Abstract
The inhibitory Fc-γ receptor FcγRIIB, expressed on myeloid and B cells, has a critical role in the balance of tolerance and autoimmunity, and is required for the antiinflammatory activity of intravenous Ig (IVIG) in various murine disease models. However, the function of FcγRIIB and its regulation by IVIG in human autoimmune diseases are less well understood. Chronic inflammatory demyelinating polyneuropathy (CIDP) is the most common treatable acquired chronic polyneuropathy, and IVIG is widely used as a first-line initial and maintenance treatment. We found that untreated patients with CIDP, compared with demographically matched healthy controls, showed consistently lower FcγRIIB expression levels on naive B cells, and failed to up-regulate or to maintain up-regulation of FcγRIIB as B cells progressed from the naive to the memory compartment. Concomitantly, the rare −386C/−120A FcγRIIB promoter polymorphism resulting in reduced promoter activity previously associated with autoimmune phenotypes was overrepresented in CIDP. Also, FcγRIIB protein expression was up-regulated on monocytes and B cells after clinically effective IVIG therapy. Thus, our results suggest that the inhibitory FcγRIIB is impaired at a critical B cell differentiation checkpoint in CIDP, and that modulating FcγRIIB expression might be a promising approach to efficiently limit antibody-mediated immunopathology in CIDP.
Keywords: autoimmunity, human, immunology, Fc receptor, CIDP
Chronic inflammatory demyelinating polyneuropathy (CIDP) is a common, although underdiagnosed, disease of the peripheral nervous system with an estimated prevalence of ≈0.5 per 100,000 children and 2 to 7 per 100,000 adults (1, 2). The clinical presentation is heterogeneous, but the most common form causes symmetrical progressive or relapsing weakness affecting proximal and distal muscles (3). Humoral immune responses are thought to have a crucial role in mediating peripheral nerve damage and represent important pharmacological targets in CIDP (2, 4). Sera and IgG antibodies from CIDP patients induce peripheral demyelination in host animals (5), can increase the permeability of the blood–nerve barrier, and impair nerve conduction in various models of peripheral neuropathies (4). Removal of humoral immune mediators by plasma exchange therapy as well as intravenous Ig (IVIG) are considered first-line treatments in patients with CIDP (6, 7).
IgG-mediated effector functions require the interaction of the Fc fragment of antibodies with their cognate cellular Fc-γ receptors (FcγR) (8). Most hematopoietic cells express both activating and inhibitory FcγR; thus, the in vivo activity of an IgG antibody results from the net effect of engaging both classes of receptors. Of the 3 classes of FcγR expressed in humans, FcγRI (CD64), FcγRII (CD32), and FcγRIII (CD16), the type II FcγRIIB (CD32B) is the only inhibitory FcγR. FcγRIIB is expressed on the cell surface of circulating B cells, on monocytes, neutrophils, as well as myeloid and plasmacytoid dendritic cells (DCs) (9). In B cells, FcγRIIB transduces an inhibitory signal upon colligation with the B cell receptor, thereby preventing B cells with low affinity or self-reactive receptors from entering the germinal center and becoming IgG positive plasma cells (8). Mice lacking FcγRIIB expression spontaneously develop autoimmune disease (10), and restoration of decreased FcγRIIB expression on activated B cells in autoimmune-susceptible mice restores immunological tolerance (8). Autoimmune prone mouse strains such as BXSB, NOD, and NZM carry a promoter polymorphism in the FcγRIIB gene, which results in decreased protein expression (11), and decreased FcγRIIB expression or nonfunctional FcγRIIB variants have been shown to be associated with the development and severity of systemic lupus erythematosus (SLE) in several human populations (8, 12). Also, this inhibitory receptor is required for antiinflammatory activity of IVIG, because disruption of this protein by genetic deletion or via blocking antibodies reverses the therapeutic effects of IVIG in various autoimmune animal models (13–16). Here, we investigated the expression profile of the inhibitory FcγRIIB on peripheral blood monocytes and B cells, its regulation after IVIG therapy, and the presence of FcγRIIB promoter polymorphisms and allelic variants, as a possible pathomechanism in patients with CIDP.
