Abstract
Nitrosothiols (RSNO), formed from thiols and metabolites of nitric oxide (•NO), have been implicated in a diverse set of physiological and pathophysiological processes, although the exact mechanisms by which they are formed biologically are unknown. Several candidate nitrosative pathways involve the reaction of •NO with O2, reactive oxygen species (ROS), and transition metals. We developed a strategy using extracellular ferrocyanide to determine that under our conditions intracellular protein RSNO formation occurs from reaction of •NO inside the cell, as opposed to cellular entry of nitrosative reactants from the extracellular compartment. Using this method we found that in RAW 264.7 cells RSNO formation occurs only at very low (<8 μM) O2 concentrations and exhibits zero-order dependence on •NO concentration. Indeed, RSNO formation is not inhibited even at O2 levels <1 μM. Additionally, chelation of intracellular chelatable iron pool (CIP) reduces RSNO formation by >50%. One possible metal-dependent, O2-independent nitrosative pathway is the reaction of thiols with dinitrosyliron complexes (DNIC), which are formed in cells from the reaction of •NO with the CIP. Under our conditions, DNIC formation, like RSNO formation, is inhibited by ≈50% after chelation of labile iron. Both DNIC and RSNO are also increased during overproduction of ROS by the redox cycler 5,8-dimethoxy-1,4-naphthoquinone. Taken together, these data strongly suggest that cellular RSNO are formed from free •NO via transnitrosation from DNIC derived from the CIP. We have examined in detail the kinetics and mechanism of RSNO formation inside cells.
Keywords: iron, nitrosation, reactive nitrogen species, reactive oxygen species, chelatable iron
Nitric oxide (nitrogen monoxide, •NO) is a ubiquitous signaling molecule that originally was thought to exert its effects solely through interaction with transition metal ligands of proteins, most notably the heme group of soluble guanylate cyclase. However, it is now recognized that •NO is capable of affecting cell physiology by inducing oxidative and covalent modification of protein amino acid residues. One such modification, S-nitrosation, the addition of a nitroso group to a thiol to form a nitrosothiol (RSNO), has received considerable attention as a potentially important posttranslational protein modification. S-nitrosated products are found ubiquitously in vivo (1), and thiol nitrosation has been found to alter the activity of a diverse set of proteins and may therefore represent an important concept in cellular and organismal biology (2).
A central issue in this paradigm is understanding the routes by which RSNO are formed inside cells. Importation of low molecular weight (LMW) RSNO through LAT transporters, followed by transnitrosation reactions, have been demonstrated to be a potent route for intracellular RSNO formation (3). However, mechanisms of de novo synthesis from free •NO are less clear. Proposed mechanisms include the reaction of •NO with O2 (autoxidation), either in the aqueous phase (4) or in an accelerated manner in hydrophobic phases (5); reaction of •NO with a thiol in the presence of a cellular electron acceptor (6); reaction between •NO and superoxide (O2•−) in the presence of excess •NO (7), and the interaction between •NO and transition metals and/or metalloproteins (8–14).
In this study, we examine the contributions of potential nitrosative pathways in RAW 264.7 murine macrophages. We present evidence suggesting that dinitrosyliron complexes (DNICs) formed at least in part from the cellular chelatable iron pool (CIP), through an O2-independent transnitrosative process, are responsible for the majority of RSNO formation in cells, and that reactive oxygen species (ROS) enhance RSNO formation, possibly via increases in DNICs.
Results
•NO Exposure Results in Intracellular RSNO Formation from Reactions of •NO Inside the Cell.
Fig. 1A presents a scheme depicting our strategy for selectively detecting RSNO formation caused by intracellular reactions of free •NO. The presence of external ferrocyanide (FCN), which rapidly reacts with •NO2 (15), will prevent the formation of nitrosative species in the external compartment, thus preventing their entry into the cells. RAW 264.7 macrophages were incubated 60 min with •NO donor [10 μM N-4–1-3-aminopropyl-2-hydroxy-2-nitrosohydrazinobutyl-1,3-propane-diamine (sper/NO), t1/2 = 39 min] with or without FCN (1 mM). Fig. 1B shows that there is no statistical difference between intracellular RSNO formation with or without FCN. However, nitrosation of an extracellular target (BSA) was nearly completely inhibited (85.8 ± 6.4%). These results indicate that under our conditions the RSNO formed inside the cell are primarily caused by the diffusion of •NO into the cell and reaction with cellular components.
Fig. 1.
Formation of intracellular RSNO is not caused by the autoxidation of •NO in the extracellular space. (A) Scheme of extracellular versus intracellular nitrosative processes. External addition of FCN will prevent cellular nitrosation from entry of nitrosative species formed extracellularly from •NO autoxidation, resulting in RSNO formation from only intracellular •NO. See Results for details. (B) Cells were exposed to sper/NO (10 μM) ± FCN (1 mM) for 60 min, and RSNO level in lysates was determined as described in Methods. (C) Same as A except nitrosation of external BSA (0.075 mg/mL) was measured. n.s., not significant. *, P < 0.05 compared with control.
Time Course of RSNO, •NO, and O2 Levels.
