Abstract
For plants, the tradeoff between resource investment in defense and increased growth to out-compete neighbors creates an allocation dilemma. How plants resolve this dilemma, at the mechanistic level, is unclear. We found that Arabidopsis plants produced an attenuated defense phenotype under conditions of crowding and when exposed to far-red (FR) radiation, a light signal that plants use to detect the proximity of neighbors via the photoreceptor phytochrome. This phenotype was detectable through standard bioassays that measured the growth of Spodoptera frugiperda caterpillars. Two possible explanations for the effect of FR are: (i) a simple by-product of the diversion of resources to competition, and (ii) a specific effect of phytochrome on defense signaling. The first possibility was ruled out by the fact that the auxin-deficient sav3 mutant, which fails to induce growth responses to FR, still responded to FR with an attenuated defense phenotype. In support of the second hypothesis, we found that phytochrome inactivation by FR caused a strong reduction of plant sensitivity to jasmonates, which are key regulators of plant immunity. The effects of FR on jasmonate sensitivity were restricted to certain elements of the pathway. Supporting the idea that the FR effects on jasmonate signaling are functionally significant, we found that FR failed to increase tissue quality in jar1, a mutant impaired in jasmonate response. We conclude that the plant modulates its investment in defense as a function of the perceived risk of competition, and that this modulation is effected by phytochrome via selective desensitization to jasmonates.
Keywords: far-red, herbivory, shade-avoidance syndrome, Arabidopsis, insect
Adaptive physiological responses to competition and insect herbivory are initiated and finely tuned by a number of information-acquiring systems that monitor environmental and internal signals and implement functional “decisions” that increase plant fitness (1–3). Light, perceived by the phytochromes, is the key warning signal used by plants to detect the proximity of competitors. Phytochromes can sense the reduction in the red to far-red (R:FR) ratio of sunlight caused by the proximity of chlorophyll-containing tissues (4). Low R:FR ratios result in a depletion of the active form of phytochrome (Pfr) (5), which unleashes the expression of the shade-avoidance syndrome (SAS) (5, 6). The SAS is characterized by rapid elongation of stems and petioles, increased leaf angles, and reduced branching, all benefiting the plants by increasing their ability to compete for light (7, 8). Chemical elicitors in the oral secretions of the herbivores and mechanical damage to tissue are used by the plant to sense an imminent risk of defoliation (9). These herbivory cues engage various signaling pathways and lead to increased production of defense-related hormones, including a group of potent lipid regulators, the jasmonates (10, 11). Upon perception of these hormonal signals, the plant cell responds with a massive reprogramming of gene expression (12, 13), and activates an array of defense mechanisms (induced defenses). Chemical defenses typically include a wide variety of secondary metabolites and defense-related proteins (14, 15).
Plastic responses entail opportunity costs: resource allocation to competition can limit investment in defense, thereby increasing vulnerability to herbivores; and allocation to defense can reduce competitive ability against neighboring plants (16–18). This allocation compromise between growth and defense is known as the “dilemma” of plants (19). Previous studies indicated that plants that are induced to express the SAS by physiological (18, 20) or genetic (18, 21) means display an attenuated defense phenotype, and demonstrated that competition signals (FR radiation) can elicit a down-regulation of plant defenses (18). Thus, when light signals herald a period of intense competition, the plant's allocation priorities appear to be placed on maintaining resource capture in the presence of competitors, rather than on reducing resource losses caused by herbivore attack. The mechanistic basis of this critical strategic decision has not been elucidated. We studied the functional connections between phytochrome and defense signaling using the reference plant Arabidopsis thaliana.
Results and Discussion
SAS Expression Is Associated with Increased Plant Quality for Insect Herbivores in Arabidopsis.
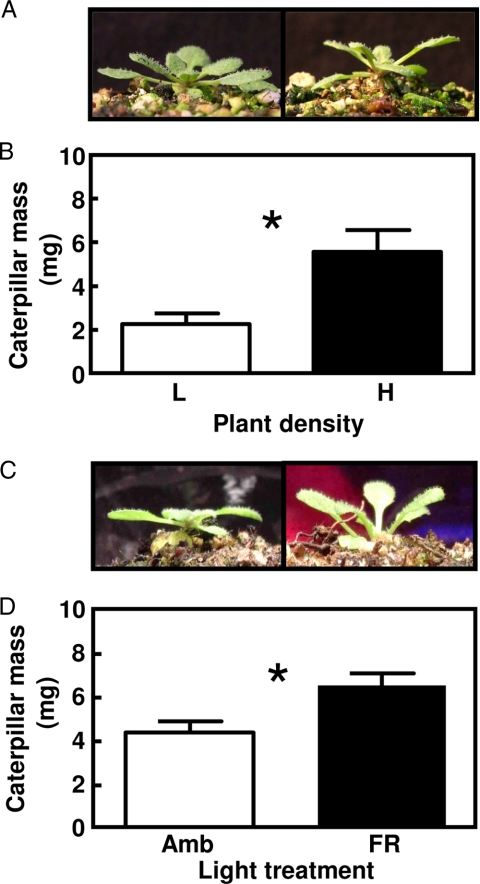
Using established protocols to manipulate neighbor proximity and R:FR ratio, and a standard bioassay based on the growth of Spodoptera frugiperda caterpillars to evaluate tissue quality, we found that: (i) crowding induced Arabidopsis plants to express the expected SAS phenotype [Fig. 1A, and supporting information (SI) Fig. S1], and simultaneously resulted in the production of leaf tissue that was of better quality for herbivores (Fig. 1B); (ii) these effects of crowding did not require competition for resources between roots of neighboring plants; and (iii) these effects of crowding could be fully mimicked by exposing the plants to FR radiation, without exposing the plants to actual competitors (Fig. 1 C and D). The effects of crowding and FR on tissue quality are likely to be at least partially mediated by a depletion in the Pfr levels of phytochrome B, although the residual tissue quality responses seen in the phyB mutants clearly indicate that other phytochromes play a role (see Figs. S1 and S2). We conclude that competition signals can increase Arabidopsis quality to insect herbivores even in the absence of competition.