Results
Selective Dysregulation of FcγRIIB Expression on B Cells in CIDP.
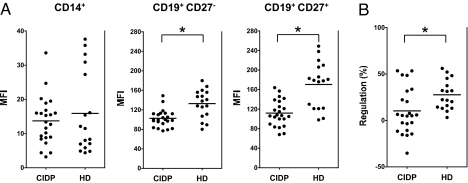
Expression of the inhibitory FcγIIB receptor was determined on circulating monocytes and B cells in untreated patients with CIDP and demographically matched healthy blood donors (Table 1). Both patients and controls were of Caucasian descent. Because previous studies showed that FcγRIIB expression changes with B cell maturation (12), CD19+CD27− naive and CD19+CD27+ memory B cells and plasma cells were analyzed separately. No statistically significant differences were detectable in either the frequencies of monocytes (mean percentage of PBMC ± SEM: 7.5 ± 1.5 for CIDP and 5.8 ± 1.0 for HD) or B cells (mean percentage of PBMC ± SEM: 9.0 ± 1.5 for CIDP and 8.5 ± 1.1 for HD) in the peripheral blood or in the distribution of CD27− (mean percentage of PBMC ± SEM: 85 ± 1.9 for CIDP and 79 ± 3.3 for HD) and CD27+ (mean percentage of PBMC ± SEM: 14 ± 1.8 for CIDP and 19 ± 3.3 for HD) B lymphocytes between patients and healthy volunteers. As seen in Fig. 1A, the level of FcγRIIB, as reflected by the mean fluorescence intensity (MFI) staining, was equivalent on CD14+ monocytes in both groups (MFI ± SEM 13.7 ± 1.5 for CIDP and 15.9 ± 2.9 for HD, P = 0.8). In contrast, naive and memory B cells from CIDP patients showed significantly lower surface expression of FcγRIIB compared with normal controls (naive B cells MFI ± SEM 102.4 ± 3.7 for CIDP and 132.7 ± 7.0 for HD, P = 0.002; memory B cells per plasma cells MFI ± SEM: 111.8 ± 5.5 for CIDP and 170.5 ± 11.5 for HD, P = 0.0002).
Table 1.
Demographic and clinical characteristics of CIDP patients and controls
| CIDP n = 23 | Controls n = 26 | |
|---|---|---|
| Age | 56.4 ± 8.6 | 42.1 ± 8.6 |
| (Years ± SD; range) | (41↔73) | (41↔65) |
| Duration of symptoms | 1.0 ± 1.4 | N/A |
| (Years ± SD; range) | (0↔6) | — |
| Male to female ratio | 2.3 | 2.5 |
| Fulfilling modified AAN criteria, % | 23, 100 | N/A |
| Fulfilling EFNS/PNS criteria, % | 23, 100 | N/A |
| Clinical course* | — | N/A |
| RR, n; %* | 16, 70 | — |
| PP, n; %† | 3, 13 | — |
| Monophasic, n; % | 4, 17 | — |
| CIDP subtype‡ | — | N/A |
| CIDP, n; % | 15, 66 | — |
| CIDP-MGUS, n; % | 1, 4 | — |
| DADS, n; % | 3, 13 | — |
| MADSAM, n; % | 4, 17 | — |
| Treatment response, n; %§ | 21, 91.3 | N/A |
N/A, not applicable.
*Relapsing-remitting.
†Primary-progressive.
‡MGUS, monoclonal gammopathy of uncertain significance; DADS, distal acquired demyelinating sensory polyneuropathy; MADSAM, multifocal acquired demyelinating sensori-motor polyneuropathy.