To determine the effects of O2 on cellular RSNO formation, we measured RSNO and O2 in cell suspensions exposed to •NO donor. Because of the dual effects of •NO on O2 consumption (16) and O2 on •NO consumption (17) it is essential to also measure the •NO concentration because varying the concentration of one will necessarily affect the other. When cells are added in the presence of sper/NO, O2 levels rapidly decline, reaching 8 μM in ≈15 min, after which they decline slowly to ≈3 μM at the end of the 60-min incubation (Fig. 2A). Subsequent addition of dithionite results in immediate disappearance of O2, indicating that the •NO at these low O2 levels is inhibiting respiration. Levels of •NO remain low (< 20 nM) during the first 20 min of incubation, and then begin rising as O2 declines to <5 μM (Fig. 2 A and B). The very low levels of detectable •NO during the initial 20 min, despite constant •NO release from the donor, can be attributed to O2-dependent •NO consumption as shown in several cell types (17, 18). By the end of the 60-min incubation, •NO levels are 771 ± 141 nM. Despite ongoing •NO formation and rapid cellular consumption during the first 20 min, RSNO formation is not observed during this time (Fig. 2B). The mechanism of O2-dependent •NO consumption by cells thus does not involve nitrosative chemistry. In addition, nitrosation is observed only when free •NO begins to appear. Subsequent to the appearance of •NO, the instantaneous rate of RSNO formation is constant despite a steady increase in •NO concentration. These surprising and unexpected results indicate that the rate of intracellular RSNO formation is independent of the concentration of •NO.
Fig. 2.
Time course of RSNO formation and •NO and O2 concentrations. (A) Cells were added to PBSD containing sper/NO (10 μM) and 1 mM FCN, and O2 concentration was monitored. (Inset) Scale expansion showing the slow decrease in O2 at longer times. At arrow, sodium dithionite was added. Representative data from 2 experiments are shown. (B) Conditions as in A except at 0, 12, 24, 36, 48, and 60 min cells were removed and processed for RSNO content (■). In parallel experiments, aliquots were removed at the indicated time points and analyzed for •NO concentration (△). (C) Cells were incubated with sper/NO and FCN as in A for 60 min, after which oxymyoglobin (20 μM) was added to scavenge •NO. Samples were collected 0, 20, or 60 min later and processed for intracellular RSNO content.
Cellular RSNO Formation Does Not Require O2.
To test whether O2 is required for RSNO formation under our conditions, cells were added to buffer containing 1 mM FCN in a reaction chamber containing both an O2 and an •NO electrode. The chamber was sealed and the cells were allowed to respire until the O2 level was below the detection limit of the O2 electrode (< 100 nM), a process that took 5–10 min. Thereupon argon-purged sper/NO (5 μM) was added and the cells were incubated with stirring for an additional 60 min. After sper/NO addition there is an immediate increase in •NO, and peak •NO levels are ≈700 nM (Fig. 3A), similar to the experiments begun under aerobic conditions (Fig. 2B). Surprisingly, RSNO levels in anaerobic experiments are 65.8 ± 7.9 pmol/mg (Fig. 3B), again similar to experiments begun under aerobic conditions. There is a small increase in O2 after sper/NO addition, although O2 levels remain below •NO levels for the duration of the experiment (Fig. 3A). These data show another unexpected result, namely, that intracellular RSNO formation is virtually independent of O2.
Fig. 3.
RSNO formation during anoxia. (A) •NO and O2 electrode traces for anaerobic experiments. Cells (2 × 107 cells/mL) were allowed to respire for 5–10 min until O2 levels reached 0 (t = 0), at which point 1 mM FCN and 5 μM sper/NO (argon-purged) were added. Cells were then incubated for 60 min. (B) RSNO determination in anaerobically-incubated cells.
Size of Cellular RSNO.
Cellular nitrosative chemistry may be targeting either LMW thiols, primarily glutathione (GSH), or thiols on high molecular weight (HMW) proteins. To determine which pool is the primary target for nitrosative chemistry, the LMW and HMW fractions from lysates of sper/NO-exposed cells were separated by using filters (10,000-kDa cutoff). After treatment with 10 μM sper/NO and 1 mM FCN, the RSNO content in the retentate is 73.4 ± 17.6% of unfractionated cell extracts. Likewise, in cells treated with 2,3-dimethoxy-1,4-naphthoquinone (DMNQ) (see below), 89.6 ± 8.1% of the mercury-labile signal is found in the retentate. These results demonstrate that the RSNO formed from •NO under our conditions are of large molecular weight.
Stability of Cellular RSNO.
To assess the stability of RSNO formed from exogenously-generated •NO, cells were incubated with sper/NO (10 μM) for 60 min and then MbO2 (20 μM) was added to scavenge the remaining •NO. Cells were incubated further for 0, 20, or 60 min and RSNO content was determined. As shown in Fig. 2C, the level of RSNO is relatively stable until 20 min post-MbO2 and then RSNO level begins to decline slowly (≈40%) during the subsequent 40-min time period. The relatively stable level of RSNO is consistent with other studies (1, 19, 20). These results show that the mechanism responsible for RSNO formation is only slowly reversible and that the increase in RSNO levels (Fig. 2B) is caused primarily by the increased rate of formation as opposed to decreased rate of disappearance.
Cellular RSNO Formation Is Largely Mediated by the CIP.
Exposure of cells to •NO results in the formation of EPR-visible dinitrosyliron complexes (DNICs) (21), which in acellular systems are capable of nitrosative chemistry (8, 9). DNICs in cells exposed to •NO are formed from the CIP (22), representing a small (≈5%) fraction of total iron thought to be in transit between the various cellular iron pools (23). To test the involvement of the CIP in cellular nitrosation, sper/NO-exposed cells were incubated in the presence of the cell-permeant CIP chelator salicylaldehyde isonicotinoyl hydrazone (SIH) (23) and assayed for RSNO. As is also true for DNIC formation (22), the addition of SIH inhibits RSNO formation in a dose-dependent fashion (Table 1), reaching a maximum inhibition of ≈60% at 100 μM SIH. As a positive control, cells were enriched with iron by preincubation with FeSO4 (100 μM), and subsequently treated without or with SIH (100 μM) during sper/NO treatment. Iron enrichment results in a substantial increase in cellular RSNO, which is completely reversed by SIH to the level of unenriched cells treated with chelator (Table 1). These data indicate an important role for the CIP in the formation of RSNO from free •NO in cells, possibly via DNIC.
