Abstract
Similar selective pressures can lead to independent origin of similar morphological structures in multiple evolutionary lineages. Developmental mechanisms underlying convergent evolution remain poorly understood. In this report, we show that similar sex comb morphology in closely related Drosophila species is produced by different cellular mechanisms. The sex comb is a recently evolved, male-specific array of modified bristles derived from transverse bristle rows found on the first thoracic legs in both sexes. “Longitudinal” sex combs oriented along the proximo-distal leg axis evolved independently in several Drosophila lineages. We show that in some of these lineages, sex combs originate as one or several transverse bristle rows that subsequently rotate 90° and align to form a single longitudinal row. In other species, bristle cells that make up the sex combs arise in their final longitudinal orientation. Thus, sex combs can develop through either sex-specific patterning of bristle precursor cells or male-specific morphogenesis of sexually monomorphic precursors. Surprisingly, the two mechanisms produce nearly identical morphology in some species. Phylogenetic analysis shows that each of these mechanisms has probably evolved repeatedly in different Drosophila lineages, suggesting that selection can recruit different cellular processes to produce similar functional solutions.
Keywords: Convergent evolution, Morphogenesis, Sexual dimorphism
Morphological traits under strong selection can undergo rapid diversification and can display wide disparity among closely related species. Because such traits evolve on relatively short time scales and reflect independent modifications of shared ancestral states, they provide powerful comparative models for addressing two key questions in evolutionary developmental biology: namely, what genetic and developmental changes underlie rapidly evolving phenotypes, and whether common genetic and developmental mechanisms are responsible for the evolution of similar traits in different lineages.
In recent years, an increasing number of studies have taken advantage of such models to characterize genetic and developmental changes associated with independent evolution of similar morphologies (1–8). An emerging picture from these studies is that evolution of similar features among closely related species is often caused by changes in the same developmental pathways. These findings suggest that genetic changes in response to selection may follow the “path of least resistance”—in other words, that mutations in some genetic pathways provide the most rapid or effective response to a particular selective pressure (9). There are, however, counterexamples in which similar traits arose through different developmental mechanisms (10–14).
In this report, we examine developmental mechanisms underlying the morphological diversification of Drosophila sex combs. The sex comb is a male-specific array of modified mechanosensory bristles found on the prothoracic leg of some Drosophila species. This structure is a recent evolutionary innovation restricted to the melanogaster and obscura species groups (subgenus Sophophora). Outside of this lineage, males and females have identical leg bristle patterns. Sex combs play important and diverse roles in courtship and mating (15–17), suggesting that their evolution was driven by sexual selection. As a result, closely related species can differ dramatically in sex comb morphology (8, 18, 19). The size of the sex comb ranges from only two “teeth” at the distal end of the proximal tarsal segment (ta1) to more than 50 teeth arrayed along the entire length of the first and second tarsal segments (ta1 and ta2), while the teeth themselves show major differences in size, shape, and color [supporting information (SI) Fig. S1].
Perhaps the most intriguing feature of sex comb morphology involves their orientation. Some species have “transverse” sex combs arranged in several rows perpendicular to the proximo-distal axis of the leg, whereas in other species the sex combs are “longitudinal,” that is, oriented along the proximo-distal leg axis (Fig. S1). Phylogenetic analysis suggests that longitudinal sex combs evolved repeatedly in multiple independent lineages (8). This system provides an excellent opportunity to examine the developmental changes underlying evolutionary divergence and convergence of a rapidly evolving morphological structure.
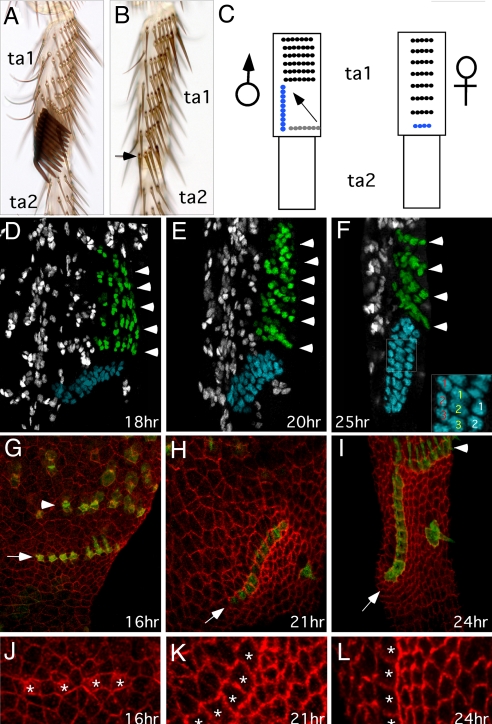
D. melanogaster has a single longitudinal sex comb on the distal ta1 (Fig. 1A), which develops by male-specific modification of precursor bristles present in both sexes. Bristles on the distal tibia and ta1 of the prothoracic leg are arranged into regular transverse bristle rows (TBRs) (Figs. 1A and 1B). During metamorphosis, the most distal TBR in males rotates 90° from an initial transverse to the final longitudinal position (20, 21) (Fig. 1C). In females, the homologous distal-most TBR remains in place. The number of bristles in this TBR is also sexually dimorphic. In males, the sex comb typically contains nine to 11 teeth, whereas the homologous female TBR has only three to five bristles (Figs. 1A and 1B) (20).