§All 23 patients received immunosuppressive or immunomodulatoring treatment, i.e., IVIGs, corticosteroids, azathioprine, and/or mycophenolate mofetil after sample collection for FcγRIIB expression analysis.
Fig. 1.
Decreased level of FcγRIIB expression on B cells in CIDP. PBMCs were stained with monoclonal antibodies specific for CD14, CD19, and CD27, and FcγRIIB compared with an isotype matched control antibody. (A) Shown are MFI of FcγRIIB expression levels on monocytes (CD14+), naive B cells (CD19+CD27−), and memory B cells (CD19+CD27+) in 23 untreated patients with CIDP and 17 age-matched healthy blood donors after subtracting the MFI from the control antibody. Horizontal lines indicate mean expression values within 1 group. The asterisk indicates a significant difference between 2 groups. There is no difference in FcγRIIB expression on monocytes, but there is decreased expression of FcγRIIB on both naive (P < 0.002; Mann–Whitney U test) and memory (P < 0.0002; Mann–Whitney U test) B cell compartments in untreated patients with CIDP. (B) Shown are percentage changes in FcγRIIB expression in memory compared with naive B cells in patients with CIDP. In contrast to patients with CIDP, healthy donors show an increase of FcγRIIB expression as B cells progressed from the naive to the memory compartment (P < 0.02; Mann–Whitney U test).
The reduction in FcγRIIB expression was stronger in the CD19+CD27+ memory compared with CD19+CD27− naive B cell compartment due to a failure of CIDP patients to up-regulate or to maintain up-regulation of FcγRIIB as B cells become memory cells or as a consequence of deregulated apoptotic elimination of FcγRIIBlow vs. FcγRIIBhigh cells in CIDP. Whereas all healthy controls showed significantly increased levels of FcγRIIB on memory compared with naive B cells (P < 0.02), only 14 out of 23 CIDP patients showed higher FcγRIIB expression levels on memory B cells (Fig. 1B) with no statistically significant differences between both B cell compartments. Thus, although the basal level of FcγRIIB expression is unchanged on myeloid cells, naive and memory B cells show decreased expression levels in untreated patients with CIDP.
Induction of FcγRIIB Expression in Memory B Cells in CIDP Patients Responding to IVIG Therapy.
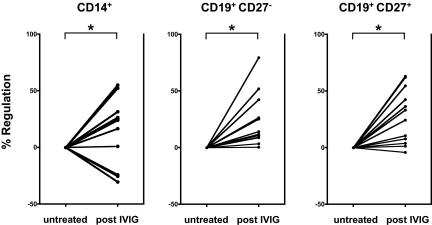
IVIG is widely used as a first-line initial and maintenance treatment for CIDP. Several prospective placebo-controlled clinical trials consistently demonstrated that administration of IVIG improves neurologic disability (17–19), and provides long-term benefits to patients with CIDP (7). IVIG is thought to act through several pathways, including complement inactivation and neutralization of idiotypic antibodies (6). Studies in various mouse autoimmune models provided solid evidence that the antiinflammatory activity of IVIG crucially depends on the presence and up-regulation of FcγRIIB (14, 17, 19). Therefore, we determined FcγRIIB expression levels on circulating monocytes and B cells in treatment-naive CIDP patients before and 2–3 weeks after IVIG administration (2-g/kg body weight over 5 days). All patients responded to IVIG treatment as defined by an improvement of disability within 4 weeks after IVIG therapy (2, 20). Compared with baseline levels, IVIG led to a significant up-regulation of FcγRIIB expression on naive B cells in 12/12 patients (P < 0.0001), and on memory B cells in 11/12 patients (P < 0.0001) (Fig. 2). FcγRIIB expression levels were also induced on monocytes in 9/12 patients (P < 0.03) after IVIG therapy. These data indicate that the impaired expression of the inhibitory FcγRIIB in CIDP can, at least partially, be restored by clinically effective IVIG treatment.
