Studies of the childhood neurological disorder Rett syndrome and methyl-CpG-binding protein 2 (MeCP2) taught us that MeCP2 performs a balancing act in modulating neurological functions. Rett syndrome, characterized by cognitive deficits, motor impairments, autistic-like features, seizures, and stereotyped repetitive hand movements is caused by loss of MeCP2 function (1). On the other hand, a syndrome with overlapping features including autism, mental retardation, seizures, motor impairments, and repetitive movements results from gain of MeCP2 function due to doubling or tripling the protein level (2–4). In animal models, neuronal loss of MeCP2 leads to reduced glutamatergic synaptic strength due to reduced synapse numbers and gain of MeCP2 in neurons results in increased synaptic strength due to increased synapse numbers (5). Until recently it was believed that MeCP2 was exclusively expressed in neurons in the central nervous system (6, 7), we now know MeCP2 is also expressed in astrocytes and that MeCP2 deficient astrocytes cannot support neuronal dendritic arborization (8). At the molecular level, several studies have shown that MeCP2 functions as a transcriptional repressor by binding to methylated CpG dinucleotides and recruiting co-repressor proteins to silence gene expression (9, 10). However, in vivo studies showed that loss of MeCP2 leads to reduced expression of thousands of genes suggesting that MeCP2 may be a transcriptional modulator important for decreasing the expression of some genes and enhancing the expression of others (11). In this issue of PNAS, we learn about yet other balancing forces in modulating MeCP2 function: the differential phosphorylation of MeCP2 in response to neuronal activity (12). Such phosphorylation events may be one key mechanism by which MeCP2 modulates gene expression.
Fig. 1.
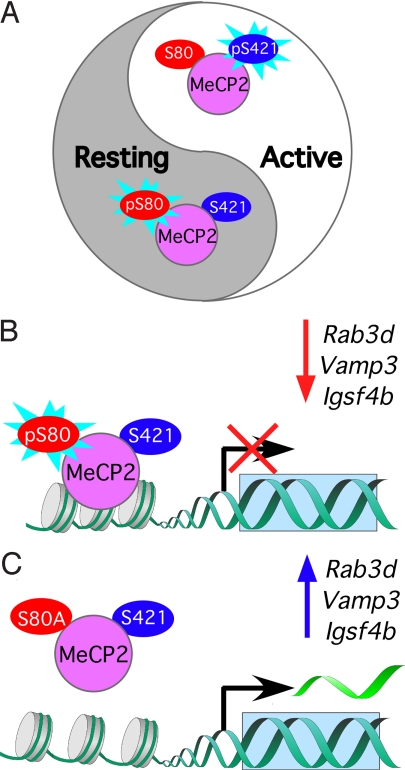
MeCP2 phosphorylation: a balancing act in neurons. A. MeCP2 in resting neurons (+TTX) is predominately phosphorylated at S80 (pS80) and dephosphorylated at S421. MeCP2 in active neurons (+ KCl) is predominately dephosphorylated at S80 and phosphorylated at S421 (S421). B. In resting neurons (+TTX) phosphorylation of S80 leads to increased MeCP2 binding to the promoters and reduced transcription of Rab3d, Vamp3, and Igsf4b. C. MeCP2 carrying the phosphorylation-deficient S80A mutation exhibits reduced binding to Rab3d, Vamp3, and Igsf4b promoters and increased transcription in resting neurons (+TTX).
Protein phosphorylation is an important posttranslational modification that can modulate the function of a protein via the addition of a phosphate group to serine, tyrosine, or threonine residues. Prior studies showed that depolarizing cultured neurons with potassium chloride (KCl) led to reduced MeCP2 association with the promoter of Brain derived neurotrophic factor (Bdnf) and a corresponding increase in Bdnf transcription (13, 14). Zhou and colleagues identified that the activity-dependent phosphorylation of serine 421 (S421) in MeCP2 leads to transcriptional induction of Bdnf (15). Together, these studies provided the initial evidence suggesting that phosphorylation of MeCP2 integrates neuronal activity with transcription of a target gene.
The new study by Tao et al. achieves a key milestone in elucidating the dynamic balance between site-specific dephosphorylation and phosphorylation that enables MeCP2 to control transcription of specific target genes (12). The authors surveyed phosphorylated serine, threonine, and tyrosine residues of rat and mouse MeCP2. They determined that these residues are all conserved, but not necessarily similarly phosphorylated across species. Further analysis in mouse brain samples revealed that serine 80 (S80) and serine 399 are the two major phosphorylation sites under resting conditions. Two residues showed specific activity-dependent phosphorylation, serine 424 (S424) and the previously identified S421.