Fig. 1.
Sex comb morphogenesis in D. melanogaster. Distal is down and ventral is to the right in all panels. (A, B) First tarsal segment (ta1) of the male and female prothoracic leg, respectively. Arrow in (B) indicates the most distal TBR, which is homologous to the male sex comb. (C) Schematic diagram showing the rotation of sex comb from the initial transverse position (gray) to its final longitudinal position. The corresponding leg segments in the female are also shown. The sex comb and the homologous TBR are shown in blue. (D–F) α-Cut immunostaining of the male ta1 during pupal development. Each bristle develops from a single progenitor cell, which gives rise to the shaft, socket, neuron, and sheath cells. α-Cut antibody labels all cells in all developing sensory organs. The sex comb and TBRs are pseudocolored in blue and green, respectively. (D) At 18 hours AP, the presumptive sex comb is found in a transverse orientation, slightly offset anteriorly from the other TBRs. Arrowheads point to individual TBRs. (E) At 20 hours AP, the sex comb is rotated halfway. (F) By 25 hours AP, the sex comb has reached its final longitudinal position. Inset shows an enlarged view of the boxed area. Cells comprising each bristle are indicated by the same number (1–3). Font colors indicate the shaft (yellow), socket (white), and neuron or sheath (red) cells. (G–I) α-Fmi immunostaining (red) of the ventral distal ta1 in neur-Gal4; UAS-ActGFP (green) males. Arrows indicate the sex comb. Only the socket cells and some shaft cells are visible in the focal planes shown. The central bristle located ventrally to the sex comb (H, I) is derived from the same TBR as the sex comb, and is “left behind” during its rotation (21). (J–L) Enlarged areas of (G–I). Asterisks mark sex comb teeth. (G, J) At 16 hours AP, cells distal and proximal to the sex comb have similar shapes. (H, K) At 21 hours AP, cells distal to the rotating sex comb are smaller than other epithelial cells. Cells proximal to the sex comb are elongated in the direction of sex comb rotation. Anti-phosphoHistone3 staining indicates that size and shape differences between the proximal and distal cells are not due to localized or oriented cell division (data not shown). (I, L) At 24 hours AP, cells that used to be distal, and are now ventral to the sex comb, are flattened against the rotated sex comb. Protruding shafts are visible in some bristles.
To elucidate the developmental basis of morphological convergence, we compared sex comb morphogenesis in several Drosophila species that may have evolved longitudinal sex combs independently (Fig. S1). Aside from the similarity in orientation, these species differ extensively in the size and position of sex combs. Our results reveal that longitudinal sex combs can develop by two distinct mechanisms, a male-specific epithelial movement or sex-specific patterning of bristle precursor cells. Surprisingly, both processes can generate similar sex comb morphologies. Phylogenetic analysis suggests that one or both developmental mechanisms evolved more than once. Thus, Drosophila sex combs present one of the few known cases where different developmental mechanisms produce similar morphological traits among closely related species.
Results
Sex Comb Morphogenesis in D. melanogaster.
The sex comb of D. melanogaster rotates 90° from an initial transverse to the final longitudinal position between 16 and 24 hours AP (20, 21) (Fig. 1C). To characterize this movement in more detail, we labeled bristle cells with an antibody against the transcription factor Cut. Each bristle consists of four cells (shaft, socket, neuron, and sheath) derived from a single sensory organ precursor (SOP) by asymmetric divisions (22, 23). Cut is expressed in all SOPs and their daughter cells (24). At 18 hours AP, shortly after adult leg epidermis detaches from the pupal cuticle, the future sex comb and TBRs are visible as parallel rows of Cut-positive cells perpendicular to the proximo-distal leg axis (Fig. 1D). The SOPs have gone through at least one round of division as strings of Cut-positive cells, representing single bristles, contain two to three cells (Fig. 1D). The sex comb contains nine to 10 bristles, compared with three to five bristles in the distal-most TBR in females (not shown). At 20 hours AP, the sex comb has rotated ≈45° from its original position, and almost all bristles contain three or four Cut-positive cells (Fig. 1E). By 25 hours, rotation is complete and the sex comb assumes its final longitudinal orientation, while the more proximal rows of bristles remain transverse (Fig. 1F).