Fig. 1.
Crowding and FR radiation trigger the SAS and improve plant tissue quality for herbivores. (A) Effects of crowding on plant morphology. (B) Effect of crowding on tissue quality for S. frugiperda caterpillars. Plants were grown in individual pots to prevent below-ground competition and were maintained for 5 weeks at the indicated density before the start of the feeding bioassay. L = 288 plants m−2; H = 615 plants m−2. (C) Effects of FR supplementation on plant morphology. (D) Effect of FR supplementation on tissue quality. Plants were exposed for 4 days to the indicated light treatments before the start of the feeding bioassay. Amb, ambient light; FR, FR added to ambient light to simulate the effect of neighbor proximity in a canopy of low-leaf area index. In all panels, thin bars indicate 1 SEM. * indicates P < 0.05 (n = 4 replicate trays per density level in the competition experiment or 15 individual plant replicates per treatment in the FR supplementation experiment). For the full data set for WT (Col) and phyB plants, see Figs. S1 and S2.
The Effects of FR on Plant Defense Are Independent of the SAS Morphology.
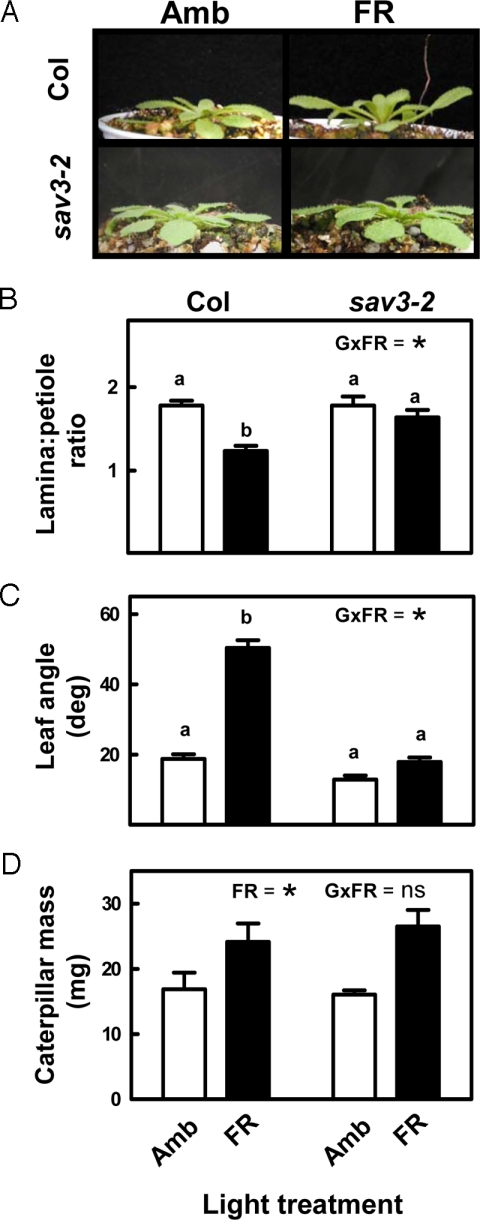
The effects of FR and the phyB mutation improving caterpillar growth could reflect a simple by-product of SAS expression or, alternatively, a specific down-regulation of plant defense caused by the depletion of Pfr. In the first scenario, the reduced investment in defense and increased tissue quality in FR-exposed plants, or phyB mutants (see Fig. 1 and Figs. S1 and S2), could be viewed as an unavoidable consequence of the diversion of resources to SAS responses (17). To address this possibility, we tested the effect of FR on tissue quality in a SAS mutant, sav3–2. This mutant has a WT phenotype in full sun, normal phytochrome content, and normal up-regulation of early SAS genes, such as ATHB-2 by FR, but fails to induce the SAS phenotype because it is impaired in a SAS-specific pathway of auxin biosynthesis (22). Despite the fact that the SAS response was absent in sav3–2 plants exposed to FR radiation (Fig. 2 A–C), the effect of FR improving tissue quality was completely conserved (Fig. 2D). This result demonstrates, unequivocally, that the effect of FR radiation as a down-regulator of defense in Arabidopsis is not merely a by-product of the expression of the morphological component of the SAS.
Fig. 2.
The effects of FR down-regulating plant defense do not require expression of the morphological component of the SAS. (A–C) Effects of FR supplementation on the morphology of Col and sav3–2 plants. The decrease in the lamina-to-petiole length ratio and the increase in leaf angle are typical SAS responses in Arabidopsis; both are missing in sav3–2. (D) Effect of FR supplementation on tissue quality in Col and sav3–2 plants. Amb, ambient light; FR, FR added to ambient light to simulate the effect of neighbor proximity in a canopy of low leaf area index. The significance of the relevant terms of the analysis of variance (FR, FR radiation; G, genotype; G × FR, G-by-FR interaction term) is indicated in each panel. Different letters indicate significant differences between means in cases in which the interaction term was significant. Ns, not significant; * indicates P < 0.05. Thin bars indicate 1 SEM (n = 15 individual plant replicates per genotype and treatment).
FR Down-Regulates the Expression of Induced Defenses in Arabidopsis.
