenon is independent of the activity of the depot adjuvant used, but instead depends on the qualities of haptenated versus native proteins. The ability of haptenated proteins to induce robust MyD88-independent adaptive responses was shared by multiple haptenated protein preparations, suggesting that this phenomenon is not specific to a particular haptenated protein preparation. Finally, haptenation converted nonimmunogenic, PAMP-free HSA into a highly immunogenic antigen that induced strong anti-hapten responses, but weak anti-protein responses.
Our data thus show that haptenated proteins are immunogenic, whereas native proteins are nonimmunogenic. This immunogenicity is TLR-independent, but results in qualitatively different T and B cell responses as compared with immunizations with native proteins plus TLR ligands—although immunizations with protein plus TLR ligands induce T and B cell responses to the protein with which they are mixed, haptenated proteins induce predominantly hapten-specific T and B cell responses and only very weak protein-specific responses (25). Thus, the nature of the immunogenicity of haptenated proteins appears to differ from the immunogenicity conferred by PRR ligands. Because haptenated proteins possess a unique immunogenicity and induce qualitatively different responses as compared with native proteins, their use can complicate studies of adjuvant requirements by occluding the activities of immunogenic components of an adjuvant. Furthermore, comparisons of studies using native proteins and studies using haptenated proteins must be made with the unique immunogenicity of haptenated proteins in mind. This consideration is especially important for B cells, which have a remarkable ability to integrate multiple signals that collectively determine the efficiency of antibody production (27).
In a recent report, Gavin et al. immunized MyD88 and TRIF double-deficient mice with trinitrophenyl (TNP;16)-keyhole limpet hemocyanin (KLH) in multiple adjuvants and concluded that TLRs play no role in the control of adaptive immunity (19, 20). Meyer-Bahlburg et al. (21) examined the role of TLRs on B cells, using nitrophenyl (NP;37)-chicken gamma globulin (CGG) in alum, and concluded that TLRs on B cells amplify early antibody responses, but are not required for robust IgG responses. These conclusions were based on the incorrect assumption that the requirements for antibody responses to haptenated and native proteins are identical. However, our data show that haptenated proteins possess a unique, TLR-independent immunogenicity that can obscure adjuvant requirements. Thus, the conclusions made by Gavin et al. (19) and by Meyer-Bahlburg et al. (21) are only applicable to haptenated proteins and cannot be generalized to native proteins or natural infections.
Common adjuvants have 2 types of activities, both of which are required to induce a robust adaptive immune response to soluble proteins. One is a depot activity that prevents the dispersion of soluble antigens and promotes their uptake by antigen presenting cells, and the other is an immunostimulatory activity that engages the innate immune system through the triggering of PRRs. In the case of Complete Freund's Adjuvant, for example, mineral oil acts largely to provide a depot, whereas heat-killed mycobacteria act to trigger the innate immune system (28). A possible explanation for the TLR-independent adjuvant effect reported by Gavin et al. (19) is simply the depot effect of the adjuvants, which is naturally not mediated by TLRs or any other PRRs, combined with the inherent immunogenicity of the haptenated antigens used in these experiments.
Although IgG1 responses to haptenated proteins appeared relatively unchanged between WT and MyD88-deficient mice, this does not necessarily mean that TLR ligands do not alter adaptive responses to haptenated proteins. Indeed, CD4+ T cell responses to haptenated proteins remained partially MyD88-dependent; further, in contrast to the report by Gavin et al. (19), we observed that IgG2c production in response to haptenated proteins was largely MyD88-dependent. Notably, B cell-intrinsic TLR signaling has been shown to be required for IgG2a/c production in multiple systems, including in response to native proteins (10), haptenated proteins (this article and ref. 21), virus like particles (15), influenza virus (13), and polyoma virus (14). We conclude that haptenated proteins, under certain conditions, bypass the requirement for TLRs in the induction of CD4+ T cell and IgG1 responses, but that the addition of PAMPs can still potentiate or otherwise alter adaptive immune responses to haptenated proteins, such as class switching to IgG2c.
