Although it has long been recognized that steroid hormones primarily exert their effects on neuronal function through their ability to modulate gene transcription in the nucleus (1), an array of physiological and behavioral effects of glucocorticoids have been documented to occur in a fashion that cannot be explained by genomic regulation (2). These findings have prompted the hypothesis that glucocorticoids (in addition to all other major classes of steroids) possess membrane-associated receptors through which nongenomic signaling may evoke rapid effects on physiology and behavior (2, 3). Research from Miles Orchinik and Frank Moore (4) in the early 1990s clearly demonstrated the presence of glucocorticoid receptors in the neuronal membrane of an amphibian species that evoked observable effects on neuronal signaling and behavior, but progress in this field has been hampered by a lack of comparable discoveries in mammalian species. However, recent data in a study in this issue of PNAS (5) sheds new light on the rapid effects of glucocorticoids in mammals by revealing a putative nongenomic role of glucocorticoids in regulating emotional learning in rodents through a coordinated induction of endocannabinoid signaling.
The report by Campolongo et al. (5) focuses on the role of endocannabinoid signaling in the amygdala in the consolidation of emotionally-aversive memory (5). Previous research has convincingly demonstrated that the consolidation of aversive memories is facilitated by cross-talk between glucocorticoids and noradrenergic signaling in the basolateral nucleus of the amygdala (BLA; ref. 6). The current data add a new player to this game by demonstrating that intra-BLA administration of a cannabinoid CB1 receptor agonist or antagonist immediately after inhibitory avoidance training dose-dependently enhanced or impaired emotional memory consolidation, respectively (5). More interesting, however, was the fact that intra-BLA administration of a CB1 receptor antagonist, at a dose previously ineffective in modulating memory consolidation, was capable of abrogating the ability of systemically administered corticosterone to facilitate aversive memory consolidation (5). These data suggest that glucocorticoids recruit endocannabinoid signaling in the BLA to modulate aversive memory processes and in turn may shed new light on the rapid mechanism by which glucocorticoids modulate synaptic function in this circuit.
As mentioned, glucocorticoids are believed to modulate neurophysiology and behavior through both genomic and nongenomic pathways (1, 2). Although the ability of steroids to regulate gene transcription is a well-characterized process of direct binding of homodimers or heterodimers of glucocorticoid receptors to nuclear DNA, or through protein–protein interactions with transcription factors (1), the nongenomic mechanisms of glucocorticoid action are poorly understood (2). The studies by Orchinik and Moore (4) demonstrated that in the Taricha granulosa (a rough-skinned newt) glucocorticoid receptors were present in neuronal membranes and associated with G proteins to modulate intracellular signaling. In the newt, a clear bioassay was established in which glucocorticoids were found to dampen stimulus-induced neuronal activation of medullary neurons, which resulted in a reduction of courtship clasping behavior, all of which occurred in a time span of <10 min (4). These exciting findings stimulated similar research in rodents, the result of which was an array of mixed findings that were far less conclusive than what the newt studies produced (e.g., ref. 7). The search for the mammalian membrane-bound glucocorticoid receptor effectively came to a halt, until an elegant study was published from the laboratory of Jeffrey Tasker in 2003 (8). In that report, Tasker and colleagues (8) used in vitro electrophysiological recording of parvocellular neurons in the paraventricular nucleus of the hypothalamus of the rat. Using this model, they demonstrated that glucocorticoids rapidly suppressed glutamatergic release onto parvocellular neurons through a mechanism that involved postsynaptic activation of a membrane-bound glucocorticoid receptor. Activation of this receptor launched a G protein signaling cascade that induced synthesis of endocannabinoid ligands, which in turn traversed back across the synapse where they bound to presynaptic CB1 receptor localized on glutamatergic terminals and inhibited subsequent glutamate release. Thus, a clear-cut pathway was defined in which glucocorticoids elicited a nongenomic induction of endocannabinoids, which in turn was the workhorse for glucocorticoids to modulate local neuronal transmission. In full circle, Moore and coworkers (9) then took this model to their newt preparation and demonstrated that the ability of glucocorticoids to inhibit sensory-evoked stimulation of medullary neurons and courtship clasping was mediated by an endocannabinoid intermediary. Collectively, these studies revealed a novel mechanism of glucocorticoid activation of a membrane-bound G protein receptor, which induces endocannabinoid synthesis, diffuses to local neuronal populations, and inhibits neurotransmitter release.