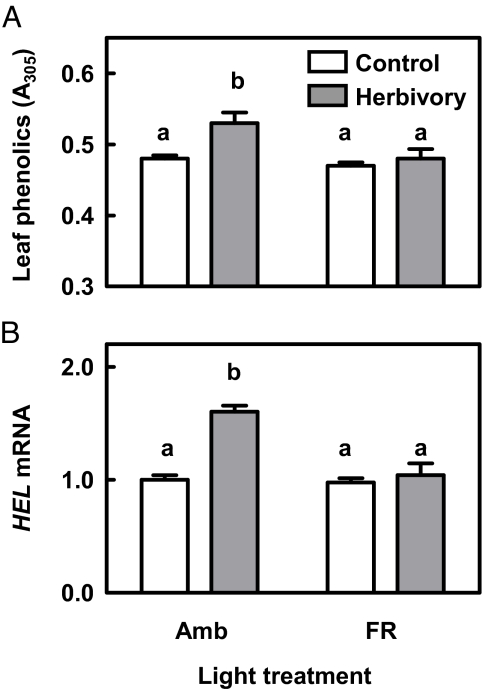
Having rejected the SAS morphology hypothesis, we focused on the effects of FR radiation and phyB on induced defenses. WT (= Col) plants of the “ambient” light treatment responded to S. frugiperda elicitation with increased accumulation of leaf phenolics (Fig. 3A) and expression of defense-related genes, such as HEVEIN-LIKE (HEL) (Fig. 3B) and beta amylase (At4g15210; not shown), among others. All of these induced responses were absent or greatly attenuated when plants were exposed to FR, simulating the proximity of other plants (see Fig. 3 A and B), or in plants of the phyB mutant (Fig. S3).
Fig. 3.
Phytochrome inactivation by FR radiation down-regulates the expression of induced defenses. (A) Effect of FR on the induction of phenolic compounds in response to simulated herbivory (mechanical damage with addition of S. frugiperda oral secretions to the wounds). Soluble phenolic compounds were measured 72 h after elicitation. (B) Effect of FR on the induction of HEL in response to simulated herbivory. The same experiment as in (A) except that HEL transcript abundance was measured as the response variable 7 h after simulated herbivory, and expressed relative to the level detected in the Ambient × Control combination. Amb, ambient light; FR, FR added to ambient light to simulate the effect of neighbor proximity in a canopy of low-leaf area index. Thin bars indicate 1 SEM (n = 4 replicates, see Materials and Methods). The FR × Herbivory interaction is significant in both panels (P < 0.05); different letters indicate significant differences between treatment means. For comparison with the response in the phyB mutant, see Fig. S3.
FR Reduces Plant Sensitivity to Jasmonates.
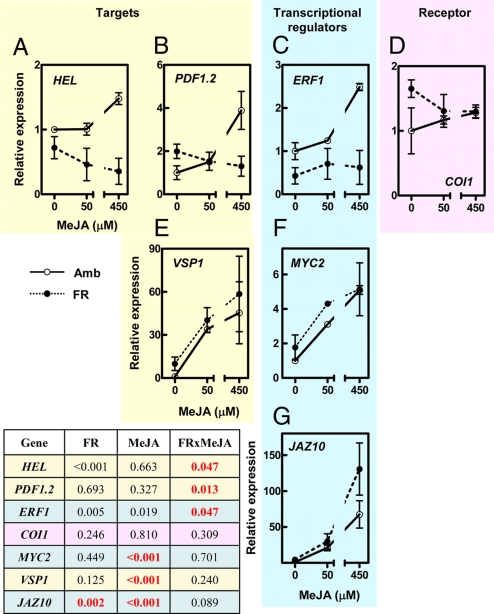
Because HEL and At4g15210 are known to be inducible by jasmonate and related oxylipins (11, 13), and jasmonates are the principal signaling molecules involved in plant defense to chewing insects, we investigated the effects of phytochrome on jasmonate response. In plants of the ambient-light treatment, applications of exogenous methyl jasmonate (MeJA) induced HEL gene-expression responses that were similar to those induced by S. frugiperda (Fig. 4A). In contrast, in plants exposed to FR, simulating the proximity of neighboring plants (see Fig. 4A), or in plants of the phyB mutant (Fig. S4), the effects of MeJA inducing expression of HEL were no longer detectable. Both FR and the phyB mutation also eliminated the response to MeJA of 2 other jasmonate markers: PATHOGEN INDUCIBLE PLANT DEFENSIN (PDF1.2); and ETHYLENE RESPONSE FACTOR1 (ERF1) (Fig. 4 B and C, and Fig. S4). PDF1.2 is typically induced by jasmonates and biotic stress, and ERF1 is a key transcription factor that regulates the expression of HEL and PDF1.2 (11). MeJA treatment induced accumulation of leaf phenolics (resembling the effect of S. frugiperda elicitation), and this response was also abrogated by FR and the phyB mutation (Fig. S5, see Fig. 3A). These results demonstrate that phytochrome inactivation reduces the sensitivity of the tissues to MeJA.
Fig. 4.
Phytochrome inactivation by FR radiation reduces the sensitivity to MeJA elicitation of selected components of the jasmonate response. (A–C) FR reduces the response of HEL, PDF1.2, and their regulator, ERF1. (D) Neither FR nor MeJA affect the expression of COI1. (E and F) The responses of MYC2 and its target VSP1 to MeJA stimulation are not affected by FR. (G) FR up-regulates JAZ10, one of the transcriptional regulators that repress jasmonate responses (for data on other JAZ genes, see Fig. S6). Amb, ambient light; FR, FR added to ambient light to simulate the effect of neighbor proximity in a canopy of low-leaf area index. The inset table shows a summary of the statistical significance of the main effects of FR and MeJA, and the FR × MeJA interaction. Red lettering highlights relevant statistical effects. Expression data are normalized to the expression level detected in the Ambient × Control combination. Thin bars indicate ± SEM (n = 4 replicates, see Materials and Methods). The same patterns of response were observed in samples taken 24 h after elicitation (data not shown).