Haptens were defined by Landsteiner as small molecules that become immunogenic only after association with a carrier protein (26). Landsteiner and others, through empirical examination of various reactive chemicals conjugated to a variety of peptides and proteins, identified a number of highly immunogenic (as well as many less immunogenic) hapten-carrier conjugates. These conjugates became antigens of choice for immunologists largely because of their uniquely potent immunogenic activity (29). Thus, the selection of specific haptenated proteins as immunogens of choice may parallel the selection of many components of adjuvants that were chosen empirically for their immunogenicity.
The mechanism behind the immunogenicity of haptenated proteins remains unclear. However, a number of possible mechanisms can be imagined. One possibility is that haptenated proteins stimulate adaptive immunity primarily through efficient triggering of the Ag receptors, and thereby bypass the requirement for innate instruction. One way in which haptenated proteins could trigger antibody production is through their multivalent nature. Indeed, highly multivalent antigens, such as T-independent type 2 (TI-2) antigens, bypass the requirement for T cell help through efficient cross-linking of the B cell receptor. TI-1 antigens, in contrast, bypass the requirement for T cell help through activation of innate immune receptors. However, haptenated proteins appear to represent neither such scenario; instead, B cell responses to haptenated proteins remain T cell-dependent (22). Furthermore, T cell responses are presumably impervious to the effects of valency, yet we observed robust T cell responses to haptenated proteins in MyD88-deficient animals. Finally, B cell responses to physiologically relevant multivalent antigens, such as polyoma virus (14), influenza virus (13) and viral like particles (15), are largely MyD88-dependent. Therefore, it seems unlikely that valency alone accounts for the immunogenicity of haptenated proteins.
Differences in the affinities, avidities, and precursor frequencies of antigen specific cells can also affect the requirements for initiation of adaptive immunity. Because haptenated proteins induce mainly anti-hapten responses, and protein plus LPS induces strong anti-protein responses, it is not possible to compare the effect of haptenation on lymphocyte responses to identical epitopes. Differences in antigen specific lymphocyte precursor frequency or antigen receptor affinities or avidities could therefore contribute to some of the differences in immunogenicity and specificity we observe between native and haptenated proteins. The process of haptenation also likely denatures protein antigens, thus promoting their aggregation. This could also contribute to the altered immunogenicity of haptenated antigens and lead to a loss of native protein epitopes for antibody recognition.
Another possible explanation for the unique, TLR-independent immunogenicity of haptenated proteins is that they engage an alternative innate pathway to adaptive immunity. Indeed, the T cell response to DNP-HSA is IL-6-dependent (Fig. S1), implicating innate control of adaptive responses to haptenated proteins. Both TLR-dependent and TLR-independent paths to adaptive immunity have been described (3, 7, 9–11, 30–32). Haptenated proteins may mimic, either in structure or activity, naturally encountered ligands that trigger adaptive immunity through a TLR-independent innate pathway. Indeed, reactive haptens are known to stimulate the innate immune system during the induction of T cell-dependent contact hypersensitivity (CHS) (33). The inflammatory cytokine Interleukin-1β (IL-1β) is critical for CHS (34). IL-1β family members require processing by Caspase-1 for their activation and secretion. Caspase-1 activation occurs in a protein complex, referred to as an inflammasome, containing a Nod-like receptor, such as NALP3, the adaptor protein ASC, and Caspase-1 itself (35). Recently, NALP3 and ASC, through Caspase-1 and IL-1β, were also shown to be critical for CHS (36, 37). Furthermore, NALP3 and Caspase-1 were also recently shown to be critical for an immunostimulatory activity of alum (38–41). Therefore, haptenated proteins might similarly induce immunity through Caspase-1 and IL-1β. However, we found that both CD4+ T cell and IgG responses to DNP-HSA appeared normal in Caspase-1 deficient mice (Fig. S2) and NALP3-deficient mice (Fig. S3). Thus, if haptenated proteins induce adaptive immunity through the induction of an alternate innate immune pathway, it must be distinct from the NALP3, Caspase-1, IL-1β dependent pathway that is involved in CHS. We did, however, observe partial defects in T cell and Ab responses to haptenated proteins in mice deficient for the inflammasome adaptor ASC (Fig. S4). These defects were specific for responses to haptenated proteins because responses to native protein and LPS in ASC-deficient mice were normal. ASC-dependent, Caspase-1 independent activities, including the induction of critical inflammatory cytokines such as IL-6, have also been reported by others (42, 43).