In principle, sex comb rotation could occur either by invasive migration of bristle cells through the surrounding epithelium or by rearrangement of the entire epithelial sheet around the sex comb. To distinguish between these mechanisms, we examined changes in cell shapes in and around the sex comb. Cell shapes were visualized using an antibody against the transmembrane protein Flamingo (25), while bristle cells were marked with Actin-GFP fusion protein (see Materials and Methods). We focused on the bristle socket cells, as they lie within the plane of epidermis and are in contact with the surrounding epidermal cells, whereas the shaft cells are subepidermal. At 16 hours AP, the sex comb socket cells and the surrounding epidermal cells have largely uniform shape and size (Figs. 1G and 1J). The socket cells of the future sex combs and TBRs are separated by one or two epidermal cells at this stage. At 21 hours AP, epidermal cells immediately distal to the sex comb are smaller than surrounding cells and appear compressed against the socket cells (Figs. 1H and 1K). In contrast, epidermal cells just proximal to the sex comb are elongated in the direction of sex comb rotation. At this stage, the socket cells of the sex comb and TBRs have assumed rectangular shapes and are packed into tight rows with no epidermal cells in between. Bristle cells comprising the sex comb do not show any changes in shape, such as directional actin-based projections, that might be indicative of invasive migration (26, 27) (Figs. 1H and 1K). At 24 hours AP, when sex comb rotation is complete, epidermal cells that used to be distal and proximal to the sex comb maintain their distinctive shapes (Figs. 1I and 1L). This pattern of cell shape changes suggests that sex comb rotation is caused by a coordinated epithelial movement and not by the migration of individual bristle cells.
Double Oblique Sex Combs in Distant Lineages Develop by a Common Mechanism.
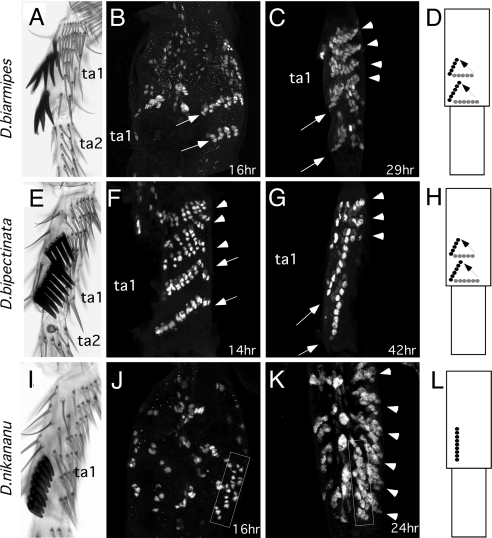
D. biarmipes and D. bipectinata belong to different lineages in the melanogaster species group but have similar sex comb morphologies (Fig. 2 and Fig. S1). Both species have sex combs in ta1 consisting of two oblique rows of bristles (Figs. 2A and 2E). α-Cut staining at early developmental stages shows that sex combs in both species arise as two separate distal-most TBRs on ta1, parallel to the more proximal TBRs (Figs. 2B and 2F). These sex combs rotate in unison by ≈70–80° to reach their final oblique positions (Fig. 2C and 2G). These observations show that, despite the distant phylogenetic relationship of these species, their sex combs develop by a similar mechanism.
Fig. 2.
Morphogenesis of small and medium sex combs. (A–D) D. biarmipes. (E–H) D. bipectinata. (I–L) D. nikananu. (A, E, I) First and second tarsal segments (ta1, ta2) of the male prothoracic leg of each species. (B, C, F, G, J, K) α-Cut immunostaining of pupal male legs. At early stages, presumptive sex combs of D. biarmipes and D. bipectinata are found as two transverse bristle rows (arrows in B and F). At later stages (C, G), the sex combs of both species have reached their final oblique positions (arrows). Arrowheads indicate proximal TBRs. (J) Immediately after pupal apolysis, the presumptive sex comb of D. nikananu is already found as a loose row of bristle cells arranged along the proximo-distal leg axis (box). TBRs are not yet visible at this stage. (K) By 24 hours AP, sex comb teeth in this species are aligned in a single longitudinal row (box), and TBRs are forming on the ventral and proximal sides of the sex comb (arrowheads). (D, H, L) Schematic diagrams of sex comb morphogenesis showing the initial (gray) and final (black) positions in each species.
Longitudinal Sex Combs in the rhopaloa and obscura Lineages Develop by Rotation.
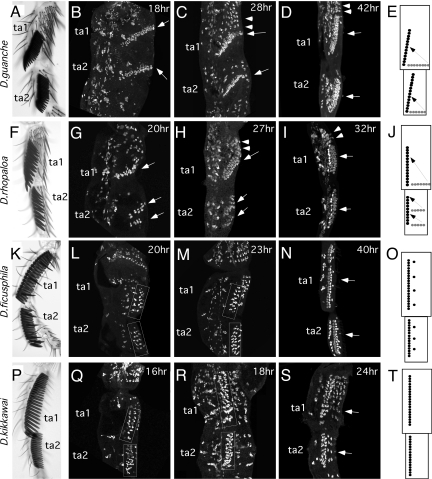
Some Drosophila species have longitudinal sex combs on ta1 and ta2 occupying almost the entire length of each segment. This type of sex comb is found in the montium, ficusphila, and rhopaloa subgroups of the melanogaster species group and in the obscura species group (Fig. S1, Figs. 3A, 3F, 3K, and 3P). Because these groups are distantly related, we tested whether their sex combs develop in a similar fashion and whether they use the same developmental mechanism as the species with smaller longitudinal sex combs.
Fig. 3.