The Effects of FR Reducing the Sensitivity to Jasmonates Are Restricted to Certain Elements of the Response Pathway.
We tested the interactive effects of FR and MeJA on the expression of additional genes implicated in current models of jasmonate response (23–25). Neither the MeJA treatment nor the FR treatment affected the expression of CORONATINE-INSENSITIVE1 (COI1) (Fig. 4D), the assumed jasmonate receptor (26, 27). As expected, MeJA up-regulated the bHLH (basic helix–loop–helix) MYC2 transcription factor and the MYC2-regulated gene VEGETATIVE STORAGE PROTEIN1 (VSP1). However, these effects of MeJA were not depressed by the FR treatment (Fig. 4 E and F). Genes of the 12 JASMONATE ZIM-domain (JAZ) transcriptional repressors present in the Arabidopsis genome were up-regulated by MeJA, as expected (24). FR did not reduce the expression of these genes; on the contrary, it significantly increased the transcript levels of JAZ10 (Fig. 4G), JAZ8, and JAZ9, and the response of JAZ4 and JAZ7 to MeJA stimulation (for the response of all JAZ genes, see Fig. S6).
These experiments (Fig. 4 and Fig. S6) demonstrate that the jasmonate response of some (but not all) of the components of the jasmonate-signaling cascade is abrogated by FR radiation, the signal that triggers the SAS. This effect of phytochrome inactivation could be mediated by several mechanisms, including direct effects of phytochrome signaling elements on members of the jasmonate response cascade (such as positive and negative transcriptional regulators), and indirect effects involving interaction with other phytochrome-regulated plant hormones (such as gibberellins, ethylene, and salicylic acid). A clear effect of phytochrome inactivation on ERF1 transcription is demonstrated in Fig. 4C and Fig. S4. ERFs have been proposed to be important integrators of diverse signals that modulate plant defense (11, 28), but light regulation of ERF1 expression had not been documented to date. This phytochrome effect might be mediated via ATHB-2, as the ERF1 (but not the MYC2) promoter contains an ATHB-2-binding site motif (29). ATHB-2 is rapidly up-regulated in response to FR radiation and encodes a homeodomain-Leu zipper transcription factor that blocks the transcription of its target genes (30–32). Members of the JAZ family of proteins are key repressors of jasmonate responses associated with defense induction (at least JAZ1 and JAZ3) (23, 24) and growth retardation (at least JAZ10) (25). Their expression is controlled by jasmonates (24), but the regulation of this gene family by light or other ecological factors has not been investigated. Our finding of up-regulation of some JAZ genes by FR (see Fig. 4 and Fig. S6) suggests a possible mechanism of jasmonate desensitization under low R:FR scenarios, particularly in the light of recent evidence indicating that some JAZ10 splice variants are resistant to jasmonate-induced degradation (33). Jasmonate sensitivity was also shown to be impaired in mutants lacking DELLA proteins (34), which are growth repressors whose degradation is promoted by gibberellins (35). Low R:FR ratios and high canopy density promote DELLA degradation (36). Therefore, although the mechanism whereby DELLAs control jasmonate response is still unknown, these previous findings suggest a possible indirect connection between jasmonate sensitivity and phytochrome signaling. Finally, because several of the downstream effectors of jasmonate response (including ERF1) are also controlled by other plant hormones, such as ethylene and salicylic acid (11), and because ethylene (37, 38) and salicylate signaling (39, 40) can be regulated by phytochrome (at least under certain physiological conditions), other points of crosstalk between light and jasmonate signaling are likely to play a role in the desensitization phenomenon that we have documented.
The Interaction between Phytochrome and Jasmonate Signaling Is Functionally Significant for the Effect of FR as an Inhibitor of Plant Defense.
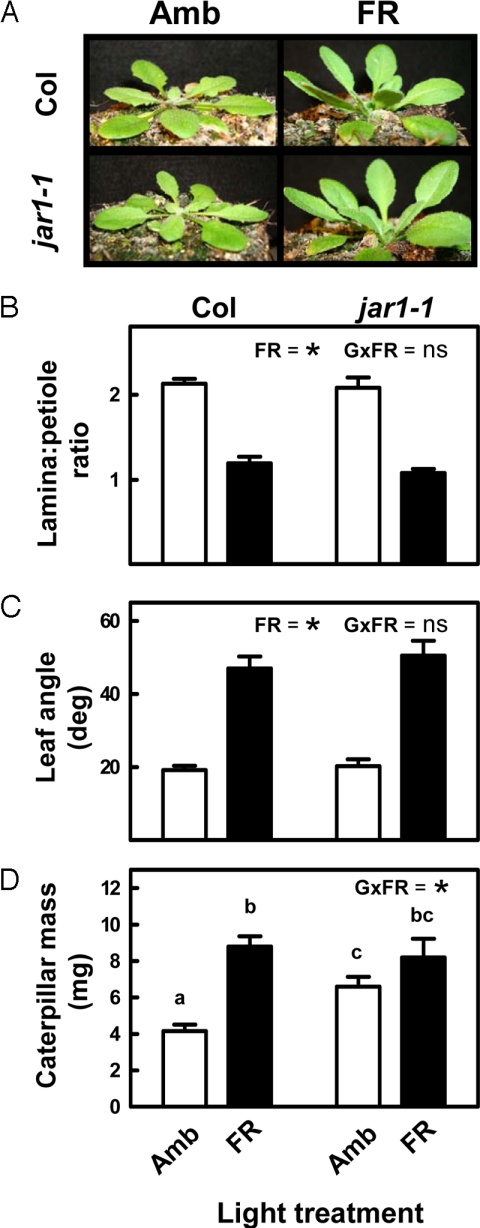
FR radiation induces many changes in plants besides altering the jasmonate response. It is therefore relevant to ask whether the reduced sensitivity to jasmonates is the functional explanation for the reduced expression of plant defense in plants exposed to FR radiation. To address this question, we tested the effect of FR on the jasmonate resistant mutant jar1–1. This mutant fails to activate a normal jasmonate signaling cascade because it is deficient in the enzyme that generates the jasmonyl-isoleucine (JA-Ile) conjugate (41) that engages the COI1 pathway (24). Genetic evidence also suggests that JAR1 is involved in some responses mediated by phytochrome (42, 43). Although these studies suggest a connection to phytochrome A only (and phytochrome A is not involved in the responses to R:FR ratio), we characterized, in addition to the defense phenotype of the mutant, the effects of jar1–1 on the growth phenotype under our simulated competition treatment. Morphologically, jar1–1 plants showed a completely normal FR response, inducing the standard SAS phenotype (Fig. 5 A–C). This observation demonstrates that JAR1 is not required for shade-avoidance responses mediated by B-like phytochromes. In the S. frugiperda bioassays, jar1–1 plants had an impaired defense phenotype, supporting more