Fig. 2.
Up-regulation of FcγRIIB expression in CIDP patients responding to IVIG treatment. FcγRIIB expression was determined in samples taken before and 1–3 weeks after IVIG therapy (2 g/kg body weight) in 12 previously untreated patients with CIDP. Shown are IVIG-induced changes in FcγRIIB expression compared with baseline levels. Clinically effective IVIG therapy led to significant changes in FcγRIIB expression levels in monocytes (P < 0.03; Mann Whitney U test), naive B cells (P < 0.0001; Mann Whitney U test), and memory B cells (P < 0.0001; Mann Whitney U test) in patients with CIDP. Dots represent expression values before and after treatment connected by lines for individual patients, the asterisk indicates a significant difference.
Increased Frequency of the −386C/−120A FcγRIIB Promotor Variant in CIDP.
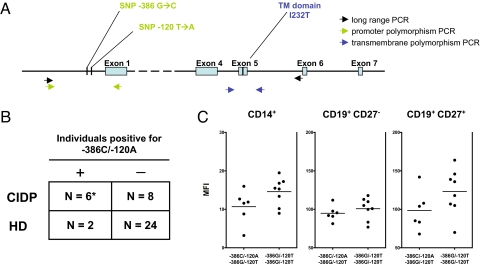
To gain an insight into the possible mechanism of dysregulated FcγRIIB expression, we next investigated whether CIDP patients show increased frequencies of functionally relevant SNPs in the FcγRIIB promoter that have previously been associated with autoimmune phenotypes, i.e., SLE (9, 21–23). These polymorphisms are located at −386 or −120 base pairs upstream of the first exon of FcγRIIB and form 2 distinct haplotypes (Fig. 3A). The majority of the Caucasian healthy population (> 90%) carries a guanine residue at position −386 (−386G) and a thymidine residue at position −120 (−120T), whereas <10% carry the −386C/−120A variant (21). However, the latter haplotype is overrepresented in Caucasian patients with SLE (14.4% in SLE patients vs. 9.4% in controls according to ref. 21), and SLE patients homozygous for this allelic variant show reduced FcγRIIB surface expression levels on activated B cells (23). An additional nonsynonymous polymorphism in the transmembrane domain of FcγRIIB, in which an isoleucine residue in the transmembrane domain is replaced by a threonine residue (I232T), is also enriched in SLE patients from Asian populations (24), and might also be overrepresented in Caucasian patients with SLE.
Fig. 3.
Increased frequency of the −386C/−120A FcγRIIB promotor polymorphism in CIDP. (A) Schematic representation of the FcγRIIB gene and promoter region including the primer sites used for determination of the SNPs in the FcγRIIB promoter region and in the region encoding the transmembrane domain. (B) SNPs were determined in 14 patients with CIDP and 26 healthy controls. None of the patients and <5% of healthy controls (1/26) tested for FcγRIIB promoter SNPs were homozygous for the rare −386C/−120A haplotype. In contrast, 43% of CIDP patients (6/14), but <5% (1/26) of the healthy controls were heterozygous for the −386C/−120A variant (*, P < 0.02 for comparing −386C/−120A frequencies between patients and controls; Fisher's exact test). (C) FcγRIIB surface expression tended to be lower in patients heterozygous for the −386C/−120A variant (n = 6), but the overall difference was not statistically significant compared with patients homozygous for the 386G/−120T haplotype (n = 8). Horizontal lines represent mean expression values.