Morphogenesis of large sex combs. (A–E) D. guanche, (F–J) D. rhopaloa, (K–O) D. ficusphila, (P–T) D. kikkawai. (A, F, K, P) First and second tarsal segments (ta1, ta2) of the adult legs of each species. (B–D, G–I, L–N, Q–S) α-Cut immunostaining of pupal male legs. (B) At 18 hours AP, sex combs of D. guanche are found as single transverse rows on ta1 and ta2 and are already slightly rotated (arrows). Each row contains the final number of sex comb teeth for that segment. (C) By 28 hours AP, the sex combs are rotated halfway and TBRs are beginning to appear proximally in ta1 (arrowheads). (D) By 42 hours AP, the sex combs have reached their final angle (arrows). (G) At 20 hours AP in D. rhopaloa, there is one transverse row of presumptive sex comb teeth in ta1 and two rows in ta2 (arrows). Most sex comb SOPs have gone through one cell division. (H) By 27 hours AP, all three rows have rotated partially, and proximal TBRs are visible (arrowheads). (I) By 32 hours AP, the sex comb in ta1 is in its final longitudinal position, and the two rows in ta2 have aligned to form a single longitudinal sex comb. (L, Q) At early developmental stages in D. ficusphila and D. kikkawai, sex comb bristles are found in loose longitudinal strips in ta1 and ta2 (boxes). Each bristle precursor contains only one or two cells. (M, R) Sex comb precursors (boxed) have divided further so that most bristles consist of three cells. (N, S) At later stages, sex comb bristles become organized more tightly into straight longitudinal rows (arrows). D. ficusphila also has several isolated sex comb teeth ventral to the main row (N, O). (E, J, O, T) Schematic diagrams of sex comb morphogenesis in each species.
Males of D. guanche (obscura species group) have sex combs with more than 20 teeth occupying the distal half to three quarters of each segment; the more proximal portion of each segment carries two to four TBRs (Fig. 3A). In contrast, females have more TBRs, but the distal-most TBRs in ta1 and ta2 contain only two to four bristles (not shown). We find that immediately after pupal–adult apolysis (18 hours AP), each presumptive sex comb is visible as a single long transverse row of Cut-positive cells at the distal end of ta1 and ta2 (Fig. 3B). At 28 hours AP, both sex combs are undergoing rotation (Fig. 3C), reaching their final angle by 42 hours AP (Fig. 3D). Thus, the ta1 and ta2 sex combs of D. guanche develop in a similar fashion, and each sex comb arises by rotation of a single distal-most TBR that has a greatly expanded number of SOPs relative to the homologous TBR in females.
In D. rhopaloa (rhopaloa subgroup), the ta1 sex comb develops from a single distal-most TBR composed of 10–12 SOPs (Fig. 3G). In contrast, the ta2 sex comb arises as two separate TBRs containing four to six SOPs each (Fig. 3G). At 27 hours AP, all three rows are rotated 50–60° from their initial position (Fig. 3H). By 32 hours AP, the short ta2 TBRs align precisely and merge to form a single longitudinal sex comb (Fig. 3I). The single ta1 TBR also completes its rotation to align along the proximo-distal leg axis (Fig. 3I). Thus, although both sex combs in D. rhopaloa develop by rotation of distal TBRs, recruitment of bristles and tissue rotation differ between the two tarsal segments. Similar to D. guanche, the pattern of bristle precursors in D. rhopaloa is sexually dimorphic, with males having fewer TBRs but more bristles in the distal TBRs. The ta1 sex comb in particular has 12–15 teeth, whereas the homologous female TBR has only three or four bristles (not shown).
Longitudinal Sex Combs in the montium and ficusphila Subgroups Develop Without Rotation.
SOPs visualized immediately after pupal–adult apolysis in males of D. ficusphila (ficusphila subgroup) and D. kikkawai (montium subgroup) are not arranged in transverse rows (Figs. 3L, 3Q). Instead, bristle precursors corresponding to the future sex comb are arrayed in a longitudinal strip running along the entire length of the ta1 and ta2 segments (Figs. 3L and 3Q). Within this strip, SOPs are not yet arranged into a single, tightly packed row as seen in adults but appear as a loose row two bristles wide. SOPs have divided no more than once by this time, indicating that they are still at an early stage of differentiation (Figs. 3L and 3Q). Later in development (23–40 hours AP), the presumptive sex combs retain their longitudinal orientation as SOPs go through further divisions and undergo a local rearrangement to form a narrower, single bristle-wide row (Figs. 3M, 3N, 3R, and 3S). This rearrangement coincides with leg elongation, which is thought to occur by interdigitation of epidermal cells from circumferential to proximo-distal positions (28). This interdigitation presumably explains the lining up of the bristles in the sex comb, suggesting that sex comb development in D. ficusphila and D. kikkawai does not require any special morphogenetic movements. Thus, differences between males and females in these species are due to sexually dimorphic specification of bristle precursors, rather than to sex-specific morphogenesis. We refer to such sex combs as “prespecified.”