caterpillar growth than WT plants (Fig. 5D). This result suggests that, as found in wild tobacco (44) and in moth oviposition studies with Arabidopsis (45), JA-Ile conjugation is necessary for full activation of defenses to chewing insects. More importantly, whereas FR had the expected effect of improving tissue quality in WT plants, this effect was significantly attenuated in the jar1–1 mutant (see Fig. 5D). JAR1 expression in WT plants was not affected by the FR treatment (Fig. S7), again indicating that this gene is not controlled by R:FR ratio in light-grown plants. This observation also suggests that FR is unlikely to regulate jasmonate signaling by reducing the availability of bioactive jasmonates. Although this hypothesis needs further confirmation, and various desensitization mechanisms proposed in the previous section must be experimentally tested, the data in Fig. 5D clearly indicate that the effect of FR attenuating plant defense requires a fully functional jasmonate-signaling pathway. Therefore, regardless of the mechanism, this result implies a key role for the interaction between phytochrome and jasmonate signaling in the modulation of defense responses by signals of competition.
Fig. 5.
The effects of FR down-regulating plant defense require a functional JA-Ile cascade. (A–C) Effects of FR supplementation on the morphology of WT (Col) and jar1–1 plants. (D) Effect of FR supplementation on tissue quality in Col and jar1–1 plants. Amb, ambient light; FR, FR added to ambient light to simulate the effect of neighbor proximity in a canopy of low-leaf area index. The significance of the relevant terms of the analysis of variance (FR, FR radiation; G, genotype; G × FR, G-by-FR interaction term) is indicated in each panel. Different letters indicate significant differences between means in cases in which the interaction term was significant. Ns, not significant; * indicates P < 0.05. Thin bars indicate 1 SEM (n = 15 individual plant replicates per genotype and treatment).
Conclusions
Collectively, our findings suggest that, in physiological time, plants solve the dilemma of competition vs. antiherbivore defense allocation by modulating the sensitivity to jasmonates using information on the risk of competition sensed by phytochrome. Previous studies showed that the 2 best characterized plastic responses to low R:FR ratio (SAS and early-flowering) are triggered using different pathways (46). We conclude, based on analysis of the sav3–2 mutants (see Fig. 4), that the effect of phytochrome on plant defense also involves the activation of an SAS-divergent signaling circuit. Phytochrome modulation of induced defenses appears to be a general phenomenon (18, 20, 21), and is likely to be a master control of resource allocation in shade-intolerant plant species (47). Selective desensitization to jasmonates should save plant resources by curtailing investment in defense and, at the same time, avoid the inhibitory effects of jasmonates on cell growth (25). These inhibitory effects could be maladaptive under conditions where the plant has to elongate rapidly to escape shading by its neighbors. Therefore, the phenomenon described in this article may be essential for proper SAS expression, providing a major selective advantage for plants growing in the wild, but could increase vulnerability to insect pests and negatively impact crop yield in high-density plantings typical of modern agriculture.
Materials and Methods
Plant Material.
The Columbia (Col) ecotype of Arabidopsis thaliana was used as the WT control in all experiments. The phyB, jar1–1, and sav3–2 mutants were all in the Col background. Seedlings were generated and grown in a glasshouse, as previously described (45). Plants were maintained in individual pots (0.3 L) on 1:1:1 vermiculite: perlite: peat mixture and watered daily with 1× solution of Hakaphos Rojo 18–18-18 (Compo, Spain). Plants were grown under short day conditions (≈10/14 h light/dark cycles); daily temperatures fluctuated between 9 and 28 °C. Peak levels of photosynthetically active radiation (PAR) were 1,000 μmol m−2 s−1. Morphological measurements used to evaluate expression of the SAS phenotype (leaf angle from the horizontal plane, lamina:petiole length ratio) were obtained on the youngest fully expanded leaf of each plant, typically at the start of the tissue quality bioassays (see below).
Light Treatments.
For light treatments, 5-week-old Arabidopsis plants were placed in front of banks of incandescent lamps covered with either opaque screens (ambient treatment) or FR-transmitting filters (FR treatment). The experimental setup for light treatments was as described previously (18). The individual plants were randomly located in front of the light sources and used as replicates for growth- and tissue-quality evaluations. The FR treatment simulated the effect of neighbor proximity in a canopy of leaf area index = 0.5 (R:FR of horizontal radiation = 0.55), where mutual shading among neighbors is negligible (48). Neither air temperature nor the level of PAR received by the plants were affected by the FR treatment. Plants were exposed to the light treatments for 4 days before they were used in the different experiments (simulated herbivory, MeJA elicitation) or for evaluation of SAS expression and measurements of tissue quality in bioassays.