The cytosolic PRRs NOD1 and NOD2, which signal through the protein kinase Receptor-interacting protein 2 (RIP2), are also involved in control of adaptive immunity (31, 44). However, MyD88 and RIP2 double-deficient mice showed no defect in antibody responses to haptenated proteins (Fig. S5). The Receptor for Advanced Glycation Endproducts (RAGE), which is implicated in inflammation and control of adaptive immunity, also could potentially be involved in adaptive responses to haptenated proteins (45, 46), but we did not observe any defects in T cell responses to DNP-HSA in RAGE-deficient mice (Fig. S6).
Regardless of the mechanism by which haptenated proteins induce TLR-independent adaptive responses, it is clear that haptenated proteins differ from native proteins in multiple ways, including the nature of the responses they induce and the innate requirements for induction of those responses.
Both antigen recognition and innate immune instruction are required for the activation of adaptive immune responses mediated by conventional lymphocytes. Several innate immune pathways that are sufficient to induce an adaptive immune response are currently known. The requirement for each of these pathways in the induction of adaptive immunity naturally depends on the combination of immunostimulatory components in a particular pathogen or the immunostimulatory components included in an immunization. Adaptive immune responses are TLR-dependent when TLR ligands are the main innate immunogenic components of adjuvants or pathogens; this is the case for immunizations using native protein plus TLR ligands (9, 10) and for numerous pathogens of multiple classes (13, 30). However, conditions under which TLRs are less important or completely dispensable for the induction of adaptive immunity certainly also exist (3, 7, 31, 32), and the full spectrum of immunogenic signals and receptors that can lead to adaptive immunity remains to be determined. Our data suggest that haptenated proteins represent one such immunogenic signal.
Materials and Methods
Mice.
All mice were bred and maintained at the Yale University School of Medicine and were used in accordance with Yale Animal Research and Care guidelines. ASC-deficient mice were made by J. Bertin and A. Coyle and were a kind gift of Millenium Pharmaceuticals through R.A.F.. All mice were backcrossed to the C57BL/6 background at least 7 times and were used at 8–14 weeks of age, and MyD88-deficient mice were backcrossed to the C57Bl/6 background 10 times.
Reagents and Antibodies.
HSA (low endotoxin), OVA, LPS and IFA were purchased from Sigma–Aldrich. Haptenated proteins of the indicated substitution ratios were purchased from Biosearch Technologies. Imject alum (aluminum hydroxide) was purchased from Pierce. Antibodies for ELISAs were purchased from Southern Biotechnology.
Immunizations.
Mice were immunized in the rear footpads with 50–100 μg of protein or haptenated protein with or without 5–10 μg of LPS in PBS emulsified 1:1 with IFA. Immunizations with 100 μg of protein or haptenated protein with or without 10 μg of LPS in alum were performed i.p.
Enzyme Linked Immunosorbent Assay.
Antigen specific ELISAs were performed on sera isolated from immunized mice on day 14. Nunc MaxiSorp 96-well plates were coated with native protein to measure anti-protein responses or a haptenated protein not used for immunization (DNP (31)-BSA) to measure anti-hapten responses. For each serum sample eight 3-fold serial dilutions starting at 1:100 were probed. Antibodies were detected using isotype specific biotinylated antibodies, Streptavidin-Horseradish Peroxidase (E bioscience) and O-phenyldiamine (Sigma).
T Cell Proliferation Assay.