Table 1.
Iron-dependent intracellular RSNO formation
| Treatment | RSNO content, pmol/mg |
|---|---|
| Control | 52.1 ± 4.3 (14) |
| SIH (30 μM) | 30.7 ± 2.5* (3) |
| SIH (100 μM) | 21.2 ± 3.3† (7) |
| FeSO4 | 140.1 ± 4.5‡ (3) |
| FeSO4 + 100 μM SIH | 17.1 ± 4.1§ (3) |
| BSO | 86.8 ± 9.1¶ (4) |
| BSO + 100 μM SIH | 57.1 ± 7.3‖ (4) |
Cells ± 100 μM FeSO4 (1 h) or 100 μM BSO (22–24 h) were treated for 1 h with 1 mM FCN and 10 μM sper/NO ± SIH. After treatment the cells were collected and assayed for RSNO content. Number of cells is indicated by the numbers in parentheses.
*P = 0.044 vs. control.
†P = 0.00017 vs. control.
‡P = 2.0 × 10−7 vs. control.
§P = 0.0027 vs. control, P = 3.6 × 10−5 vs. FeSO4.
¶P = 0.002 vs. control.
‖P = 0.58 vs. control, 0.044 vs. BSO.
Oxidative Stress Increases Cellular RSNO Formation.
Oxidative stress can result in increases in nitrosative chemistry as assessed by using fluorescent probes (7). To establish whether this chemistry will result in cellular RSNO formation, we treated cells with DMNQ, a cell-permeable redox cycling compound that produces O2•− and hydrogen peroxide (H2O2) (24). Unlike other quinone-based redox cyclers, semiquinones produced by DMNQ reduction will not form adducts (25) and DMNQ is therefore thought to exert its effects on cells exclusively through the production of ROS. As shown in Fig. 4A confocal microscopy reveals that exposure of cells to 10 μM DMNQ results in a robust increase (>2-fold) in fluorescence of the probe dichlorofluorescein (DCF), confirming that the redox cycler increases ROS. Fig. 4B shows that cellular RSNO are increased by DMNQ in a dose-dependent fashion. To confirm that DMNQ increases RSNO because of intracellular production of ROS, we performed DMNQ experiments in the presence of extracellular superoxide dismutase (SOD) (1,000 units/mL). There is no difference in RSNO in DMNQ-treated cells in the presence or absence of SOD (125.1 ± 31.7 without SOD vs. 140.6 ± 25.1 pmol/mg with SOD; P = 0.71).
Fig. 4.
Effect of ROS on cellular RSNO formation. (A) Cells preloaded with DCFH-DA were exposed to DMNQ (0 or 10 μM) for 30 min, and DCFH oxidation was determined as described in Methods. (Magnification: 20×.) (B) Cells were exposed to sper/NO and FCN as in Fig. 1 and DMNQ at the indicated concentrations, and cells were analyzed for RSNO content.
We attempted to verify that the DMNQ effect on cellular RSNO is attributable to ROS by testing the effect of manganese tetrakis (4-benzoic acid) porphyrin (MnTBAP), a cell-permeable scavenger of reactive oxygen and nitrogen intermediates (26). As shown in Table 2, MnTBAP decreases RSNO production in the presence of DMNQ to levels significantly lower than controls. Although these results could indicate that the effects of MnTBAP are caused by scavenging of ROS, as described in Discussion, the exact mechanisms are not clear. In particular, as shown in Table 2, SIH reverses the effect of DMNQ on cellular RSNO formation, thus indicating that the enhanced RSNO formation from DMNQ involves the CIP.
Table 2.
ROS-dependent intracellular RSNO formation
| Treatment | RSNO content, pmol/mg |
|---|---|
| Control | 73.2 ± 5.4 (7) |
| MnTBAP | 65.9 ± 6.9* (4) |
| DMNQ | 180.2 ± 16.0† (7) |
| MnTBAP + DMNQ | 10.6 ± 3.2‡ (4) |
| SIH + DMNQ | 79.9 ± 21.5§ (4) |
Cells were treated with 1 mM FCN and 10 μM sper/NO, ± 100 μM MnTBAP, 10 μM DMNQ, or 100 μM SIH as indicated. After treatment cells were collected and assayed for RSNO content. Number of cells is indicated by the numbers in parentheses.
*P = 0.22 vs. control.
†P = 2.5 × 10−5 vs. control.
‡P = 3.0 × 10−8 vs. control, P = 2.8 × 10−5 vs. DMNQ.
§P = 0.68 vs. control, P = 0.0045 vs. DMNQ.
Oxidative stress can occur either via increased production of ROS or decreased antioxidant defenses. To test the effect of the latter on RSNO formation, we incubated cells with the GSH synthesis inhibitor l-buthionine sulfoximine (BSO). Treatment of cells for 20–22 h with BSO diminished the total pool of GSH [GSH + glutathione disulfide (GSSG)] to 19.1 ± 6.2% of controls as assessed by the GSH recycling assay. LMW reduced thiol was undetectable (<6% total control thiol). Treatment of BSO-treated cells with sper/NO results in a significant increase in RSNO (Table 1), confirming the conclusions from the DMNQ experiments that oxidative stress in cells is pronitrosative. These results also reveal that GSH is not involved in the CIP-dependent nitrosative pathway because SIH still reduces cellular RSNO in BSO-treated cells, in net amount comparable to its effect on control cells (Table 1).
Cellular RSNO Are Formed by DNICs.