Tao and colleagues discovered that neuronal activity-induced calcium influx through L-type voltage gated calcium channel triggers calcium/calmodulin-dependent protein kinase IV (CamK IV) to phosphorylate S421. When either neuronal activity or calcium influx is blocked pharmacologically, S421 is dephosphorylated. In contrast to S421, S80 is the most constitutively phosphorylated residue in resting neurons and is dephosphorylated with neuronal activity. Based on the S421 results, could S80 dephosphorylation be mediated by a phosphatase, such as calcineurin that is found in the highest concentrations in the brain? The phosphatase activity of calcineurin increases in response to calcium influx (16), thus it would be interesting to determine whether calcineurin inhibitors such as cyclosporin and tacrolimus inhibit the activity-dependent dephosphorylation of S80.
To explore the in vivo biological consequences of the key phosphorylation events, the authors generated two phosphorylation deficient knockin mouse models, one carrying a single mutant, S80A MeCP2, and the other expressing a MeCP2 with two mutations, S421A/S424A, to demonstrate that the phosphorylation of S80, S421, and S424 have biological consequences in vivo. Interestingly, mice expressing the single mutant S80A exhibit weight gain and decreased locomotor activity, which is suggestive of possible decreased MeCP2 function. Hypoactivity and weight gain are observed in various mouse models that have complete or partial loss of MeCP2 function (17–21). In contrast, mice carrying the S421A/S424A double mutation have normal weight and increased locomotor activity. The mouse studies suggest that these phosphorylation sites have different and perhaps opposing effects on MeCP2 function. Such findings imply that interference with the phosphorylation at any of these sites might lead to neurological abnormalities in humans.
An intriguing finding is that although mouse MeCP2 contains several residues that are evolutionarily conserved and undergo activity-dependent phosphorylation, only one of these sites, S421, undergoes activity-dependent phosphorylation in the rat. This suggests that the activity-dependent phosphorylation of MeCP2 may not be uniformly conserved between rat and mouse at all sites, which raises the question of how much we can translate what we learned from the S421A/S424A mice to rat or even human MeCP2. In this context, it would be critical to determine the extent the behavioral changes in the S421A/S424A mice result from either mutation alone or together. Exploring these cross-species differences will be important for understanding how MeCP2 normally functions in humans and how alteration in phosphorylation might contribute to the pathogenesis of MeCP2-related disorders.
To gain insight about the physiological and molecular consequences of interfering with MeCP2 phosphorylation at S80, Tao and colleagues resorted to cultured mouse cortical neurons and used lentiviruses to express either wildtype or S80A MeCP2. The investigators performed microarray analysis using RNA from these cells and examined the association of S80A MeCP2 with various gene promoters after treatment with tetrodotoxin (TTX), a sodium channel blocker. They showed that preventing phosphorylation of S80 led to reduced association of MeCP2 with several target genes: Pomc, Gtl2, Rab3d, Vamp3, and Igsf4b. The expression levels of Rab3d, Vamp3, and Igsf4b increased in conjunction with the reduced MeCP2 binding. Surprisingly, however, the expression levels of Pomc and Gtl2 were not significantly altered despite the robust reduction of MeCP2 binding to their promoters. These results imply that the effect of MeCP2 phosphorylation may also be dependent on the nature of the target gene.
Rab3d modulates the vesicular release machinery (22). Vamp3 is in astrocytic vesicles and mediates SNARE-dependent exocytosis (23). Igsf4b is a neural-tissue specific cell adhesion molecule that is important for axon-glia interaction in myelination (24). The fact that Vamp3 and Igsf4b are important in regulating glial function is exciting in light of recent findings showing a role for MeCP2 in astrocytes (8). Perhaps genes that are critical for activity-dependent regulation of glial and synaptic function are more dependent on MeCP2 phosphorylation.
Tao and colleagues show that phosphorylation of S80 increases binding to the promoters of specific target genes. However can phosphorylation of S80 decrease MeCP2 binding to the promoters of other target genes? Future studies using the S80A and S421A/S424A knockin mice are necessary to uncover how many and which genes are dependent on such opposing phosphorylation events. Importantly, detailed characterization of the consequences of S80A and S421A/S424A mutations will reveal the cellular and behavioral phenotypes affected by such phosphorylation events. Such studies can define the extent to which the S80A mutation recapitulates loss of function phenotypes and whether the S421A/S424A double mutant displays phenotypes observed in the gain of function model. It will also be interesting to determine the independent effects of the S421A and S424A mutations separately. Does a single residue largely determine the behavioral phenotypes or is an additive effect of both residues required?