CD4+ T cells from immunized mice were purified from popliteal and inguinal lymph nodes on day 7–10 after immunization, using MACS anti-CD4 beads (L3T4; Miltenyi Biotec) and an AutoMACS sorter. Purified T cells (>95% CD4+; 1 × 105) were cultured in 96-well plates with irradiated (1,000 rad) wild-type splenocytes (3 × 105) and titrating doses of antigen for 72–84 h. Proliferation was measured by [3H]thymidine incorporation during the last 12–16 h of incubation.
Supplementary Material
Acknowledgments.
We thank D. Gray, A. Unni, C. Pasare, D. Schenten, D. Stetson, and I. Brodsky for useful discussions and/or for critical review of this manuscript; F. Sutterwalla (University of Iowa, Iowa City) and R.A.F. for the NALP3-deficient mice; R.A.F. for the Caspase-1 deficient mice; and R.A.F., John Bertin, and Anthony Coyle for the ASC-deficient mice. R.M. is an investigator of the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0809403105/DCSupplemental.
References
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Brown GD, Gordon S. Immune recognition. A new receptor for beta-glucans. Nature. 2001;413:36–37. doi: 10.1038/35092620. [DOI] [PubMed] [Google Scholar]
- 3.LeibundGut-Landmann S, et al. Syk- and CARD9-dependent coupling of innate immunity to the induction of T helper cells that produce interleukin 17. Nat Immunol. 2007;8:630–638. doi: 10.1038/ni1460. [DOI] [PubMed] [Google Scholar]
- 4.Acosta-Rodriguez EV, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 5.Kanneganti TD, Lamkanfi M, Nunez G. Intracellular NOD-like receptors in host defense and disease. Immunity. 2007;27:549–559. doi: 10.1016/j.immuni.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kato H, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 7.Koyama S, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. doi: 10.4049/jimmunol.179.7.4711. [DOI] [PubMed] [Google Scholar]
- 8.Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- 9.Pasare C, Medzhitov R. Toll-dependent control mechanisms of CD4 T cell activation. Immunity. 2004;21:733–741. doi: 10.1016/j.immuni.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 10.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438:364–368. doi: 10.1038/nature04267. [DOI] [PubMed] [Google Scholar]
- 11.Schnare M, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 12.Ruprecht CR, Lanzavecchia A. Toll-like receptor stimulation as a third signal required for activation of human naive B cells. Eur J Immunol. 2006;36:810–816. doi: 10.1002/eji.200535744. [DOI] [PubMed] [Google Scholar]
- 13.Heer AK, et al. TLR signaling fine-tunes anti-influenza B cell responses without regulating effector T cell responses. J Immunol. 2007;178:2182–2191. doi: 10.4049/jimmunol.178.4.2182. [DOI] [PubMed] [Google Scholar]
- 14.Guay HM, Andreyeva TA, Garcea RL, Welsh RM, Szomolanyi-Tsuda E. MyD88 is required for the formation of long-term humoral immunity to virus infection. J Immunol. 2007;178:5124–5131. doi: 10.4049/jimmunol.178.8.5124. [DOI] [PubMed] [Google Scholar]
- 15.Jegerlehner A, et al. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol. 2007;178:2415–2420. doi: 10.4049/jimmunol.178.4.2415. [DOI] [PubMed] [Google Scholar]
- 16.Groom JR, et al. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leadbetter EA, et al. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 18.Marshak-Rothstein A. Toll-like receptors in systemic autoimmune disease. Nat Rev Immunol. 2006;6:823–835. doi: 10.1038/nri1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314:1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemazee D, Gavin A, Hoebe K, Beutler B. Immunology: Toll-like receptors and antibody responses. Nature. 2006;441:E4. doi: 10.1038/nature04875. discussion E4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meyer-Bahlburg AKS, Rawlings DJ. B cell intrinsic TLR signals amplify but are not required for humoral immunity. J Exp Med. 2007 doi: 10.1084/jem.20071250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchison NA. The carrier effect in the secondary response to hapten-protein conjugates. I. Measurement of the effect with transferred cells and objections to the local environment hypothesis. Eur J Immunol. 1971;1:10–17. doi: 10.1002/eji.1830010103. [DOI] [PubMed] [Google Scholar]