If DNICs are involved in RSNO formation, it would be expected that our manipulations (iron chelation, ROS production) would have effects on cellular DNIC pool similar to those on the RSNO pool. As shown in Fig. 5, this is indeed the case. Changes in DNIC content with SIH and/or DMNQ demonstrate strong correlation with the extent of RSNO formation. Thus, the extent of RSNO formation is predicted by DNIC content. To verify that the tri-iodide chemiluminescence (CL) assay for RSNO is not measuring DNIC, samples processed as for CL (see Methods) were analyzed for DNIC by EPR. There was no decrease in the intensity of the EPR DNIC signal upon 20-min incubation with HgCl2/sulfanilamide as compared with sulfanilamide alone, indicating that the mercury-resistant signal in the CL assay, commonly interpreted as RSNO, is not from DNIC.
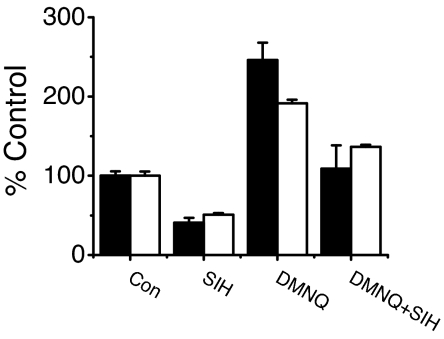
Fig. 5.
Correlation between DNIC and RSNO. Cells were exposed to 10 μM sper/NO and 1 mM FCN for 60 min, ± SIH and/or 10 μM DMNQ, and samples were analyzed for RSNO content (closed bars) or DNIC (open bars) as described in Methods. Values for RSNO are taken from Tables 1 and 2 and plotted in graphical form as percentage of control.
Room temperature EPR analysis reveals anisotropic DNIC spectra under both basal (sper/NO alone; also see ref. 22) and ROS-stimulated (sper/NO + DMNQ) conditions (Fig. S1), indicating that in both cases, DNICs (like RSNO) are associated with proteins. Together, these data strongly suggest that cellular protein-associated DNIC formation from •NO and the CIP is the primary nitrosative pathway from free •NO.
Discussion
We have used ferrocyanide to probe mechanisms of intracellular protein RSNO formation from •NO. When concurrent [O2], [•NO], and RSNO are measured after addition of cells and •NO donor, a lag occurs in the appearance of both •NO and RSNO, which corresponds to a decline in [O2] to very low levels (<10 μM) (Fig. 2). We attribute the lag in •NO appearance, in the face of constant •NO liberation from donor, to cellular •NO consumption, which we have shown is O2 dependent (17). Eventually, as [O2] decreases, •NO liberation inhibits mitochondrial O2 consumption, resulting in a plateau of [O2]. At this point both •NO and RSNO appear and, importantly, the instantaneous rate of RSNO formation is independent of [•NO]. This zero-order kinetic dependence argues against either autoxidation (4) or direct •NO/thiol reaction with subsequent oxidation (6) as the mechanism of RSNO formation because both processes are second order with respect to [•NO]. The zero-order kinetics suggests that the rate-limiting step is the formation of either a short-lived or a low-abundance (and thus limiting) species.
One of the most surprising results from this study is the observation that RSNO formation from physiological •NO concentrations (<1 μM) is not inhibited even under conditions of very low (< 300 nM) O2 (Fig. 3). RSNO formation under low O2 has been reported (27, 28); however, high concentrations of •NO donor (50–100 μM) were used. Our experiments establish that under any physiologically-relevant condition O2 will not be limiting for RSNO formation [although at very low levels it may become limiting for •NO generation by NO synthase (NOS) as discussed below]. These results also point out that RSNO may be readily formed in subcellular organelles, such as mitochondria, that may experience extremely low O2 tensions.
RSNO formation involves the participation of the CIP, as demonstrated by the inhibitory effects of SIH (Table 1). More specifically, the species involving the CIP appears to be the DNIC, because the extent of RSNO formation correlates with the intensity of DNIC under a variety of conditions, including SIH-inhibitable DNIC and SIH-resistant DNIC (Fig. 5). We have shown (22) that there appears to be mutually-exclusive quantitative conversion of the CIP to either DNIC (upon •NO pretreatment) or conversion to an EPR-visible chelate complex (pretreatment with deferroxamine). These experiments were carried out under different conditions, in particular with much higher •NO donor doses. Overall, however, similar to results presented here (Fig. 5), SIH does not completely prevent DNIC formation from •NO and SIH-resistant DNIC may well be capable of transnitrosating thiols. Other studies have demonstrated SIH-inhibitable and SIH-resistant DNIC formation in cells (29, 30).
We propose that the major mechanism for RSNO formation is via transnitrosation from DNIC to thiol. This mechanism, which has been suggested by Vanin et al. (9) based on in vitro chemical studies, would explain the surprising independence on both [•NO] and [O2]. Zero-order kinetics with regard to [•NO] can be explained by rapid nitrosylation of the CIP by •NO to form DNIC (22) followed by the slower transnitrosation to thiol. The O2 independence is explained by the fact that neither DNIC formation nor transnitrosation involves O2. However, nitrosative chemistry requires a net 1-electron oxidation; in the case of DNIC-mediated nitrosation it has been suggested that the iron may facilitate the net transfer of an electron from one •NO to another, producing HNO and a nitrosonium equivalent (9). Thus, in this scheme, the oxidant would be •NO itself. Alternatively, transition metal centers other than DNIC might be nitrosating species; several metalloproteins are known to catalyze RSNO formation in vitro (10, 13, 14, 31), and it is unlikely that SIH will be able to chelate tightly-bound metals in prosthetic groups.