Plant Density Treatment.
In the crowding experiments, 1-week-old plants grown in individual pots were grouped to form canopies of either 288 or 615 plants m−2 (low- and high-density treatments, respectively). There were 4 replicate canopies (27 × 27 cm) for each density and genotype (WT and phyB), which were randomly distributed along the greenhouse bench and used as replicates in the statistical analyses. Plants were grown for 5 weeks at the indicated density before they were used for evaluation of SAS expression and measurements of tissue quality in bioassays. Only the plants located at the center of each canopy were used in the evaluations.
Tissue Quality.
Because plant defense is a complex trait and cannot be evaluated using measurements of a single compound or morphological attribute, we used insect growth bioassays to obtain a meaningful evaluation of the defense phenotype expressed by the plants of the various genotypes and treatments. Bioassays were based on the growth of Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) caterpillars. Three neonates were placed on each plant. Caterpillar growth was measured after 7 to 9 days of feeding. We used insect mass increase as a measure of insect performance because it is strongly correlated with potential fecundity (49). The S. frugiperda colony was established from eggs collected near Proimi (Tucumán, Argentina), and maintained in our laboratory on an artificial diet (50). All bioassays were replicated 3 or more times using independent cohorts of plants and insects.
Simulated Herbivory and MeJA Treatments.
Induced defenses were elicited using treatments of simulated herbivory (wounding plus S. frugiperda oral secretions) or MeJA. Typically, the plants used for the experiments were 5 weeks old and had 14 to 18 leaves. To simulate the effects of herbivory, 1 leaf per plant (the youngest fully-expanded) was damaged with a razor blade (a single 6- to 7-mm cut parallel to the mid-vein). In plants of the herbivory treatment, 3.5 μl of diluted (10%) S. frugiperda regurgitate were applied to the wound immediately after the damage. Plants of the control treatment received 3.5 μl of distilled water. Regurgitate was collected from third to fifth instar larvae fed on an artificial diet, centrifuged, and stored at –80 °C until dilution and application. Chemical induction was performed by dipping 5-week-old plants in an aqueous solution containing 0-, 50-, or 450-μM MeJA (Sigma) for 5 seconds. Although the MeJA treatments were effective in inducing typical phenolic and gene expression responses, none of the doses caused visible growth inhibition in these greenhouse plants grown at high PAR. Plants were harvested at different times after the induction treatments and immediately frozen in liquid nitrogen for analysis of gene expression or leaf chemistry.
Leaf Chemistry and Gene Expression.
Determination of soluble leaf phenolics was carried out using the techniques described by Izaguirre et al. (18). Transcript levels were evaluated by means of quantitative real-time PCR. Total RNA was extracted from 100 mg of plant tissue as described previously (51). A fraction of purified RNA was DNA-decontaminated with a RQ1 RNase-Free DNase treatment according to manufacturer's instructions (Promega). For cDNA production, 1 μg of total RNA were mixed with 0.5 μg of oligo dT. The mix was denatured at 70 °C for 5 min and rapidly chilled on ice. M-MLV 5× Reaction Buffer, dNTPs 10 mM, 200 units of M-MLV RT (Promega), and nuclease-free water were finally added to a final volume of 20 μl. The reaction was performed at 42 °C for 60 min, and it was stopped by heating at 70 °C for 15 min. The resulting cDNA was subsequently used as DNA template for amplification of specific genes using real-time PCR. Primers were designed using the Primer Express 1.5 software (Applied Biosystems). PCR reactions were carried out in a 7500 PCR Real System (Applied Biosystems) with SYBR Green Master Mix (Applied Biosystems) using the primers listed below at a final concentration of 3.6 μM, and 1.0 μl of 50% (vol/vol) cDNA as template. PCR-cycling conditions consisted of an initial polymerase activation step at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. Expression detected for β-actin-8 (At1g49240) gene of Arabidopsis, with universal actin forward primer 5′-AGTGGTCGTACAACCGGTATTGT-3′ and specific reverse primers 5′-GAGGATAGCATGTGGAACTGAGAA-3′, was used as internal standard to normalize small differences in template amounts. Gene-specific primers are listed in Table S1. Real-time dsDNA amplification was monitored and analyzed using the Sequence Detector 1.3 program (Applied Biosystems). CT values were normalized for differences using the β-actin-8 CT values. Normalized transcript levels of the genes tested were compared between treatments and the fold-change in expression level was calculated. Gene expression results are based on 4 independent replicates. Each replicate was obtained by pooling tissue from 4 to 5 individual plants.
Statistics and Analysis.
Treatment effects and interactions between factors (light × herbivory; light × MeJA, and so forth) were evaluated by means of analysis of variance using INFOSTAT (InfoStat/Profesional Version 1.1). Appropriate transformations of the primary data were used to meet the assumptions of the analysis.
Supplementary Material
Acknowledgments.
We thank Miriam Izaguirre, Carlos Mazza, Patricia Demkura, and Mercedes Keller for discussions, Amy Austin for many helpful comments on the manuscript, Miriam Cargnel and Eduardo Virla for technical assistance and the Arabidopsis Biological Resource Center for provision of seed stocks. Supported by grants from the Agencia Nacional de Promoción Científica y Tecnológica and Universidad de Buenos Aires (to C.L.B.), and grants from the Howard Hughes Medical Institute and National Institutes of Health (to J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0900701106/DCSupplemental.