We found that none of the patients and <5% of healthy controls (1/26) tested for FcγRIIB promotor SNPs were homozygous for the rare −386C/−120A haplotype. In contrast, 43% of CIDP patients (6/14), but, again, <5% of the healthy controls (1/26) were heterozygous for the −386C/−120A variant (Fig. 3B) (P < 0.02 for comparing −386C/−120A frequencies between patients and controls). FcγRIIB surface expression tended to be lower in patients carrying this promotor variant, although the overall difference was not statistically significant compared with patients homozygous for the 386G/−120T haplotype (Fig. 3C). However, the latter result is based on a limited number of patients and clearly requires further investigation in larger cohorts of patients carrying this polymorphism. We did not detect any differences in frequencies of homozygous or heterozygous I232T carriers between patients (3/14) and controls (4/26). Due to the small sample size of our study and the rarity of this polymorphism, further studies will be necessary to investigate a possible disease association of this allele. Altogether, these data suggest that heterozygous −386C/−120A carriers are overrepresented in patients with CIDP.
Discussion
Our study provides evidence for a selective dysregulation of the inhibitory FcγRIIB on B cells in CIDP, the most common treatable acquired chronic polyneuropathy. Untreated patients with CIDP show lower FcγRIIB expression levels on naive B cells, and failed to up-regulate or to maintain FcγRIIB as B cells progressed from the naive to the memory compartment. Moreover, functionally relevant FcγRIIB promotor polymorphisms that were previously associated with the development or severity of SLE and lead to a decreased expression of this receptor were enriched in CIDP patients (9, 12). Moreover, we found that clinically effective IVIG treatment induces FcγRIIB expression. These data suggest that FcγRIIB may play a pivotal role in the pathogenesis of CIDP.
By using either ubiquitous or B cell specific overexpression of the inhibitory FcγRIIB, it was demonstrated that increasing the threshold for B cell activation is sufficient to ameliorate autoimmune disease in SLE prone mouse strains, such as NZM and BXSB, and in induced models of autoimmune disease such as collagen induced arthritis (CIA) (25, 26). Also, it was suggested that the antiinflammatory activity of IVIG essentially depended on the presence or up-regulation of the inhibitory FcγRIIB. Animals deficient in FcγRIIB are no longer protected by IVIG in models of immune thrombocytopenic purpura, rheumatoid arthritis, and nephrotoxic nephritis (13–16). In addition, IVIG administration resulted in an up-regulation of FcγRIIB surface expression on effector macrophages or the enhanced recruitment of FcγRIIB positive myeloid cells at the site of inflammation in vivo (13–15). Consistent with these observations, FcγRIIB was up-regulated on circulating B cells and monocytes in patients responding to IVIG treatment, indicating that IVIG might also work by increasing the FcγRIIB expression level in humans. These data suggest that FcγRIIB expression mediates immunomodulatory effects of IVIG by raising the activation threshold for B lineage and myeloid cells. Within the inflamed peripheral nerve, recruitment of FcγRIIB expressing monocytes and induction of FcγRIIB expression in resident myeloid cells might additionally contribute to the beneficial effects of IVIG, because macrophages are main local effector cells in CIDP (4, 27, 28). These data provide evidence that the observations obtained in murine model systems with respect to the mechanism of IVIG activity in vivo might be transferable to humans.
In addition to our protein expression analysis, we could show that a certain FcγRIIB promoter haplotype, for which an association with the development and severity of SLE in humans has been previously reported (21–23), was significantly enriched in CIDP patients. The −386 GC haplotype (sometimes also referred to as the 343 haplotype; see refs. 22, 23) shows a particular strong association with human SLE. Recent studies suggest that this polymorphism leads to the displacement of activating transcription factors, such as AP1, by other transcriptional regulators; thus, offering a potential explanation for the lower expression level of FcγRIIB (22). Although we did not find homozygous CIDP patients for this SNP, one functionally impaired allele of FcγRIIB might be sufficient to result in a decreased expression level or functionally impaired regulation of expression during B cell development. However, not all patients carried this promoter variant, suggesting that other additional factors might be involved in the deregulated expression of the inhibitory FcγRIIB. Also, given the low allelic frequencies of FcγRIIB polymorphisms, many patients and controls need to be analyzed to allow definite conclusions on frequencies of FcγRIIB allelic variants such as CA promoter polymorphism and their impact for FcγRIIB expression levels and B cell function in CIDP.