One alternative possibility for the inhibition of RSNO formation by SIH is that the chelator is directly reacting with •NO or a derived nitrosating species. However, we feel that this possibility is unlikely given that RSNO levels in the presence of SIH are the same in both Fe-unenriched and Fe-enriched cells (Table 1), indicating that SIH is affecting RSNO levels through an iron-dependent pathway. Additionally, SIH actually decreases the rate at which cells scavenge •NO (Fig. S2), opposite of what would be expected were SIH directly reacting with •NO.
Prooxidant treatments such as BSO (to lower GSH levels) and DMNQ (a well-established redox cycler) both substantially increase RSNO formation (Fig. 4 and Tables 1 and 2), but the effect is related to the CIP because it is almost completely reversed by SIH (Tables 1 and 2). The portion that is not reversed by SIH may still be caused by DNIC because there may be 2 pools of iron involved in DNIC formation and protein nitrosation, one chelatable and one resistant to SIH chelation. ROS and RNS have been shown to modulate intracellular iron (32). The effect of BSO also shows that the CIP-dependent nitrosative pathway is unaffected by changes in GSH or other LMW thiol (Table 1), indicating that GSH-DNICs (33) are not obligatory intermediates, nor are other products of the interaction between GSH and the CIP.
Another possibility for the BSO effect is that decreased GSH will result in decreased S-nitrosoglutathione (GSNO) reductase (34) activity. Assuming an equilibrium between GSNO and protein RSNO as suggested by Liu et al. (35), this effect could tend to increase cellular RSNO by inhibiting the GSNO reductase-mediated enzymatic breakdown pathway. However, the slow decline in RSNO upon removal of •NO (Fig. 2C) suggests that the mechanism of action of BSO is not on decreased breakdown via transnitrosation to GSH and subsequent GSNO breakdown via GSNO reductase.
One interesting feature of DMNQ-mediated RSNO formation is that it does not exhibit a “bell shape” with a DMNQ dose that is expected for O2•−-dependent nitrosative chemistry (7). The bell-shape derives from the fact that O2•− potentiates nitrosation only in conditions where O2•− flux is below •NO flux, because O2•− will consume •NO, and nitrosation requires free •NO (36). Assuming a similar process inside cells, the observed plateau could be a result of saturation of ROS production caused by rate-limiting DMNQ reduction. Additionally, if the DMNQ effect on nitrosation is caused by mobilization of nitrosative iron (as suggested above), rather than a direct reaction of RNS derived from the O2•−/•NO with thiols, then a bell-shaped curve would not be predicted.
We attempted to test the involvement of ROS on RSNO formation by using the scavenger MnTBAP. MnTBAP has no effect on RSNO formation in the absence of DMNQ (Table 2); however, with DMNQ it dramatically reduces RSNO to below controls. However, interpretation of these results is problematic, because manganese porphyrinic oxidant scavengers such as MnTBAP display a wide spectrum of reactivity and are capable of reaction with several stronger oxidants than O2•− (26). In addition, it has been recently demonstrated that commercial preparations of MnTBAP are contaminated by multiple “noninnocent” impurities (26). Further work on the precise action of commercial and purified manganese porphyrinic scavengers is needed before we can make useful speculations on the mechanistic basis of their effects on RSNO formation.
We observe RSNO formation only at O2 levels where iNOS and nNOS activity will be less than maximal because of the relatively high Km for O2 for these enzymes. However, although enzymatic activity will be lower than maximal below the Km, it will not be 0 because appreciable •NO production from NOS isozymes does indeed occur well below the Km (37). The zero-order dependence of RSNO formation on [•NO] implies an interesting possibility for the in vivo condition, that at low O2 levels, RSNO formation will be independent of the effects of O2 on NOS, e.g., decreasing O2 from 10 to 5 μM will decrease the activity of the NOS, but have no effect on RSNO formation. Indeed, Bryan et al. (1) have determined that RSNO levels in vivo are relatively unaffected by global hypoxia, and in some tissues they are actually increased.
The results of this study are interesting in light of 3 other reports investigating mechanisms of nitrosation in cells. Kim et al. (11) showed that both DNIC and RSNO could be increased in RAW cells by iron loading, and that both of these species correlated to the size of the nonheme iron pool. Our work, using intracellular iron chelators, further implicates the CIP as an important mechanistic link between DNIC and RSNO. Yang and Loscalzo (20) reported evidence to suggest that mitochondria act as a source of ROS, which potentiate cellular RSNO formation. Our results with DMNQ and GSH depletion are consistent with this conclusion and suggest a specific mechanism, i.e., increasing DNIC. It is also possible that mitochondria regulate RSNO formation by regulating O2 levels; decreased mitochondrial O2 consumption could attanuate RSNO levels by increased O2-dependent •NO consumption (Fig. 2). Espey et al. (38) studied intracellular nitrosation of the probe diaminofluorescein (DAF) and compared the mechanism to that for aqueous solution. They conclude that the intracellular mechanism is different from in aqueous solution, based primarily on the first-order rate of increase of DAF nitrosation in the presence of cells, compared with the second-order rate in the absence of cells or with cell lysate. However, their kinetic results could reflect the appearance and disappearance of •NO in their reaction system, both of which will be first-order under the conditions they use.