In principle, the sex combs of D. ficusphila and D. kikkawai could arise as TBRs but rotate much earlier than in other species (before 18 hours AP). However, this is unlikely, as leg epidermis detaches from the pupal cuticle only shortly before this time, and it is difficult to imagine how epithelial movement could occur while cells are attached to the overlying cuticle. To confirm that sex comb development in these species does not involve tissue rotation, we examined cell shape changes during sex comb morphogenesis in D. kikkawai starting immediately after pupal–adult apolysis. In D. melanogaster, sex comb rotation is accompanied by characteristic changes in the shape of epidermal cells flanking the sex comb (Fig. 1G–3I). In contrast, in D. kikkawai, epidermal cells surrounding the sex comb maintain similar shapes from the earliest stage until sex comb maturation, and there is no visible difference between the cells on the ventral and dorsal sides of the sex comb until the alignment is complete (Fig. S2). Thus, there is nothing to indicate that the sex comb had rotated at an earlier time.
Morphogenesis of Melanogaster-Like Sex Combs in the montium Subgroup Without Rotation.
Most species of the montium subgroup have large longitudinal sex combs similar to that of D. kikkawai. One of the few exceptions is found in D. nikananu, which represents a derived lineage in the montium subgroup and has a small sex comb restricted to the distal ta1, similar to D. melanogaster (Fig. S1, Fig. 2I). Immediately after pupal-adult apolysis (16 hours AP), the sex comb of D. nikananu already has a longitudinal orientation similar to D. kikkawai (Fig. 2J). Thus, sex comb development in this species occurs by sex-specific patterning of SOPs and does not involve tissue rotation.
Independent Origin of Similar Developmental Mechanisms.
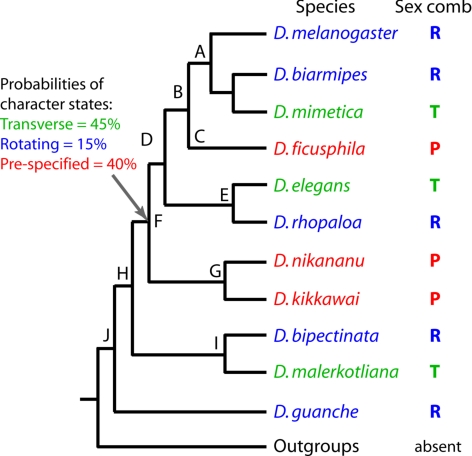
Our results show that longitudinal sex combs use different developmental mechanisms, which involve either sex-specific patterning of SOPs or sex-specific morphogenetic movement. Phylogenetic relationships in the melanogaster and obscura species groups (8) indicate that species with similar modes of sex comb development do not cluster together, suggesting that one or both mechanisms have evolved more than once (Fig. 4). We can envision two general scenarios of sex comb evolution. First, the last common ancestor of the melanogaster species group may have had a transverse sex comb. This scenario would require two independent origins of prespecified sex combs (nodes C and G in Fig. 4) and several origins of rotating sex combs. Alternatively, that last common ancestor may have had a longitudinal sex comb. This would involve several independent transitions between rotating and prespecified sex combs, as well as multiple origins of transverse sex combs. The first scenario can best be described as convergent morphological evolution, whereas the second implies multiple instances of developmental system drift (preservation of an ancestral phenotype despite the divergence of underlying developmental pathways) (29). Despite the different hypotheses of morphological change, both scenarios involve independent origin and loss of particular modes of development over short evolutionary distances.
Fig. 4.
Alternative scenarios of sex comb evolution. Only the species included in this study are shown. Probabilities of ancestral character states at Node F were determined by Bayesian estimation (see SI). T, transverse sex combs; R, rotating sex combs; P, prespecified sex combs. All nodes have posterior probabilities of 100% based on combined sequences of 14 loci (8).
Bayesian character reconstruction indicates that the two scenarios are equally likely, and the ancestral mode of development has been obscured by the large number of subsequent transitions (see SI Text). However, some evolutionary changes are unambiguously convergent. For example, the sex combs of D. melanogaster and D. nikananu present a case of convergent morphological evolution through different developmental mechanisms. Similarly, the rotating sex combs of D. biarmipes and D. bipectinata have evolved independently, inasmuch as the last common ancestor of the ananassae species subgroup (node I in Fig. 4), of which D. bipectinata is a derived lineage, had a transverse sex comb (M. Matsuda, C.-S. Ng, M. Doi, A.K., and Y. Tobari, unpublished data). Thus, any plausible scenario of evolution involves convergent changes in both morphology and development.
Discussion
Evolution of Similar Sex Combs by Distinct Developmental Mechanisms.
We have identified two different modes of sex comb development. In D. guanche, D. biarmipes, D. bipectinata, D. rhopaloa, and D. melanogaster, longitudinal sex combs are formed by rotation of one or several bristle rows (TBRs) that initially arise perpendicular to the proximo-distal leg axis. In contrast, in D. ficusphila, D. kikkawai, and D. nikananu, bristle precursors that give rise to the sex comb are specified close to their final longitudinal position. These two modes of development must be based on different molecular changes. Coordinated epithelial movement in species with rotating sex combs is likely to require sex-specific regulation of cytoskeletal and cell adhesion molecules. On the other hand, development of prespecified sex combs probably involves changes in the early spatial patterning of SOPs by proneural genes (31, 32). Despite these fundamental differences, the two mechanisms can generate very similar adult structures, for example in D. kikkawai and D. rhopaloa, or in D. nikananu and D. melanogaster. These findings suggest that selection can recruit distinct developmental pathways to meet similar functional requirements in different species.