In conclusion, we identified a deficiency in CIDP patients in the expression of a specific inhibitory receptor known to have a role in peripheral tolerance in antigen-activated B cells. Because IVIG, which up-regulates and acts through FcγRIIB expression, is an effective first-line initial and maintenance treatment for this autoimmune disease, previously undescribed strategies specifically targeting FcγRIIB (29) might have therapeutic merit in CIDP.
Materials and Methods
Patients and Healthy Blood Donors.
Twenty-three untreated patients with CIDP fulfilling both the modified AAN and the EFNS/PNS diagnostic guidelines (2, 3, 30), and 26 demographically matched healthy blood donors were included in this study (Table 1). FcγRIIB expression on circulating blood cells was determined in all patients and compared with 17 matched controls; 12 patients were followed longitudinally to investigate FcγRIIB expression expression levels before and 2–3 weeks after IVIG treatment (2-g/kg body weight). All of these patients showed a clinical response to IVIG as defined by an improvement of disability, as assessed by the modified Rankin disability scale (2, 20), within 4 weeks after IVIG treatment. FcγRIIB genotyping was performed in 14 patients and 26 healthy controls. Patients and healthy blood donors were recruited from the Department of Neurology at the Phillips University of Marburg. The study was approved by the local Institutional Review Board, and all subjects provided informed consent.
Antibodies and Flow Cytometry.
The mAb 2B6 that selectively recognizes the inhibitory FcγRIIB as shown in prior studies by ELISA, surface plasmon resonance, and FACS staining of cell lines and transfectants (12) was coupled to Alexa Fluor 647. An IgG1 isotype control-APC antibody (clone MOPC-21) as well as CD14-Pacific Blue (clone M5E2), CD19-FITC (clone HIB19), CD27-PE (clone M-T271), and CD138-PerCPCy5.5 (clone MI15) antibodies were purchased from BD Bioscience. Paired prae-IVIG and post-IVIG samples from patients with CIDP were analyzed at a later time point and on a different LSR II flow cytometer than paired samples form patients and healthy donors. To display the data in a more comparable format, we analyzed and display relative changes in FcγRIIB expression in memory compared with naive B cells (Fig. 1B), and after IVIG treatment compared with baseline levels (Fig. 2) instead of MFI values. Peripheral blood mononuclear cells (PBMCs) were purified from whole blood by density gradient centrifugation by using Ficoll-Hypaque. 2 × 106 PBMCs were incubated with the indicated mAbs for 45 min on ice. Cells were washed twice with PBS and resuspended in 200 μL FACS buffer (0.01% sodium azide in PBS) before FACS analysis. The PBMC samples were analyzed on an LSR II flow cytometer gating on PBMC excluding cell duplets based on size and monocytes and B cells on being CD14+ or CD19+ cells, and within the B cell population on being CD27− and CD27+ populations. FcγRIIb was expressed on CD19+ CD138+ plasma cells, but the low frequency of CD138+ cells (< 1% of circulating B cells) precluded a thorough evaluation of FcγRIIb expression levels on plasma cells. Cell duplets were excluded based on size. Gating and calculations for precursor frequencies were performed with FlowJo (Tree Star) software.
FcγRIIB Genotyping.
Due to the high sequence homology between FcγRIIB, FcγRIIA, and FcγRIIC, we used a 2 step PCR protocol to specifically amplify FcγRIIB as described before (21). Briefly, a long-range PCR was performed initially with a set of FcγRIIB specific primers and by using the Qiagen LongRange PCR Kit. The amplified 15-kb PCR product was gel-purified (Qiagen Gel purification Kit) and used as template for the nested PCR to amplify the promoter region with primers as published before. Last, the 2-kb PCR product was sequenced to determine any polymorphisms in the promoter sequence. To analyze the allelic variant with the amino acid exchange in the transmembrane domain (I232T variant), the nested PCR was performed with a set of primers that amplified this region. The sense (5′-cctgcctgctcacaaatgta-3′) and antisense primers (5′-cactgctctccccaagac-3′) were chosen to flank the polymorphism in intron 5. As before, the resulting 750-bp PCR product was gel purified and sequenced.