We should point out several caveats to this work. First, we measure overall cellular nitrosative chemistry, rather than nitrosation reactions within specific spatially or functionally distinct groups. Both protein structure and intracellular compartmentalization will affect susceptibility of individual cysteine residues to nitrosation. It is also possible that a mechanism accounting for a small proportion of cellular RSNO, perhaps not detectable here, will have functional consequences far beyond its contribution to overall nitrosation, because of the importance of the target proteins in cellular processes. We also note that our work is restricted to the formation of RSNO from free •NO inside cells, rather than the importation of LMW RSNO from the extracellular space. LMW RSNO have been shown to be imported into the cell and cause massive protein nitrosation (3), and it is possible this mechanism is as important, if not more important, in vivo. Additionally, we have used •NO donor rather than •NO from endogenous formation. It may be, for example, that relatively long-term •NO exposure during up-regulation of inducible NOS results in •NO-induced modifications in protein expression and/or iron homeostatic responses to modifications in the CIP (39). Finally, our proposal that •NO affects nitrosation via DNIC formation and subsequent transnitrosation to thiols is based on indirect evidence (kinetics, O2 independence, CIP dependence) and the direct correlation between RSNO and DNIC with different experimental manipulations. It may be, however, that the correlation is simply reflective of some underlying mechanism that produces RSNO and DNIC by separate and nonoverlapping processes. However, given the relative sizes of the CIP, RSNO, and DNIC (22) pools and the aforementioned kinetic observations, the simplest (and thus most likely) explanation is that DNIC and RSNO formation are causally linked. In our previous work (22), we have ruled out the possibility that cellular DNICs are formed from RSNO. Thus, if a cause and effect relationship exists, as seems probable, it must be that DNICs form RSNO.
Our results may provide insight into another question of major importance to NO signaling biology, namely as to how protein specificity is achieved in nitrosation reactions. Factors that have been suggested to be important are thiol pKa, identity and configuration of neighboring amino acid residues, and accessibility (2). We suggest that another factor that may contribute to specificity of nitrosation is iron-dinitrosyl coordination near the target thiol. Alternatively, once the DNIC is formed on a protein separate from the target thiol, specificity is imparted by protein–protein recognition mechanisms.
Methods
Materials.
DMNQ was from Oxis, and sper/NO and MnTBAP were from Alexis. SIH was a generous gift from Prem Ponka (Lady Davis Institute for Medical Research, Montreal). DMEM, FBS, and penicillin/streptomycin were from Fisher. All other reagents were from Sigma.
Cell Culture.
Murine RAW 264.7 macrophages were cultured as described (22). Before experiments, cells were rinsed twice and collected in HBSS supplemented with 10 mM Hepes, pH 7.4. Cells were collected by scraping and washed in fresh HBSS/Hepes before use. For iron loading, cells were preloaded by incubation with 100 μM FeSO4 in HBSS/Hepes for 1 h. Excess iron was then removed by washing 3 times with HBSS/Hepes.
Cell •NO Exposure.
Cells were added to reaction vials (to a final density of 107 cells per mL, unless otherwise indicated) containing PBS with 100 μM diethylenetriaminepentacetic acid (DTPA) at pH 7.4 (PBSD) with sper/NO (10 μM, unless otherwise indicated) and (where indicated) FCN (1 mM) and concentrations of SIH, DMNQ, and/or MnTBAP as indicated. Vials were capped with rubber septa and incubated with zero headspace with rotation at 37 °C in the dark for 60 min. For anaerobic and time-course experiments, MbO2 (20–30 μM) was added at the end of the exposure to scavenge remaining •NO. Cells were processed as described by Zhang and Hogg (3), modified as follows. Cells were collected by centrifugation, washed twice with ice-cold PBSD, and resuspended in ice-cold thiol blocking buffer (50 mM KPi, 50 mM N-ethylmaleimide, 100 μM DTPA, pH 7.4) for RSNO determination or in 200 μL of PBSD for EPR analysis. EPR samples were transferred to EPR tubes and frozen at −80 °C until analysis. Samples to be analyzed for RSNO content were lysed by freeze-thaw, followed by brief sonication on ice. Insoluble material was removed by centrifugation and lysates were stored at −80 °C until analysis for RSNO content. Cells treated with 20 μM nitrite had barely-detectable RSNO levels (2.6 ± 0.8 pmol·mg−1 protein), indicating that RSNO formed from sper/NO were caused by the release of •NO.
RSNO and •NO Determination.
RSNO levels were determined by triiodide-dependent CL as described by Feelisch et al. (40), using an EcoMedics CLD 88 sp detector. •NO concentration during sper/NO treatment was analyzed by removing aliquots from reaction vials using a gas-tight syringe and adding them to the CL reaction chamber containing 1 M NaOH to prevent further release of •NO from the sper/NO. Signals were integrated by using ChromProcessor software (ACD Labs) against nitrite standards. For anaerobic experiments, •NO was measured by using an •NO-specific electrode (ISO-NOP; World Precision Instruments).
O2 Measurements.
O2 concentration of cell suspensions was determined in a sealed, stirred chamber at 37 °C containing an O2 electrode (Oxygraph; Oroboros). The electrode was calibrated at room air and at zero O2 by the addition of sodium dithionite.
GSH and Reduced Thiol Measurements.
For GSH determination, cells were rinsed twice with PBSD and lysed with PBSD containing Triton X-100 (0.1%). Lysates were vortexed vigorously and centrifuged 15,800 × g at 4 °C. Supernatants were reserved for GSH and total thiol analysis. Total GSH (GSH + GSSG) was measured by using the GSH recycling assay (41). Total and LMW reduced thiol levels were assessed by DTNB before and after protein precipitation by trichloroacetic acid (42).
Imaging of DCF Fluorescence.
Intracellular oxidants were measured by using the probe dichlorofluoroscin diacetate and confocal microscopy as described (43).
EPR Spectroscopy.
EPR spectra were obtained and analyzed as described (22).
Protein Determination:
Protein content was determined by using the bicinchoninic acid method (Pierce).
Statistical Analysis.
All measures reflect mean ± SEM, with n ≥ 3. Means between groups were compared by using paired Student's t test. P < 0.05 were considered significant.
Supplementary Material
Acknowledgments.