Building on preceding MeCP2 phosphorylation studies, this new study by Tao and colleagues reveals another layer of complexity in fine-tuning MeCP2 function. The interplay between specific phosphorylation sites of MeCP2 orchestrates its response to changes in the state of neuronal activity and the transcription of certain genes. This latest insight about the posttranslational regulation of MeCP2 activity opens a potential therapeutic avenue to restore balanced neuronal activity in MeCP2-related disorders by targeting the associated phosphatases and kinases.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4882.
References
- 1.Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- 2.Van Esch H, et al. Duplication of the MECP2 region is a frequent cause of severe mental retardation and progressive neurological symptoms in males. Am J Hum Genet. 2005;77:442–453. doi: 10.1086/444549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meins M, et al. Submicroscopic duplication in Xq28 causes increased expression of the MECP2 gene in a boy with severe mental retardation and features of Rett syndrome. J Med Genet. 2005;42:e12. doi: 10.1136/jmg.2004.023804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Gaudio D, et al. Increased MECP2 gene copy number as the result of genomic duplication in neurodevelopmentally delayed males. Genet Med. 2006;8:784–792. doi: 10.1097/01.gim.0000250502.28516.3c. [DOI] [PubMed] [Google Scholar]
- 5.Chao HT, Zoghbi HY, Rosenmund C. MeCP2 controls excitatory synaptic strength by regulating glutamatergic synapse number. Neuron. 2007;56:58–65. doi: 10.1016/j.neuron.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jung BP, et al. The expression of methyl CpG binding factor MeCP2 correlates with cellular differentiation in the developing rat brain and in cultured cells. J Neurobiol. 2003;55:86–96. doi: 10.1002/neu.10201. [DOI] [PubMed] [Google Scholar]
- 7.Kishi N, Macklis JD. MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci. 2004;27:306–321. doi: 10.1016/j.mcn.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Ballas N, Lioy DT, Grunseich C, Mandel G. Noncell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nan X, et al. Transcriptional repression by the methyl-CpG binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. doi: 10.1038/30764. [DOI] [PubMed] [Google Scholar]
- 10.Jones PL, et al. Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet. 1998;19:187–191. doi: 10.1038/561. [DOI] [PubMed] [Google Scholar]
- 11.Chahrour M, et al. MeCP2, a key contributor to neurological disease, activates and represses transcription. Science. 2008;320:1224–1229. doi: 10.1126/science.1153252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tao J, et al. Phosphorylation of MeCP2 at Serine 80 regulates its chromatin association and neurological function. Proc Natl Acad Sci USA. 2009;106:4882–4887. doi: 10.1073/pnas.0811648106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinowich K, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science. 2003;302:890–893. doi: 10.1126/science.1090842. [DOI] [PubMed] [Google Scholar]
- 14.Chen WG, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science. 2003;302:885–889. doi: 10.1126/science.1086446. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, et al. Brain-specific phosphorylation of MeCP2 regulates activity-dependent Bdnf transcription, dendritic growth, and spine maturation. Neuron. 2006;52:255–269. doi: 10.1016/j.neuron.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibasaki F, Hallin U, Uchino H. Calcineurin as a multifunctional regulator. J Biochem. 2002;131:1–15. doi: 10.1093/oxfordjournals.jbchem.a003063. [DOI] [PubMed] [Google Scholar]
- 17.Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. doi: 10.1038/85899. [DOI] [PubMed] [Google Scholar]
- 18.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet. 2001;27:327–331. doi: 10.1038/85906. [DOI] [PubMed] [Google Scholar]
- 19.Shahbazian M, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35:243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 20.Fyffe SL, et al. Deletion of Mecp2 in Sim1-expressing neurons reveals a critical role for MeCP2 in feeding behavior, aggression, and the response to stress. Neuron. 2008;59:947–958. doi: 10.1016/j.neuron.2008.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Samaco RC, et al. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–1727. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schlüter OM, Schmitz F, Jahn R, Rosenmund C, Südhof TC. A complete genetic analysis of neuronal Rab3 function. J Neurosci. 2004;24:6629–6637. doi: 10.1523/JNEUROSCI.1610-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bezzi P, et al. Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat Neurosci. 2004;7:613–620. doi: 10.1038/nn1246. [DOI] [PubMed] [Google Scholar]
- 24.Park J, et al. Disruption of Nectin-like 1 cell adhesion molecule leads to delayed axonal myelination in the CNS. J Neurosci. 2008;28:12815–12819. doi: 10.1523/JNEUROSCI.2665-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]