Statistics.
Statistical analyses were performed by using commercial software (PRISM 4, GraphPad). FcγRIIB expression levels and frequencies of circulating monocytes and B cells in MS patients and healthy donors were compared by using the nonparametric Mann–Whitney U rank sum test. FcγRIIB haplotype frequencies were compared by using Fisher's exact test.
Acknowledgments.
We thank our patients for their continuous cooperation, Dr. Christian Münz (Laboratory of Viral Immunobiology, The Rockefeller University) for critically reviewing this manuscript, and Petra Breiden (Institute for Neuroimmunology and Clinical Multiple Sclerosis Research, University Medical Center Eppendorf, Hamburg) for excellent technical assistance. The B26 antibody was generously provided by Macrogenics, Inc (Rockville, MD). I.J. is supported by the Deutsche Forschungsgemeinschaft (JE 530/1-1). The Institute for Neuroimmunology and Clinical Multiple Sclerosis Research is supported by the Gemeinnützige Hertie Stiftung. F.N. is supported by the Deutsche Forschungsgemeinschaft and the Bavarian Genome Research Network (BayGene). J.D.L. is a recipient of the Dana Foundation and Irvington Institute's Human Immunology fellowship provided by the Cancer Research Institute.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Koller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. N Engl J Med. 2005;352:1343–1356. doi: 10.1056/NEJMra041347. [DOI] [PubMed] [Google Scholar]
- 2.Tackenberg B, et al. Classifications and treatment responses in chronic immune-mediated demyelinating polyneuropathy. Neurology. 2007;68:1622–1629. doi: 10.1212/01.wnl.0000260972.07422.ea. [DOI] [PubMed] [Google Scholar]
- 3.Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle Nerve. 2001;24:311–324. doi: 10.1002/1097-4598(200103)24:3<311::aid-mus1001>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 4.Meyer zu Horste G, Hartung HP, Kieseier BC. From bench to bedside—experimental rationale for immune-specific therapies in the inflamed peripheral nerve. Nat Clin Pract Neurol. 2007;3:198–211. doi: 10.1038/ncpneuro0452. [DOI] [PubMed] [Google Scholar]
- 5.Yan WX, Taylor J, Andrias-Kauba S, Pollard JD. Passive transfer of demyelination by serum or IgG from chronic inflammatory demyelinating polyneuropathy patients. Ann Neurol. 2000;47:765–775. [PubMed] [Google Scholar]
- 6.Dalakas MC. Mechanisms of action of IVIg and therapeutic considerations in the treatment of acute and chronic demyelinating neuropathies. Neurology. 2002;59:S13–S21. doi: 10.1212/wnl.59.12_suppl_6.s13. [DOI] [PubMed] [Google Scholar]
- 7.Hughes RA, et al. Intravenous immune globulin (10% caprylate-chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE study): A randomised placebo-controlled trial. Lancet Neurol. 2008;7:136–144. doi: 10.1016/S1474-4422(07)70329-0. [DOI] [PubMed] [Google Scholar]
- 8.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 9.Su K, et al. Expression profile of FcgammaRIIb on leukocytes and its dysregulation in systemic lupus erythematosus. J Immunol. 2007;178:3272–3280. doi: 10.4049/jimmunol.178.5.3272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bolland S, Ravetch JV. Spontaneous autoimmune disease in Fc(gamma)RIIB-deficient mice results from strain-specific epistasis. Immunity. 2000;13:277–285. doi: 10.1016/s1074-7613(00)00027-3. [DOI] [PubMed] [Google Scholar]
- 11.Pritchard NR, et al. Autoimmune-prone mice share a promoter haplotype associated with reduced expression and function of the Fc receptor FcgammaRII. Curr Biol. 2000;10:227–230. doi: 10.1016/s0960-9822(00)00344-4. [DOI] [PubMed] [Google Scholar]