References
- 1.Karban R. Plant behaviour and communication. Ecol Lett. 2008;11:727–739. doi: 10.1111/j.1461-0248.2008.01183.x. [DOI] [PubMed] [Google Scholar]
- 2.Aphalo PJ, Ballaré CL. On the importance of information-acquiring systems in plant-plant interactions. Funct Ecol. 1995;9:5–14. [Google Scholar]
- 3.Trewavas A. Aspects of plant intelligence. Ann Bot. 2003;92:1–20. doi: 10.1093/aob/mcg101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ballaré CL, Scopel AL, Sánchez RA. Far-red radiation reflected from adjacent leaves: an early signal of competition in plant canopies. Science. 1990;247:329–332. doi: 10.1126/science.247.4940.329. [DOI] [PubMed] [Google Scholar]
- 5.Smith H. Phytochromes and light signal perception by plants-an emerging synthesis. Nature. 2000;407:585–591. doi: 10.1038/35036500. [DOI] [PubMed] [Google Scholar]
- 6.Ballaré CL. Keeping up with the neighbours: phytochrome sensing and other signalling mechanisms. Trends Plants Sci. 1999;4:97–102. doi: 10.1016/s1360-1385(99)01383-7. [DOI] [PubMed] [Google Scholar]
- 7.Dorn LA, Pyle EH, Schmitt J. Plasticity to light cues and resources in Arabidopsis thaliana: testing for adaptive value and costs. Evolution. 2000;54:1982–1994. doi: 10.1111/j.0014-3820.2000.tb01242.x. [DOI] [PubMed] [Google Scholar]
- 8.Bell DL, Galloway LF. Population differentiation for plasticity to light in an annual herb: Adaptation and cost. Am J Bot. 2008;95:59–65. doi: 10.3732/ajb.95.1.59. [DOI] [PubMed] [Google Scholar]
- 9.Mithöfer A, Boland W. Recognition of herbivory-associated molecular patterns. Plant Physiol. 2008;146:825–831. doi: 10.1104/pp.107.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farmer EE, Ryan CA. Octadecanoid precursors of jasmonic acid activate the synthesis of wound-inducible proteinase inhibitors. Plant Cell. 1992;4:129–134. doi: 10.1105/tpc.4.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorenzo O, Solano R. Molecular players regulating the jasmonate signalling network. Curr Opin Plant Biol. 2005;8:532–540. doi: 10.1016/j.pbi.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Reymond P, Weber H, Damond M, Farmer EE. Differential gene expression in response to mechanical wounding and insect feeding in Arabidopsis. Plant Cell. 2000;12:707–719. doi: 10.1105/tpc.12.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Vos M, et al. Signal signature and transcriptome changes of Arabidopsis during pathogen and insect attack. Mol Plant Microb Interactions. 2005;18:923–937. doi: 10.1094/MPMI-18-0923. [DOI] [PubMed] [Google Scholar]
- 14.Walling LL. The myriad plant responses to herbivores. J Plant Growth Regul. 2000;19:195–216. doi: 10.1007/s003440000026. [DOI] [PubMed] [Google Scholar]
- 15.Howe GA, Jander G. Plant immunity to insect herbivores. Annu Rev Plant Biol. 2008;59:41–66. doi: 10.1146/annurev.arplant.59.032607.092825. [DOI] [PubMed] [Google Scholar]
- 16.Baldwin IT. Jasmonate induced responses are costly but benefit plants under attack in native populations. Proc Natl Acad Sci USA. 1998;95:8113–8118. doi: 10.1073/pnas.95.14.8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cipollini D. Stretching the limits of plasticity: can a plant defend against both competitors and herbivores? Ecology. 2004;85:28–37. [Google Scholar]
- 18.Izaguirre MM, Mazza CA, Biondini M, Baldwin IT, Ballaré CL. Remote sensing of future competitors: impacts on plant defenses. Proc Natl Acad Sci USA. 2006;103:7170–7174. doi: 10.1073/pnas.0509805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herms DA, Mattson WJ. The dilemma of plants: to grow or defend. Q Rev Biol. 1992;67:283–335. [Google Scholar]
- 20.Kurashige NS, Agrawal AA. Phenotypic plasticity to light competition and herbivory in Chenopodium album (Chenopodiaceae) Am J Bot. 2005;92:21–26. doi: 10.3732/ajb.92.1.21. [DOI] [PubMed] [Google Scholar]
- 21.McGuire R, Agrawal AA. Trade-offs between the shade-avoidance response and plant resistance to herbivores? Tests with mutant Cucumis sativus. Funct Ecol. 2005;19:1025–1031. [Google Scholar]
- 22.Tao Y, et al. Rapid synthesis of auxin via a new tryptophan-dependent pathway is required for shade avoidance in plants. Cell. 2008;133:164–176. doi: 10.1016/j.cell.2008.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chini A, et al. The JAZ family of repressors is the missing link in jasmonate signalling. Nature. 2007;448:666–671. doi: 10.1038/nature06006. [DOI] [PubMed] [Google Scholar]
- 24.Thines B, et al. JAZ repressor proteins are targets of the SCFCOI1 complex during jasmonate signalling. Nature. 2007;448:661–665. doi: 10.1038/nature05960. [DOI] [PubMed] [Google Scholar]
- 25.Yan Y, et al. A downstream mediator in the growth repression limb of the jasmonate pathway. Plant Cell. 2007;19:2470–2483. doi: 10.1105/tpc.107.050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katsir L, Chung HS, Koo AJK, Howe GA. Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol. 2008;11:428–435. doi: 10.1016/j.pbi.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chico JM, Chini A, Fonseca S, Solano R. JAZ repressors set the rhythm in jasmonate signaling. Curr Opin Plant Biol. 2008;11:486–494. doi: 10.1016/j.pbi.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 28.Brown RL, Kazan K, McGrath KC, Maclean DJ, Manners JM. A role for the GCC-box in jasmonate-mediated activation of the PDF1.2 gene of Arabidopsis. Plant Physiol. 2003;132:1020–1032. doi: 10.1104/pp.102.017814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Davuluri RV, et al. Arabidopsis gene regulatory information server, an information resource of Arabidopsis cis-regulatory elements and transcription factors. BMC Bioinformatics. 2003;4:25. doi: 10.1186/1471-2105-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jiao Y, Lau OS, Deng XW. Light-regulated transcriptional networks in higher plants. Nat Rev Genet. 2007;8:217–230. doi: 10.1038/nrg2049. [DOI] [PubMed] [Google Scholar]