Not only can different mechanisms produce similar morphology, but one or both of these mechanisms have evolved independently in two or more separate lineages. Similarities in the adult appearance between rotating and prespecified sex combs may reflect either convergent morphological evolution or developmental system drift (29). However, any conceivable scenario of sex comb evolution requires multiple transitions between transverse, rotating, and prespecified modes of development, demonstrating that complex developmental pathways can be highly plastic on short evolutionary time scales.
Alternative modes of sex comb development raise intriguing questions about homology at different levels of biological organization. Sex combs of all species are homologous as morphological structures, in the sense that the last common ancestor of the obscura and melanogaster species groups had a sex comb. However, the bristles that make up the sex comb in species with rotating vs. prespecified sex combs are not derived by descent with modification from the same ancestral cell population, as they arise in different parts of the leg.
Distinct cellular mechanisms contrast with a recurrent type of molecular change. Evolution of both rotating and prespecified sex combs is associated with sexually dimorphic expression of the HOX gene Sex comb reduced (Scr) during their development (8). On the other hand, species with transverse sex combs, or no sex combs, have sexually monomorphic Scr expression. Phylogenetic analysis shows that sex-specific expression of Scr has been gained and lost multiple times, correlating with the gain and loss of longitudinal sex combs (8). Scr is an essential upstream regulator of sex comb development that affects both SOP patterning and subsequent morphogenesis (8, 33, 34). It is generally accepted that homologies at genetic and morphological levels are dissociable, so that homologous genes can control the development of non-homologous structures and vice versa (35, 36). In the case of sex comb evolution, we observe a close association between the morphological and (upstream) genetic levels, whereas the intermediate tier of biological organization, i.e., cell differentiation, shows drastic divergence.
Developmental Complexity and the Mechanisms of Phenotypic Evolution.
The finding that different developmental mechanisms are responsible for the evolution of similar sex comb morphologies can be contrasted with other systems where changes in the same genetic pathways are associated with independent evolution of similar traits in different species (2, 3, 5–7, 37). This contrast may be attributed to the fact that most previous studies examined either loss or reduction of characters (3, 5, 37) or gain of simple traits such as pigmentation (6, 7). Indeed, cases in which different ontogenetic processes underlie similar adult traits tend to involve complex features such as organ size and shape. A classical example comes from the evolution of elongated body shape in burrowing salamanders. In some species, this phenotype is caused by the lengthening of individual vertebrae, whereas in others the same outward appearance is produced by an increased number of vertebrae (12, 38). In Drosophila, differences in wing size are caused largely by changes in cell number in some populations, whereas differences in cell size make a larger contribution in others (11, 39). Floral morphology in legumes (13) and vulval development in nematodes (10) offer further examples in which similar adult structures are produced despite major differences in development. The greater the developmental complexity of a trait, the more diverse the options for altering the phenotype. Evolution of Drosophila sex combs shows that even on short evolutionary time scales, different developmental processes can be modified by selection to give rise to similar morphological structures.
Materials and Methods
White prepupae were collected, sexed by the presence or absence of testes, and aged at 25 °C to desired stages. Developmental timing was determined empirically for each species. Pupae were removed from puparia, and the dorsal half and the posterior third of the body were cut away in insect saline solution (156 mM NaCl, 6.9 mM KCl, 8.2 mM CaCl2, 4.0 mM MgCl2). Remaining tissues were fixed for 30 minutes in 4% formaldehyde in the fix buffer (0.1 M Pipes pH = 6.9, 1 mM EGTA pH = 7.0, 2 mM MgCl, 1% Triton X-100), followed by two washes in the wash buffer (50 mM Tris pH = 6.8, 150 mM NaCl, 0.5% Nonidet P-40, 1 mg/ml bovine serum albumin [BSA]). Further dissections were carried out in the wash buffer. For the early stages, prothoracic legs were cut off at the femur/tibia boundary and left inside the pupal cuticle. For the later stages, legs were removed from the pupal cuticle by first tearing the cuticle around the dorsal femur/tibia boundary and then pulling out the tibial and tarsal segments. Samples were blocked in the block buffer (50 mM Tris pH = 6.8, 150 mM NaCl, 0.5% Nonidet P-40, 5 mg/ml BSA) for 1 hour to overnight at 4 °C and incubated in the primary antibodies for 1 or 2 nights at 4 °C. Legs were then washed in the wash buffer four times and incubated in the secondary antibodies overnight at 4 °C. The primary antibodies used were mouse anti-Cut 2B10 (1:10; Developmental Study Hybridoma Bank) (30) and mouse anti-Flamingo (25) (1:10; Developmental Study Hybridoma Bank). The secondary antibodies were AlexaFluor 488 and 594 used at 1:200 (Invitrogen). In D. melanogaster, bristle precursor cells were also visualized using GFP-tagged actin expressed in neur-Gal4A101/UAS-Act5C:GFP pupae. Stained samples were washed four times in the wash buffer and mounted in Fluoromount 50 (SouthernBiotech).