- 12.Mackay M, et al. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 14.Bruhns P, Samuelsson A, Pollard JW, Ravetch JV. Colony-stimulating factor-1-dependent macrophages are responsible for IVIG protection in antibody-induced autoimmune disease. Immunity. 2003;18:573–581. doi: 10.1016/s1074-7613(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–797. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko Y, Nimmerjahn F, Ravetch JV. Anti-inflammatory activity of immunoglobulin G resulting from Fc sialylation. Science. 2006;313:670–673. doi: 10.1126/science.1129594. [DOI] [PubMed] [Google Scholar]
- 17.Vermeulen M, et al. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: A double blind, placebo controlled study. J Neurol Neurosurg Psychiatry. 1993;56:36–39. doi: 10.1136/jnnp.56.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double-blind, placebo-controlled, cross-over study. Brain. 1996;119:1067–1077. doi: 10.1093/brain/119.4.1067. [DOI] [PubMed] [Google Scholar]
- 19.Mendell JR, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology. 2001;56:445–449. doi: 10.1212/wnl.56.4.445. [DOI] [PubMed] [Google Scholar]
- 20.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 21.Su K, et al. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. I. Regulatory FCGR2B polymorphisms and their association with systemic lupus erythematosus. J Immunol. 2004;172:7186–7191. doi: 10.4049/jimmunol.172.11.7186. [DOI] [PubMed] [Google Scholar]
- 22.Olferiev M, Masuda E, Tanaka S, Blank MC, Pricop L. The role of activating protein 1 in the transcriptional regulation of the human FCGR2B promoter mediated by the −343 G -> C polymorphism associated with systemic lupus erythematosus. J Biol Chem. 2007;282:1738–1746. doi: 10.1074/jbc.M605808200. [DOI] [PubMed] [Google Scholar]
- 23.Blank MC, et al. Decreased transcription of the human FCGR2B gene mediated by the −343 G/C promoter polymorphism and association with systemic lupus erythematosus. Hum Genet. 2005;117:220–227. doi: 10.1007/s00439-005-1302-3. [DOI] [PubMed] [Google Scholar]
- 24.Kyogoku C, et al. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: Contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 25.Brownlie RJ, et al. Distinct cell-specific control of autoimmunity and infection by FcgammaRIIb. J Exp Med. 2008;205:883–895. doi: 10.1084/jem.20072565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGaha TL, Sorrentino B, Ravetch JV. Restoration of tolerance in lupus by targeted inhibitory receptor expression. Science. 2005;307:590–593. doi: 10.1126/science.1105160. [DOI] [PubMed] [Google Scholar]
- 27.Hafer-Macko C-E, et al. Immune attack on the Schwann cell surface in acute inflammatory demyelinating polyneuropathy. Ann Neurol. 1996;39:625–635. doi: 10.1002/ana.410390512. [DOI] [PubMed] [Google Scholar]
- 28.Bonetti B, et al. Human peripheral nerve macrophages in normal and pathological conditions. J Neurol Sci. 1993;118:158–168. doi: 10.1016/0022-510x(93)90105-8. [DOI] [PubMed] [Google Scholar]
- 29.Anthony RM, et al. Recapitulation of IVIG anti-inflammatory activity with a recombinant IgG Fc. Science. 2008;320:373–376. doi: 10.1126/science.1154315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hughes RA, et al. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2006;13:326–332. doi: 10.1111/j.1468-1331.2006.01278.x. [DOI] [PubMed] [Google Scholar]