- 31.Steindler C, et al. Shade avoidance responses are mediated by the ATHB-2 HD-zip protein, a negative regulator of gene expression. Development. 1999;126:4235–4245. doi: 10.1242/dev.126.19.4235. [DOI] [PubMed] [Google Scholar]
- 32.Franklin KA, et al. Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiol. 2003;131:1340–1346. doi: 10.1104/pp.102.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chung HS, Howe GA. A critical role for the TIFY motif in repression of jasmonate signaling by a stabilized splice variant of the JASMONATE ZIM-domain protein JAZ10 in Arabidopsis. Plant Cell. 2009 doi: 10.1105/tpc.108.064095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro L, et al. DELLAs control plant immune responses by modulating the balance of jasmonic acid and salicylic acid signaling. Curr Biol. 2008;18:650–655. doi: 10.1016/j.cub.2008.03.060. [DOI] [PubMed] [Google Scholar]
- 35.Hirano K, Ueguchi-Tanaka M, Matsuoka M. GID1-mediated gibberellin signaling in plants. Trends Plants Sci. 2008;13:192–199. doi: 10.1016/j.tplants.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Djakovic-Petrovic T, de Wit M, Voesenek LACJ, Pierik R. DELLA protein function in growth responses to canopy signals. Plant J. 2007;51:117–126. doi: 10.1111/j.1365-313X.2007.03122.x. [DOI] [PubMed] [Google Scholar]
- 37.Finlayson SA, Lee I-J, Morgan PW. Phytochrome B and the regulation of circadian ethylene production in Sorghum. Plant Physiol. 1998;116:17–25. doi: 10.1104/pp.119.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pierik R, Visser EJW, De Kroon H, Voesenek LACJ. Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ. 2003;26:1229–1234. [Google Scholar]
- 39.Genoud T, Buchala AJ, Chua N-H, Metraux J-P. Phytochrome signalling modulates the SA-perceptive pathway in Arabidopsis. Plant J. 2002;31:87–95. doi: 10.1046/j.1365-313x.2002.01338.x. [DOI] [PubMed] [Google Scholar]
- 40.Griebel T, Zeier J. Light regulation and daytime dependency of inducible plant defenses in Arabidopsis: phytochrome signaling controls systemic acquired resistance rather than local defense. Plant Physiol. 2008;147:790–801. doi: 10.1104/pp.108.119503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staswick PE, Tiryaki I. The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell. 2004;16:2117–2127. doi: 10.1105/tpc.104.023549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hsieh H-L, et al. FIN219, an auxin-regulated gene, defines a link between phytochrome A and the downstream regulator COP1 in light control of Arabidopsis development. Genes Dev. 2000;14:1958–1970. [PMC free article] [PubMed] [Google Scholar]
- 43.Riemann M, Riemann M, Takano M. Rice JASMONATE RESISTANT 1 is involved in phytochrome and jasmonate signalling. Plant Cell Environ. 2008;31:783–792. doi: 10.1111/j.1365-3040.2008.01790.x. [DOI] [PubMed] [Google Scholar]
- 44.Kang J-H, Wang L, Giri A, Baldwin IT. Silencing threonine deaminase and JAR4 in Nicotiana attenuata impairs jasmonic acid-isoleucine-mediated defenses against Manduca sexta. Plant Cell. 2006;18:3303–3320. doi: 10.1105/tpc.106.041103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Caputo C, Rutitzky M, Ballaré CL. Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia. 2006;149:81–90. doi: 10.1007/s00442-006-0422-3. [DOI] [PubMed] [Google Scholar]
- 46.Cerdán PD, Chory J. Regulation of flowering time by light quality. Nature. 2003;423:881–885. doi: 10.1038/nature01636. [DOI] [PubMed] [Google Scholar]
- 47.Ballaré CL. Illuminated behavior. Phytochrome as a key regulator of light foraging and plant anti-herbivore defense. Plant Cell Environ. 2009 doi: 10.1111/j.1365-3040.2009.01958.x. [DOI] [PubMed] [Google Scholar]
- 48.Ballaré CL, Sánchez RA, Scopel AL, Casal JJ, Ghersa CM. Early detection of neighbour plants by phytochrome perception of spectral changes in reflected sunlight. Plant Cell Environ. 1987;10:551–557. [Google Scholar]
- 49.Klingenberg C, Spence J. On the role of body size for life-history evolution. Ecol Entomol. 1997;22:55–68. [Google Scholar]
- 50.Murúa MG, Defagó V, Virla E. Evaluación de cuatro dietas artificiales para la cría experimental de Spodoptera frugiperda (Lep., Noctuidae) destinada a mantener poblaciones experimentales de himenópteros parasitoides. Plagas. 2003;29:43–52. [Google Scholar]
- 51.Izaguirre MM, Scopel AL, Baldwin IT, Ballaré CL. Convergent responses to stress. Solar ultraviolet-B radiation and Manduca sexta herbivory elicit overlapping transcriptional responses in field-grown plants of Nicotiana longiflora. Plant Physiol. 2003;132:1755–1767. doi: 10.1104/pp.103.024323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.