Fluorescent images were taken on Olympus FluoView 1000 confocal microscope. Images were processed using Image J software and Adobe Photoshop. At least eight individuals were examined per species per stage. To assemble leg images, autofluorescence from the cuticle and trachea was removed from each confocal section, and image stacks were processed through Z-series projection using Image J.
Bayesian reconstruction of character evolution was performed as described in SI Text using the existing phylogenetic framework (8).
Supplementary Material
Acknowledgments.
We thank H. Takamori and the Tucson and Bloomington stock centers for Drosophila strains; the Developmental Studies Hybridoma Bank for antibodies; and Nicolas Gompel, Lewis Held, Theresa Orenic, Benjamin Prud'homme, and two anonymous reviewers for comments on the manuscript. This work was supported by National Science Foundation grant DEB-0548991 (to A.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807875106/DCSupplemental.
References
- 1.Mundy NI, Badcock NS, Hart T, Scribner K, Janssen K, Nadeau NJ. Conserved genetic basis of a quantitative plumage trait involved in mate choice. Science. 2004;303:1870–1873. doi: 10.1126/science.1093834. [DOI] [PubMed] [Google Scholar]
- 2.Abouheif E, Wray GA. Evolution of the gene network underlying wing polyphenism in ants. Science. 2002;297:249–252. doi: 10.1126/science.1071468. [DOI] [PubMed] [Google Scholar]
- 3.Sucena E, Delon I, Jones I, Payre F, Stern DL. Regulatory evolution of shavenbaby/ovo underlies multiple cases of morphological parallelism. Nature. 2003;424:935–938. doi: 10.1038/nature01768. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro MD, Marks ME, Peichel CL, Blackman BK, Nereng KS, et al. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428:717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- 5.Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, et al. Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet. 2006;38:107–111. doi: 10.1038/ng1700. [DOI] [PubMed] [Google Scholar]
- 6.Prud'homme B, Gompel N, Rokas A, Kassner VA, Williams TM, et al. Repeated morphological evolution through cis-regulatory changes in a pleiotropic gene. Nature. 2006;440:1050–1053. doi: 10.1038/nature04597. [DOI] [PubMed] [Google Scholar]
- 7.Hoekstra HE, Hirschmann RJ, Bundey RJ, Insel P, Crossland J.P. A single amino acid mutation contributes to adaptive color pattern in beach mice. Science. 2006;313:101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- 8.Barmina O, Kopp A. Sex-specific expression of a HOX gene associated with rapid morphological evolution. Dev Biol. 2007;311:277–286. doi: 10.1016/j.ydbio.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 9.Philippe N, Crozat E, Lenski RE, Schneider D. Evolution of global regulatory networks during a long-term experiment with Escherichia coli. Bioessays. 2007;29:846–860. doi: 10.1002/bies.20629. [DOI] [PubMed] [Google Scholar]
- 10.Kiontke K, Barriere A, Kolotuev I, Podbilewicz B, Sommer R, et al. Trends, stasis, and drift in the evolution of nematode vulva development. Curr Biol. 2007;17:1925–1937. doi: 10.1016/j.cub.2007.10.061. [DOI] [PubMed] [Google Scholar]
- 11.Zwaan BJ, Azevedo RB, James AC, Van 't Land J, Partridge L . Cellular basis of wing size variation in Drosophila melanogaster: A comparison of latitudinal clines on two continents. Heredity. 2000;84:338–347. doi: 10.1046/j.1365-2540.2000.00677.x. [DOI] [PubMed] [Google Scholar]
- 12.Parra-Olea G, Wake DB. Extreme morphological and ecological homoplasy in tropical salamanders. Proc Natl Acad Sci USA. 2001;98:7888–7891. doi: 10.1073/pnas.131203598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucker SC. Floral evolution, development and convergence: The hierarchical-significance hypothesis. Int J Plant Sci. 2003;158:s143–s161. [Google Scholar]
- 14.Moczek AP, Cruickshank TE, Shelby A. When ontogeny reveals what phylogeny hides: Gain and loss of horns during development and evolution of horned beetles. Evolution. 2006;60:2329–2341. [PubMed] [Google Scholar]
- 15.Ng C, Kopp AK. Sex combs are important for male mating success in Drosophila melanogaster. Behav Genet. 2008;38:195–201. doi: 10.1007/s10519-008-9190-7. [DOI] [PubMed] [Google Scholar]
- 16.Spieth HT. Mating behavior within the genus Drosophila (Diptera) Bull Am Mus Nat Hist. 1952;99:395–474. [Google Scholar]
- 17.Cook R. Behavioral role of the sexcombs in Drosophila melanogaster and Drosophila simulans. Behav Genet. 1977;7:349–357. doi: 10.1007/BF01077448. [DOI] [PubMed] [Google Scholar]