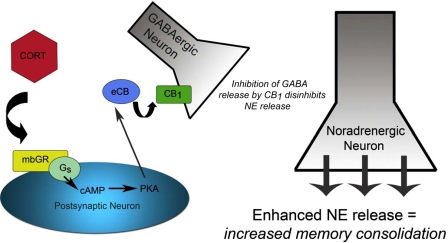
The current study by Campolongo et al. (5) adds to this model by demonstrating that glucocorticoids modulate emotional memory consolidation through an induction of endocannabinoid signaling in the BLA, providing the first in vivo evidence in mammals of the existence of this pathway. In addition, these data help us to understand the neuronal mechanisms by which glucocorticoids modulate emotional memory consolidation. Previous work from Roozendaal et al. (6) demonstrated that glucocorticoids interact with noradrenergic transmission in the BLA such that glucocorticoids evoke a rapid facilitation of the noradrenergic signal, which in turn is responsible for the enhancing effects on memory consolidation. Previously, these effects were attributed to a putative mechanism in which glucocorticoids altered the G protein signaling evoked by the β-adrenoreceptor in the BLA by amplifying β-adrenoreceptor induction of the cAMP/PKA cascade (10). Specifically, the facilitating effects of glucocorticoids on aversive memory consolidation were ablated by preadministration of a PKA inhibitor into the BLA (10). When viewed in light of the current data, however, a new model can be proposed (see Fig. 1). In this model, glucocorticoids bind to membrane-bound receptors that activate a G protein signaling cascade that induces endocannabinoid synthesis. The ensuing release of endocannabinoid ligands that follows could diffuse to local GABAergic terminals and inhibit GABAergic release onto noradrenergic terminals in the BLA, as proposed by Campolongo et al. (5). The end result of this process would be an enhancement of noradrenergic release into the BLA, and subsequently an enhancement of emotional memory consolidation. Given that Tasker's laboratory (11) has suggested that this membrane-bound glucocorticoid receptor is coupled to the Gs–cAMP–PKA pathway, the ability of PKA inhibitors to prevent the facilitating effects of glucocorticoids on memory consolidation may not reflect an interruption of the β-adrenoreceptor signaling cascade, but moreso may prevent the ability of the membrane-bound glucocorticoid receptor to induce endocannabinoid synthesis. In line with this model, the in vitro work of Tasker's laboratory (11) has demonstrated that inhibition of PKA can prevent the ability of glucocorticoids to induce endocannabinoid synthesis. Furthermore, this model is also supported by the presence of glucocorticoid receptors in the postsynaptic membranes of neurons in the lateral amygdala (12) and the fact that glucocorticoids have been found to rapidly impair GABAergic neurotransmission in the BLA (13).
Fig. 1.
Corticosterone (CORT) binds to a yet-uncharacterized membrane-bound glucocorticoid receptor (mbGR) that activates the Gs–cAMP/PKA pathway to induce endocannabinoid (eCB) synthesis. Endocannabinoids are released into the synapse where they bind to CB1 receptors on GABAergic terminals inhibiting GABA release. This inhibition of GABA release disinhibits norepinephrine (NE) release and increases NE activation of postsynaptic β-adrenoreceptors, increasing the consolidation of emotionally-aversive memories.
Taken together, these findings may provide a novel in vivo bioassay with which mammalian researchers can examine the rapid effects of glucocorticoids. Based on the current data, and the in vitro data from Tasker's laboratory (11), it can be assumed that glucocorticoid administration would result in a notable induction of endocannabinoid synthesis in the BLA. If this holds true, this response can be used as a reliable endpoint with which to map the pharmacology, and potentially, the molecular identity of the membrane-bound glucocorticoid receptor.