- 23.Adachi O, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 24.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu T, et al. T cells specific to hapten carrier but not to carrier alone assist in the production of anti-hapten and anti-carrier antibodies. Int Immunol. 2007;19:1157–1164. doi: 10.1093/intimm/dxm080. [DOI] [PubMed] [Google Scholar]
- 26.Landsteiner K. The Specificity of Serological Reactions. 3rd Ed. Cambridge, MA: Harvard Univ Press; 1962. [Google Scholar]
- 27.Lanzavecchia A, Sallusto F. Toll-like receptors and innate immunity in B-cell activation and antibody responses. Curr Opin Immunol. 2007;19:268–274. doi: 10.1016/j.coi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 28.Billiau A, Matthys P. Modes of action of Freund's adjuvants in experimental models of autoimmune diseases. J Leukoc Biol. 2001;70:849–860. [PubMed] [Google Scholar]
- 29.Neuberger A, Tatum EL. Immunogenicity. London: North Holland Publishing Company; 1972. p. 584. [Google Scholar]
- 30.Yarovinsky F, Kanzler H, Hieny S, Coffman RL, Sher A. Toll-like receptor recognition regulates immunodominance in an antimicrobial CD4+ T cell response. Immunity. 2006;25:655–664. doi: 10.1016/j.immuni.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi KS, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–734. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 32.Rivera A, et al. Innate immune activation and CD4+ T cell priming during respiratory fungal infection. Immunity. 2006;25:665–675. doi: 10.1016/j.immuni.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 33.Martin S, Weltzien HU. T cell recognition of haptens, a molecular view. Int Arch Allergy Immunol. 1994;104:10–16. doi: 10.1159/000236703. [DOI] [PubMed] [Google Scholar]
- 34.Shornick LP, et al. Mice deficient in IL-1beta manifest impaired contact hypersensitivity to trinitrochlorobenzone. J Exp Med. 1996;183:1427–1436. doi: 10.1084/jem.183.4.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Martinon F, Tschopp J. Inflammatory caspases: Linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Sutterwala FS, et al. Critical role for NALP3/CIAS1/Cryopyrin in innate and adaptive immunity through its regulation of caspase-1. Immunity. 2006;24:317–327. doi: 10.1016/j.immuni.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Watanabe H, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol. 2007;127:1956–1963. doi: 10.1038/sj.jid.5700819. [DOI] [PubMed] [Google Scholar]
- 38.Eisenbarth SC, Colegio OR, O'Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franchi L, Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38:2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Nookala S, Re F. Aluminum hydroxide adjuvants activate caspase-1 and induce IL-1beta and IL-18 release. J Immunol. 2007;178:5271–5276. doi: 10.4049/jimmunol.178.8.5271. [DOI] [PubMed] [Google Scholar]
- 41.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J Immunol. 2008;181:17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willingham SB, et al. Microbial pathogen-induced necrotic cell death mediated by the inflammasome components CIAS1/cryopyrin/NLRP3 and ASC. Cell Host Microbe. 2007;2:147–159. doi: 10.1016/j.chom.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taxman DJ, et al. Cutting edge: ASC mediates the induction of multiple cytokines by Porphyromonas gingivalis via caspase-1-dependent and -independent pathways. J Immunol. 2006;177:4252–4256. doi: 10.4049/jimmunol.177.7.4252. [DOI] [PubMed] [Google Scholar]
- 44.Fritz JH, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26:445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 45.Herold K, et al. Receptor for advanced glycation end products (RAGE) in a dash to the rescue: Inflammatory signals gone awry in the primal response to stress. J Leukoc Biol. 2007;82:204–212. doi: 10.1189/jlb.1206751. [DOI] [PubMed] [Google Scholar]
- 46.Liliensiek B, et al. Receptor for advanced glycation end products (RAGE) regulates sepsis but not the adaptive immune response. J Clin Invest. 2004;113:1641–1650. doi: 10.1172/JCI18704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.