We thank Kay Shi for excellent technical assistance, Dave Kraus for use of the oxygraph, and Dr. Rakesh Patel for insightful discussions. This work was supported in part by American Heart Association (Southeastern Affiliate) Predoctoral Fellowship 0515098B (to C.A.B.) and National Institutes of Health Grants HL71189 and HL074391 (to J.R.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0710416106/DCSupplemental.
References
- 1.Bryan NS, et al. Cellular targets and mechanisms of nitros(yl)ation: An insight into their nature and kinetics in vivo. Proc Natl Acad Sci USA. 2004;101:4308–4313. doi: 10.1073/pnas.0306706101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: Purview and parameters. Nat Rev Mol Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Hogg N. S-nitrosothiols: Cellular formation and transport. Free Radical Biol Med. 2005;38:831–838. doi: 10.1016/j.freeradbiomed.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 4.Kharitonov VG, Sundquist AR, Sharma VS. Kinetics of nitrosation of thiols by nitric oxide in the presence of oxygen. J Biol Chem. 1995;270:28158–28164. doi: 10.1074/jbc.270.47.28158. [DOI] [PubMed] [Google Scholar]
- 5.Moller MN, Li Q, Lancaster JR, Jr, Denicola A. Acceleration of nitric oxide autoxidation and nitrosation by membranes. IUBMB Life. 2007;59:243–248. doi: 10.1080/15216540701311147. [DOI] [PubMed] [Google Scholar]
- 6.Gow AJ, Buerk DG, Ischiropoulos H. A novel reaction mechanism for the formation of S-nitrosothiol in vivo. J Biol Chem. 1997;272:2841–2845. doi: 10.1074/jbc.272.5.2841. [DOI] [PubMed] [Google Scholar]
- 7.Espey MG, Thomas DD, Miranda KM, Wink DA. Focusing of nitric oxide mediated nitrosation and oxidative nitrosylation as a consequence of reaction with superoxide. Proc Natl Acad Sci USA. 2002;99:11127–11132. doi: 10.1073/pnas.152157599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boese M, Mordvintcev PI, Vanin AF, Busse R, Mulsch A. S-nitrosation of serum albumin by dinitrosyl-iron complex. J Biol Chem. 1995;270:29244–29249. doi: 10.1074/jbc.270.49.29244. [DOI] [PubMed] [Google Scholar]
- 9.Vanin AF, Malenkova IV, Serezhenkov VA. Iron catalyzes both decomposition and synthesis of S-nitrosothiols: Optical and electron paramagnetic resonance studies. Nitric Oxide. 1997;1:191–203. doi: 10.1006/niox.1997.0122. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, et al. Nitrosothiol formation catalyzed by ceruloplasmin. Implication for cytoprotective mechanism in vivo. J Biol Chem. 1999;274:27069–27075. doi: 10.1074/jbc.274.38.27069. [DOI] [PubMed] [Google Scholar]
- 11.Kim YM, Chung HT, Simmons RL, Billiar TR. Cellular nonheme iron content is a determinant of nitric oxide-mediated apoptosis, necrosis, and caspase inhibition. J Biol Chem. 2000;275:10954–10961. doi: 10.1074/jbc.275.15.10954. [DOI] [PubMed] [Google Scholar]
- 12.English AM, Wilcox DE. Effects of metal ions on the oxidation and nitrosation of cysteine residues in proteins and enzymes. Met Ions Biol Syst. 2001;38:313–350. [PubMed] [Google Scholar]
- 13.Tao L, English AM. Mechanism of S-nitrosation of recombinant human brain calbindin D28K. Biochemistry. 2003;42:3326–3334. doi: 10.1021/bi0269963. [DOI] [PubMed] [Google Scholar]
- 14.Weichsel A, et al. Heme-assisted S-nitrosation of a proximal thiolate in a nitric oxide transport protein. Proc Natl Acad Sci USA. 2005;102:594–599. doi: 10.1073/pnas.0406549102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldstein S, Czapski G. Kinetics of nitric oxide autooxidation in aqueous solution in the absence and presence of various reductants. The nature of the oxidizing intermediates. J Am Chem Soc. 1995;117:12078–12084. [Google Scholar]
- 16.Brown GC. Nitric oxide and mitochondria. Front Biosci. 2007;12:1024–1033. doi: 10.2741/2122. [DOI] [PubMed] [Google Scholar]
- 17.Thomas DD, Liu X, Kantrow SP, Lancaster JR., Jr The biological lifetime of nitric oxide: Implications for the perivascular dynamics of NO and O2. Proc Natl Acad Sci USA. 2001;98:355–360. doi: 10.1073/pnas.011379598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner PR, Martin LA, Hall D, Gardner AM. Dioxygen-dependent metabolism of nitric oxide in mammalian cells. Free Radical Biol Med. 2001;31:191–204. doi: 10.1016/s0891-5849(01)00569-x. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Hogg N. Formation and stability of S-nitrosothiols in RAW 264.7 cells. Am J Physiol. 2004;287:L467–L474. doi: 10.1152/ajplung.00350.2003. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Loscalzo J. S-nitrosoprotein formation and localization in endothelial cells. Proc Natl Acad Sci USA. 2005;102:117–122. doi: 10.1073/pnas.0405989102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henry YA, Guissani A, Ducastel B. Nitric Oxide Research from Chemistry to Biology: EPR Spectroscopy of Nitrosylated Compounds. Austin, TX: Landes Bioscience; 1997. [Google Scholar]