On a broader level, these data reiterate the growing notion of the endocannabinoid system as a rapid mediator of responses to stress and stress hormones (14). Endocannabinoid signaling is known to regulate a diversity of physiological functions such as hunger, emotion, energy balance, nociception, and motivated behaviors (14–16). All of these behaviors and processes are known to be rapidly modulated by stress, and consistently, endocannabinoid signaling has been found to contribute to stress-induced modulation of many of these processes (9, 16, 17). With the emergence of the study from Moore and coworkers (9) and the current report by Campolongo et al. (5), we can gain further understanding that these rapid responses may be evoked by a nongenomic glucocorticoid mechanism. It has been proposed by Mary Dallman (2) that the rapid effects of steroids may be ancient, primordial functions that are evolutionarily important to improve fitness under conditions of stress given their presence in nonprimate mammalian vertebrate and invertebrate species. Interestingly, endocannabinoid signaling appears to be an evolutionarily-conserved signaling mechanism that is present throughout evolutionary phylogeny (18). Potentially, glucocorticoid and endocannabinoid cross-talk has been occurring throughout evolution to regulate and modulate rapid responses to stress. Until now, glucocorticoids have taken the spotlight on these processes; perhaps it is time for endocannabinoids to step out of the shadows and take their place as a putative regulator of rapid physiological and behavioral responses to stress.
Footnotes
The authors declare no conflict of interest.
See companion article on page 4888.
References
- 1.de Kloet ER. Stress in the brain. Eur J Pharmacol. 2000;405:187–198. doi: 10.1016/s0014-2999(00)00552-5. [DOI] [PubMed] [Google Scholar]
- 2.Dallman MF. Fast glucocorticoid actions on brain: Back to the future. Front Neuroendocrinol. 2005;26:103–108. doi: 10.1016/j.yfrne.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 3.McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore FL, Orchinik M. Membrane receptors for corticosterone: A mechanism for rapid behavioral responses in an amphibian. Horm Behav. 1994;28:512–519. doi: 10.1006/hbeh.1994.1049. [DOI] [PubMed] [Google Scholar]
- 5.Campolongo P, et al. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation: Involvement of the glucocorticoid system. Proc Natl Acad Sci USA. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roozendaal B, et al. Glucocorticoids interact with emotion-induced noradrenergic activation in influencing different memory functions. Neuroscience. 2006;138:901–910. doi: 10.1016/j.neuroscience.2005.07.049. [DOI] [PubMed] [Google Scholar]
- 7.Orchinik M, et al. High-affinity binding of corticosterone to mammalian neuronal membranes: Possible role of corticosteroid binding globulin. J Steroid Biochem Mol Biol. 1997;60:229–236. doi: 10.1016/s0960-0760(96)00191-4. [DOI] [PubMed] [Google Scholar]
- 8.Di S, et al. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: A fast feedback mechanism. J Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coddington E, et al. Endocannabinoids mediate the effects of acute stress and corticosterone on sex behavior. Endocrinology. 2007;148:493–500. doi: 10.1210/en.2006-0740. [DOI] [PubMed] [Google Scholar]
- 10.Roozendaal B, et al. Glucocorticoids interact with the basolateral amygdala β-adrenoreceptor-cAMP/PKA system in influencing memory consolidation. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 11.Malcher-Lopes R, et al. Opposing cross-talk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci. 2006;26:6643–6650. doi: 10.1523/JNEUROSCI.5126-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson LR, et al. Localization of glucocorticoid receptors at postsynaptic membranes in the lateral amygdala. Neuroscience. 2005;136:289–299. doi: 10.1016/j.neuroscience.2005.06.050. [DOI] [PubMed] [Google Scholar]
- 13.Duvarci S, Pare D. Glucocorticoids enhance the excitability of principal basolateral amygdala neurons. J Neurosci. 2007;27:4482–4491. doi: 10.1523/JNEUROSCI.0680-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tasker JG. Rapid glucocorticoid actions in the hypothalamus as a mechanism of homeostatic integration. Obesity. 2006;14:259S–265S. doi: 10.1038/oby.2006.320. [DOI] [PubMed] [Google Scholar]
- 15.Moreira FA, Lutz B. The endocannabinoid system: Emotion, learning, and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 16.Hohman AG, et al. An endocannabinoid mechanism for stress-induced analgesia. Nature. 2005;435:1108–1112. doi: 10.1038/nature03658. [DOI] [PubMed] [Google Scholar]
- 17.Hill MN, et al. Endocannabinoids modulate stress-induced suppression of hippocampal cell proliferation and activation of defensive behaviors. Eur J Neurosci. 2006;24:1845–1849. doi: 10.1111/j.1460-9568.2006.05061.x. [DOI] [PubMed] [Google Scholar]
- 18.McPartland JM, et al. Evolutionary origins of the endocannabinoid system. Gene. 2006;370:64–74. doi: 10.1016/j.gene.2005.11.004. [DOI] [PubMed] [Google Scholar]