- 18.Kopp A, True JR. Evolution of male sexual characters in the oriental Drosophila melanogaster species group. Evol Dev. 2002;4:278–291. doi: 10.1046/j.1525-142x.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- 19.Kopp A, Barmina O. Evolutionary history of the Drosophila bipectinata species complex. Genet Res. 2005;85:23–46. doi: 10.1017/s0016672305007317. [DOI] [PubMed] [Google Scholar]
- 20.Tokunaga C. Cell lineage and differentiation on the male foreleg of Drosophila melanogaster. Dev Biol. 1962;4:489–516. doi: 10.1016/0012-1606(62)90054-4. [DOI] [PubMed] [Google Scholar]
- 21.Held LI, Jr, Grimson MJ, Du Z . Proving an old prediction: The sex comb rotates at 16 to 24 hours after pupariation. Dros Inf Serv. 2004;87:76–78. [Google Scholar]
- 22.Lai EC, Orgogozo V. A hidden program in Drosophila peripheral neurogenesis revealed: Fundamental principles underlying sensory organ diversity. Dev Biol. 2004;269:1–17. doi: 10.1016/j.ydbio.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Bodmer R, Carretto R, Jan YN. Neurogenesis of the peripheral nervous system in Drosophila embryos: DNA replication patterns and cell lineages. Neuron. 1989;3:21–32. doi: 10.1016/0896-6273(89)90112-8. [DOI] [PubMed] [Google Scholar]
- 24.Blochlinger K., Jan LY., Jan YN . Postembryonic patterns of expression of cut, a locus regulating sensory oragn identity in Drosophila. Development. 1993;117:441–450. doi: 10.1242/dev.117.2.441. [DOI] [PubMed] [Google Scholar]
- 25.Usui T, Shima Y, Shimada Y, Hirano S, Burgess RW, et al. Flamingo, a seven-pass transmembrane cadherin, regulates planar cell polarity under the control of Frizzled. Cell. 1999;98:585–595. doi: 10.1016/s0092-8674(00)80046-x. [DOI] [PubMed] [Google Scholar]
- 26.Kaltschmidt JA, Lawrence N, Morel V, Balayo T, Fernandez BG, et al. Planar polarity and actin dynamics in the epidermis of Drosophila. Nat Cell Biol. 2002;4:937–944. doi: 10.1038/ncb882. [DOI] [PubMed] [Google Scholar]
- 27.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nature Cell Biol. 2002;4:715–719. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- 28.Held LI., Jr A high-resolution morphogenetic map of the second-leg basitarsus in Drosophila melanogaster. Roux Arch. 1979;187:129–150. doi: 10.1007/BF00848267. [DOI] [PubMed] [Google Scholar]
- 29.True JR, Haag ES. Developmental system drift and flexibility in evolutionary trajectories. Evol Dev. 2001;3:109–119. doi: 10.1046/j.1525-142x.2001.003002109.x. [DOI] [PubMed] [Google Scholar]
- 30.Blochlinger K, Bodmer R, Jan LY, Jan YN. Patterns of expression of cut, a protein required for external sensory organ development in wild-type and cut mutant Drosophila embryos. Genes Dev. 1990;4:1322–1331. doi: 10.1101/gad.4.8.1322. [DOI] [PubMed] [Google Scholar]
- 31.Orenic TV, Held LI, Jr, Paddock SW, Carroll SB. The spatial organization of epidermal structures: Hairy establishes the geometrical pattern of Drosophila leg bristles by delimiting the domains of achaete expression. Development. 1993;118:9–20. doi: 10.1242/dev.118.1.9. [DOI] [PubMed] [Google Scholar]
- 32.Joshi M, Buchanan K, Shroff S, Orenic TV. Delta and Hairy establish a periodic prepattern that positions sensory bristles in Drosophila legs. Dev Biol. 2006;293:64–76. doi: 10.1016/j.ydbio.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Shroff S, Joshi M, Orenic TV. Differential Delta expression underlies the diversity of sensory organ patterns among the legs of the Drosophila adult. Mech Dev. 2007;124:43–58. doi: 10.1016/j.mod.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 34.Randsholt NB, Santamaria P. How Drosophila change their combs: The Hox gene sex combs reduced and sex comb variation among Sophophora species. Evol Dev. 2008;10:121–133. doi: 10.1111/j.1525-142X.2008.00219.x. [DOI] [PubMed] [Google Scholar]
- 35.Abouheif E, Akam M, Dickinson WJ, Holland PW, Meyer A, et al. Homology and developmental genes. Trends Genet. 1997;13:432–433. doi: 10.1016/s0168-9525(97)01271-7. [DOI] [PubMed] [Google Scholar]
- 36.Hall BK. Descent with modification: The unity underlying homology and homoplasy as seen through an analysis of development and evolution. Biol Rev Camb Philos Soc. 2003;78:409–433. doi: 10.1017/s1464793102006097. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro MD, Bell MA, Kingsley DM. Parallel genetic origins of pelvic reduction in vertebrates. Proc Natl Acad Sci USA. 2006;103:13753–13758. doi: 10.1073/pnas.0604706103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wake DB. Homoplasy: The result of natural selection, or evidence of design limitations? Am Naturalist. 1991;138:543–567. [Google Scholar]
- 39.James AC, Azevedo RB, Partridge L. Genetic and environmental responses to temperature of Drosophila melanogaster from a latitudinal cline. Genetics. 1997;146:881–890. doi: 10.1093/genetics/146.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.