- 22.Toledo JC, Jr, et al. Nitric oxide-induced conversion of cellular chelatable iron into macromolecule-bound paramagnetic dinitrosyliron complexes. J Biol Chem. 2008;283:28926–28933. doi: 10.1074/jbc.M707862200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glickstein H, El RB, Shvartsman M, Cabantchik ZI. Intracellular labile iron pools as direct targets of iron chelators: A fluorescence study of chelator action in living cells. Blood. 2005;106:3242–3250. doi: 10.1182/blood-2005-02-0460. [DOI] [PubMed] [Google Scholar]
- 24.Shi MM, Kugelman A, Iwamoto T, Tian L, Forman HJ. Quinone-induced oxidative stress elevates glutathione and induces γ-glutamylcysteine synthetase activity in rat lung epithelial L2 cells. J Biol Chem. 1994;269:26512–26517. [PubMed] [Google Scholar]
- 25.Gant TW, Rao DN, Mason RP, Cohen GM. Redox cycling and sulphydryl arylation: Their relative importance in the mechanism of quinone cytotoxicity to isolated hepatocytes. Chem Biol Interact. 1988;65:157–173. doi: 10.1016/0009-2797(88)90052-x. [DOI] [PubMed] [Google Scholar]
- 26.Reboucas JS, Spasojevic I, Batinic-Haberle I. Pure manganese(III) 5,10,15,20-tetrakis(4-benzoic acid)porphyrin (MnTBAP) is not a superoxide dismutase mimic in aqueous systems: A case of structure–activity relationship as a watchdog mechanism in experimental therapeutics and biology. J Biol Inorg Chem. 2008;13:289–302. doi: 10.1007/s00775-007-0324-9. [DOI] [PubMed] [Google Scholar]
- 27.Foster MW, Stamler JS. New insights into protein S-nitrosylation. Mitochondria as a model system. J Biol Chem. 2004;279:25891–25897. doi: 10.1074/jbc.M313853200. [DOI] [PubMed] [Google Scholar]
- 28.Jourd'heuil D, Jourd'heuil FL, Feelisch M. Oxidation and nitrosation of thiols at low micromolar exposure to nitric oxide. Evidence for a free radical mechanism. J Biol Chem. 2003;278:15720–15726. doi: 10.1074/jbc.M300203200. [DOI] [PubMed] [Google Scholar]
- 29.Lewandowska H, Meczynska S, Sochanowicz B, Sadlo J, Kruszewski M. Crucial role of lysosomal iron in the formation of dinitrosyl iron complexes in vivo. J Biol Inorg Chem. 2007;12:345–352. doi: 10.1007/s00775-006-0192-8. [DOI] [PubMed] [Google Scholar]
- 30.Meczynska S, Lewandowska H, Sochanowicz B, Sadlo J, Kruszewski M. Variable inhibitory effects on the formation of dinitrosyl iron complexes by deferoxamine and salicylaldehyde isonicotinoyl hydrazone in K562 cells. Hemoglobin. 2008;32:157–163. doi: 10.1080/03630260701699821. [DOI] [PubMed] [Google Scholar]
- 31.Luchsinger BP, et al. Routes to S-nitroso-hemoglobin formation with heme redox and preferential reactivity in the beta subunits. Proc Natl Acad Sci USA. 2003;100:461–466. doi: 10.1073/pnas.0233287100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mladenka P, Simunek T, Hubl M, Hrdina R. The role of reactive oxygen and nitrogen species in cellular iron metabolism. Free Radical Res. 2006;40:263–272. doi: 10.1080/10715760500511484. [DOI] [PubMed] [Google Scholar]
- 33.Cesareo E, et al. Nitrosylation of human glutathione transferase P1–1 with dinitrosyl diglutathionyl iron complex in vitro and in vivo. J Biol Chem. 2005;280:42172–42180. doi: 10.1074/jbc.M507916200. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, et al. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410:490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 35.Liu L, et al. Essential roles of S-nitrosothiols in vascular homeostasis and endotoxic shock. Cell. 2004;116:617–628. doi: 10.1016/s0092-8674(04)00131-x. [DOI] [PubMed] [Google Scholar]
- 36.Lancaster JR., Jr Nitroxidative, nitrosative, and nitrative stress: Kinetic predictions of reactive nitrogen species chemistry under biological conditions. Chem Res Toxicol. 2006;19:1160–1174. doi: 10.1021/tx060061w. [DOI] [PubMed] [Google Scholar]
- 37.Robinson MA, Baumgardner JE, Good VP, Otto CM. Physiological and hypoxic O2 tensions rapidly regulate NO production by stimulated macrophages. Am J Physiol. 2008;294:C1079–C1087. doi: 10.1152/ajpcell.00469.2007. [DOI] [PubMed] [Google Scholar]
- 38.Espey MG, Miranda KM, Thomas DD, Wink DA. Distinction between nitrosating mechanisms within human cells and aqueous solution. J Biol Chem. 2001;276:30085–30091. doi: 10.1074/jbc.M101723200. [DOI] [PubMed] [Google Scholar]
- 39.Pantopoulos K, Weiss G, Hentze MW. Nitric oxide and oxidative stress (H2O2) control mammalian iron metabolism by different pathways. Mol Cell Biol. 1996;16:3781–3788. doi: 10.1128/mcb.16.7.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feelisch M, et al. Concomitant S-, N-, and heme-nitros(yl)ation in biological tissues and fluids: Implications for the fate of NO in vivo. FASEB J. 2002;16:1775–1785. doi: 10.1096/fj.02-0363com. [DOI] [PubMed] [Google Scholar]
- 41.Moellering DR, et al. Induction of glutathione synthesis by oxidized low-density lipoprotein and 1-palmitoyl-2-arachidonyl phosphatidylcholine: Protection against quinone-mediated oxidative stress. Biochem J. 2002;362:51–59. doi: 10.1042/0264-6021:3620051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 43.Zmijewski JW, et al. Oxidized LDL induces mitochondrially associated reactive oxygen/nitrogen species formation in endothelial cells. Am J Physiol. 2005;289:H852–H861. doi: 10.1152/ajpheart.00015.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.